Abstract
The Hardangerfjord is one of the largest salmon-farming areas in Norway, with an annual production of approximately 70,000 metric tonnes. The regional impact of fin-fish farming in a fjord environment was studied during 2008–2010. Ecological conditions in intertidal macroalgal and benthic deep basin communities were studied in addition to measurements of nutrients and chlorophyll-a values. Macroalgal communities in the intertidal zone and the deep water fauna communities showed a high ecological status in the intermediate part of the fjord and a good status in the inner part of the fjord. Faunal communities in the outermost basin indicate that the assimilative capacity for farm waste of this deep basin could be limited. Nutrients and chlorophyll-a values were within national thresholds defined as high water quality. The good ecological conditions of the parameters studied in the fjord show little evidence of a regional impact from the fish farming industry despite the intensive production level.
Introduction
Salmon farming has grown rapidly in Norway since its onset in the 1970s and approximately one million metric tonnes are produced yearly. Waste from fish farming in open cages will have a large impact on the bottom communities in the area close to the farm and the release of nutrient and fine particulate material may also have a local influence in the shallow water communities in the near farm area. This local footprint of fish farming is well recognized and is subject to different types of monitoring in different area in the world (Ervik et al. Citation1997, 2004; Hansen et al. Citation2001; Read & Fernandes Citation2003).
However, intense fish farming in an area may inflict regional impacts on marine ecosystems such as eutrophication, impact on shore communities and major changes in the environmental conditions for bottom communities. In order to develop environmentally sustainable fin-fish farming it is important to understand if such activities might have an impact beyond the immediate production area. The regional impact of intensive marine fish farming has been little studied although some investigations do exist, mainly on the impact of dissolved nutrients (Gowen & Ezzi Citation1994; Soto & Norambuena Citation2004; Pitta et al. Citation2006; Kaymakci Basaran et al. Citation2010; Skejić et al. Citation2011).
The main fraction of the waste released during farming is bound in faeces and sinks rapidly (4–9 cm s−1) towards the sea bed (Chen et al. Citation2003). In low dynamic environments an increased flux of organic matter to the sea bed and co-occurring changes in infauna community structure are therefore mainly observed within 100 m of the farm's perimeter (Kutti et al. Citation2007b). Once settled, organic particles may be transported further in resuspension–deposition loops (Thomsen & Gust Citation2000), spreading the farm waste beyond the farm′s perimeter. In western Norwegian fjords, near-bed current speeds above the threshold at which salmon faecal pellets are resuspended, i.e. 10 cm s−1 (Cromey et al. Citation2002), occur episodically in connection with intermediate water exchange (density-driven current) and episodic deep-water inflows (Aure et al. Citation2007). In Bjørnafjord (Norway), farm waste was traced and signs of a moderately enriched infauna community were observed in an accumulation area 1 km away from a fish farm (Kutti et al. Citation2007a, Citation2007b). In intensive salmon farming areas where several farms have been active in the same body of water for an extended period of time, wider impacts of the release of organic waste should be expected.
Norwegian coastal waters are normally nitrogen-limited in the euphotic zone during summer (Aure & Johannessen Citation1997). Salmon growth is usually highest at this time of year and the emissions of nutrients will thus be high when nitrogen is naturally limited. An addition of dissolved nitrogen can stimulate phytoplankton growth and plankton blooms (Gowen et al. Citation1992; Boynton et al. Citation1996; Pedersen & Borum Citation1996; Bricker et al. Citation2003). If the production is not recirculated in the euphotic zone but settles on the sea bed, increased organic loading may occur and in extreme situations oxygen depletion in the basin water (Gowen & Bradbury Citation1987; Best et al. Citation2007). Nitrogen addition can also change the seaweed communities in the littoral zone by stimulating growth of annual, rapidly growing species which might out-compete perennial habitat-building species and cause shifts from highly diverse macroalgal communities dominated by perennial brown algae to low-diversity communities dominated by opportunists and annual species (Rueness & Fredriksen Citation1991; Bokn et al. Citation1992; Munda Citation1996; Pihl et al. Citation1999; Worm & Sommer Citation2000; Krause-Jensen et al. Citation2007).
