Abstract
Paracatenula galateia sp. nov. is a mouthless marine catenulid platyhelminth with bacterial intracellular endosymbionts. The worms live in shallow back-reef sands in the Belize Barrier Reef system and are distinguished from the four previously described members of the genus by their large size combined with a ribbon-shaped body and characteristic bipartite inclusions in cells, which are interpreted as sperm. The bacteria are presumed to be sulphur-oxidizing chemoautotrophs. They are found in bacteriocytes which fill the body region (‘trophosome region’) posterior to the brain, whereas the anterior part of the worm (rostrum) is bacteria-free. Phalloidin staining reveals a delicate system of subepitheliar circular and longitudinal muscles and dorsoventral fibres. The serotonergic nervous system consists of a brain at the base of the rostrum and longitudinal fibres extending both anteriorly and posteriorly, the latter being concentrated in a structure called the ‘dorsal cord’.
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
Introduction
Catenulida are an order of small, free-living Platyhelminthes (‘Turbellaria’, Tyler et al. Citation2006–2010). Originally thought to occur exclusively in freshwater except for the questionable Tyrrheniella sigillata (Riedl, Citation1959), they were first reported from marine sandy bottoms by Sterrer (Citation1966). Sterrer & Rieger (Citation1974) described nine marine species from NE and W Atlantic coasts, belonging to two new genera (Retronectes and Paracatenula) for which they erected the family Retronectidae, named for their ability to swim backward by reversing the ciliary beat. Usually found only as isolated, fragile specimens, and difficult to identify for their paucity of consistent morphometric features, retronectids are characterized by an often polylithophorous statocyst (which may be lacking in some species or specimens), and a simple, anterio-dorsally opening mixed gonad. Reproductive biology is as yet unresolved: oocytes were observed in only one, and a mature egg in another species of Retronectes of at least 50 specimens studied (Sterrer & Rieger 1974). A small proportion of specimens contained cells with distinctly shaped inclusions that have been interpreted as spermatozoa and their nuclei; spermatids contain ciliary rudiments (Rieger Citation1978). In Paracatenula, distinct gonads are apparently lacking; in fact, no oocytes or eggs have ever been found. Instead, cells containing characteristic rod-, ribbon-, banana- or even spicule-shaped inclusions were found along a strand of tissue named ‘dorsal cord’ (Sterrer & Rieger 1974), which extends throughout the body in median position. These inclusions often clustered behind the brain (in a vesicula seminalis?), where a dorsal pore to the outside may be located.
The peculiar retronectid gonad with its large, oocyte-like sperm prompted Sterrer & Rieger (1974) to suggest ‘a completely new mode of reproduction – such as parthenogenesis from sperm’ along the transformation of male cells into oocytes described by Borkott (Citation1970) for the freshwater catenulid Stenostomum; see also Schuchert & Rieger (Citation1990) – and further ‘underlines the isolated position of the Catenulida within the Turbellaria and the Platyhelminthes’ (Rieger 1978). Recent multilocus phylogenetic studies corroborate the placement of the genus Paracatenula within the Catenulida and also the status of the Catenulida as the basal taxon of Platyhelminthes (Larsson & Jondelius Citation2008).
While members of Retronectes have a mouth, ciliated pharynx and gut lumen, those of Paracatenula were described to lack mouth, pharynx and gut lumen; rather, the tissue filling most of the body volume consists of large turgescent cells containing ‘granular bodies’. These have been identified as intracellular bacteria in P. erato Sterrer and Rieger, 1974 (Ott et al. Citation1982). Subsequent investigations have shown that this is the case for all members of the genus Paracatenula (own unpublished observations). The bacteria are presumably symbiotic sulphur-oxidizers, since the bacteria from two Paracatenula species have been shown to possess a reverse dissimilatory sulphite reductase (DsrAB) gene, an important gene in bacterial sulphur metabolism, which clustered with the sequences of other sulphur-oxidizing bacteria (Loy et al. Citation2009). In addition, the bacteria contain highly refractive granules, appearing white in incident light, which resemble the elemental sulphur storage granules known from many sulphur-oxidizing bacteria (Pasteris et al. Citation2001). Members of Paracatenula have a symbiont-free anterior body region (rostrum) and a posterior body region filled by symbiont-containing cells (bacteriocytes), which together form a distinct organ, which we call ‘trophosome’ in analogy to the symbiont-containing organ in the vestimentiferan annelids.
