Abstract
Hardangerfjord is the second largest fjord in Norway and is situated on the southwest coast. During the last century the fjord has been influenced by a variety of anthropogenous activities such as industry, hydro-electrical power plants and since 1980 an increase in fish farming. This study was carried out in order to investigate whether changes in the macroalgal communities of Hardangerfjord have taken place since the 1950s. The macroalgal composition at a number of stations investigated in 2008–2009 was compared to recordings from the same stations during the 1950s. While the distribution and abundance of dominant groups (fucoids, kelps) showed a high resilience when compared to recordings from the 1950s, some changes in the macroalgal communities in the fjord were evident. The present study showed higher species richness and a higher frequency of species with a warm-water affinity. Since the first part of the 1990s an increase in summer sea surface temperatures has taken place in the area, and the observed algal changes suggest a pronounced temperature effect on macroalgal communities. A number of red algal species was observed to protrude further into the fjord in the present study than in the 1950s, probably caused by altered salinity regime due to the electrical power industry.
Introduction
Ecosystems in the coastal zone worldwide are under increasing pressure due to human activities. The coastal shores may be impacted by eutrophication, pollution, habitat destruction, increased sedimentation and establishment of introduced species. It can often be hard to discriminate the most important factors causing changes in the marine ecosystems. The lack of good baseline studies and poor knowledge about natural variations in marine communities can make it difficult to demonstrate if changes have taken place. The effects of climate variations and ocean warming may complicate the picture even further and must therefore be considered carefully in studies of anthropogenic impact on marine ecosystems.
Seaweed communities on rocky shores experience large fluctuations in a number of environmental factors and will normally show a high degree of stability and persistence, when not subjected to strong grazing pressure (Davison & Pearson Citation1996; Allison Citation2004; Sjøtun et al. Citation2006). Kelp and fucoids are the common providers of three-dimensional habitats in the shallow coastal waters of the cold–temperate zone, but major stress events may alter this community structure. Such stress events may involve extreme temperature events, changes in salinity and current patterns, increased nutrient load and sedimentation rates, grazing pressure, or establishment of novel and competitive algae species (Sagarin et al. Citation1999; Britton-Simmons Citation2004; Casas et al. Citation2004; Aguilera and Rautenberg, Citation2011). The understorey and epiphytic algal communities are often composed of a variety of smaller and ephemeral species, which may respond differently to environmental fluctuations than kelp and fucoids (Worm & Sommer Citation2000; Hemmi et al. Citation2005).
Hardangerfjord is the second largest fjord in Norway and is situated on the southwest coast. The sea surface area inside the sill is approximately 800 km2. The fjord penetrates 165 km inland from the coast and is divided in several fjord branches and is surrounded by mountains with a steeper slope inwards of the fjord. It is strongly influenced by freshwater runoff from the mountains and the Folgefonna glacier. The precipitation mainly comes as snow in the winter, which melts resulting in a spring flood from May, persisting through the summer months. Although the freshwater runoff may vary considerably from year to year, it normally creates a brackish layer throughout the fjord in the summer, while winter conditions are more saline. Only the inner parts of the fjord branches are brackish all year round. The brackish layer varies from 3 to 5 m deep. The water exchange in the fjord is considered to be good and is driven by wind, tidal waves and density currents (Asplin et al. Citation2014), and the retention time for surface water is approximately one month (Ervik et al. Citation2008).
The human population around Hardangerfjord has been decreasing during the last decades, and is currently around 23,000 inhabitants (Statistics Norway). The area is one of the most intense fish-farming areas in Norway. Since the salmon production started up in 1979, the annual production has been steadily increasing and is currently 60000–70000 t per year (Directorate of Fisheries). The high production level is resulting in emissions of approximately 130 t of DIP (dissolved inorganic phosphorous) and 770 t of DIN (dissolved inorganic nitrogen) annually (calculated by Ancyllus-MOM based on 2010 biomass (Stigebrandt et al. Citation2004)). The inner fjord area is also impacted from past metal industry, and the water flow in many of the rivers to the fjord is regulated by hydro-electric power plants. One of the fjord branches, Sørfjorden, is still influenced by discharges from a former smelter. The industry discharged approximately 50 t of black waste lime from dicyandiamide production into the water each year in the late 1990s, resulting in extreme low oxygen conditions, high nitrate levels and toxic flagellate blooms (Aure & Pettersen Citation1998). Piles of black waste lime still remain on the bottom close to the factory in the inner part of the fjord branch and are continuously reactive, consuming oxygen and producing nitrate. The factory opened in 1908 and was closed down in 2002 and the area has partly recovered over the last decade (Ruus et al. Citation2006). It is uncertain whether the impact from the factory was the same in the 1950s, as no environmental concern were raised before 1996, and there are no data on the historical emissions.
Historical data have been used in order to provide evidence of changes in marine communities on several occasions (Simkanin et al. Citation2005; Lima et al. Citation2007; Husa et al. Citation2008). The aim of this study is to use historical data from the Hardangerfjord area to detect temporal changes in the seaweed communities due to anthropogenic impact during the last 60 years. The algal vegetation in Hardangerfjord area was studied by Jorde & Klavestad (Citation1963) during the period 1955–1960. In order to study temporal changes in the macroalgal communities in Hardangerfjord, 22 sites along the fjord previously examined in the period 1955–1960 were reinvestigated in the summers of 2008 and 2009 using the same methods as in the historical study (Jorde & Klavestad Citation1963). In addition, data on the distribution of dominating functional groups such as kelp, sea urchins and filamentous algae have been obtained from video recordings.