The European Water Framework Directive (WFD, 2000/60/EC) establishes a framework for monitoring and, when required, the improvement of European coastal waters. WFD is incorporated into the Norwegian Water Management Regulations that have an objective of reaching at least a good status for all Norwegian waters by 2021. The assessment of the ecological quality within coastal waters is based on a combination of both biological and physical–chemical quality elements. The biological elements include assessments of phytoplankton biomass (i.e. concentrations of chlorophyll-a), macroalgae (maximum depth distribution of selected species and diversity in the littoral algal communities) and soft bottom fauna (i.e. diversity and NQI1 (Norwegian Quality Index 1)). The biological quality elements are considered as the most important indicators for determining water quality, as they are a direct measurement of the impact from pollution on organisms and may also indicate the long-term effects of pollutants in ecosystems, even when values beyond thresholds are difficult to detect (Bermejo et al. Citation2012). Development, calibration and testing of tools and criteria for different water types in Norwegian coastal waters are still in progress.
The objective of this study is to assess regional impacts of intensive salmon farming by a classical monitoring programme of nutrients and chlorophyll-a values, but also by the use of novel tools (benthic bottom fauna and macroalgal communities) provided by the European Water Framework Directive (WFD, 2000/60/EC).
Material and methods
The study area
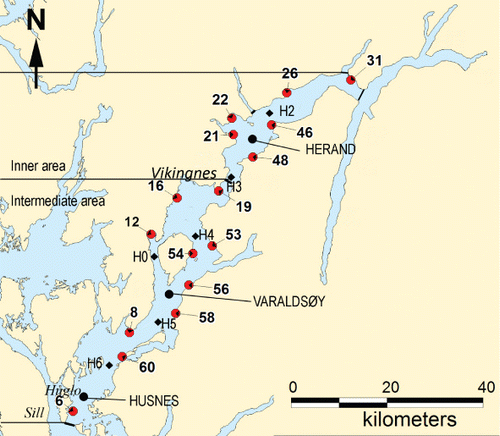
The Hardangerfjord is the second largest fjord in Norway, penetrating 179 km inland in a northeasterly direction, and it is the third largest fjord in the world (). The sea surface area inside the main sill is approximately 800 km2. The main sill is 150–200 m deep and is situated near the island of Huglo as indicated in , and the depth increases inwards in the fjord, reaching the greatest depth of 890 m in the inner basin of the fjord inside Vikingnes. Previous studies estimate a natural sedimentation rate of 5 mm per day in the inner area, mainly of fine material discharged by rivers with a high content of silt (30–80%; Holtedahl Citation1975). Water exchange in the surface layers of the fjord is high due to tidal waves, intermediate water exchange and fresh water run-off into the fjord. Near-bed current speeds may thus be low in many areas (Aure et al. Citation2007; Asplin et al. Citation2014). The retention time for surface water in the fjord is approximately one month (Ervik et al. Citation2008). Freshwater discharge from the drainage area, the Folgefonna glacier and rivers create a brackish surface layer throughout the fjord mainly during summer after snow melting. The inner fjord branches experience a brackish surface layer (salinity ≤25) in all seasons.
The Hardangerfjord is one of the most intensively used salmon-farming areas in the world, with an annual production of approximately 70,000 metric tonnes (Taranger et al. Citation2011). The production takes place in open net-cages and dissolved nutrients and organic wastes are released directly into the surrounding environment. Calculated using the Ancylus–MOM Fish-model (Stigebrandt et al. Citation2004), 127 metric tonnes of DIP (dissolved inorganic phosphorous) and 280 tonnes of POP (particulate organic phosphorous) from the production are released into the environment each year. Estimated annual nitrogen emissions are 770 metric tonnes of DIN (dissolved inorganic nitrogen) and 1756 metric tonnes of PON (particulate organic nitrogen). The production of 70,000 metric tonnes of salmon will produce around 7000 metric tonnes of particulate organic waste and contribute substantially to the total annual flux of organic matter to the infauna communities (Kutti Citation2008).