Only three more species of Retronectidae have been described since Sterrer & Rieger's (1974) original nine: one new genus, Myoretronectes paranensis Noreña-Jansson & Faubel, Citation1996 from Argentina, and two species of Retronectes: R. sterreri Faubel, Citation1976 from the North Sea and R. atypica Doe & Rieger, Citation1977 from North Carolina. During investigations of the meiofauna in back-reef sediments of the Mesoamerican barrier reef system at Belize, a large, conspicuous new species was found to be common in subtidal sands close to the field station of the US National Museum of Natural History (Washington, DC) on the island of Carrie Bow Cay (Rützler & Macintyre Citation1982). This species is currently subject to intensive studies of its biology and ecology. Here we present the description of this species new to science.
Materials and methods
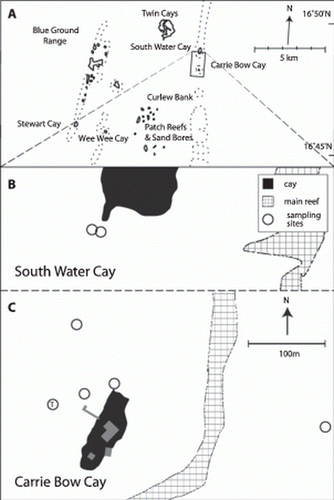
Specimens were collected between 2007 and 2010 at several locations in the vicinity of the field station of the US National Museum of Natural History (Washington, DC) on the island of Carrie Bow Cay (). The worms were extracted by shaking sand samples in seawater and pouring the supernatant through a 32-µm pore-size mesh. Individual specimens were picked by hand and inspected using a dissecting microscope. Squeeze preparations of live animals were analysed using bright field and phase contrast microscopy at 16–1000× magnification. Digital photomicrographs were collected and used for measurements with the analysis tool of Adobe Photoshop CS5.
Figure 1. A. Map of the southern part of the Belize Barrier Reef. B and C. Sample locations in the vicinity of Carrie Bow Cay where Paracatenula galateia sp. nov. was found. T, type location.
Fluorescent staining of whole mounts
Musculature in whole mounts of individual worms was made visible by staining F-actin with fluorescently labelled phalloidin (Alexa Fluor 568; Invitrogen, Austria); the nervous system by staining of serotonergic nerves with an anti-serotonin antiserum produced in rabbit (Sigma Aldrich, Austria) followed by a staining with an Alexa Fluor 568-conjugated secondary antibody. Freshly collected specimens were first relaxed in a magnesium chloride solution isotonic to seawater, fixed for 12 h in 4% (w/v) formaldehyde at 4°C, rinsed in phosphate-buffered saline (PBS) and stored in PBS at 4°C (for phalloidin staining) or in methanol at −20°C (for serotonin staining). For phalloidin staining the specimens were washed and permeabilized in PBS with 0.2% (v/v) Triton X-100 (PBS-T) and then stained for 30 min with phalloidin-Alexa 568 diluted 1:200 in PBS-T. After three washes in PBS and a DAPI staining for 5 min, the specimens were mounted on slides in Vecta shield (Vector Laboratories). For antibody staining the fixed animals were transferred into PBS-T and digested for 6 min at room temperature in 0.1 mg/ml proteinase K in PBS-T. Digestion was stopped by adding 2 N hydrochloric acid. Animals were then washed in PBS-T and blocked in BSA-T (bovine serum albumin (BSA) plus 0.2% Triton X-100) for 30 min. Serotonin was stained with the anti-serotonin antiserum 1:2000 diluted in BSA-T overnight at 4°C. After three washing steps in PBS-T, an Alexa 547 conjugated secondary antibody was applied for 1 h at room temperature at 1:300 dilution in BSA-T. DAPI staining and mounting was done as described above. Slides were either scanned with a confocal laser-scanning microscope (Zeiss LSM 510) or examined and photographed with an epifluorescence microscope (Zeiss Axio Imager).