Material and methods
Sampling and data collection
In the present study, the macroalgal community composition was investigated at 22 of 34 sites () examined in 1955–1960, for which complete species lists exist (see in Jorde & Klavestad Citation1963). The reinvestigated stations were located from the outer to the inner parts of Hardangerfjord, including the fjord branches. An overview of stations, sampling years and activity is given in . The positions of the original stations were localized from illustrations and descriptions in the field diary of I. Jorde (deposited at the University Museum of Bergen). In cases where the original site was difficult to locate precisely, the closest site with similar inclination and substrate composition as described in the field diary was selected. At two of the stations (37 Lofthus, 64 Borgund), the sampling sites had to be moved (up to 200 m) from the original sites, due to construction of a quay and a boat house, respectively. All sampling and data collection was carried out during 9–13 June 2008 and 22 June–3 July 2009 as the main collections of algae in 1955–1960 were during these summer months. Samples were collected from 7 stations in 2008 and from 15 in 2009 ().
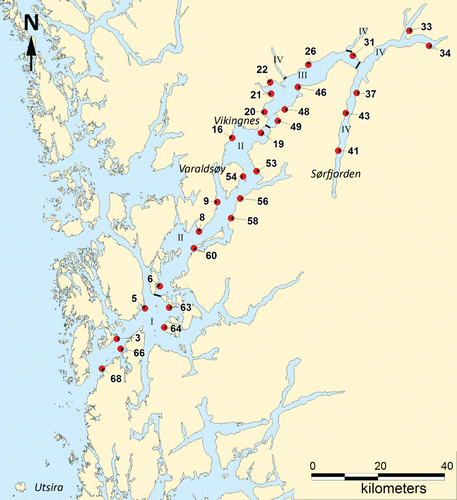
Table I Overview of stations, with data on sampling and data collection, investigated in Hardangerfjord in 1955–1960 and reinvestigated in 2008–2009. * The station is slightly moved due to a construction.
The sampling methods described by Jorde & Klavestad (Citation1963) were followed closely in the reinvestigation of the macroalgal community composition, and included sampling by hand and by rake in the intertidal and by dredging in the subtidal areas. Dredging in the shallow subtidal zone (normally to 5 m depth) was done by using a small handheld triangular dredge (50×50×50 cm) from a small boat, while a larger triangular dredge (70×70×70 cm) was used to collect algal samples from deeper waters. The dredgings at each station were made as close as possible to the dredging depths from the first investigation, based on information from Jorde & Klavestad (Citation1963) and information from the original field diary. Algal material was also sampled by scuba diving from 15 m depth to the surface at those localities where material was collected by divers in 1960 (Stations 16 and 22).
Kelp, fucoids and easily identifiable species were recorded in situ, while the rest of the algal material were sorted on the boat and samples of each species and unidentified material were preserved in 4% formaldehyde in seawater for later identification in the laboratory. The occurrence of all taxa was recorded as present or absent at each station. Taxa were identified to lowest possible taxonomic levels by the use of relevant literature (Dixon & Irvine Citation1977; Kornmann & Sahling Citation1977; Rueness Citation1977; Prud'Homme van Reine Citation1982; Irvine Citation1983; Forward & South Citation1985; Maggs & Hommersand Citation1993; Irvine & Chamberlain Citation1994; Brodie & Irvine Citation2003; Brodie et al. Citation2007). The nomenclature follows Guiry & Guiry (Citation2012). Genera with an unresolved taxonomic status were identified to genus level. Voucher samples of each species found in the investigation in 2008–2009 are kept at the University of Bergen, and a collection of herbarium specimens are deposited at the University Museum of Bergen. The division of algae into biogeographic groups follows Brattegard and Holthe, which divides the Norwegian coast into 26 sectors. Southern species are defined as species with a northern boundary on the coast, northern species are species with a southern boundary and pansectorial species occur in all sectors.
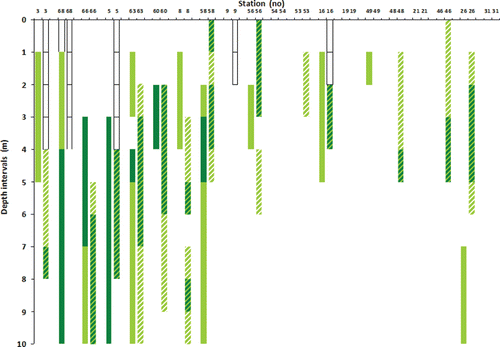
To explore depth variations in macroalgae and sea urchin distribution, video recordings were made between 0 and 25 m depth in June 2009, at 21 of the stations previously investigated by Jorde & Klavestad (Citation1963) (). An underwater video system (UVS 5080), deployed from a surface vessel through a connection cord, was used for this purpose. The video camera was moved at a constant speed of 0.5–1 knot, 0.5–1 m above the seafloor, along vertical transects at a 90° angle to the shoreline. The abundances of Laminaria spp. and Saccharina latissima were recorded in depth intervals of 1 m between 0 and 10 m depth at each station, in the following categories: (1) no kelp, (2) scattered kelps, (3) dense kelp vegetation. In order to explore possible seasonal variations in sea urchin and algae, additional video recordings were made at stations 9, 16, 48 and 20 (, ) in February 2010, and compared to recordings made at the same stations in June 2009. For each video transect the approximate average coverage of kelp and turf forming or filamentous algae and the approximate density of sea urchins was estimated at fixed depth intervals.
Statistical methods
Multidimensional scaling ordination (MDS) based on the Bray–Curtis Similarity Index was used to examine temporal differences in community structure (Bray & Curtis Citation1957; Shepard Citation1962; Kruskal Citation1964). The two sets of recordings of macroalgal taxa were adjusted before the analyses by excluding a number of small genera which are difficult to identify, such as encrusting and calcareous maerl species. Genera which included species that were difficult to identify were included as genera in the analyses. Similarity percentage analyses (SIMPER) were used to identify which species contributed to similarity in each investigation and which species contributed to the dissimilarity in species composition between the investigations. The analyses were conducted using Primer software (Plymouth Routines in Multivariate Ecological Research 5.2.9). Temporal differences in species number, the percentage share of southern species and species in each algal group were tested on data from pooled sites by non-parametric analysis (Mann–Whitney U-test, Analyze-it Standard edition).