Most of the fish production takes place in the intermediate and outer part of the fjord, while the inner part of the fjord harbours fewer farms. In the intermediate and outer area the fish production is the main contributor to nutrient enrichment, while in the inner area the main contributor is natural run-off from land (Anon. Citation2011). The maximum allowed fish biomass in the intermediate area is high relative to the sea surface area (190 metric tonnes of fish/km2), while in the inner area there is relatively low biomass of fish compared to the sea surface area (56 metric tonnes of salmonid fish/km2; Norwegian Directorate of Fisheries). The annual production of fish is approximately 10–20% lower than the maximum allowed biomass because not all farms are in operation at the same time. The fish farms are generally situated 100–300 m from land along the steep hard rock shores of the fjord. In the fjord branches there is no fish production and these are not included in this study; nor is the outer area of the fjord, where we find a more coastal dynamic environment considered to be less sensitive to effluents from fish farming. In this study we have focused on the inner and intermediate area of the fjord (; detailed information on sampling sites is given in Supplementary Material A). The intermediate area has a rather narrow entrance to the inner area and the deep basins of the two areas are partly separated by a deep sill at approximately 500 m depth, close to Vikingnes ().
Nutrients and fluorescence
Six locations, two in the inner area and four in the intermediate area, were selected for sampling of physico-chemical parameters (). The sites were situated at least 500 m away the shore and more than 1 km from fish farms to ensure that the water was representative of the fjord water and not influenced by local emissions of nutrients. Water samples were collected from depths of 0, 2, 5, 7 and 10 m approximately monthly in the period March 2008–August 2010. Salinity, temperature and fluorescence (chlorophyll-a) were measured by a CTD (SD 200 W, SAIV A/S). Measurements were recorded every second while the CTD was lowered at a rate of<0.5 ms−1 from the surface to 30 m depth. Water samples were stored in a cool, dark area and taken to the laboratory and were principally analysed for nitrate, nitrite, phosphate and silicate within 24 h. In some cases when this was not possible, the nutrient samples were immediately fixed with 0.02 ml chloroform for later analysis. Water samples for the analysis of total N and P were taken in February 2009 and monthly in the period June to August 2010. Dissolved inorganic nutrients were analysed according to standard methods (Parsons et al. Citation1992). Nutrient values were calculated as mean values for each station in surface water at 0–10 m depth for the summer months (June–August) and winter months (December–February). Chlorophyll-a values were calculated as mean values for each station in surface waters (0–10 m) during summer months (June–August). We have used the national thresholds for nutrient and chlorophyll-a values given by the Norwegian Climate and Pollution Directorate (Molvær et al. Citation1997) for determining water quality with these parameters.
Intertidal macroalgal communities
To assess the condition of the quality element of macroalgae required by the Water Framework Directive, we have used methods described in Guidelines for classification of ecological quality in water, which are still under development for Norwegian waters (Anon. Citation2009).
The multimetric index for macroalgae communities gives a value for normalized shore diversity (reference condition), which gives the characteristics for the macroalgal communities on a pristine shore. The normalized shore diversity is a list of species that is commonly associated with undisturbed conditions (reduced species list, RSL) and is specific for a geographic region. The water type in Hardangerfjord is characterized as Ns4A (strongly freshwater-influenced fjord, North Sea, Lindesnes–Korsfjorden). The multimetric index for macroalgal intertidal communities is only developed and validated for the water types Ns1B, and Ns2B and is suggested for the water types No1A and No1B, which are the areas north of Korsfjorden on the Norwegian west coast (Anon. Citation2009). Currently there is no available index for waters that are strongly influenced by fresh water run-off such as fjords. With the lack of such a fjord index we have chosen to use the available index to assess the condition of the macroalgal communities in the fjord environment.