DNA extraction, PCR amplification and sequencing
DNA was extracted from ten worms using the Blood and Tissue DNA extraction kit (Qiagen, Germany) and 2 µl of each extraction was used as PCR template. Fragments of the 18S and 28S rRNA gene (1750 and 1350 nt long, respectively) were amplified for each worm by PCR with the general eukaryotic primers 1f (5′-CTGGTTGATYCTGCCAGT-3′) and 2023r (5′-GGTTCACCTACGGAAACC-3′) for 18S (Pradillon et al. Citation2007) and the Primers D1a (5′-CCC(C/G)CGTAA(T/C)TTAAGCATAT-3′) and D5b2 (5′-CGCCAGTTCTGCTTACC-3′) initially developed for Arthropoda for 28S (von Reumont et al. Citation2009). Cycling conditions for both genes were: 94°C for 3 min followed by 40 cycles of 94°C for 45 s, 49°C for 30 s, 72°C for 1 min, and a final elongation step of 72°C for 10 min. The PCR products obtained were purified using the MinElute PCR purification kit (Qiagen) and directly sequenced with the PCR primers. All sequences were deposited in Genbank, accession numbers HQ231330–HQ231344.
rRNA genes-based phylogenetic analysis
18S and 28S rRNA gene data sets were constructed with our sequences and selected Catenulida sequences available in Genbank. The 18S and 28S rRNA gene data sets were separately aligned using MAFFT Q-INS-I, which considers the secondary structure of RNA (Katoh et al. Citation2005). The 5′ and 3′ ends of both alignments were trimmed, final length of alignments were 1816 nt (18S) and 1676 nt (28S). The nucleotide sequence identity between individuals was calculated based on these alignments using Geneious 5 (Drummond et al. Citation2010). The alignments were concatenated and we reconstructed the phylogenies using maximum likelihood- (PHYML at the phylogeny.fr web service; Guindon & Gascuel Citation2003; Dereeper et al. Citation2008) and Bayesian inference-based (MrBayes; Ronquist & Huelsenbeck Citation2003) algorithms. Substitution models for both genes were evaluated using MrModeltest 2.3 (Nylander Citation2008). The GTR + I+G model was chosen using the Akaike information criterion. MrBayes was run for five Mio generations using four chains. Convergence was evaluated by plotting the generations versus logL and the burn-in was set to two Mio generations. Node stability was evaluated using posterior probabilities (pp, Bayesian inference) and aLRT (maximum likelihood; Anisimova & Gascuel Citation2006; Guindon et al. Citation2010). Sequences of Macrostomida served as out-group.
Taxonomy
Paracatenula galateia sp. nov.
Material
Of more than 100 individuals from back-reef sediments at a variety of sampling sites in the vicinity of Carrie Bow Cay (Belize) (see ), 19 adults were studied live in squeeze preparation, six by serial semi-thin sections, and about 10 each as whole mounts stained with phalloidin and serotonin antibody, respectively.
Type specimens
Holotype: 1 specimen fixed in formaldehyde 4% and mounted on a microscope slide embedded in glycerol; USNM 1154145.
Paratype: 2 specimens fixed in Bouin's fluid in separate vials; USNM 1154146 and 1154147.
Type locality
Carrie Bow Cay, Belize Barrier Reef (16°48′10.50′′N, 88°04′56.30′′W). Subtidal sand in approximately 1 m water depth, 50 m west of the island in front of the pier ().
Etymology
Galateia refers to the silky, milky white appearance of the worm under incident light. In Greek mythology this is an attribute of the skin of the nymph Galateia (Greek Γαλατϵíα, ‘the milky white’).
Diagnosis
Ribbon-shaped Paracatenula up to 6 mm long, with or without a statocyst. Inclusions bipartite, with conical to ladyfinger-shaped parts, 12–19 µm long and 3 µm wide.
External appearance
Paracatenula galateia is a ribbon-shaped worm up to 6 mm long (A, 3). The length of the animals is extremely variable because the fragile worms tend to break, probably due to the extraction process. Only rarely are individuals found that are apparently complete and show an undamaged posterior end. Many worms show constrictions or an irregular outline (C). The width of the trophosome region, which makes up the largest part of the animal, is 225–315 µm (271±30.6 µm, n=19); its dorsoventral height, however, is much smaller, and difficult to measure or even estimate in living worms. Fixed specimens have a width/height ratio of approximately 4.6–5.5. The trophosome is pinkish-white in incident light and has a characteristic silky appearance (A). The transparent dorsal cord appears to divide the trophosome into two parts along the midline ( and A).