Results
Temporal changes in community composition and species richness
The MDS plot of similarity between individual stations in Hardangerfjord shows the temporal differences in community structure between the two studies, and illustrates how the temporal dissimilarity increases with distance to the fjord mouth (). A pair-wise comparison of the Bray–Curtis Similarity Index from all stations in 1955–1960 and 2008–2009 reveals that the highest temporal dissimilarity was found in the fjord branches. The average dissimilarity between macroalgal communities at all stations in 1955–1960 and in 2008–2009 was 44.05% (SIMPER) and the taxa contributing to this dissimilarity are shown in . The macroalgal community at all stations in the fjord showed a slightly higher average similarity with each other in 2008–2009 (68.54%) than in 1955–1960 (61.14%) (SIMPER).
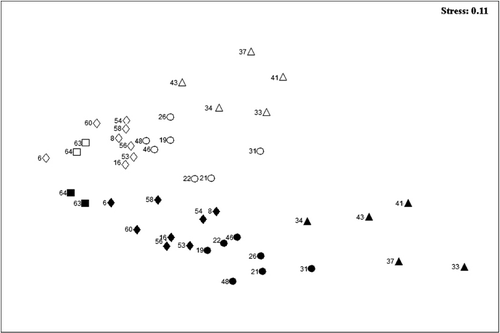
Table II Average abundance (reflectsing the number of stations where the species were found) of species in Hardangerfjord (based on present/absent data) contributing to approximately 30% of the net dissimilarity in macroalgal assemblages between years 1955–1960 and 2008–2009 determined by SIMPER analysis. 10% of the rarest species are excluded before the analysis. Species distribution: S, southern species (species with a southern distribution in Norway); X, pansectorial species (species that occurs along the entire Norwegian coastline).
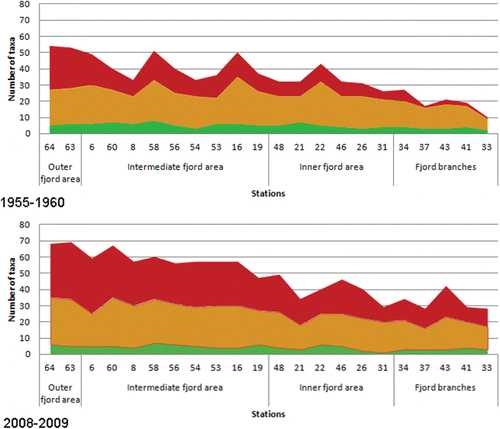
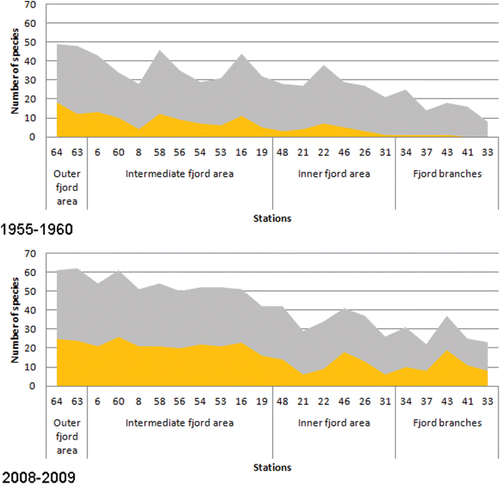
A total number of 109 taxa were identified in Hardangerfjord in 2008–2009, whereas a total number of 88 taxa were recorded in the 1950s (see Online Supplementary Material). The number of taxa recorded at each station was significantly higher in 2008–2009 than in 1955–1960 (Mann–Whitney U-test, p<0.05; ). There was also a significant increase in the percentage share of red algal taxa in 2008–2009 (Mann–Whitney U-test, p<0.05) compared to the 1950s (). A significant temporal increase in the percentage share of southern species compared to the number of pansectorial species in each study was also evident (Mann–Whitney U-test, p <0.05; ).
A number of species was recorded in only one of the two studies. While most of the species observed in the 1950s were also found in 2008–2009, a number of species new to Hardangerfjord were recorded for the first time in the present study (). Bryopsis hypnoides J. W. Lamouroux was not present in the 1950s, but abundant, growing on mussels, at eight sites in 2008–2009. The rare species Bryopsis plumosa (Hudson) C. Agardh was also found at one locality in Varaldsøy in 2009. The brown alga Acrothrix gracilis Kylin is considered rare on the Norwegian coast and was found at only one site in the outer part of the fjord in 1955–1960, but was present at nine sites in 2008–2009. The brown alga Sphacelaria plumula Zanardini, regarded as rare in Scandinavia, was recorded at one site (Stn 16 Skjerring) in the 2008–2009 study. Several red algal species with few previous recordings from Norway were also found in 2008–2009: Compsothamnion thuyoides (Smith) Nägeli, Halurus flosculosus (J. Ellis) Maggs & Hommersand and Schmitzia hiscockiana Maggs & Guiry. Schmitzia hiscockiana, a species not yet described at the time of the first investigation, was found at one site (Stn 43 Aga in Sørfjorden) both in 2009 and on a revisit at this locality in 2010. Notable was also the emergence and high occurrence of several red algal species which did not appear in the fjord in the 1950s, such as Cystoclonium purpureum (Hudson) Batters, Rhodophyllis divaricata (Stackhouse) Papenfuss, Scagelia pylaisaei (Montagne) M. J. Wynne and Seirospora interrupta (Smith) F. Schmitz.
Table III Species only found in Hardangerfjord either in 1955–1960 or in 2008–2009. R, number of records.
