Abstract
The Arctic is of special importance to the world, and it is changing rapidly. Uncovering the relationship between drivers of change and biological responses in the Barents Sea is therefore crucial for understanding the potential effects of climate change on the ecosystem in general and on commercially important species in particular. This thematic review provides an overview of the discussions related to long- and short-term variations in climate in the Barents Sea, what these physical changes really are, and how they may develop in the future. Furthermore, questions related to how these predicted climate-driven physical changes may alter ecosystems and the implications and future challenges that this represents for the management of resources in the area are raised. There is no doubt that to better understand the structure and function of an ecosystem, as well as to investigate the possible effects of climate changes, there is a need for thorough monitoring and data collection. The Barents Sea Ecosystem Survey (BESS) is used in several of the studies highlighted in this review. Therefore, we can provide a detailed description of the BESS and relate BESS research activities to other research initiatives in a thematic context.
Published in collaboration with the Institute of Marine Research, Norway
Introduction
The Barents Sea (BS) is a productive area with more than 200 species of fish, thousands of benthic invertebrate species and diverse communities of plankton, seabirds and marine mammals inhabiting or visiting the area (see Jakobsen & Ozhigin Citation2011 and references therein). Only a limited number of species from the different taxa are of commercial interest. Nonetheless, a couple of species provide the basis for some of the largest fisheries in the world, and in 2010 the catches of capelin Mallotus villosus (Müller, 1776), polar cod Boreogadus saida (Lepechin, 1774), cod Gadus morhua Linnaeus, 1758, haddock Melanogrammus aeglefinus (Linnaeus, 1758), redfish Sebastes spp., Greenland halibut Reinhardtius hippoglossoides (Walbaum, 1792) and shrimp Pandalus borealis Krøyer, 1838 were reported to be close to 2.9 million tonnes (ICES Citation2010, Citation2011a, Citation2011b). Marine mammals are also harvested, although on a smaller scale (Haug et al. Citation2011). Human activities such as shipping, tourism, mining and oil and gas exploration also influence the ecosystem.
The ocean circulation pattern in the Barents Sea is well described in the literature (see Sakshaug et al. Citation2009; Stiansen et al. Citation2009; Jakobsen & Ozhigin Citation2011). In addition, the Barents Sea climate has strong variability (Loeng et al. Citation1992). The highest temperatures since the beginning of the twentieth century were observed in the period from 2004 to 2007 (). The associated decrease in sea ice cover has allowed ships access to previously ice-covered regions (). During the same period, changes in the distribution and abundance of various species have also been observed. This has led to several studies dealing with questions related to how these climate-driven changes may alter ecosystems and the implications and future challenges that this represents for the management of resources in the area. In the following article, results from these studies will be reviewed and put into a broader context to better understand the ecosystem structure and function of the Barents Sea Ecosystem Survey (BESS). Special focus will be given to the collection of the data used in scientific investigations of climate change and ecosystem functioning, because a prerequisite for all these studies is thorough monitoring and data collection.
Figure 1. Time period of the Barents Sea Ecosystem Survey and start time of the predecessor surveys (for details see Michalsen et al. Citation2011), and the temporal development in ocean temperature (at the Kola section, black line) and summer ice in the northern (77–82°N, 20–50°E) Barents Sea (dotted line). Note the reversed axis for ice area.
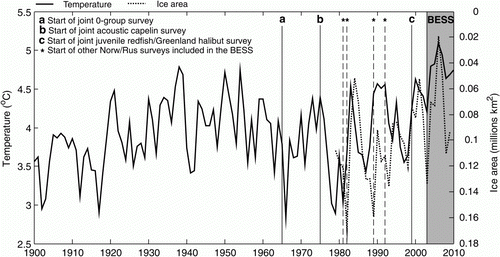
Even though there are several other surveys conducted in the Barents Sea, the joint Russian–Norwegian BESS is an example of a comprehensive survey with near-synoptic collection of information about the physical and biological components of the ecosystem, which should allow for an improved understanding of the processes in the BS ecosystem. The survey covers the whole BS shelf from August to early October, the months with the least sea ice cover. The time period described here (2003–2010) also coincided with the peak of a long-term warming period (). This makes this survey a highly suitable platform for observing the system structure and functioning during a warming of the Barents Sea. The synoptic sampling of many components in the same survey allows for an increased understanding of the processes of the Barents Sea ecosystem. Compared to the preceding surveys, much more information about the ecosystem is now being sampled.
The gear used for collection (gear selection), the frequency of the samples (variability in time) and the cruise track (spatial distribution and geographic variation) are all factors that affect the collected data. Detailed knowledge of how the data are collected is therefore of the utmost importance to ensure valid analysis, correct parameterizations of models and reliable conclusions.
This review provides a description of the BESS and how the data are used in scientific investigations of climate change and ecosystem functioning. Further, the review tries to relate BESS research activities to other research platform initiatives in a thematic context. Based on eight years of experience, recommendations for further improvements to the ecosystem surveys are suggested.
A brief description of BESS
The Norwegian–Russian cooperation
The ecosystem monitoring in the BS is a joint effort between Norway and Russia based on collaboration between the two countries dating back to 1954 (Røttingen et al. Citation2007). A strong decrease of the spawning stock of Northeast Arctic cod stock in the 1950s and the Norwegian spring spawning herring stock Clupea harengus Linnaus, 1758 in the 1960s led to the total collapse of the fisheries. In order to avoid similar situations occurring in the future, the ICES Herring Committee recommended initiating the investigation of 0-group fish (Gjøsæter Citation1999; Dragesund et al. Citation2008; Eriksen & Prozorkevich Citation2011). An overview of previous surveys conducted in the Barents Sea in summer and autumn is given in Michalsen et al. (Citation2011; ). Due to international agreements and attempts to improve the efficiency and enhance the ecological focus and scientific impetus of these surveys, they were gradually merged to form the joint BS Ecosystem Survey (BESS). The current survey design is thus a compromise between available economic resources and sufficient data quality required for assessment while maintaining consistency in the long time series that includes data from preceding surveys.
Table I. Overview of the scientific investigations carried out during the joint ecosystem survey in the Barents Sea. Component monitored, gear used, samples taken, variable measured and application for these are given (from Michalsen et al. 2011). ADCP=Acoustic Doppler Current Profiler; CTD=conductivity, temperature and depth; SA=backscattering; TS=target strength.
Implementation of the survey
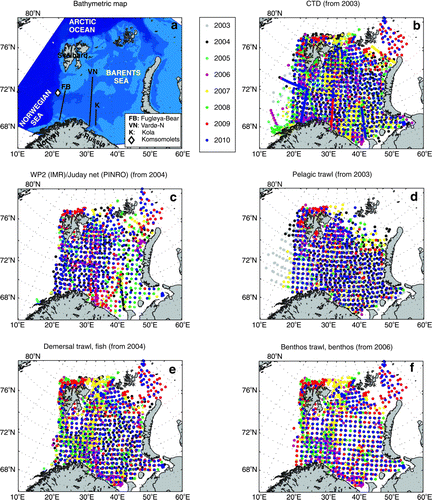
Beginning in 2003, the BESS has been conducted from August to October and covers the ice-free part of the BS and the Svalbard shelf (). Five vessels normally operate in the region: three Norwegian and two Russian. Most aspects of the ecosystem are included, from physical and chemical oceanography, phytoplankton and zooplankton to fish (both young and adults), benthic invertebrates, sea mammals, seabirds and contaminant levels. A range of methods and gear are applied, from water sampling, using conductivity, temperature and depth (CTDs), to plankton nets, pelagic and demersal trawls, grabs and sledges, echo sounders and direct visual observations. More detailed information about the components monitored, gear used, samples collected, variables measured and the applications of the information are provided in .
