Abstract
Freshwater and marine growth of anadromous brown trout (Salmo trutta) and Atlantic salmon (Salmo salar) from the River Etneelva was analysed in relation to river, fjord and ocean temperatures during the periods 1976–1982 and 2000–2007. Anadromous brown trout grew more slowly through their first and second summers in the sea during the last observation period compared to the first period, and there were more growth checks in the scales sampled from the last period. The reduced growth in length corresponds to a weight reduction of about 20–40%. In fresh water, there was no change in growth rate of the trout parr, while in Atlantic salmon, growth rates of both parr and post-smolts increased from the first to the second period, possibly due to the corresponding increase in temperatures in the river and the marine environments. Anadromous brown trout stay in the fjord during the whole marine feeding period, and therefore the reduced growth in the sea must be caused by factors operating in the fjord environment. Although changes in food availability may also play a part in the observed changes, the negative trend for anadromous brown trout is most likely related to the high infection levels of salmon lice (Lepeophtheirus salmonis) observed in the central and outer parts of the Hardangerfjord.
Introduction
Temperature, food availability and quality have important controlling effects on food consumption and metabolism in fish, thereby also affecting their growth rates (Wootton Citation1999). Anadromous species like brown trout (Salmo trutta Linnaeus, 1758) and Atlantic salmon (Salmo salar Linnaeus, 1758) are influenced by temperature conditions in both fresh water and in the sea and changes in temperature in one or both environments are therefore expected to affect the annual growth rates of these species in several ways. According to Elliott & Hurley (Citation2000), the temperature giving the maximum growth rate of brown trout increases from 13.9°C for individuals feeding on invertebrates to 17.0°C for piscivorous trout. Atlantic salmon parr have their maximum growth at higher temperatures than anadromous trout parr, and are also better at converting food to body mass at high temperatures (Forseth et al. Citation2001). Depending on the size of the Atlantic salmon post-smolt, optimal temperatures for growth may be in the range of 12.8–14°C (Handeland et al. Citation2008). As there seems to be a positive relationship between post-smolt growth and the return rate of SW Atlantic salmon (Friedland et al. Citation2000), temperature may also indirectly affect survival. Over the past few decades, reduced growth during the post-smolt year has been observed in Atlantic salmon from Norwegian rivers (Jonsson & Jonsson Citation2004; McCarthy et al. Citation2008).
Norway and the Norwegian Sea have experienced higher mean air and ocean temperatures during the past decade than during the early 1980s (IPCC Citation2007; Todd et al. Citation2008), and therefore growth conditions for anadromous species may also have changed. This study analysed river and marine growth rates of cohorts of Atlantic salmon and anadromous brown trout from the River Etneelva, based on mature fish captured in 1983 and 2008. Thus, the study represents several cohorts for each period, and a time span of 25 years. Our hypothesis is that annual growth rates of both anadromous brown trout and Atlantic salmon in freshwater and in the marine environment correlate positively with temperature, and thus exhibit higher growth rates during the last observation period.
Material and methods
The study area
The River Etneelva is located on the southwestern coast of Norway, in the County of Hordaland (UTM zone 32, 331719 E, 6619892 N) (). Including a large tributary, the total river stretch available for anadromous salmonids is 13 km. The river has a longstanding reputation as excellent for salmon angling. The fishing season has been between 1 June and 15 September. Due to decreasing spawning populations, a bag limit of two fish per rod per day was introduced in 2008, of which only one could be anadromous brown trout. Fishing was closed in 2010 and 2011 because of a continued decline in spawning populations of both Atlantic salmon and anadromous brown trout ().
The outer and central regions of the Hardangerfjord system, of which the Etnefjord forms a part, are known to have some of the highest infection rates of salmon lice (Lepeophtheirus salmonis Krøyer, 1837) on wild salmonids in Norway (Bjørn et al. Citation2011).