Abundance of dominant functional groups
A separate similarity analysis of the presence of habitat building large brown algae in the intertidal and shallow subtidal zone in 2008–2009 showed that the intertidal community at these stations had a high similarity with the recordings in 1955–1960. When comparing the Bray–Curtis Similarity Indices from all stations in 1955–1960 and 2008–2009 pairwise, all sites showed a higher similarity than 80% except for site 19 Ljonestangen, which had a similarity of 72.7%. The species accounting for the negligible temporal dissimilarity at all sites are given in . At site 19 Laminaria digitata and Pelvetia canaliculata were not recorded in 2008–2009. Halidrys siliquosa was not recorded at site 19 in 1955–1960, but was abundant in 2008–2009. Some additional minor changes were evident in the present investigation, e.g. the intertidal Fucus spiralis was found in somewhat greater abundances further into the fjord, and P. canaliculata was absent from some localities in the inner part of the fjord compared to the 1950s.
Table IV Average abundance (reflecting the number of stations where the species were found) of large brown macroalgae in the intertidal and shallow subtidal zone at 22 localities in Hardangerfjord determined by SIMPER analysis (based on present/absent data).
Laminaria spp. occurred most commonly between the upper subtidal down to 4 m depth from station 63 and inwards of the fjord (). Saccharina latissima had a more scattered distribution in the intermediate and innermost parts of the fjord, but was found to be abundant or common down to 5 m depth (). The abundance of Laminaria hyperborea and L. digitata were not separated in the present study like Jorde & Klavestad (Citation1963) did, as these species proved difficult to distinguish from each other in the video recordings. A comparison with results from the 1950s showed that Laminaria spp. and Saccharina latissima were most abundant in the outermost part of Hardangerfjord during both investigations, and that both genera had a similar depth occurrence and showed a sporadic occurrence towards the innermost part of the fjord in both investigations ( and ).
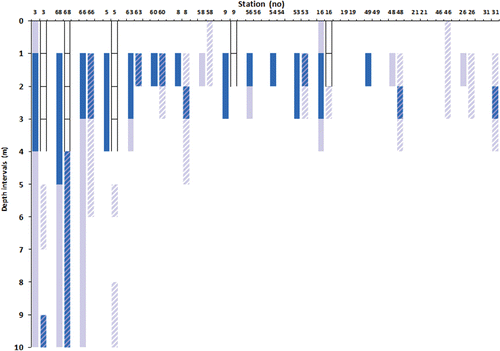
Echinus acutus Lamarck 1816 was the dominant sea urchin in Hardangerfjord and the abundance of the species was high at many stations. The density of sea urchins increased with increasing depth down to 25 m, and their distribution varied geographically with the highest abundances being recorded at stations at the middle part of the fjord. Four of the video stations where sea urchins had been particularly abundant during June 2009 were revisited in February 2010, for analysis of seasonal variation in abundance of algal functional groups (in this case kelp and turf or filamentous algae) and sea urchins (). The upper depth limit of sea urchins had extended upwards from 8 m depth in June 2009 to 2 m depth in February 2010.
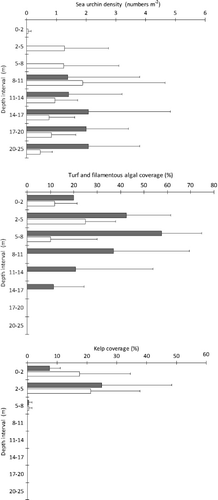
In June 2009 the turf or filamentous algal group was dominated by brown algae extending down to 17 m depth and on average covering more than 50% of the substratum between 5 and 8 m. By February 2010 the dominance of this functional group had shifted towards turf-forming red algae, and the distribution limited to depths shallower than 8 m. Kelp abundance showed little seasonal variation, being largely confined to the upper 5 m in both June and February.
Discussion
Temporal changes in community composition and species richness
The macroalgae communities in the Hardangerfjord have changed since the 1950s, and these changes were more pronounced towards the inner part of the fjord. The main changes in the community structure were identified as increased species richness, increase in red algal taxa recorded at each site and increase in the abundance of species with warm-water affinity.
The main fraction of species contributing to the temporal dissimilarity between investigations had an increased average abundance in the later study. One major contributor to this temporal dissimilarity was the introduced red alga Heterosiphonia japonica. The species was first registered in Norway in 1996 and has spread rapidly along the Norwegian coast (Lein 1996; Husa et al. Citation2004). In 2008–2009 H. japonica was recorded from the sea mouth of the fjord to the innermost fjord branches. The species was absent from only three sites and was very common when dredging deeper than 5 m. In the video survey dense establishments of H. japonica were observed on sandy/mixed bottoms at 10–20 m depth.
Another introduced red alga Bonnemaisonia hamifera was only recorded at three sites in the outer fjord area during the 1950s, but was close to omnipresent in the recent study. Although this species most commonly occurred at deeper intervals, dense associations were recorded in the shallow subtidal zone at several sites. Gametophytes of B. hamifera, a life stage with few previous recordings from the Norwegian coast, were found at three sites in the outer parts of the fjord. The development of gametophytes of B. hamifera is highly dependent on short days and high temperatures (Breeman Citation1988; Breeman & Guiry Citation1989) which we only experience at our latitude in years with prolonged periods of elevated sea temperatures during autumn months. Such optimal temperatures in autumn have occurred frequently the last decade (Husa et al. Citation2008; hydrographic data from www.imr.no; accessed 27 Sept 2013).