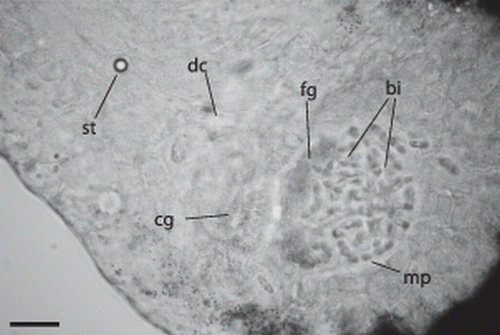
Figure 2. Paracatenula galateia sp. nov. A. Habitus of live specimens. B. Drawings of bipartite inclusions showing the conical to ladyfinger-shaped bodies (cb) connected by a short strand (ss) and surrounded by a dense matrix (dm). C. Morphology and organization of the rostrum showing the ciliated epidermis (ci), the monolithophorous statocyst (st), the dorsal cord (dc), the trophosome (tr), the dorsal opening (do) with surrounding gland cells with coarse granules (cg) adjacent to the muscular pouch (mp) which is filled with bipartite inclusions (bi) and has gland cells with fine granules (fg). The positions where measurements of width (arrowheads) and length (bars) were taken are indicated. Scale bars indicate 250 µm (A), 10 µm (B) and 50 µm (C).
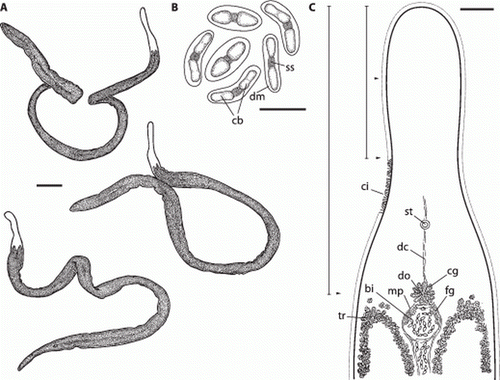
Figure 3. Paracatenula galateia sp. nov. Micrographs of live specimens. A. Incident light showing smooth silky appearance of trophosome. B. Transmitted light showing characteristic shape of extended rostrum and dorsal chord. C. Specimen with irregular outline and constriction. D. Small (juvenile?) specimen. Note position of the statocysts in inserts to B and D. A–D at same scale, bar indicates 100 µm.
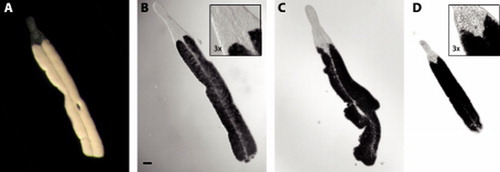
The rostrum is 330–460 µm (398±37.5 µm, n=19) long and has a characteristic shape. The anterior part is cylindrical or club-shaped, with a rounded tip (A,C and 3). Its diameter is 100–176 µm (129±17.7 µm, n=19). The posterior part is conical and widens to match the width of the trophosome region. This widening begins at about 60% of the length of the rostrum, where it has its minimum diameter of 94–145 µm (119±14.9 µm, n=19). In 5 of 19 specimens studied in detail, the rostrum contained a monolithophorous statocyst () of 12.8±0.63 µm in diameter; the diameter of the statolith was 6.5±0.43 µm. In small (juvenile?) worms the statocyst lies close to the brain (D), which nestles between the anterior tips of the two trophosome parts, whereas in large (adult?) animals it is in a more forward position (B).
Anatomy
A cross-section in the trophosome region () shows a thin (3.7–4.7 µm thick), ciliated epidermis. The nuclei of the epidermis cells are sunk into the underlying muscle layer that consists of fine longitudinal and even finer circular muscle fibres (A,B). Numerous dorsoventral muscle fibres run through the body. The ‘dorsal cord’ is a muscular strand that also contains the major longitudinal nerves. The remainder of the body is filled with the bacteriocytes. Approximately 50 bacteriocytes can be distinguished in a cross-section; each bacteriocyte contains numerous bacteria. The symbionts show different shapes in the section (). When squeezed out of the worm, they attain a coccoid shape with a diameter of 8.26±0.63 µm (n=10). The refractive granules are contained in vacuoles and are approximately 0.5 µm in diameter.