Data for evaluation of the environmental condition of the macroalgal communities at 16 sites in Hardangerfjord () were compiled from the investigations of the macroalgal communities in the fjord during the summer of 2008–2009 (Husa et al. Citation2014). The sites were not randomly selected because this study was a reinvestigation of sites that were studied in the period 1955–1960 (Jorde & Klavestad Citation1963). Macroalgal communities in the intertidal and shallow subtidal zone were studied at 6 sites in the inner area and 10 sites in the intermediate area of the fjord during low tide in June 2008 and 2009. The sites were mainly situated on protruding land with a rocky substrate and the closest distance to a fish farm was 800 m and as such they should be characteristic of the intertidal macroalgal community in the fjord.
Normalized shore diversity was calculated for each site based on a scoring system evaluating parameters such as substrate, grazers, filter feeders and habitat complexity of the shore. An ecological quality ratio (EQR value) for the combined parameters (1) proportion of Chlorophyta taxa, (2) proportion of Rhodophyta taxa, (3) proportion of opportunistic taxa, (4) the ratio between late successional or perennial taxa (ESG 1 species) and opportunistic or annual taxa (ESG 2 species) at each individual site was calculated as described in the guidelines (Anon. Citation2009).
Soft bottom infauna and oxygen
Three sites were selected for deep-water infauna sampling in Hardangerfjord: Herand (837 m deep), Varaldsøy (642 m deep) and Husnes (455 m deep) (). The three sites were situated at least 3 km from any farms in separate deep basins distributed in the inner and intermediate part of the fjord. At all sites five replicate grab samples of infauna (> 1 mm) were collected using a 0.1-m2 van Veen grab, following the international standards given for soft bottom surveys (ISO 16665:2005). On board, the samples were labelled and preserved in 4% formaldehyde. In the laboratory the fauna samples were sorted and transferred to 70% ethanol. The infauna was identified to the lowest possible taxonomic level and counted. Before analysis the species list was checked to remove species that are not quantitatively sampled with the grab, i.e. pelagic organisms and highly mobile fauna. Infauna abundance (N), total number of species (S), diversity (H′), AZTI Marine Biotic Index (AMBI; Borja et al. Citation2003) and Norwegian Quality Index 1 (NQI1; Anon. Citation2009) were calculated for each sample separately. The AMBI values were calculated using the M-AMBI software (http://ambi.azti.es/index.php?lang=en). The NQI1 values were calculated according to the following formula:
The average values for H′ and NQI1 of the five replicate samples were used to assess the ecological status of the sites. N, S and the AMBI value are not used in the classification but only in the calculation of NQI1. As there are no fjords without fish farms, the ecological status was assessed against the standard reference conditions for Norwegian coastal waters, i.e. 0.78 for NQI1 and 4.4 for H′ (Anon. Citation2009), corresponding to undisturbed pristine conditions (Borja et al. Citation2004). Thus, EQRs for NQI1 were created by dividing the present value by the reference value of 0.78. The thresholds for each of the ecological status levels for H were:>0.86=high, 0.68–0.86=good, 0.43–0.68=moderate, 0.2–0.43=poor,<0.2=bad and for NQI1:>0.92=high, 0.81–0.92=good, 0.63–0.81=moderate, 0.4–0.63=poor,<0.4=bad (Anon. Citation2009). Since most intercalibration work has been focused on the NQI1, main focus should be given to this element when evaluating the ecological status of a given body of water in Norway (Anon. Citation2009). Oxygen saturation in the near-bottom layer was measured in the deep basin of the inner area of Hardangerfjord at Herand and in the intermediate area at Husnesfjorden using a CTD with an oxygen sensor and Winkler titration, respectively.
Results
Chlorophyll-a and nutrient values
Nutrient values and chlorophyll-a were within the thresholds of a high water quality according to national guidelines () at all six sites in the inner and intermediate area of the Hardangerfjord. Chlorophyll-a values typically reached levels of 3–6 µg l−1 during spring bloom with a peak in March (). The highest mean values of chlorophyll-a were detected at station H0 and station H5 during spring bloom. Mean summer values at all sites ranged from 0.97 to 1.15 µg l−1, which is within the threshold indicating a high quality (threshold high quality <2.0 µg l−1) ().