One of the major changes to the macroalgal vegetation in Hardangerfjord since the 1950s was the observed increase of southern species. This was the case for several species such as Acrothrix gracilis Kylin, Striaria attenuata, Stictyosiphon soriferus, Brongniartella byssoides, Griffithsia corallinoides (Linnaeus) Trevisan, Bonnemaisonia asparagoides and Seirospora interrupta, which also showed increased abundance in a similar investigation along the Norwegian coast in 2003 (Husa et al. Citation2008). All of these species are restricted to the southern part of Norway (Brattegaard & Holthe, Citation1997) either due to lethal winter temperatures in the north or by too low summer temperatures for growth or reproduction (Breeman, Citation1988). Warm summers and prolonged periods of high temperature in autumn are likely to stimulate populations of species living close to their northern temperature boundary (Harley et al. Citation2006, Citation2012). For some species the temperature optima are not known and the reason for their increased abundance might be ambiguous, but for some – for example Striaria attenuata and Seirospora interrupta – a warm summer would be a clear advantage, as they need 15°C to reproduce (Plattner & Nichols Citation1977; Peters & Breeman Citation1993).
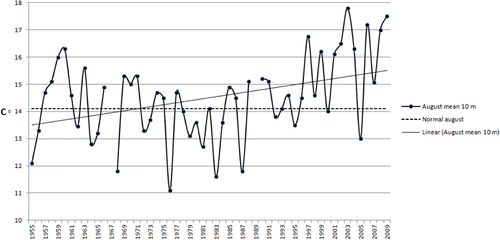
A rapid response to climate changes has recently been reported for macroalgae in other coastal areas as well (Lima et al. Citation2007; Berecibar et al. Citation2009; Wernberg et al. Citation2011a, Citation2011b). Most studies report northward migration of macroalgae with a warm-water affinity. Husa et al. (Citation2008) showed an increased temporal diversity in local macroalgal communities due to a higher occurrence of warm-water species. An increase in mean summer temperatures has been measured at Utsira () during recent decades. Long-term temperature data from Hardangerfjord are missing, but the measurements made at Utsira can give a good indication of the temperatures in Hardangerfjord, because the fjord is strongly influenced by this coastal water (Aure et al. Citation2007). However, sea-water temperatures in Hardangerfjord may diverge from the coastal temperatures depending on wind, currents and melting of snow in the mountains (Asplin et al. Citation2014).
Several red algal species that were only present in the outer part of the fjord in the 1950s were now abundant along the entire fjord and some of them even protruding into the fjord branches. This migration pattern was observed for both southern and pansectorial red algae and was particularly pronounced for relatively large species like Phycodrys rubens Batters, Chondrus crispus, Polysiphonia elongata, Rhodomela confervoides, Coccotylus truncatus and several smaller filamentous red algae. Increased richness in red algal species was evident along the entire fjord but most pronounced in the fjord branches. One example of such a shift in community structure was observed at station 43 Aga in Sørfjorden, where only the three red algal species Hildenbrandia rubra, Polyides rotundus and Polysiphonia stricta were recorded in 1955–1960, while 19 red algal taxa were recorded in 2008–2009.
Jorde & Klavestad (Citation1963) proposed decreased wave exposure and increased stress caused by seasonal variations in salinity as the two most likely factors contributing to the decline in species numbers inwards of the fjord. Of these, change in salinity stress is the most likely one to have influenced temporal differences in species distribution in the inner part of the fjord. Since the 1950s the water flow of a number of large rivers in the area has been regulated, and it is possible that the altered hydrographical regime in the fjord has resulted in less salinity stress due to a less-pronounced spring freshwater runoff, and higher species richness in macroalgal communities in the inner part of the fjord. The impact of an altered salinity regime on macroalgal communities has been little studied. However, an increase in species number, and in particularly for red algal species, has been observed when moving towards higher salinity in the Baltic Sea (Nielsen et al. Citation1995).
Only a few species were less abundant in the recent investigations than in the 1950s. Stictyosiphon tortilis (Ruprecht) Reinke was recorded as common across the fjord by Jorde & Klavestad (Citation1963), even forming associations at several stations, particularly in the inner part of the fjord. Association forming populations of S. tortilis were not recorded in 2008–2009, although the species was present at almost all sites and quite common in many. The small brown alga Protohalopteris radicans was observed making dense carpets in the lower part of the littoral zone and down to 4–5 m depth at several localities in the 1950s, but was not detected in the recent survey. The filamentous brown alga Dictyosiphon foeniculaceus was also not recorded in 2008–2009. The changed abundance of these species does not have a clear explanation.
Two factors – favourable temperatures and changed salinity – seem to work together, causing the observed changes in biodiversity in Hardangerfjord. The increase in southern species was not merely caused by an increased number of red algae at stations, but also more frequent recordings of several brown algae with warm-water affinity. Likewise, the increased number of red algal taxa was not only caused by southern red algae, but also red algal species with a pansectorial distribution on the Norwegian coast.
Abundance of dominant functional groups
This study shows that the distribution of fucoids and kelps in Hardangerfjord had only changed marginally since the 1950s. Along the coast of southern Norway a large-scale decline in Saccharina latissimahas been reported (Moy & Christie Citation2012), but a recovery of S. latissima populations has been observed on the southwest coast of Norway since 2008 (Moy & Christie Citation2012). They hypothesized that this phenomenon might be due to eutrophication, high summer temperatures, increased sedimentation rates or disturbances in the marine communities due to overfishing of large predators. In this study we found that the geographical and vertical distribution of S. latissima had changed little since the 1950s; however, in 2008–2009 the species was found to have scattered populations at several stations in the inner part of the fjord, where it was not present in the 1950s.