Figure 5. Paracatenula galateia sp. nov. Semi-thin cross-section through trophosome region showing the thin epidermis (ep), the dorsal cord (dc), the bacteriocytes (bc) filling most of the body and the symbiotic bacteria (ba) within the bacteriocytes. Scale bar 50 µm.
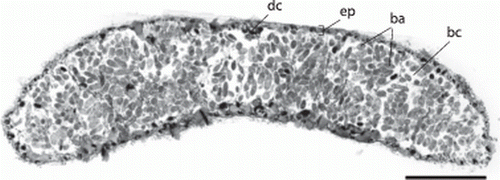
Figure 6. Paracatenula galateia sp. nov. Phalloidin staining of muscles. A. Anterior region showing rostrum muscles (rm) and dorsal cord muscles (dm) B. Detail showing longitudinal fibres (lm), thin circular fibres (cm), nuclei of the epidermis (nu) sunken into the muscle sheath and dorsoventral fibres (dv). C. Muscular layer (ml) surrounding the pouch that contains the bipartite inclusions (bi). Scale bars 50 µm (A) and 5 µm (B, C).
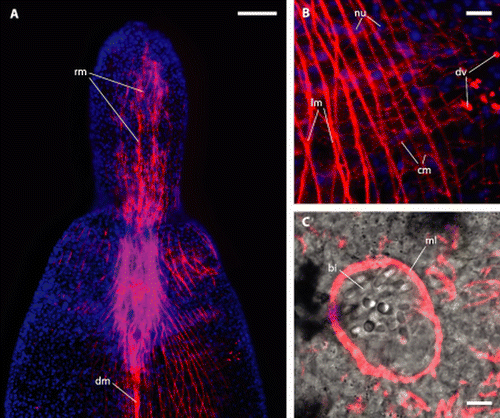
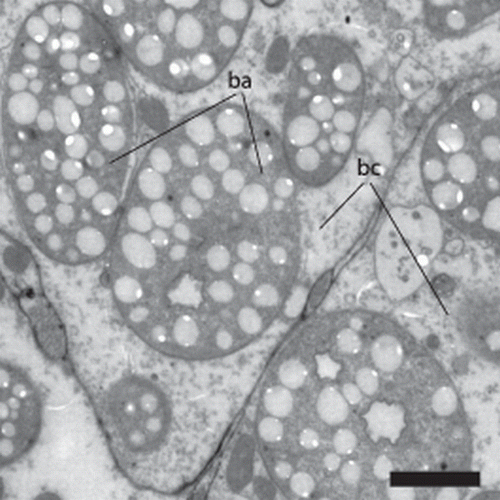
Figure 7. Paracatenula galateia sp. nov. symbiotic bacteria. TEM section of the trophosome region with several bacteria (ba) localized in a bacteriocyte (bc). Scale bar 2 µm.
The serotonergic nervous system () is centralized in a brain, which is located at the boundary of trophosome and rostrum. Originating in the brain, there are four nerve cords innervating the rostrum in an anterior direction, two on the dorsal and two on the ventral side. In the median of the DV-axis we find two prominent nerves originating in the brain that extend laterally and innervate the subepidermal or submuscular nerve nets. The region posterior to the brain has two kinds of nerves showing strong serotonin signals. There is a very strong staining of two main nerve cords that are direct extensions of the dorsal parts of the brain. These main nerves are associated with the ‘dorsal cord’ and extend through the entire posterior part of the worm. The other type of serotonergic nerve in the posterior part of the animal is the subepidermal or submuscular nerve net, which is ubiquitous and evenly distributed.