Data collection
Age and growth rates of the fish were determined by scale readings. Scale samples of Atlantic salmon and anadromous brown trout caught in the river in 1983 originate from a study by Bjerknes & Waatevik (Citation1985), while samples from 2008 were obtained partly from local anglers and partly from research catches in the Etnefjord. A total of 175 Atlantic salmon and 112 anadromous brown trout scale samples from 1983 were studied. In 2008, scale samples of 41 Atlantic salmon were used, 31 of which had been taken in the river and 10 in a bag net in the Etnefjord. Four individuals that were recorded as Atlantic salmon by the fishermen were identified as anadromous brown trout after the scale readings. A total of 40 anadromous brown trout were used from 2008, of which 4 were caught in the river, while the rest were captured by gill netting in the Etnefjord. The low number captured in the river this year is partly due to the bag limit, resulting in a low total catch. Thus, the trout sample had to be supplemented by fish captured in the fjord. Because there is only one small creek in the Etnefjord that produces anadromous brown trout beside the River Etne, it is most likely that individuals belonging to the river population were captured.
All the scales were sampled from the area above the lateral line, behind the dorsal fin. In the laboratory, impressions of the scales were made in strips of celluloid and subsequently analysed in a Minox microfilm reader. All circuli and annuli from the magnified scale as seen on the screen were transcribed and marked on a paper strip. The freshwater growth zones of both species were identified by the relatively close spacing of the circuli, while the sea stage was identified by a marked increase in circulus spacing (Dahl Citation1910). The annuli were identified by the relative decrease in circulus spacing and by forking of the circuli (Dahl Citation1910). False annuli, or growth checks, were identified according to the description given by MacLean et al. (Citation2000).
Scale lengths and the annual length increments marked on the paper strip were later measured in mm. The formula of Lea-Dahl was used to back-calculate the annual length increments of individual fish (Dahl Citation1910). The total number of circuli deposited in the post-smolt year was also used as a measure of growth for both species, as described by Peyronnet et al. (Citation2007). Fulton's condition factor of fish captured in June was taken as an indicator of the general length–weight relationship of the fish (Ricker Citation1975).
The distribution of the sampled individuals of Atlantic salmon and anadromous brown trout according to 1+ parr and post-smolt year is shown in . Years with fewer than three samples from a given cohort of either species were excluded from further analysis, but all fish were included when the period as a whole was analysed.
Table I. Annual number of 1+ parr and post-smolts of Atlantic salmon and anadromous brown trout from the River Etneelva used in the growth analysis, according to the scales sampled in 1983 and 2008.
Temperature and river discharge data were obtained from the Norwegian Water Resources and Energy Directorate (NVE), which has a station just below the outlet of the lake Stordalsvatnet (UTM zone 32 W, 331719 E, 6619892 N). Ocean surface temperatures at weather station Mike in the Northeast Atlantic (UTM zone 33 W, −86617 E, 7381055 N) were used to represent the temperature conditions experienced by the Atlantic salmon post-smolts. As the temperature recordings in the fjord were incomplete, data from the closest air temperature station, at Nedre Vats (UTM zone 33 W, −22808 E, 6630422 N), were used as a proxy. The ocean surface and air temperature data were obtained from the weather service eKlima (available at http://sharki.oslo.dnmi.no). Temperature and water discharge data for the three summer months of June, July and August were used. Because of incomplete data, especially for 1975–1982, average temperatures for each month and the whole summer (June–August) were used instead of degree days.
Statistical methods
The statistical program Minitab 15 was used to analyse the data. t-Tests were used to test whether two data sets were significantly different. Some of the data sets were not normally distributed, in which case Mann–Whitney U-tests were used. To test for relationships between data sets, correlations were used. For all tests, a significance level of α =0.05 was accepted.
Results
Temperature and parr growth in fresh water
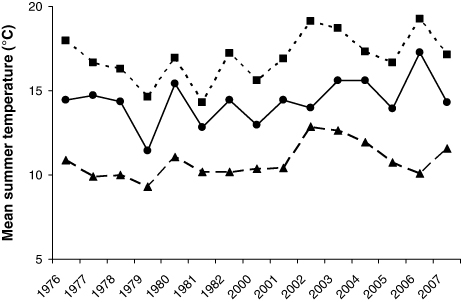
For the period June–August as a whole, there was a significant increase in mean temperature in the river, from 14.0°C during the first period (1976–1982) to 14.8°C for the second period (2000–2007) (t-test; p<0.01) (). There was no significant change in water discharge in July (t-test; p=0.30) and August (t-test; p=0.11), while in June there was a marginal difference (t-test; p=0.06). Water discharge differences for the period June–August as a whole was also close to being significant (t-test; p=0.07).