Figure 2. Bathymetric map (a) and the joint Norwegian–Russian stations performed during the Barents Sea Ecosystem Survey 2003–2010. The hydrographic (b) and pelagic fish (d) investigations have been joint since 2003, the zooplankton (c) and demersal fish (e) investigations since 2004 and the benthic investigations (f) since 2006. For details of the sampling, see Michalsen et al. (Citation2011). CTD=measurements of conductivity, temperature and depth.
Traditionally, data from the scientific surveys in the BS have been used to monitor the status of and changes in the environment and to calculate the abundance and recruitment of commercially important fish stocks. However, the near-synoptic sampling of a range of ecosystem components during the same survey also allows for ecological studies that will enable an increased understanding of biological communities and processes in the BS ecosystem.
Monitoring
Environmental monitoring
The environmental monitoring performed during BESS is essential when reporting climate status to ICES working groups (Oceanic hydrography working group, Arctic Fisheries working group, Working group of Northern Pelagic and Blue Whiting, working group for Regional Ecosystem Description), the Norwegian Management Plan for the BS and the Joint Norwegian–Russian environmental status report (e.g. Stiansen et al. Citation2009). Data from both the regional coverage and the two Norwegian and three Russian standard sections sampled during the BESS are used by these working groups. The Russian Kola section has been sampled since 1900 and the Norwegian Vardø-N section since 1953. This makes the data from these sections the only continuous series from the open-ocean BS, including data from multiple cold and warm periods and they are, therefore, crucial for integrated analyses of long-term climate variability and change (e.g. Ingvaldsen et al. Citation2003; Boitsov et al. Citation2012; Mathisov et al. Citation2012).
The northeastern BS is only monitored during BESS, while the northwestern BS is also monitored during the Russian October–December survey. During the rest of the year, these areas are covered with ice and are not accessible for sampling. The BESS gathers data that are needed for monitoring the seasonal ice-covered water masses, the dense bottom water formed during the preceding winter, and the inflow of water entering the BS from the north. These data are traditionally used for process studies or projects with a specific scientific focus, but in recent years, this information has also been published in the status reports mentioned above.
Monitoring contaminants, e.g. the levels of hydrocarbons, persistent organic pollutants (PCB, DDT, HCH, HCB) and radionuclides, is also performed every third year during the BESS. Results are reported in Stiansen et al. (Citation2009) and Green et al. (Citation2010). In addition, the area around the wreck of the Russian submarine ‘Komsomolets’ is monitored for radionuclides every year (NRPA Citation2011).
Because of the increased solubility of CO2 in cold water, polar oceans are likely to be among the first marine environments to exhibit effects from increased acidity. A scenario that includes increased ocean acidification (OA) and global warming in particular is expected to affect marine organisms in the polar regions and thus the structure and functioning of such regional ecosystems. The BESS initiated monitoring of the carbonate system in the BS in 2010.
Trends in the abundance of fish stocks, shrimp and zooplankton
A major output from the survey is the stock sizes of key species in the BS ecosystem. Central to the stock assessment for a species is the time series of stock size estimates by age. In most cases, the time series of stock size indices are what actually enter the assessment, and it is important that the indices are calculated consistently from year to year. However, for capelin Mallotus villosus this is different because the measured stock size is not an index, but an absolute estimate of stock size. The capelin time series is used primarily as an input to long-term simulations used for studying sustainable yield and harvest control rules (Hamre & Tjelmeland 1985; Røttingen & Tjelmeland Citation2009). Therefore, it is important that the capelin stock size estimate is unbiased and accurate every year.
Data on the shrimp obtained during the ecosystem survey form the basis for calculations of the abundance of the stock (ICES Citation2010, Citation2011c; Hvingel & Berenboim Citation2011). The BESS covers a much larger geographical area than earlier shrimp surveys, and shrimp have been caught in more than 70% of bottom trawl hauls during these cruises.
The consumption of capelin by cod, based on stomach samples and temperature measurements, is another parameter estimated from the survey data (ICES Citation2011a). The amount of capelin consumed by cod is central to the management of capelin fisheries, as the induced mortality of capelin in the assessment model heavily affects the quotas of capelin (Gjøsæter et al. Citation2002, Citation2012). In the current version of the capelin assessment model, it is primarily predation by cod during the winter months that is important, but work is currently being undertaken to include predation during the summer months as well.
Cod cannibalism and predation by cod on juvenile haddock are presently used as inputs to the assessments of those species, and to a great extent, predation by cod determines the natural mortality of juvenile fish of these species (Yaragina et al. Citation2009). In this case, an important amount of dietary information comes from the BESS because the summer/autumn season is a crucial feeding period, especially for cod.
Average zooplankton biomass is estimated based on BESS data and from standard sections in the BS. Biomass data are also obtained from the standard sections for periods other than the BESS season (up to 5 times per year). In addition, the species composition of zooplankton is monitored and exchanged between IMR and PINRO for two standard sections in the western (Fugløya – Bear Island) and central (Kola) regions of the sea. The distribution and abundance data are used to monitor zooplankton biomass, species composition and the availability of food for higher trophic levels and to identify the primary feeding areas for key fish species. Zooplankton, copepods and euphausiids in particular, are ecologically important components of the BS ecosystem. These key zooplankton groups are predominantly herbivorous, thus important in transferring energy from the lower trophic levels, e.g. phytoplankton, to the higher levels, e.g. fish such as capelin and young cod (Dalpadado & Bogstad Citation2004; Orlova et al. Citation2010). The zooplankton biomass and species indicators could serve as early warning signals with respect to shifts occurring in the ecosystem due to, e.g. climate change.
0-group indices and recruitment
In August and September, the majority of 0-group fish (5–7 months old) are distributed within the upper 60 m of the water column. An exception is the Greenland halibut, which is distributed deeper and hence will most likely be underestimated by the survey methods used. Since 1980, relative abundance indices have been calculated for capelin, Norwegian spring-spawning herring, Northeast Arctic cod, Northeast Arctic haddock Melanogrammus aeglefinus, saithe Pollachius virens (Linnaeus, 1758), redfishes, Greenland halibut, long rough dab Hippoglossoides platessoides (Fabricius, 1780), wolffishes Anarhichas spp., and polar cod in the BS. These indices are calculated based on a stratified sample mean (Dingsør Citation2005; Eriksen et al. Citation2009). The indices for capelin, cod, haddock and herring are provided with corrections for length-dependent capture efficiency, but this is not done for any other species. The 0-group indices of capelin and herring are used in fish stock assessments, and the other indices and 0-group data are commonly applied in fish recruitment studies.