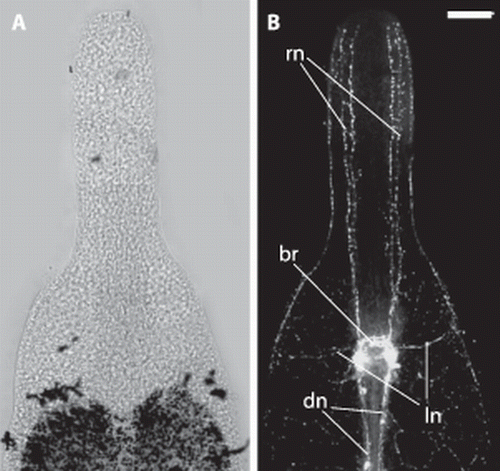
Figure 8. Paracatenula galateia sp. nov. Serotonin staining of the nervous system in the anterior region. A. Light micrograph of anterior end. B. Projection of several immunofluorescent micrographs of the same specimen showing the brain (br), rostrum nerves (rn), lateral nerves (ln) and dorsal cord nerves (dn). Scale bar 50 µm.
Cells containing characteristic bipartite inclusions (B, 4, 6C, 9A,B), which could be interpreted as spermatozoa, were encountered in five of 19 large specimens. They are distributed throughout the trophosome region, especially along the dorsal cord (A), but are concentrated behind the brain, between the anterior ends of the trophosome lobes, in a round pouch enclosed by a muscle layer ( C, 9A), which we interpret as a vesicula seminalis. The rostral wall of the pouch is made up of fine-grained gland cells. Anterior and connected to this pouch there is a circular dorsal opening to the outside (genital pore?), which is surrounded by gland cells containing coarse granules (A).
Figure 9. Paracatenula galateia sp. nov. bipartite inclusions and rhabdoids. A, Anterior end of trophosome (tr) region. Focus on dorsal side showing the bipartite inclusions (bi) along the dorsal chord and concentrated in the circular pouch with finely granulated glands (fg), the dorsal opening (do) and the surrounding glands with coarse granules (cg). B, Bipartite inclusion in a semi-thin section showing two slightly cone-shaped bodies (cb) connected by a short strand (ss) surrounded by a dense matrix (dm). C, Same region as A; focus on ventral side, showing bundles of rhabdoids (rh). Scale bars 25 µm (A, C) and 10 µm (B).
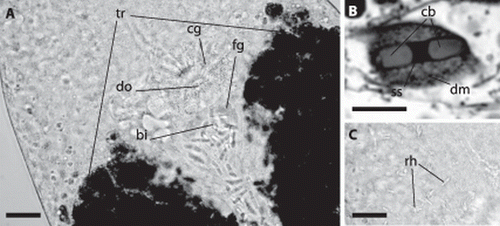
The inclusion containing cells are oval, 20–24 µm long and 7–15 µm wide. The inclusions are made up of a pair of conical or ladyfinger-shaped bodies (B, 4, 6C, 9A,B) that are arranged in either a straight line or at a 160° angle, without touching each other but joined together by a short bond; each pair measures 11.2–13.5 µm (mean 12.2 µm) in length and 2.6–3.6 µm (mean 3.0 µm) in width. The conical or ladyfinger-shaped bodies are 3–6.1 µm long; the connecting bond is a 1.6–3.3 µm long electron-dense strand.
Bundles of 2–6 rhabdoids, rounded rods 10–12 µm long and 1–2 µm wide, may be found throughout the animal (C).
Molecular phylogenetic analysis
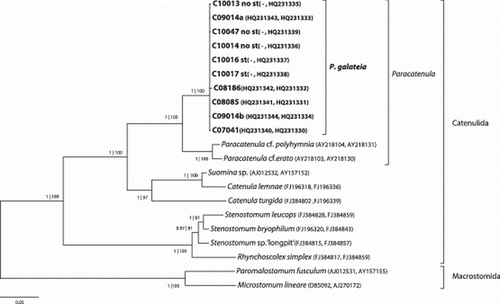
Sequences of the 18S and 28S rRNA gene were obtained from 10 individuals of Paracatenula galateia sp. nov. They are highly similar, with 99.9% and 99.7% pairwise identity. Phylogenetic analysis based on a concatenated alignment of 18S and 28S rRNA genes from Paracatenula galateia and selected Catenulida as well as Macrostomida () shows that (1) our reconstruction of the Catenulida internal phylogeny supports the phylogeny presented by Larsson et al. (2008); (2) all sequences from the genus Paracatenula form one clade within the Catenulida; (3) sequences from P. galateia form a highly supported and well-separated cluster within the genus meriting the designation of a new species; and (4) there is no separation of sequences from individuals with statocyst to individuals without statocyst, showing that all specimens studied belong to the same species.