Anadromous brown trout parr in age-class 1+ displayed a slightly reduced length growth from the first to the second period, but the difference was not significant (t-test; p=0.15; ). Moreover, the length and age of anadromous brown trout smolt did not change from the first to the second period (Mann–Whitney; p=0.6 and p=0.92, respectively).
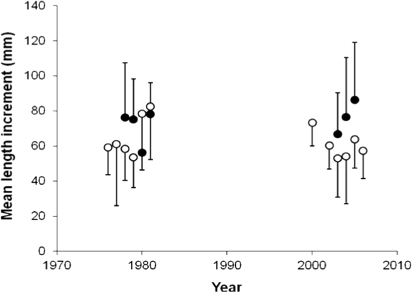
The length growth of 1+ Atlantic salmon parr increased from the first to the second period, but the change was marginally not significant (Mann–Whitney; p=0.06; ). Smolt length increased significantly from the first to the second period, by a mean of 8 mm (t-test; p=0.05), but there was no significant change in smolt age (Mann–Whitney; p=0.17). Of the salmon caught in 2008, 51% had become smolt after 2 years, while the corresponding frequency in 1983 was 40%.
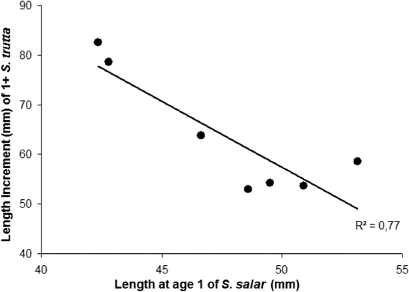
The growth in length of anadromous brown trout parr correlated negatively with the length of Atlantic salmon parr at the start of the growing season (R 2=0.77, p=0.01; ). Neither the mean summer river temperature nor the mean summer water discharge had any significant effect on the growth of 1+ anadromous brown trout (R2=0.06, p=0.44 and R2=0.09, p=0.35, respectively). In Atlantic salmon, however, there was a strong positive relationship between the growth of 1+ parr and mean summer temperature (R2=0.86, p<0.01).
Temperature and growth in the sea
Between the first and the second observation period, fjord temperatures increased significantly in July and August, but not in June (t-test; p<0.01 for July and August, and p=0.33 for June). The mean increment was 1.3°C in July and 1.5°C in August. The mean fjord temperature rose significantly by 1.1°C for the three summer months together from the first to the second period (t-test; p<0.01; ), but temperature displayed larger fluctuations during the second period. The ocean surface temperatures for June–August also rose significantly from the first to the second period (t-test; p<0.05 for all three months). The mean increases were 0.7°C in June, 1.3°C in July and 1.2°C in August. Like the fjord temperature proxy, the mean summer ocean temperature has increased by 1.1°C from the first to the second period (t-test; p<0.01; ).
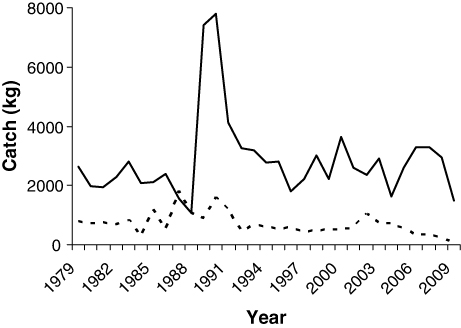
There was no significant relationship between summer fjord temperature proxies and growth rates of anadromous brown trout post-smolts (R 2=0.13, p=0.34), nor between ocean surface temperatures during the summer and growth rates of Atlantic salmon post-smolts (R 2=0.30, p=0.91). However, there was a significant decrease in the growth rate of anadromous brown trout post-smolts from the first to the second period (Mann–Whitney; p<0.01), from a median length increment at 129 mm in the first period, to a median increment at 91 mm in the last period. The growth during the second season in the fjord is also reduced from the first to the second period, by a mean of 25 mm (Mann–Whitney; p<0.01; ). During the third and fourth growth season there was a falling trend in growth, but the differences were not significant (Mann–Whitney; p=0.37 and p=0.46, respectively). Growth checks were observed in 12% of the scales of anadromous brown trout caught in 2008, but in only 3.6% of the scale samples from the 1983 catch.