Monitoring of other ecosystem components
Time series for krill, pelagic amphipods (hyperiids), jellyfish and non-commercial fishes (family level) have been developed based on the 0-group data (Eriksen & Dalpadado Citation2011; Eriksen et al. Citation2012b). The distribution and biomass data for these groups are used to monitor the food availability (krill, hyperiids) for higher trophic levels, species interactions and the effects of climate change.
The spatial coverage of the BS by demersal trawls during the autumn was limited and variable before the initiation of the BESS, but with the new time series established, it is now possible to develop estimates of the abundance and distribution of benthos, polar cod, demersal fish, seabirds and marine mammals. Distribution data from the BESS on all fish records have been reported in the form of a fish atlas (Wienerroither et al. Citation2011).
Studies of the benthos from BESS data have revealed several faunal groups or communities of benthic invertebrates in the BS, that are spatially structured primarily by depth and temperature (Anisimova et al. Citation2011). Monitoring benthic invertebrate abundances and biomass are critical to our understanding of the benthic–pelagic coupling, particularly related to seasonally ice-covered regions, and to enhance our knowledge of the seabed as a feeding and nourishing ground for demersal species. Changes in the distribution of benthic indicator species could provide early warning signals of climate change. In addition, the snow crab (Chionoecetes opilio (Fabricius, 1788)), a new species of uncertain origin in the BS ecosystem (introduced or invasive), was first recorded in 1996 (Alvsvåg et al. Citation2009; Agnalt et al. Citation2010, Citation2011). The snow crab population has since increased, and its expansion and abundance along with its potential effects on other ecosystem components are now key elements of the BESS monitoring programme. Data from the benthic time series are used in the Pan-Arctic monitoring programme under the Arctic Council, in the Norwegian Management Plan for the BS and the Joint Norwegian–Russian environmental status report (e.g. Stiansen et al. Citation2009).
Investigation into the diseases and pathology of commercial marine organisms was initiated by PINRO in 1999. This is a monitoring programme in which the primary goal is to develop a system to describe the well-being of the commercial fish populations. The main purpose of this fish pathology research is the annual estimation and control of the epizootic state of cod, flatfish and wolffish and the completion of a database on fish diseases and pathology (Karasev et al. Citation2011).
Studies of ecological processes and ecosystem functioning
The near-synoptic sampling of many ecosystem components during BESS has greatly enhanced the possibility of investigating dynamic large-scale ecosystem processes. During the study period, temperatures have increased and the northern areas have become ice-free, coinciding with a collapse and recovery of the capelin stock. The BESS provides the data needed to investigate the responses to these perturbations at the species, community and ecosystem levels. In the following, several studies will be reviewed and put in a broader context.
The impact of capelin fluctuations
Collapses in the capelin stock have had profound effects on the BS ecosystem. The collapse of the capelin stock in 1984 had impacts on different trophic levels: (a) the growth of cod decreased and cannibalism increased, (b) seabird recruitment failed, and (c) harp seals Pagophilus groenlandicus (Erxleben, 1777) in poor condition invaded coastal areas (Gjøsæter et al. Citation2009 and references therein). However, the two most recent capelin stock collapses had less profound effects on the top predator community (Gjøsæter et al. Citation2009). Thus, the trophic interactions and dynamics of the BS system appear to have changed during the last decade (Johannesen et al. Citation2012b).
The synoptic BESS data on macrozooplankton (krill and pelagic amphipods), 0-group fish, pelagic fish, cod, seabirds and marine mammals can be used to investigate the trophic structure of the pelagic ecosystem during the most recent capelin collapse (2003–2007, Skern-Mauritzen, pers. comm.). Johannesen et al. (Citation2012c) used data from BESS to investigate the responses of cod to changes in distribution of capelin (2004–2009). The results from these studies suggest that, among the top predators, only cod appeared to maintain a rather strong spatial association with capelin during the years of low capelin abundance. Consistent distributions of top predators in summer, despite dramatic fluctuations in one key prey species, suggest that predators are associated with specific habitats during this season. In these persistent habitats, the reduced availability of one type of prey, such as capelin, may be buffered by a sufficient abundance of other prey species.
Climate variations and their effects on species and species interactions
The climate variability in the BS is substantial, and data from the BESS and preceding surveys have revealed a positive temperature trend in the BS over the last four decades (). The extent of the warm Atlantic region has increased (Johannesen et al. Citation2012b), thereby expanding the thermal habitat of juvenile cod, haddock and herring (Eriksen et al. Citation2012a). Simultaneously, the extent of cold Arctic waters has declined, causing a reduction in Arctic zooplankton (Dalpadado et al. Citation2012).
The data from the BESS have also revealed an accelerating temperature trend in the northern BS in the 2000s caused by an increased inflow of subsurface Atlantic water to the BS from the north (Lind & Ingvaldsen Citation2012) and a reduction in the formation of cold bottom water in the last decade (Årthun et al. Citation2011). Such data are also an important source for validating general ocean circulation models.
Based on the BESS data, climate impacts on zooplankton (Eriksen & Dalpadado 2011; Orlova et al. Citation2011; Dalpadado et al. Citation2012), shrimp recruitment (Aschan & Ingvaldsen Citation2009), the abundance, length and distribution of 0-group fish (Eriksen et al. Citation2012a), the distribution of baleen whales (Skern-Mauritzen et al. Citation2011) and capelin–prey and cod–prey interactions in the BS ecosystem (Orlova et al. Citation2010; Johannesen et al. Citation2012c) have been evaluated. In the future, BESS data will also form the basis for studies addressing productivity (from plankton to top predators) and climate impacts on the structure and functioning of the ecosystem.
Changes in temperature throughout the water column in the BS are known to affect the distributions of both fish and key pelagic invertebrates (Drinkwater et al. Citation2010). Data from historical surveys before the BESS have been used to study changes in the geographical distribution of capelin (Gjøsæter & Loeng Citation1984; Gjøsæter Citation1999; Fauchald et al. Citation2006; Eriksen et al. Citation2012a; Carscadden et al. Citation2013; Ingvaldsen & Gjøsæter, Citation2013). Studies have revealed that both abundance and temperature are important for the geographical distribution of capelin, but also link the northward expansion of observed distributions in recent years to the higher temperatures in the northern BS. In addition, the data illustrate that when species change their distributional range and/or migration patterns, this might in turn affect species interactions, as the spatiotemporal overlap between predators and prey or among competitors may change. A long-term data set for an area is therefore of the utmost importance.
Hop and Gjøsæter (Citation2013) compare and contrast these two central players in the BS and elsewhere in the Arctic based on data from the BESS as well as a review of the relevant literature. Both species represent high-energy prey (lipids) for upper trophic levels and constitute important links between plankton in the central and northern areas and predatory fish, sea mammals and seabirds. The authors suggest that global warming, with associated reductions in sea ice and increases in temperature, is expected to affect these two species differently. Polar cod will likely lose the ice-associated part of their life cycle and become more restricted in their pelagic distribution during the summer, whereas the capelin stock may expand to the north and east, although with considerable interannual fluctuations.