Figure 10. 18S and 28S rRNA-gene based phylogenetic reconstruction showing the position of the sequenced individuals of P. galateia sp. nov. in the Catenulida. The tree is based on the most likely PHYML tree (GTR + I+G model of substitution). Support in MrBayes and PHYML analysis is indicated (pp | aRLT) for each node. Microstomidae were used as outgroup. GenBank accession numbers are given in parentheses (first 18S and then 28S rRNA gene). Scale bar represents 5% estimated sequence divergence. st=statocyst.
Discussion
The lack of mouth and pharynx together with the molecular data define the new species as belonging to the genus Paracatenula. P. galateia sp. nov. is the most massive species described so far. The width of the similarly long P. urania Sterrer and Rieger, 1974 is only a tenth of that of the new species. A comparison of biometric data is given in . Furthermore, P. galateia sp. nov. is clearly distinguished from the four species described by Sterrer & Rieger (1974) by the structure of its ‘sperm nucleus’, which is spindle-shaped in P. urania and P. erato, spicule-shaped in P. polyhymnia Sterrer & Rieger, 1974, and possibly ribbon-shaped in the lesser-known P. kalliope Sterrer & Rieger, 1974. The fact that in P. galateia sp. nov. the two ‘ladyfingers’ often diverge at a 160° angle might suggest an affinity with P. polyhymnia where the two arms of the spicules as a rule enclose a 160° angle (Sterrer & Rieger 1974, fig. 13g). However, the nature of the peculiar cells containing the variously shaped inclusions is still unclear. In P. galateia sp. nov. the location of the cells, and their concentration in a muscular pouch with a dorsal opening, strongly suggests them to be sperm. Attempts to stain the presumed nucleus with DAPI, however, failed. Most puzzling are the spicule shaped inclusions in P. polyhymnia and several other not yet described species (own unpublished observations) that seem to have a mineral nature.
Table I. Biometric comparison of the Paracatenula species.
Editorial responsibility: Tomas Cedhagen
Acknowledgements
This work was supported by the Austrian Science Fund (FWF) project 20394-B03 (JO, HG, NL and UD). We thank Renate Hodinka for suggesting the species name. Part of this work was carried out by using the resources of the Computational Biology Service Unit from Cornell University which is partially funded by The Microsoft Corporation. This is contribution 899 from the Carrie Bow Cay Laboratory, Caribbean Coral Reef Ecosystem Program, NMNH, Washington, DC.
Additional information
Notes on contributors
Harald R. Gruber-Vodicka
These authors contributed equallyNotes
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
References
- Anisimova , M and Gascuel , O. 2006 . Approximate likelihood-ratio test for branches: A fast, Accurate, and powerful alternative . Systematic Biology , 55 : 539 – 52 .
- Borkott H. 1970 . Geschlechtliche Organisation, Fortpflanzungsverhalten und Ursachen der sexuellen Vermehrung von Stenostomum sthenum; nov. spec . (Turbellaria, Catenulida) . Zoomorphology 67 : 183 – 262 .
- Dereeper A , Guignon V , Blanc G , Audic S , Buffet S , Chevenet F , et al. 2008 . Phylogeny.fr: Robust phylogenetic analysis for the non-specialist . Nucleic Acids Research 36 : W465 – 69 .
- Doe D , Rieger RM. 1977 . A new species of the genus Retronectes (Turbellaria, Catenulida) from the coast of North Carolina, U.S.A . Mikrofauna des Meeresbodens 66 : 1 – 10 .
- Drummond A , Ashton B , Buxton S , Cheung M , Cooper A , Heled J , et al. 2010 . Geneious v5.1 , available from http://www.geneious.com/.Computerprogram .
- Faubel , A. 1976 . Eine neue Art der Gattung Retronectes (Turbeliaria, Catenulida) aus dem Küstengrundwasser der Nordseeinsel Sylt . Zoologica Scripta , 5 : 217 – 20 .