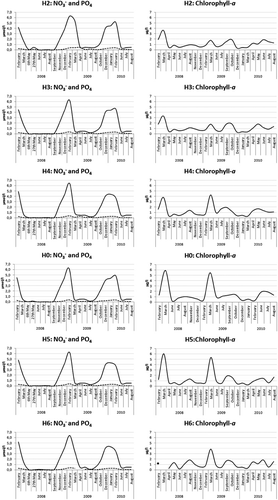
Nitrate values at all sites were low during summer, varying from not detectable to 0.5 µmol l−1 (threshold high quality <0.85 µmol l−1). During winter, nitrate levels reached a maximum value of 6.5 µmol l−1 in February, just before the spring bloom (threshold high quality <6.43 µmol l−1). Phosphate summer values in the fjord were mainly within the threshold of high water quality (threshold high quality <0.13 µmol l−1), with the exception of measurements in June 2009, where the values were slightly higher (0.14–0.16 µmol l−1) at all sites. Winter mean values of phosphate reached a maximum of 0.40 µmol l−1 in February (threshold high quality <0.52 µmol l−1). At the innermost station in H2 the phosphate values remained high, with a maximum of 0.44 µmol l−1 also during March and April in 2009 ().
Winter values (only one measurement in February 2009) of Total P varied between 0.53 and 0.59 µmol l−1 (threshold high quality <0.68 µmol l−1) and the corresponding Total N values varied between 12.9 and 14.2 µmol l−1, with the highest concentration at the outermost station in the fjord (threshold high quality <21.07 µmol l−1). Summer values measured from June 2010 to August 2010 showed Total P values between 0.27 and 0.40 µmol l−1 (threshold high quality <0.39 µmol l−1) and the corresponding Total N values ranged between 8.9 and 13.6 µmol l−1 (threshold high quality <17.86 µmol l−1) and both were within the national threshold of high water quality.
Macroalgae
The nine macroalgal sites examined in the intermediate part of the fjord all showed a high ecological status (). The number of species and opportunistic species varied little between sites and a high abundance of opportunistic species was not found at any of these sites. The littoral zone in this area was dominated by the fucoids Ascophyllum nodosum (Linnaeus) Le Jolis, Fucus vesiculosus Linnaeus and Fucus serratus Linnaeus, with a narrow zone of Fucus spiralis Linnaeus at most sites. The supra-littoral brown alga Pelvetia canaliculata (Linnaeus) Decaisne & Thuret appeared scattered at four of the sites. The kelp Saccharina latissima (Linnaeus) C.E. Lane, C. Mayes, Druehl & G.W. Saunders was common in the shallow subtidal at all sites except from Haukanes (54) in this area and Laminaria digitata (Hudson) J.V. Lamouroux was common in the shallow subtidal zone at six sites (a detailed species list (RSL) is to be found in Supplementary Material B).
Table I Species richness and calculated EQR values (ecological quality ratio) showing ecological status for the biological quality element intertidal macroalgae studied at 16 sites in Hardangerfjord during the summers of 2008 and 2009.
Macroalgal communities at five of the six sites examined in the inner area of the fjord were classified as being of a high quality ecological status, while the innermost site (31) was classified as being of a good ecological status (). Fewer species were recorded at the two innermost sites than at the four sites located further out in the fjord (). Site 31 had a particularly low species number as it was almost completely dominated by blue mussels (Mytilus edulis Linnaeus, 1758). The dominant brown algae at the innermost sites were A. nodosum, F. vesiculosus and F. serratus. The fucoid F. spiralis was recorded as common at the two outermost sites, but was only sporadically found at the innermost sites. The supralittoral brown alga P. canaliculata was not present in the inner area of the fjord. The kelps L. digitata and S. latissima were more scarce in this area, but were common in the shallow subtidal zone at sites 26 and 46 (detailed species list (RSL) in Supplementary Material B).