Bogstad et al. (Citation2013) used data for the period from 1913 to the present to investigate temperature–recruitment relationships for these stocks as a foundation for the use of such relationships in single- and multispecies population models, which are relevant for management. The analysis revealed that the relationships between temperature and the recruitment of cod, haddock and herring in the BS, as well as the co-variability in the recruitment of these three species, vary over the temperature range. At low temperatures, the recruitment of cod and haddock are highly variable, vary in synchrony and increase with increasing temperature, but at high temperatures they do not. The differences among the species might be caused by different thermal adaptations, but differences in spawning area, drift routes of eggs and larvae or life histories might also play a role. The article also found that the variability in recruitment declined towards the end of the period from 1950 to the present for all species, in particular for cod. The reasons for this decline were not clear, but the authors suggested a combined effect of enhanced management and higher and thus more favourable temperatures.
Ecosystem functioning
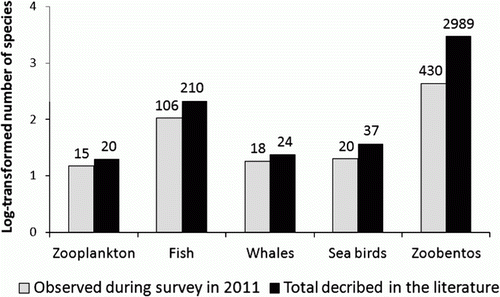
The management of marine living resources depends on the reliable identification of all marine species in the area, both to understand their population structure and distribution and to assess the relative importance of different species as either predators or prey. The number of species recorded during the BESS in 2011 is close to the total number of species described as regularly occurring in the BS in the literature (). However, today's sampling equipment used for benthic invertebrates can leave the infauna severely undersampled. This will influence the number of species recorded dramatically. The same can be argued with respect to zooplankton. Tiny zooplankton that is smaller than the standard mesh size used during the survey will not be recorded. It is important to note that there are large spatial differences in the BS due to the transition zone between Atlantic and Arctic waters, which support boreal and Arctic faunas, respectively. These faunas are partly decoupled and have different adaptations and are thus likely to respond differently to climatic changes (Johannesen et al. Citation2012a). The interactions between species also change seasonally and over time. Johannesen et al. (Citation2012c) studied the interactions between cod and capelin, shrimp, herring, polar cod and hyperiids during the summer from 2004 to 2009. They concluded that although the prey species were found in spatially segregated habitats, the cod were found in all prey habitats except for the deepest areas. However, the analyses also suggest that the cod were spatially constrained and that their prey differed in composition. Cod density was higher in areas occupied by capelin and herring and lower in the northeastern areas occupied by hyperiids and polar cod. Although feeding success increased with local polar cod abundance, polar cod had a spatial refuge in the north-eastern part of the BS, which cod did not enter.
Figure 3. Overview of the number of species recorded during the Barents Sea Ecosystem Survey and in the literature. This provides an illustration of the biodiversity in the Barents Sea. Real numbers are included above each column.
Further studies on the impacts of biodiversity and trophic interactions on ecosystem functioning and resilience have been initiated (Planque et al. Citation2010, Citation2012). These studies rely on data collected by the BESS and explicitly examine how the functional diversity of fish and benthos varies spatially and how the resilience to climate change varies in different regions of the BS.
Model data can thus make an important contribution to understanding and identifying key processes within an ecosystem. As an example, a simplified biogeochemical model corresponded well with observational data of the BS carbonate system only when the influence of organic matter was taken into account (Yakushev & Sørensen Citation2013). This indicates that the production and decay of organic matter (i.e. the biological components) play a dominant role in the temporal dynamics of the carbonate system and thus for seasonal changes in pCO2 and pH in the BS.
Orlova et al. (Citation2013) use data from the BESS and other surveys to elucidate how the functioning of the trophic structure among the central players in the ecosystem has changed in the recent warming period. They conclude that the warming of the BS has influenced which species dominate, as well as how those species affect each other. However, the authors maintain that predictions about the further development of these trophic structures, if sea temperatures continue to rise in the future, should be made with caution. Further changes will not necessarily be a simple extrapolation of the changes observed thus far.
Søreide et al. (Citation2013) use stable isotopes (δ13C and δ15N) and fatty acid trophic markers (FATMs) to assess the carbon flow and trophic structures of sympagic, pelagic and benthic communities in three regions in the north and northeastern high-Arctic Svalbard. Biological ‘hot spots’ were only revealed in Arctic water in the northeastern region that was covered by dense sea ice for >7 months. Here, both the pelagic and benthic biomasses were typically high. It should, however, be noted that these ‘hot spots’ were situated in close proximity to land, where ice conditions appear to persist and remain stable for a longer period compared to more open shelf waters. One interesting finding was that the omnivorous–carnivorous ice fauna could have high proportions of Calanus-FATMs, indicating that a significant portion of their diet and energy comes from the pelagic realm. This observation was also supported by stable isotope analysis and could mean that in a global warming context, some ice-associated fauna could have alternative energy pathways when the extent of multiyear fast-ice decreases. Another issue concerns pelagic–benthic coupling. Following sea ice retreat and the loss of ice algal production, pelagic secondary production will likely benefit from increased primary production. Following such a scenario, the benthic community structure, which has been demonstrated to be strongly dependent on the sedimentation of fresh ice-algae, could dramatically change, giving a boost to opportunistic species.
The results of this study arise from dedicated surveys in which there is a possibility of conducting focused field experiments compared to the traditional monitoring work typical of the BESS. It illustrates that process studies at a more specific level, that can be difficult to undertake during a regular monitoring survey, are valuable and necessary complements to the BESS approach.
Management implications
Ecosystem-based Approach to Fisheries (EAF) involves developing an all-embracing plan for managing the ecosystem as a whole, where the goal is not merely to maintain a high level of commercial resource harvesting in the long term, but also to prevent human activity from having detrimental effects on the fish stocks and the rest of the ecosystem (Garcia et al. Citation2003). This means that, to a greater extent than in the past, it is necessary to understand the structure and function of the ecosystem to be able to predict the consequences of human activity. For this reason, it is fundamental to invest more in the monitoring of and research on ecosystem processes, and to identify key ecosystem processes.
Howell et al. (Citation2013) provide an example of how temperature-dependent recruitment in a modelled cod stock provides different predictions of future stock development. This is a challenge for management, as different stock projections may point to different management regimes. However, integrating, for example, climatic effects with fish stock assessments through correlations between climate indices and stock population processes is complicated and may introduce additional sources of error, as these correlations tend to break down over time (Myers Citation1998, Howell et al. Citation2013). Hence, for implementation in fish stock assessment and management advice, a good understanding of underlying processes rather than mere correlations is required (e.g. Howell et al. Citation2013). BESS monitoring and associated research is highly relevant in this respect.