- Guindon , S , Dufayard , J-F , Lefort , V , Anisimova , M , Hordijk , W and Gascuel , O. 2010 . New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0 . Systematic Biology , 59 : 307 – 21 .
- Guindon , S and Gascuel , O. 2003 . A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood . Systematic Biology , 52 : 696 – 704 .
- Katoh , K , Kuma , K-i , Toh , H and Miyata , T. 2005 . MAFFT version 5: Improvement in accuracy of multiple sequence alignment . Nucleic Acids Research , 33 : 511 – 18 .
- Larsson , K and Jondelius , U. 2008 . Phylogeny of Catenulida and support for Platyhelminthes . Organisms Diversity & Evolution , 8 : 378 – 87 .
- Loy , A , Duller , S , Baranyi , C , Mussmann , M , Ott , J Sharon , I . 2009 . Reverse dissimilatory sulfite reductase as phylogenetic marker for a subgroup of sulfur-oxidizing prokaryotes . Environmental Microbiology , 11 : 289 – 99 .
- Noreña-Janssen , C and Faubel , A. 1996 . Myoretronectes paranaensis n. gen.et sp., a new freshwater genus of the family Retronectidae (Turbellaria, Catenulida) from the Paraná, Argentina . Hydrobiologia , 330 : 111 – 18 .
- Nylander JAA. 2008 . MrModeltest v2.3 , Program distributed by the author, Evolutionary Biology Centre, Uppsala University. Computer program .
- Ott , JA , Rieger , G , Rieger , R and Enderes , F. 1982 . New mouthless interstitial worms from the sulfide system: Symbiosis with Prokaryotes . Pubblicazioni Stazione Zoologica Napoli I: Marine Ecology , 3 : 313 – 33 .
- Pasteris , JD , Freeman , JJ , Goffredi , SK and Buck , KR. 2001 . Raman spectroscopic and laser scanning confocal microscopic analysis of sulfur in living sulfur-precipitating marine bacteria . Chemical Geology , 180 : 3 – 18 .
- Pradillon , F , Schmidt , A , Peplies , J and Dubilier , N. 2007 . Species identification of marine invertebrate early stages by whole-larvae in situ hybridisation of 18S ribosomal RNA . Marine Ecology Progress Series , 333 : 103 – 16 .
- Riedl , R. 1959 . Turbellarien aus submarinen Höhlen. I. Archoophora . Pubblicazioni Stazione Zoologica Napoli Supplement , 30 : 178 – 208 .
- Rieger , PM. 1978 . Multiple ciliary stuctures in developing spermatozoa of marine Catenulida (Turbellaria) . Zoomorphology , 89 : 229 – 36 .
- Ronquist , F and Huelsenbeck , JP. 2003 . MrBayes 3: Bayesian phylogenetic inference under mixed models . Bioinformatics , 19 : 1572 – 74 .
- Rützler , K and Macintyre , I. 1982 . The Atlantic barrier reef ecosystem at Carrie Bow Cay, Belize, I. Structure and communities , Washington, DC : Smithsonian Institution Press. 539 pages .
- Schuchert , P and Rieger , RM. 1990 . Ultrastructural examination of spermatogenesis in Retronectes atypica (Catenulida, Platyhelminthes) . Journal of Submicroscopical Cytology & Pathology , 22 : 379 – 87 .
- Sterrer , W. 1966 . New polylithophorus marine Turbellaria . Nature , 210 : 436
- Sterrer , W and Rieger , RM. 1974 . “ Retronectidae – A new cosmopolitan marine family of Catenulida (Turbellaria) ” . In Biology of the Turbellaria , Edited by: Riser , N and Morse , M . New York, NY : McGraw-Hill, p 63–92 .
- Tyler S , Schilling S , Hooge M , Bush L. 2006 – 2010 . Turbellarian taxonomic database Version 1.6 , available at http://turbellaria.umaine.edu .
- von Reumont , B , Meusemann , K , Szucsich , N , Dell'Ampio , E , Gowri-Shankar , V Bartel , D . 2009 . Can comprehensive background knowledge be incorporated into substitution models to improve phylogenetic analyses? A case study on major arthropod relationships . BMC Evolutionary Biology , 9 : 119