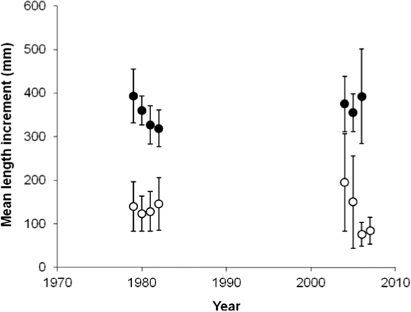
Unlike the anadromous brown trout post-smolts, the growth in length of Atlantic salmon post-smolts was significantly higher in the last period compared to the first (t-test; p=0.01; ), with a mean increase in length from 334 mm (SD±40 mm) to 375 mm (SD±83 mm). In addition, the number of circuli deposited during the post-smolt year increased significantly, by a mean of nine circuli, from the first to the second period (t-test; p<0.01). The growth in length during the second year at sea, on the other hand, was significantly reduced from the first to the second period, by a mean of 50 mm (t-test; p<0.01; ). In addition, the condition factor of Atlantic salmon captured in June decreased significantly from the first to the second period (t-test; p<0.01). In the 1983 sample, only 4.5% of the Atlantic salmon had growth checks, while 65% of the individuals caught in 2008 had false annuli in the scales during their first year at sea. The trend appears to have started in 2005, as few (4.5% in 2003) or none (in 2004) of the fish that were post-smolts in previous years displayed growth checks, whereas 53–100% of the post-smolts in 2005–2007 had summer growth checks.
Discussion
Contrary to what was expected as a result of higher fjord temperatures, there was a significant reduction in the growth of anadromous brown trout in the marine environment over the past 25 years. A reduction in length of about 4 cm after the first summer in the sea may correspond to a reduction in weight of about 100–200 g or more, equivalent to a weight reduction of about 20–40%. A long-term study of anadromous brown trout from the Norwegian river Imsa has revealed a positive correlation between sea growth and survival (Jonsson & Jonsson Citation2009a). Thus, it is most likely that the observed reduction in growth has caused reduced survival and recruitment in anadromous brown trout from the River Etneelva as well. Similar to the present study, Jonsson & Jonsson (Citation2009a) failed to find a correlation between sea temperature and post-smolt growth, which they explained by the ability of anadromous brown trout to partly select its thermal habitat in the fjord. Our data suggest that other factors may override the effect of temperature on growth. The reduced growth during the first two summers in the fjord, combined with an increase in the frequency of summer growth checks, from 3.9% in the 1983 catch to 12% in fish captured in 2008, point towards harsher conditions for the anadromous brown trout in the fjord in recent years. Changes in the availability of food may possibly influence growth rates of the anadromous brown trout, but local changes in food availability alone can hardly explain the declining trend in anadromous brown trout populations along large parts of the Norwegian coast (Anon. Citation2009). For post-smolts of anadromous brown trout, high infection rates of salmon lice can force infected fish to an early return to estuaries and rivers, and in turn lead to reduced growth, as well as increased mortality (Birkeland & Jakobsen Citation1997; Heuch et al. Citation2005). A study from the River Lønningdalselva, also in Hordaland county, showed that post-smolts that had re-entered the river lost almost a quarter of their body mass before they returned to the fjord (Birkeland Citation1996). The major expansion in salmon farming in the Hardangerfjord, together with the first epidemics of salmon lice, appeared several years after the first observation period. Therefore, the reduced growth of anadromous brown trout from the River Etneelva during the marine migration, combined with the increased occurrence of growth checks in the scales, is most likely due to the high infection levels observed in parts of the Hardangerfjord. Furthermore, Skår (Citation2010) did not find any reductions in growth of anadromous brown trout from the River Granvinselva, located in the inner parts of the Hardangerfjord, where infection levels of salmon lice have been lower than in the outer parts. A study from the River Guddalselva, located in central parts of the Hardangerfjord, found that smolts of anadromous brown trout treated with Substance EX obtained a higher survival than untreated groups, indicating that salmon lice have a regulatory effect on anadromous brown trout populations in the region (Skaala et al. Citation2014). Correspondingly, prophylactic treatments of Atlantic salmon smolts against salmon lice led to higher return rates than those of untreated control groups (Krkošek et al. Citation2013), indicating that salmon lice may affect growth and survival of both anadromous brown trout and Atlantic salmon.