Jorde & Klavestad (Citation1963) observed that kelp normally did not extend below 4–5 m depth in the fjord proper, and that a zone of filamentous brown algae could be found down to 8–10 m depth. The present study shows a similar distribution of kelps and an occurrence of the same filamentous algae. Jorde & Klavestad (Citation1963) suggested that this distribution pattern was caused by sea urchin grazing in the fjord, and results from the video observations in the present study support this. Sea urchins were observed to stay below 8 m depth during summer and to move upwards during winter, and could consequently remove kelps and other algae in shallow waters during winter. This suggests that sea urchin grazing maintains the lower limit of kelp vegetation in parts of the fjord, and that this is a stable state of the benthic ecosystem, at least in parts of the fjord proper. Alternatively, the lack of kelp below 4–5 m could also be due to competition or shading from filamentous algae, which developed during the summer. The formation of ephemeral filamentous algae below the kelp zone is most likely facilitated by the release from grazing during summer. A permanent sea urchin population in the fjord has also been reported from New Zealand (Wing et al. Citation2003), where its persistent occurrence was related to fjord retention of larvae (Lamare Citation1998). Similar processes may cause the persistent occurrence of dense populations of Echinus acutus in Hardangerfjord.
Development of extensive mats of filamentous algae and disappearance of large, habitat building algae like kelps and fucoids due to eutrophication has been reported for several coastal areas (Rueness & Fredriksen Citation1991; Bokn et al. Citation1992; Munda Citation1996; Pihl et al. Citation1999; Krause-Jensen et al. Citation2007). Since the 1980s extensive fish farming has developed in Hardangerfjord, with subsequent high discharges of nutrients into the fjord. On the other hand, a high nutrient level has not been recorded in the main fjord during 2008–2010 (Husa et al. Citation2014). No quantitative collections of kelp and filamentous brown algae were made during the first investigation in Hardangerfjord during 1955–1960, and it is therefore difficult to assess if the zone of turf and filamentous algae is more conspicuous during the present study than during the 1950s. According to Jorde & Klavestad (Citation1963), the lower limit of this zone was at approximately 8–10 m depth along the fjord proper and in the side branches, while observations of the present study indicate a somewhat deeper extension. During the 1950s, Stictyosiphon tortilis and Protohalopteris radicans were dominant in this zone at a large number of stations, while several brown and red filamentous algae, such as Sphacelaria spp., Polysiphonia spp., Ectocarpus spp., Desmarestia spp., Rhodomela confervoides, Spermatochnus paradoxus and Striaria attenuata, dominated in the present study. The persistent presence of kelps and Fucus spp. in the upper part of the sublittoral zone suggest that the present level of nutrient input is not detrimental to the habitat-building brown algae in the fjord. Grazing has been shown to be important for the maintenance of habitat-forming large brown algae in eutrophicated areas, by removing rapidly growing filamentous algae (Worm et al. Citation1999; Worm & Lotze Citation2006; Guerry et al. Citation2009). There are probably few mesograzers in the sublittoral of Hardangerfjord due to the relatively low salinity (Brattegard Citation1966), and the lower limit of algal growth may be controlled by the large sea urchin populations alone.
Conclusion
The observed temporal changes in the macroflora in Hardangerfjord since the 1950s were mainly due to increased species richness, a higher number of southern species and a number of red algal species protruding further into the fjord, whereas the occurrence and distribution of the dominant functional algal groups was resilient. We suggest that increased sea-water temperatures and an altered salinity regime have been the main driving forces behind the observed changes, altering the distribution of red algal species and facilitating the establishment of more southern species throughout the fjord.
Editorial responsibility: Mirta Teichberg
smar_a_810751_sm1891.pdf
Download PDF (3.3 MB)Acknowledgements
We thank Professor Emeritus Jan Rueness and Professor Christine A. Maggs for identifying or verifying a number of species. We thank the diving group from the Institute of Marine Research and Mette Eilertsen at Rådgivende Biologer for field assistance and the crew of ‘RV Hans Brattström’ for valuable assistance during field work. We also thank Dr Ann-Lisbeth Agnalt (IMR) and two anonymous referees for valuable comments on the manuscript. The Research Council of Norway supported the study.
References
- Aguilera J, Rautenberger R. 2011. Oxidative stress tolerance strategies of intertidal macroalgae. In: Abele D, Vázquez-Medina JP, Zenteno-Savín T, editors. Oxidative Stress in Aquatic Ecosystems. Chichester: John Wiley & Sons. 524 pages.
- Allison G. 2004. The influence of species diversity and stress intensity on community resistance and resilience. Ecological Monographs 74:117–34. 10.1890/02-0681
- Asplin L, Aure J, Sandvik AD, Albretsen J, Sundfjord V, Johnsen IA, et al. 2014. Fluctuations in the physical climate of the Hardangerfjord, and its influence on salmon lice distribution. Marine Biology Research 10:216–25.
- Aure J, Pettersen R. 1998. Miljøundersøkelser i norske fjorder: Sørfjorden – Hardanger. Fisken og Havet 11/1998. 15 pages. ( in Norwegian)
- Aure J, Strand Ø. 2001. Hydrografiske normaler og langtidsvariasjoner i norske kystfarvann mellom 1936 og 2000. Fisken og Havet 13/2001. 10 pages. ( in Norwegian)
- Aure J, Asplin L, Sætre R. 2007. Coast/fjord water exchange. Chapter 9 in: Sætre R, editor. The Norwegian Coastal Current – Oceanography and Climate. Trondheim: Tapir Academic Press. 150 pages.
- Berecibar E, Wynne MJ, Barbara I, Santos R. 2009. Records of Rhodophyta new to the flora of the Iberian Atlantic Coast. Botanica Marina 52:217–28.
- Bokn T, Murray SN, Moy FE, Magnusson JB. 1992. Changes in fucoid distribution and abundances in the inner Oslofjord, Norway: 1974–80 versus 1988–90. Acta Phytogeographica Suecia 78:117–24.
- Brattegard T. 1966. The natural history of the Hardangerfjord. 7. Horizontal distribution of the fauna of rocky shores. Sarsia 22:1–54.
- Brattegaard T, Holthe T. 1997. Distribution of marine, benthic macro-organisms in Norway. Research Report for DN 1997-1. Directorate for Nature Management. 409 pages.