The implementation of EAF is thus a challenging task, as is developing and creating an optimal monitoring programme to support such an approach. Obtaining information on a range of different ecosystem components as well as the physical environment requires very different equipment and sampling strategies. According to the European Marine Strategy Framework Directive (MSFD), surveys of the marine habitat should provide information on a variety of topics described by 12 descriptors: biodiversity, non-indigenous species, commercial fish, food webs, eutrophication, sea-floor integrity, hydrographical conditions, contaminants, food safety, litter, energy and noise. The BESS covers many of these descriptors. However, some of these are not at all easy to interpret in terms of what should be measured, i.e. ‘sea-floor integrity’, ‘food safety’ and ‘energy’. In this context, Trenkel et al. (Citation2011) state that data from acoustic surveys are underutilized and provide a brief introduction to how acoustic data could be used as complementary information. However, further development of acoustics-derived descriptors or indicators is still needed. Here, new developments in acoustic wideband systems that are capable of obtaining a continuous response over broad frequency ranges are promising for classification purposes (Foote et al. Citation2005; Lavery et al. Citation2010; Stanton et al. Citation2010).
End-to-end ecosystem models integrate physical and biological processes on different scales, implement two-way interactions between ecosystem components and account for forcing by climate and human impacts at multiple trophic levels (Travers et al. Citation2007). These models often rely on unverified assumptions about key processes and often request quantitative information that traditional surveys do not collect (Handegard et al. Citation2012). Through a closer collaboration between those conducting the surveys, ecologists, modellers and managers, important processes can be identified and areas of special interest highlighted so that the survey design can be adjusted and improved to collect data that will be useful for ecological modelling (i.e. diel stations for process studies or intensified acoustic recordings in frontal regions). Survey data clearly have the potential to make a strong contribution to EAF in the future.
Challenges and new insights
The primary aim of the BESS has been to conduct a near-synoptic sampling of the components of the BS ecosystem while maintaining the old time series, but also initiate monitoring of new ecosystem components. The primary focus of the survey has varied among years (see discussion in Michalsen et al. Citation2011), but has generally been the distribution and abundance of the young and adult stages of several pelagic and demersal fish species, followed by the monitoring of environmental features, phytoplankton and zooplankton.
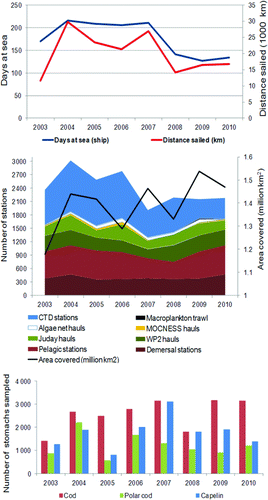
The number of days at sea has, however, been reduced over the years of the BESS (). This has reduced the time available for sampling and also constrained the ability to account for bad weather conditions. It has also influenced the time available for the inter-calibration of equipment and replicate sampling, if needed, as well as targeted trawling for validation of acoustic registrations. The total effort during BESS has varied (). Clearer objectives for the surveys and agreed-upon priorities with respect to all tasks currently undertaken during the survey should be developed in the future. Additionally, a long-term perspective for the survey is important to ensure continuity in the survey's objectives and the resources needed to perform such a coordinated effort.
Figure 4. The total effort carried out during the Joint Norwegian–Russian survey in the Barents Sea. Numbers are taken from Michalsen et al. (Citation2011).
The survey design of the BESS now consists of a uniform sampling regime with additional trawl sampling performed on acoustic registrations. This sampling design may be inadequate to capture particular features such as physical spatial gradients and/or particular patterns in organism distributions. There is also an inherent conflict between those investigations that rely on sampling at specific, predetermined stations and those that rely on data obtained from a moving vessel. Furthermore, a major challenge is the contrast between optimizing the survey design for one type of organism versus optimizing the design for an assemblage of species or ecosystem components. Ideally, further improvements to the survey should be based on identified spatial scales for all the prioritized components and processes being monitored. Since the main Russian–Norwegian survey for cod and haddock during the winter does not always cover all year-classes of cod (Johansen et al. Citation2013), and the Russian groundfish survey carried out in late autumn (October–December) will likely have increasing problems in covering the stock when the cod distribution area expands because of an increasingly warmer ocean, the BESS might become increasingly important in providing such indices. This might in turn provide further constraints on the sampling strategy during the BESS. Constructing a grid of stations that is suitable for the main investigations with a sufficient density that still covers the entire BS is not straightforward. A survey design combining a regular and an irregular sampling grid is likely to be a more feasible approach. Over the years of the BESS, the survey design and equipment have become more standardised, although additional improvements are needed.
Over the years of the BESS, warm-water species, e.g. the snake pipefish Entelurus aequoreus (Linnaeus, 1758), have peaked and declined in the BS (Rusyaev et al. 2006; Reeve Citation2008; Stiansen et al. Citation2009). Additionally, the blue whiting (Micromesistius poutassou Risso, 1827) stock that primarily feeds in the Norwegian Sea has spread considerably into the BS during the 2000s, with its highest abundance in 2004, but followed by a decrease thereafter. The appearance of this species in the western and central BS, a fringe area compared to its normal distributional range, initially led many to conclude that this is an example of how the warming of the sea induces changes to the ecosystem. Today it is realized that this sudden change was primarily caused by one or two highly abundant year-classes, for which survival was facilitated by a concurrent temperature increase (Heino et al. Citation2008; Dolgov et al. Citation2010). These examples highlight the need for large geographic-scale monitoring to identify changes in species distribution and a long-term perspective to identify temporal variability in species distributions. They also illustrate the need for future management to consider the structure and functioning of populations and ecosystems in a wider sense.
One of the significant accomplishments of BESS is the strengthened cooperation between Norwegian and Russian scientists and technicians. The well-coordinated effort in planning, implementation and reporting a survey of this size is unique in an international context. This implies that experts are forced to work together to coordinate an integrated synthesis of the information gathered during the survey. In this respect, the working environment associated with the BESS has provided a highly stimulating forum for scientific debates that are integrated across disciplines. The development of new indicators and suitable reference points for key species is another challenge for the future.
Recommendations
Based on eight years of experience with ecosystem surveys in the BS, we recommend the following for optimizing comprehensive, multipurpose surveys in general and the BESS in particular.
| • | An interdisciplinary group made up of members from the main institutions involved should define clear objectives for the surveys and agreed-upon priorities among all tasks currently undertaken during the surveys. | ||||||||||||||||
| • | A long-term perspective of the survey should be established to ensure continuity in the objectives, sampling design and the resources needed to perform a survey with such objectives. | ||||||||||||||||
| • | It should be considered whether the survey area should be expanded towards the north and east to include areas that were previously ice covered but in recent years have become progressively more ice-free due to the increased melting of ice. | ||||||||||||||||
| • | Effort should be allocated to a thorough analysis of how various survey designs fulfil the goals defined by the group mentioned above. For the BESS, the aim should be to develop and implement a new standard survey design. | ||||||||||||||||
| • | Improvements and further developments of the sampling methodology, instrumentation and equipment should be evaluated. In particular, it should be considered whether:
| ||||||||||||||||
| • | The necessary days-at-sea must be thoroughly evaluated. For the BESS, the survey effort should be maintained at the 2005–2007 level (150–200 ship days) awaiting further input based on the work suggested above before any changes are made to the survey length. | ||||||||||||||||
| • | It should be determined whether some of the investigations could be conducted less regularly, e.g. every second or third year, based on a prioritized list of key ecosystem components and the expected rates of change in target populations as well as their longevity. The necessary days-at-sea could increase, however, if decisions are made to expand the cruise when new regions become accessible due to ice retreat. | ||||||||||||||||
| • | For the BESS, it should also be considered whether some of the investigations could benefit from being conducted in other seasons of the year. | ||||||||||||||||
Editorial responsibility: Franz Uiblein
Acknowledgements
Organizing and conducting ecosystem surveys demands a tremendous effort. We are therefore grateful to the research vessel crews and the scientific staff both in Norway and Russia that have organized and participated in the surveys, and those on land who have analysed the samples. Without their dedication and professionalism, it would not have been possible to collect such a huge amount of high-quality data. This article was financially supported by the Norwegian Research Council through the BarEcoRe project (NRC contract number 200793/S30), the Russian Polar Research Institute of Marine Fisheries and Oceanography and the Institute of Marine Research in Norway.