The strong positive relationship between the mean summer river temperature and the growth of Atlantic salmon parr in the River Etneelva was not seen in the same year-classes of anadromous brown trout, although higher river temperatures in streams without Atlantic salmon parr have led to increased growth rates of brown trout parr (Elliott Citation2009). However, other factors may mask the temperature effect, at least in some rivers (Jonsson & Jonsson Citation2009b). Such factors may be competition for food, either due to high fish densities or to variable food availability (Jonsson & Jonsson Citation2009b). In an experimental study, maximum growth of Atlantic salmon parr from the River Suldalslågen, located close to the River Etneelva, was found at 16°C (Jonsson et al. Citation2001). In brown trout parr, the maximum growth rate was found at 13.9°C (Elliott & Hurley Citation2000), while at 19.5°C growth of brown trout parr may be equal to zero (Elliott Citation1975). As mean summer temperatures in the River Etneelva have been in the range of 14–17°C during 2000–2007, the growth conditions may have been more favourable for Atlantic salmon parr than for trout parr. The negative correlation between the length increment of anadromous brown trout parr and the size of the Atlantic salmon parr at the beginning of the growing season is an indication of that competition exists between the cohorts of these two species in the River Etneelva. Similar observations regarding growth interactions were seen in a study of Atlantic salmon parr and rainbow trout (Oncorhyncus mykiss Walbaum, 1792), in which parr of Atlantic salmon were better competitors than the larger rainbow trout when temperatures were high (Coghlan & Ringler Citation2005). The indirect competition effect could thus have affected the growth of anadromous brown trout parr in the River Etneelva, and thereby masked a potential direct temperature effect.
European stocks of Atlantic salmon have experienced dwindling numbers and reduced growth at sea during the last 30 years (Friedland et al. Citation2000; Todd et al. Citation2008; Otero et al. Citation2011; Skilbrei et al. in press). Two factors are often recognized as possible contributors to a decline in Atlantic salmon and anadromous brown trout abundance: the introduction of marine fish farming and climate change (Jonsson & Jonsson Citation2009b; Anon. Citation2009; Otero et al. Citation2011). Jensen et al. (Citation2011) studied the oceanic growth of Atlantic salmon from seven Norwegian rivers until 2003, and in spite of annual variations, they observed a general decline in growth during both the first and second year at sea. Likewise, Skilbrei et al. (2013) found declining recaptures of adult Atlantic salmon after releases of smolts in the River Dale, Hordaland County, during the period 1997–2009, a development that corresponded with a poor marine growth and increased age at maturity in three monitored salmon populations. Accordingly, the increased growth of the Atlantic salmon post-smolts from the River Etneelva during the past few years indicates that conditions during the first post-smolt season may have improved locally, in spite of the occurrences of growth checks. However, the reduced growth rate of the Atlantic salmon during the second year at sea, combined with the reduction in condition factor, may indicate that feeding conditions have been poor for salmon after the post-smolt stage, as also recorded by Skilbrei et al. (Citation2013). As length increments are maintained, Todd et al. (Citation2011) have suggested that the low condition factor of the mature salmon arose during the winter and the period just before return to the rivers, probably due to a bottom-up effect of changes in the zooplankton communities, affecting the availability of prey fish.
In conclusion, Atlantic salmon parr and post-smolts have responded positively to the significant increase in summer temperatures with increased growth rates from the period 1976–1982 to the period 2000–2007. On the other hand, the anadromous brown trout showed a significant reduction in growth during the two first summers in the sea, from the first to the second period, simultaneously with an increased number of growth checks in the scales. Although changes in food availability may also play a part in the observed changes, the negative trend for anadromous brown trout is most likely related to the high infection levels of salmon lice observed in the central and outer parts of the Hardangerfjord.