- Bray JR, Curtis JT. 1957. An ordination of the upland forest communities of Southern Wisconsin. Ecological Monographs 27:325–49. 10.2307/1942268
- Breemann AM. 1988. Relative importance of temperature and other factors in determining geographic boundaries of seaweeds: Experimental and phonological evidence. Helgoländer Meeresuntersuchungen 42:199–241. 10.1007/BF02366043
- Breeman AM, Guiry MD. 1989. Tidal influences on the photoperiodic induction of tetrasporogenesis in Bonnemaisonia hamifera (Rhodophyta). Marine Biology 102:5–14. 10.1007/BF00391318
- Britton-Simmons KH. 2004. Direct and indirect effects of the introduced alga Sargassum muticum on benthic, subtidal communities of Washington State, USA. Marine Ecology Progress Series 277:61–78. 10.3354/meps277061
- Brodie J, Irvine LM. 2003. Seaweeds of the British Isles. Volume 1. Rhodophyta. Part 3B. Bangiophycidae. London: The Natural History Museum. 167 pages.
- Brodie J, Maggs CA, John DM. 2007. Green Seaweeds of Britain and Ireland. London: British Phycological Society. 250 pages.
- Casas G, Scrosati R, Luz Piriz M. 2004. The invasive kelp Undaria pinnatifida (Phaeophyceae, Laminariales) reduces native seaweed diversity in Nuevo Gulf (Patagonia, Argentina). Biological Invasions 6:411–16. 10.1023/B:BINV.0000041555.29305.41
- Davison IR, Pearson GA. 1996. Stress tolerance in intertidal seaweeds. Journal of Phycology 32:197–211. 10.1111/j.0022-3646.1996.00197.x
- Dixon PS, Irvine LM. 1977. Seaweeds of the British Isles. Volume 1. Rhodophyta. Part 1. Introduction, Nemaliales, Gigartinales. London: The Natural History Museum. 252 pages.
- Ervik A, Agnalt A-L, Asplin L, Aure J, Bekkvik TC, Døskeland I et al. 2008. AkvaVis – dynamisk GIS-verktøy for lokalisering av oppdrettsanlegg for nye oppdrettsarter. Miljøkrav for nye oppdrettsarter og laks. Fisken og Havet 10/2008. 90 pages. ( in Norwegian)
- Fletcher SG, South GR. Seaweeds of the British Isles. Volume 3. Fucophyceae (Phaeophyceae). Part 1. London: The Natural History Museum. 359 pages.
- Forward SG, South GR. 1985. Observations on the taxonomy and life history of North Atlantic Acrothrix Kylin (Phaeophyceae, Chordariales). Phycologia 24:347–59. 10.2216/i0031-8884-24-3-347.1
- Guerry AD, Menge BA, Dunmore RA. 2009. Effects of consumers and enrichment on abundance and diversity of benthic algae in a rocky intertidal community. Journal of Experimental Marine Biology and Ecology 369:155–64. 10.1016/j.jembe.2008.11.011
- Guiry MD, Guiry GM. 2012. Algaebase. World-wide electronic publication. National University of Ireland, Galway. http://www.algaebase.org (accessed 23 April 2012).
- Harley CDG, Hughes AR, Hultgren KM, Miner BG, Sorte CJB, Thornber CS, et al. 2006. The impacts of climate change in coastal marine systems. Ecology Letters 9:228–41. 10.1111/j.1461-0248.2005.00871.x
- Harley CDG, Anderson KM, Demes KW, Jorve JP, Kordas RL, Coyle TA. 2012. Effects of climate change on global seaweed communities. Journal of Phycology 48:1064–78. 10.1111/j.1529-8817.2012.01224.x
- Hemmi A, Mäkinen A, Jormalainen V, Honkanen T. 2005. Responses of growth and phlorotannins in Fucus vesiculosus to nutrient enrichment and herbivory. Aquatic Ecology 39:201–11. 10.1007/s10452-004-3526-z
- Husa V, Sjøtun K, Lein TE. 2004. The newly introduced species Heterosiphonia japonica Yendo (Dasyaceae, Rhodophyta): Geographical distribution and abundance at the Norwegian southwest coast. Sarsia 89:211–17. 10.1080/00364820410006600
- Husa V, Sjøtun K, Brattenborg N, Lein TE. 2008. Changes of macroalgal biodiversity in sublittoral sites in southwest Norway: Impact of an introduced species or higher temperature? Marine Biology Research 4:414–28. 10.1080/17451000802232874
- Husa V, Kutti T, Ervik A, Sjøtun K, Hansen PK, Aure J. 2014. Assessing water-quality in a Norwegian salmon producing fjord. Marine Biology Research 10:241–52.
- Irvine LM. 1983. Seaweeds of the British Isles. Volume 1. Part 2A. Rhodophyta. Part 2A. Cryptonemiales (sensu stricto), Palmariales, Rhodymeniales. London: The Natural History Museum. 115 pages.
- Irvine LM, Chamberlain YM. 1994. Seaweeds of the British Isles. Volume 1. Rhodophyta. Part 2B. Corallinales, Hildenbrandiales. London: The Natural History Museum. 276 pages.
- Jorde I, Klavestad N. 1963. The natural history of the Hardangerfjord 4. The benthonic algal vegetation. Sarsia 9:1–100.