Notes
Published in collaboration with the Institute of Marine Research, Norway
References
- Agnalt , A-L , Jørstad , KE , Pavlov , VA and Olsen , E. 2010 . “ Recent trends in distribution and abundance of the snow crab (Chionoecetes opilio). Population in the Barents Sea ” . In Biology and Management of Exploited Crab Populations under Climate Change , Edited by: Kruse , GH , Eckert , GL , Foy , RJ , Lipcius , RN , Sainte-Marie , B Stram , DL . 81 – 90 . Alaska, Sea Grant : University of Alaska, Fairbanks .
- Agnalt A-L , Pavlov V , Jørstad KE , Farestveit E , Sundet J. 2011 . The snow crab, Chionoecetes opilio (Decapoda, Majoidea, Oregoniidae) in the Barents Sea . In : Galil BS , Clark PF , Carlton JT In the Wrong Place – Alien Marine Crustaceans: Distribution, Biology and Impacts, Invading Nature. Springer Series in Invasion Ecology 6 Springer : Dordrecht , p 283 – 300 .
- Alvsvåg , J , Agnalt , A-L and Jørstad , KE. 2009 . Evidence for a permanent establishment of the snow crab (Chionoecetes opilio) in the Barents Sea . Biological Invasions , 11 : 587 – 95 . doi: 10.1007/s10530-008-9273-7
- Anisimova NA , Jørgensen LL , Lubin P , Manushin I. 2011 . Benthos . Chapter 4.1 in : Jakobsen T , Ozhigin VK The Barents Sea. Ecosystem, Resources, Management. Half a Century of Russian–Norwegian Cooperation . Trondheim : Tapir Academic Press , p 121 – 59 .
- Årthun , M , Ingvaldsen , R , Smedsrud , B and Schrum , C. 2011 . Dense water formation and circulation in the Barents Sea . Deep-Sea Research I , 58 : 801 – 17 . doi: 10.1016/j.dsr.2011.06.001
- Aschan , M and Ingvaldsen , R. 2009 . Recruitment of shrimp (Pandalus borealis) in the Barents Sea related to spawning stock and environment . Deep-Sea Research II , 56 : 2012 – 22 . doi: 10.1016/j.dsr2.2008.11.012
- Bogstad B , Dingsør GE , Ingvaldsen RB , Gjøsæter H. 2013 . Changes in the relations between oceanographic conditions and recruitment of cod, haddock and herring in the Barents Sea . Marine Biology Research 9 : 895 – 907 .
- Boitsov , VD , Karsakov , AL and Trofimov , AG. 2012 . Atlantic water temperature and climate in the Barents Sea, 2000–2009 . ICES Journal of Marine Science , 69 : 833 – 40 . doi: 10.1093/icesjms/fss075
- Carscadden JE , Gjøsæter H , Vilhjálmsson H. 2013 . A comparison of recent changes in distribution of capelin (Mallotus villosus) in the Barents Sea, around Iceland and in the Northwest Atlantic . Progress in Oceanography. http://dx.doi.org/10.1016/j.pocean.2013.05.005
- Dalpadado , P and Bogstad , B. 2004 . Diet of juvenile cod (age 0–2) in the Barents Sea in relation to food availability and cod growth . Polar Biology , 27 : 140 – 54 . doi: 10.1007/s00300-003-0561-5
- Dalpadado , P , Ingvaldsen , RB , Stige , LC , Bogstad , B , Knutsen , T Ottersen , G . 2012 . Climate effects on the Barents Sea ecosystem dynamics . ICES Journal of Marine Science , 69 : 1303 – 16 . doi: 10.1093/icesjms/fss063
- Dingsør , GE. 2005 . Estimating abundance indices from the international 0-group fish survey in the Barents Sea . Fisheries Research , 72 : 205 – 18 . doi: 10.1016/j.fishres.2004.11.001
- Dolgov , A , Johannesen , E , Heino , M and Olsen , E. 2010 . Trophic ecology of blue whiting in the Barents Sea . ICES Journal of Marine Science , 67 : 483 – 93 . doi: 10.1093/icesjms/fsp254
- Dragesund O , Hylen A , Olsen S , Nakken O . 2008 . The Barents Sea 0-group surveys; a new concept of prerecruitment studies . In: Nakken O , Norwegian spring-spawning herring and Northeast Arctic cod – 100 Years of Research and Management . Tapir Academic Press , Trondheim , p 119 – 136 .
- Drinkwater , KF , Beaugrand , G , Kaeriyama , M , Kim , S , Ottersen , G Perry , RI . 2010 . On the processes linking climate to ecosystem changes . Journal of Marine Systems , 79 : 374 – 88 . doi: 10.1016/j.jmarsys.2008.12.014
- Eriksen E , Dalpadado P . 2011 . Long-term changes in Krill biomass and distribution in the Barents Sea: are the changes mainly related to capelin stock size and temperature conditions? Polar Biology , 34 ( 9 ): 1399 – 1409 .
- Eriksen , E , Ingvaldsen , R , Stiansen , JE and Johansen , GE. 2012a . Thermal habitat for 0-group fishes in the Barents Sea; How climate variability impacts their density, length and geographical distribution . ICES Journal of Marine Science , 69 : 870 – 79 . doi: 10.1093/icesjms/fsr210
- Eriksen , E and Prozorkevich , D. 2011 . “ 0-group survey ” . In The Barents Sea Ecosystem, Resources, Management. Half a Century of Russian–Norwegian Cooperation , Edited by: Jakobsen , T and Ozhigin , VK . 557 – 569 . Trondheim : Tapir Academic Press .
- Eriksen , E , Prozorkevich , DV and Dingsør , GE. 2009 . An evaluation of 0-group abundance indices of the Barents Sea fish stocks . The Open Fish Science Journal , 2 : 6 – 14 . doi: 10.2174/1874401X00902010006
- Eriksen E , Prozorkevich DV , Trofimov A , Howell D. 2012b . Biomass of Scyphozoan jellyfish, and its spatial association with 0-group fish in the Barents Sea . PLoS ONE 7 ( 3 ): e33050 . 10 pages. doi: 10.1371/journal.pone.0033050
- Fauchald , P , Mauritzen , M and Gjøsæter , H. 2006 . Density-dependent migratory waves in the marine pelagic ecosystem . Ecology , 87 : 2915 – 24 . doi: 10.1890/0012-9658(2006)87[2915:DMWITM]2.0.CO;2
- Foote KG , Atkins PR , Francis DTI , Knutsen T. 2005 . Measuring echo spectra of marine organisms over a wide bandwidth . In : Papadakis JS , Bjørnø L Proceedings of the International Conference on Underwater Acoustic Measurements: Technologies and Results, II . Institute of Applied and Computational Mathematics (IACM) at the Foundation for Research and Technology (FORTH) , Heraklion , Greece , 28 June–1 July 2005 , p 501 – 08 .