Editorial responsibility: Geir Ottersen
References
- Anon. 2009. Bestandsutvikling hos sjøørret og forslag til forvaltningstiltak. Notat 2009-1. Trondheim: The Directorate of Nature Management. 32 pages. ( in Norwegian)
- Birkeland K. 1996. Consequences of premature return by sea trout (Salmo trutta) infested with the salmon louse (Lepeophtheirus salmonis Krøyer): Migration, growth, and mortality. Canadian Journal of Fisheries and Aquatic Sciences 53:2808–13. 10.1139/f96-231
- Birkeland K, Jakobsen PJ. 1997. Salmon lice, Lepeophtheirus salmonis, infestation as a causal agent of premature return to rivers and estuaries by sea trout, Salmo trutta, juveniles. Environmental Biology of Fishes 49:129–37. 10.1023/A:1007354632039
- Bjerknes V, Waatevik E. 1985. Fiskeribiologiske granskingar i Etne- og Saudafjella. Bergen: Rådgivende Fiskeribiologer. 127 pages. ( in Norwegian)
- Bjørn PA, Nilsen R, Serra Llinares RM, Asplin L, Boxaspen KK, Finstad B, et al. 2011. Sluttrapport til Mattilsynet over lakselusinfeksjonen på vill laksefisk langs norskekysten i 2011. Rapport fra Havforskningen Nr 19-2011. Bergen: Institute of Marine Research. 34 pages. ( in Norwegian)
- Coghlan SM, Ringler NH. 2005. Temperature-dependent effects of rainbow trout on growth of Atlantic salmon parr. Journal of Great Lakes Research 31:386–96. 10.1016/S0380-1330(05)70270-7
- Dahl K. 1910. Alder og vekst hos laks og ørret belyst ved studier av deres skjæl. Kristiania: Centraltrykkeriet. 60 pages. ( in Norwegian)
- Elliott JM. 1975. Growth rate of brown trout (Salmo trutta L) fed on maximum rations. Journal of Animal Ecology 44:805–21. 10.2307/3720
- Elliott JM. 2009. Validation and implications of a growth model for brown trout, Salmo trutta, using long-term data from a small stream in north-west England. Freshwater Biology 54:2263–75. 10.1111/j.1365-2427.2009.02258.x
- Elliott JM, Hurley MA. 2000. Daily energy intake and growth of piscivorous brown trout, Salmo trutta. Freshwater Biology 44:237–45. 10.1046/j.1365-2427.2000.00560.x
- Forseth T, Hurley MA, Jensen AJ, Elliott JM. 2001. Functional models for growth and food consumption of Atlantic salmon parr, Salmo salar, from a Norwegian river. Freshwater Biology 46:173–86. 10.1046/j.1365-2427.2001.00631.x
- Friedland KD, Hansen LP, Dunkley DA, MacLean JC. 2000. Linkage between ocean climate, post-smolt growth, and survival of Atlantic salmon (Salmo salar L.) in the North Sea area. ICES Journal of Marine Sciences 57:419–29. 10.1006/jmsc.1999.0639
- Handeland SO, Imsland AK, Stefansson S. 2008. The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 283:36–42. 10.1016/j.aquaculture.2008.06.042
- Heuch PA, Bjørn PA, Finstad B, Holst JC, Asplin L, Nilsen F. 2005. A review of the Norwegian National Action Plan Against Salmon Lice on Salmonids: The effect on wild salmonids. Aquaculture 246:79–92. 10.1016/j.aquaculture.2004.12.027
- IPCC. 2007. Summary for policymakers. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, editors. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. 18 pages.