- Kornmann P, Sahling PH. 1977. Meeresalgen von Helgoland. Benthische Grün-, Braun und Rotalgen. Helgoländer Wissenschaftliche Meeresuntersuchungen 29: 1–289. 10.1007/BF01611137
- Krause-Jensen D, Middelboe AL, Carstensen J, Dahl K. 2007. Spatial patterns of macroalgal abundance in relation to eutrophication. Marine Biology 152:25–36. 10.1007/s00227-007-0676-2
- Kruskal JB. 1964. Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 29:1–27. 10.1007/BF02289565
- Lamare MD. 1998. Origin and transport of larvae of the sea urchin Evechinus chloroticus (Echinodermata: Echinoidea) in a New Zealand fiord. Marine Ecology Progress Series 174:107–21. 10.3354/meps174107
- Lein TE. 1999. A newly immigrated red alga (‘Dasysiphonia’, Dasyaceae, Rhodophyta) to the Norwegian coast. Sarsia 84:85–88.
- Lima FP, Ribeiro PA, Queiroz N, Hawkins SJ, Santos AM. 2007. Do distributional shifts of northern and southern species of algae match the warming pattern? Global Change Biology 13:2592–604. 10.1111/j.1365-2486.2007.01451.x
- Maggs CA, Hommersand MH. 1993. Seaweeds of the British Isles. Volume 1. Rhodophyta. Part 3A. Ceramiales. London: The Natural History Museum. 444 pages.
- Moy FE, Christie H. 2012. Large-scale shift from sugar kelp (Saccharina latissima) to ephemeral algae along the south and west coast of Norway. Marine Biology Research 8:309–21. 10.1080/17451000.2011.637561
- Munda IM. 1996. The northern Adriatic Sea. In: Scramm E, Nienhaus PH, editors. Marine Benthic Vegetation: Recent Changes and the Effects of Eutrophication. Ecological Studies, volume 123. Berlin: Springer-Verlag. 470 pages.
- Nielsen R, Kristiansen AA, Mathiesen L, Mathiesen H. 1995. Distributional index of the benthic macroalgae of the Baltic Sea area. Acta Botanica Fennica 155:1–51.
- Peters AE, Breeman AM. 1993. Temperature tolerance and latitudinal range of brown algae from temperate Pacific South America. Marine Biology 115:143–50. 10.1007/BF00349396
- Pihl L, Svenson A, Moksnes PO, Wennehage H. 1999. Distribution of green algal mats throughout shallow soft bottoms of the Swedish Skagerrak archipelago in relation to nutrient sources and wave exposure. Journal of Sea Research 41:281–95. 10.1016/S1385-1101(99)00004-0
- Plattner SB, Nichols HW. 1977. Asexual development in Seirospora seirosperma. Phytomorphology 27:371–77.
- Prud'Homme van Reine WF. 1982. A taxonomic revision of the European Sphacelariaceae (Sphacelariales, Phaeophyceae). Leiden Botanical Series 6:1–293.
- Rueness J. 1977. Norsk Algeflora. Oslo: Universitetsforlaget. 266 pages. ( in Norwegian)
- Rueness J, Fredriksen S. 1991. An assessment of possible pollution effects on the benthic algae of the outer Oslofjord, Norway. Oebalia 17:223–35.
- Ruus A, Molvær J, Schøyen M. 2006. Overvåkning av miljøforholdene i Sørfjorden i 2005. Oksygen, nitrogen og fosfor i vannmassene. SFT rapport 951/06. 27 pages. ( in Norwegian)
- Sagarin RD, Barry JP, Gilman SE, Baxter CH. 1999. Climate related change in an intertidal community over short and long time scales. Ecological Monographs 69:465–90. 10.1890/0012-9615(1999)069[0465:CRCIAI]2.0.CO;2
- Shepard RN. 1962. The analysis of proximities: Multidimensional scaling with an unknown distance function. Psychometrika 27:125–40. 10.1007/BF02289630
- Simkanin C, Power AM, Myers AA, McGrath D, Southward A, Mieszkowska N, et al. 2005. Using historical data to detect temporal changes in the abundance of intertidal species on Irish shores. Journal of Marine Biology Association of the United Kingdom 85:1329–40. 10.1017/S0025315405012506
- Sjøtun K, Christie H, Fosså JH. 2006. The combined effect of canopy shading and sea urchin grazing on recruitment in kelp forest (Laminaria hyperborea). Marine Biology Research 2:24–32. 10.1080/17451000500537418
- Stigebrandt A, Aure J, Ervik A, Hansen PK. 2004. Regulating the local environmental impact of intensive marine fish farming. III: A model for estimation of the holding capacity in the MOM system (Modelling–Ongrowing fish farm–Monitoring). Aquaculture 234:239–61. 10.1016/j.aquaculture.2003.11.029
- Wernberg T, Russell BD, Thomsen MS, Gurgel FD, Bradshaw JA, Poloczanska ES, et al. 2011a. Seaweed communities in retreat from ocean warming. Current Biology 21:1828–32. 10.1016/j.cub.2011.09.028
- Wernberg T, Thomsen MS, Tuya F, Kendrick GA. 2011b. Biogenic habitat structure of seaweeds change along a latitudinal gradient in ocean temperature. Journal of Experimental Marine Biology 400:264–71. 10.1016/j.jembe.2011.02.017
- Wing SR, Gibb MT, Lamare MD. 2003. Reproductive sources and sinks within a sea urchin, Evechinus chloroticus, population of a New Zealand fjord. Marine Ecology Progress Series 248:109–23. 10.3354/meps248109
- Worm B, Lotze HK. 2006. Effects of eutrophication, grazing, and algal blooms on rocky shores. Limnologia and Oceanographica 51:569–79. 10.4319/lo.2006.51.1_part_2.0569
- Worm B, Sommer U. 2000. Rapid direct and indirect effects of a single nutrient pulse in a seaweed–epiphyte grazer system. Marine Ecology Progress Series 200:283–88. 10.3354/meps202283
- Worm B, Lotze HK, Boström C, Engkvist R, Labanauskas V, Sommer U. 1999. Marine diversity shift linked to interactions among grazers, nutrients and dormant propagules. Marine Ecology Progress Series 185:309–14. 10.3354/meps185309