- Garcia SM , Zerbi A , Aliaume C , Do Chi T , Lasserre G. 2003 . The ecosystem approach to fisheries. Issues, terminology, principles, institutional foundations, implementation and outlook . FAO Fisheries Technical Paper 443 . 71 pages.
- Gjøsæter H. 1999 . Studies on the Barents Sea capelin (Mallotus villosus Müller), with emphasis on growth. PhD thesis . Institute of Fisheries Biology, University of Bergen . 160 pages.
- Gjøsæter , H , Bogstad , B and Tjelmeland , S. 2002 . Assessment methodology for Barents Sea capelin, Mallotus villosus (Müller) . ICES Journal of Marine Science , 59 : 1086 – 95 . doi: 10.1006/jmsc.2002.1238
- Gjøsæter , H , Bogstad , B and Tjelmeland , S. 2009 . Ecosystem effects of the three capelin stock collapses in the Barents Sea . Marine Biology Research , 5 : 40 – 53 . doi: 10.1080/17451000802454866
- Gjøsæter H , Loeng H. 1984 . Distribution and growth of the capelin in the Barents Sea in relation to water temperature in the period 1974 to 1983 . ICES CM 1984/G:16 . 15 pages .
- Gjøsæter H , Tjelmeland S , Bogstad B . 2012 . Ecosystem-based management of fish species in the Barents Sea . In : Kruse GH , Browman HI , Cochrane KL , Evans D , Jamieson GS , Livingston PA , et al. Global Progress in Ecosystem-Based Fisheries Management . Alaska Sea Grant, University of Alaska, Fairbanks , p 333 – 52 .
- Green NW , Molvær J , Kaste Ø , Schrum C , Yakushev K , Sørensen K , et al. 2010 . Tilførselsprogrammet 2009. Overvåking av tilførsler og miljøtilstand i Barentshavet og Lofotenområdet . Klima og Forurensningsdirektoratet (Klif) rapport TA 2660/2010. Norsk institutt for vannforskning (NIVA) rapport nr. 5980-2010 . 243 pages. (in Norwegian)
- Hamre J , Tjelmeland S. 1982 . Sustainable yield estimates of the Barents Sea capelin stock . ICES CM 1982/H:45 . 24 pages.
- Handegard NO , du Buisson L , Brehmer P , Chalmers SJ , De Robertis A , Huse G , et al. 2012 . Toward a global observation and modelling system for studying the ecology of the open ocean using acoustics . Fish and Fisheries . http://onlinelibrary.wiley.com/doi/10.1111/j.1467-2979.2012.00480.x/full
- Haug , T , Bjørge , A , Øien , N , Ziryanov , SV and Golikov , AP. 2011 . “ Marine mammals. Chapter 7 in ” . In The Barents Sea. Ecosystem, Resources, Management , Edited by: Jakobsen , T and Ozhigin , VK . 395 – 430 . Trondheim : Tapir Academic Press. Half a Century of Norwegian-Russian Cooperation .
- Heino , M , Engelhard , GH and Godø , OR. 2008 . Ocean climate and migrations determine the abundance fluctuations of blue whiting in the Barents Sea . Fisheries Oceanography , 17 : 153 – 63 . doi: 10.1111/j.1365-2419.2008.00472.x
- Hop H , Gjøsæter H . 2013 . Polar cod (Boreogadus saida) and capelin (Mallotus villosus) as key species in marine food webs of the Arctic and the Barents Sea . Marine Biology Research . 9 : 878 – 894 .
- Howell D , Filin AA , Bogstad B , Stiansen JE . 2013 . Unquantifiable uncertainty in projecting stock response to climate change: Example from North East Arctic cod . Marine Biology Research 9:920–931 .
- Hvingel C , Berenboim BI . 2011 . Northern shrimp . Chapter 4.4 in : Jakobsen T , Ozhigin VK The Barents Sea . Ecosystem, Resources, Management. Half a Century of Russian-Norwegian Cooperation . Trondheim : Tapir Academic Press , p 172 – 85 .
- ICES 2010 . NAFO/ICES Pandalus Assessment Group Meeting, 20–27 October 2010 . ICES Headquarters, Copenhagen, Denmark. ICES CM 2010/ACOM:14 . 79 pages.
- ICES . 2011a . Report of the Arctic Fisheries Working Group , Hamburg, 28 April–4 May 2011. ICES CM 2011/ACOM:05 . 659 pages.
- ICES . 2011b . Report of the Working Group on Widely Distributed Stocks (WGWIDE) , 23–29 August 2011 , ICES Headquarters , Copenhagen , Denmark . ICES CM 2011/ACOM:15 . 624 pages.
- ICES . 2011c . NAFO/ICES Pandalus Assessment Group Meeting , 19–26 October 2011 . ICES Headquarters , Copenhagen , Denmark . ICES CM 2011/ACOM:14 . 81 pages.
- Ingvaldsen RB , Gjøsæter H . 2013 . Impact of marine climate variability on the spatial distribution of Barents Sea capelin . Marine Biology Research 9 : 867 – 77 .
- Ingvaldsen , R , Loeng , H , Ådlandsvik , B and Ottersen , G. 2003 . Climate variability in the Barents Sea during the 20th century with focus on the 1990s . ICES Marine Science Symposium , 219 : 160 – 68 .
- Jakobsen T , Ozhigin VK . 2011 . The Barents Sea. Ecosystem, Resources, Management. Half a Century of Russian–Norwegian Cooperation . Trondheim : T. Tapir Academic Press , 825 pages.
- Johannesen E , Høines ÅS , Dolgov AV , Fossheim M . 2012a . Demersal fish assemblages and spatial diversity patterns in the Arctic–Atlantic transition zone in the Barents Sea . PLoS ONE 7 : e34924 . 14 pages . doi: 10.1371/journal.pone.0034924
- Johannesen , E , Ingvaldsen , RB , Dalpadado , P , Skern-Mauritzen , M , Stiansen , JE Eriksen , E . 2012b . The Barents Sea ecosystem state 1970–2009: Climate fluctuations, human impact and trophic interactions . ICES Journal of Marine Science , 69 : 880 – 89 . doi: 10.1093/icesjms/fss046
- Johannesen , E , Lindstrøm , U , Michalsen , K , Skern-Mauritzen , M , Fauchald , P Bogstad , B . 2012c . Feeding in a heterogeneous environment: Spatial dynamics in summer foraging Barents Sea cod . Marine Ecology Progress Series , 458 : 181 – 97 . doi: 10.3354/meps09818
- Johansen GO , Johannessen E , Michalsen K , Aglen A , Fotland Å . 2013 . Seasonal variation in geographic distribution of NEA cod – Survey coverage in a warmer Barents Sea . Marine Biology Research 9 : 908 – 919 .