- Jensen AJ, Fiske P, Hansen LP, Johnsen BO, Mork KA, Næsje TF. 2011. Synchrony in marine growth among Atlantic salmon (Salmo salar) populations. Canadian Journal of Fisheries and Aquatic Sciences 68:444–57. 10.1139/F10-156
- Jonsson B, Jonsson N. 2004. Factors affecting marine production of Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 61:2369–83. 10.1139/f04-215
- Jonsson B, Jonsson N. 2009a. Migratory timing, marine survival and growth of anadromous brown trout Salmo trutta in the River Imsa, Norway. Journal of Fish Biology 74:621–38. 10.1111/j.1095-8649.2008.02152.x
- Jonsson B, Jonsson N. 2009b. A review of the likely effects of climate change on anadromous Atlantic salmon Salmo salar and brown trout Salmo trutta, with particular reference to water temperature and flow. Journal of Fish Biology 75:2381–447. 10.1111/j.1095-8649.2009.02380.x
- Jonsson B, Forseth T, Jensen AJ, Næsje TF. 2001. Thermal performance of juvenile Atlantic salmon, Salmo salar L. Functional Ecology 15:701–11. 10.1046/j.0269-8463.2001.00572.x
- Krkošek M, Revie CW, Gargan PG, Skilbrei OT, Finstad B, Todd CD. 2013. Impact of parasites on salmon recruitment in the Northwest Atlantic Ocean. Proceedings of the Royal Society B 280(1750). 8 pages.
- MacLean JC, Smith GW, Whyte BDM. 2000. Description of marine growth checks observed on the scales of salmon returning to Scottish home waters in 1997. In: Mills D, editors. The Ocean Life of Atlantic Salmon – Environmental and Biological Factors Influencing Survival. Oxford: Fishing News Books, p 37–48.
- McCarthy JL, Friedland KD, Hansen LP. 2008. Monthly indices of the post-smolt growth of Atlantic salmon from the Drammen River, Norway. Journal of Fish Biology 72:1572–88. 10.1111/j.1095-8649.2008.01820.x
- Otero J, Jensen AJ, L'Abée-Lund JH, Stenseth NC, Storvik GO, Vøllestad LA. 2011. Quantifying the ocean, freshwater and human effects on year-to-year variability of one-sea-winter Atlantic salmon angled in multiple Norwegian rivers. PLoS ONE 6:e24005. 11 pages. 10.1371/journal.pone.0024005
- Peyronnet A, Friedland KD, O'Maoilédidigh N, Manning M, Poole WR. 2007. Links between patterns of marine growth and survival of Atlantic salmon Salmo salar L. Journal of Fish Biology 71:684–700. 10.1111/j.1095-8649.2007.01538.x
- Ricker WE. 1975. Computation and Interpretation of Biological Statistics of Fish Populations. Bulletin of the Fisheries Research Board of Canada, Department of the Environment, Fisheries and Marine Service, volume 191. 382 pages.
- Skaala Ø, Kålås S, Borgstrøm R. 2014. Evidence of salmon lice-induced mortality of sea trout (Salmo trutta) in the Hardangerfjord, Norway. Marine Biology Research 10:279–288.
- Skilbrei OT, Finstad B, Urdal K, Bakke G, Kroglund F, Strand R. 2013. Impact of early salmon louse (Lepeophtheirus salmonis) infestation, and differences in survival and marine growth of sea-ranched Atlantic salmon (Salmo salar) smolts 1997–2009. Journal of Fish Diseases 36:249–60.
- Skår B. 2010. Sjøauren i Granvinsvassdraget; vekst i ferskvatn og sjø før og etter større miljøendringar. [Sea trout (Salmo trutta) in Granvin water course; growth in freshwater and sea, before and after large environmental changes. Master's Thesis. Norwegian University of Life Sciences. 42 pages. ( in Norwegian)
- Statistics Norway. 2010. http://www.ssb.no/english (accessed 20 July 2010).
- Todd CD, Hughes SL, Marshall CT, MacLean JC, Lonergan ME, Biuw EM. 2008. Detrimental effects of recent ocean surface warming on growth condition of Atlantic salmon. Global Change Biology 14:958–70. 10.1111/j.1365-2486.2007.01522.x
- Todd CD, Friedland KD, MacLean JC, Hazon N, Jensen AJ. 2011. Getting into hot water? Atlantic salmon responses to climate change in freshwater and marine environments. In: Aas Ø, Einum S, Klemetsen A, Skurdal J, editors. Atlantic Salmon Ecology. Oxford: Wiley-Blackwell, p 409–43.
- Wootton RJ. 1999. Ecology of Teleost Fishes. 2nd edition. Dordrecht: Kluwer Academic Publishers. 386 pages.