- Karasev , E , Karaseva , A and Karlsbakk , E. 2011 . “ Diseases and parasites ” . In The Barents Sea Ecosystem, Resources, Management. Half a Century of Russian–Norwegian Cooperation , Edited by: Jakobsen , T and Ozhigin , VK . 743 – 49 . Trondheim : Tapir Academic Press .
- Lavery , AC , Chu , D and Moum , JN. 2010 . Measurements of acoustic scattering from zooplankton and oceanic microstructure using a broadband echosounder . ICES Journal of Marine Science , 67 : 379 – 394 . doi: 10.1093/icesjms/fsp242
- Lind , S and Ingvaldsen , RB. 2012 . Variability and impacts of Atlantic Water entering the Barents Sea from the north . Deep-Sea Research I , 62 : 70 – 88 . doi: 10.1016/j.dsr.2011.12.007
- Loeng , H , Blindheim , J , Ådlandsvik , B and Ottersen , G. 1992 . Climatic variability in the Norwegian and Barents Seas . ICES Marine Science Symposia , 195 : 52 – 61 .
- Mathisov G , Moiseev D , Lyubina O , Zhichkin A , Dzhenyuk S , Karamushko O , et al. 2012 . Climate and cyclic hydrobiological changes of the Barents Sea from the twentieth to twenty-first centuries . Polar Biology 35 : 1773 – 90 .
- Michalsen K , Dalpadado P , Eriksen E , Gjøsæter H , Ingvaldsen R , Johannesen E , et al. 2011 . The joint Norwegian–Russian ecosystem survey: overview and lessons learned . Proceeding, of the 15th Norwegian–Russian Symposium, Longyearbyen , Norway , 6–9 September 2011 , p 247 – 72 .
- Myers , RA. 1998 . When do environment–recruitment correlations work? . Reviews in Fish Biology and Fisheries , 8 : 285 – 305 . doi: 10.1023/A:1008828730759
- NRPA . 2011 . Radioactivity in the Marine Environment 2008 and 2009 . Results from the Norwegian National Monitoring Programme (RAME). Strålevern Rapport 2011:4. Østerås : Norwegian Radiation Protection Authority . 108 pages.
- Orlova , EL , Dalpadado , P , Knutsen , T , Nesterova , VN and Prokopchuk , IP. 2011 . “ Zooplankton ” . In The Barents Sea Ecosystem, Resources, Management. Half a Century of Russian–Norwegian Cooperation , Edited by: Jakobsen , T and Ozhigin , VK . 91 – 120 . Trondheim : Tapir Academic Press .
- Orlova EL , Dolgov AV , Renaud PE , Boitsov VD , Prokopchuk IP , Zashihina MV . 2013 . Structure of the macroplankton–pelagic fish–cod trophic complex in a warmer Barents Sea . Marine Biology Research 9 : 851 – 866 .
- Orlova , EL , Rudneva , GB , Renuad , PE , Eiane , K , Savinov , V and Yurko , AS. 2010 . Climate impact on feeding and condition of capelin Mallotus villosus in the Barents Sea: evidence and mechanisms from a 30 year data set . Aquatic Biology , 10 : 105 – 18 .
- Planque B , Certain G , Michalsen K , Wiedmann M , Kortsch S , Jørgensen LL , et al. 2012 . Ecological resilience research in practice: The experience of the Barents Sea Ecosystem Resilience project (BarEcoRe) . ICES CM 2012/A:06 . 10 pages.
- Planque B , Johannesen E , Michalsen K , Primicerio R , Fossheim M , Ingvaldsen R , et al. 2010 . Barents Sea ecosystem resilience under global environmental change . ICES CM 2012/Q:27. Poster .
- Reeve M. 2008 . A study of the distribution, biology and ecology of snakepipefish (Entelurus aequoreus) as a new species in the Barents Sea . MSc thesis. University of Bergen , Norway . 62 pages.
- Rusyaev , SM , Dolgov , AV and Karamushko , OV. 2007 . Captures of snake pipefish Entelurus aequoreus in the Barents and Greenland Seas . Journal of Ichthyology , 47 : 544 – 46 . doi: 10.1134/S0032945207070090
- Røttingen I , Gjøsæter H , Sunnset BH . 2007 . Fifty years of Norwegian–Russian scientific cooperation . Marine Research News 2007:16. ISSBN 0804-5496 . 2 pages.
- Røttingen I , Tjelmeland S . 2009 . Towards a MSY-based management of Barents Sea Capelin in an ecosystem context . ICES CM 2009/R:07 . 12 pages.
- Sakshaug E , Johansen G , Kovacs K . 2009 . Ecosystem Barents Sea . Trondheim : Tapir Academic Press . 587 pages.
- Skern-Mauritzen , M , Johannesen , E , Bjørge , A and Øien , N. 2011 . Baleen whale distributions and prey associations in the Barents Sea . Marine Ecology Progress Series , 426 : 289 – 301 . doi: 10.3354/meps09027
- Stanton , TK , Chu , D , Jech , JM and Irish , JD. 2010 . New broadband methods for resonance classification and high-resolution imagery of fish with swimbladders using a modified commercial broadband echosounder . ICES Journal of Marine Science , 67 : 365 – 78 . doi: 10.1093/icesjms/fsp262
- Stiansen JE , Korneev O , Titov O , Arneberg P . 2009 . Joint Norwegian–Russian environmental status 2008. Report on the Barents Sea Ecosystem . Part II – Complete report. IMR/PINRO Joint Report Series 3-2009 . 378 pages.
- Søreide JE , Carroll ML , Hop H , Ambrose Jr WG , Hegseth EN , Falk-Petersen S . 2013 . Trophic structures and carbon flows in Arctic and Atlantic waters around Svalbard revealed by stable isotopic and fatty acid tracers . Marine Biology Research 9 : 831 – 850 .
- Travers , M , Shin , Y , Jennings , S and Cury , P. 2007 . Towards end-to-end models for investigating the effects of climate and fishing in marine ecosystems . Progress in Oceanography , 75 : 751 – 70 . doi: 10.1016/j.pocean.2007.08.001
- Trenkel , VM , Ressler , PH , Jech , M , Giannoulaki , M and Taylor , M. 2011 . Underwater acoustics for ecosystem-based management: State of the science and proposals for ecosystem indicators . Marine Ecology Progress Series , 442 : 285 – 301 . doi: 10.3354/meps09425
- Wienerroither R , Johannesen E , Dolgov A , Byrkjedal I , Bjelland O , Drevetnyak K , et al. 2011 . Atlas of the Barents Sea Fishes . IMR/PINRO Joint Report Series 1-2011 . 272 pages.
- Yakushev EV , Sørensen K . 2013 . On seasonal changes of the carbonate system in the Barents Sea: observations and modeling . Marine Biology Research 9 : 822 – 830 .
- Yaragina , NA , Bogstad , B and Kovalev , YA. 2009 . Variability in cannibalism in Northeast Arctic cod (Gadus morhua) during the period 1947–2006 . Marine Biology Research , 5 : 75 – 85 . doi: 10.1080/17451000802512739