Soft bottom infauna and oxygen
In the inner basin of Hardangerfjord infauna samples were collected at one site only, at 837 m depth at Herand, the deepest part of the fjord (). This site was characterized by a rather low infauna abundance (67±16 SD) and good species diversity (3.42±0.26 SD). The infauna community at the site was dominated by the errant polychaete Paradiopatra fiordica (Fauchald, Citation1974) and juveniles of the bivalve Thyasira sp. (). The average EQR for NQI1 was 0.92, placing the site just at the boundary between a high and a good ecological status (). The average EQR for H′ was 0.78 and the oxygen concentration of the bottom water was 4.17 ml l−1, both supporting the classification of the site into a good ecological status.
Table II Abundance of the five most common infauna species at three deep water sites in Hardangerfjord, Husnes, Varaldsøy and Herand, in 2008–2011.
Table III Diversity of the infauna communities sampled in the three deep water sites in Hardangerfjord: Husnes, Varaldsøy and Herand. Richness = total number of species and Diversity = H′; AMBI = AZTI Marine Biotic Index; NQI1 = Norwegian Quality Index 1; EQR = ecological quality ratio. All values are given for a 0.1-m2 van Veen grab sample.
In the intermediate basin of Hardangerfjord infauna samples were collected at two sites, just south of the island of Varaldsøy at a depth of 642 m and outside Husnes at a depth of 455 m (). The Varaldsøy site was characterized by a rather high infauna abundance (116±50 SD) and high infauna diversity (4.20±0.14 SD). The bivalve Kelliella sp., the polychaetes Paradiopatra fiordica (Fauchald, Citation1974) and Chaetozone setosa Malmgren, 1867 and the ophiuroid Amphilepis norvegica (Ljungman, 1865) were dominant in all five grab samples (). The average EQR for NQI1 for the Varaldsøy site was 0.96, indicating a high ecological status of the site (). Average EQR for H′ was 0.95, arriving at the same classification. The Husnes site was characterized by an abundance of infauna similar to that found in Varaldsøy (117±46 SD) and a diversity of 4.0±0.48 SD (). The infauna community at the site was dominated by the cirratulid polychaetes Aphalochaeta sp. and Chaetozone setosa Malmgren, 1867 and the polychaetes Paramphinome jeffreysii (McIntosh, 1868) and Heteromastus filiformis (Claparède, 1864) (). In addition, nematodes were abundant (detailed species lists are given in Supplementary Material C). All grab samples from Husnesfjorden contained large amounts of spicules derived from dead demosponges from surrounding areas (20–60% of the content of the grab). The average EQR for NQI1 for the Husnes site was 0.95 and the average EQR for H′ was 0.90, indicating a high ecological status for this body of water (). The concentration of oxygen in the bottom water was 5.68 ml l−1, arriving at the same classification.
Discussion
Chlorophyll-a and nutrient values
All nutrient and chlorophyll-a values in both areas were within the thresholds for high water quality set by the national authorities, and there were no indications of elevated levels in the intermediate area, which produces the higher biomass of salmon. The Hardangerfjord is a very dynamic fjord with surface current speed normally in the range of 10–50 cm s−1 and monthly renewal of the upper fjord water (Asplin et al. Citation2014). Modelling of the distribution of nutrients in the fjord, taking into account water exchange rates, shows that nutrient emissions from fish farms will increase the natural nitrogen concentrations (Anon. Citation2011) and phytoplankton biomass by 1–5% (Skogen et al. Citation2009).
In general the chlorophyll-a values were lower in the inner area compared to the intermediate area, indicating a lower primary production in the former (). The inner area experienced weak spring blooms compared to the intermediate area, particularly in 2009 and 2010. The spring bloom in 2010 was not very pronounced in any of the areas studied. Braarud (Citation1974a, Citation1974b) studied the phytoplankton communities in Hardangerfjord during the period 1955–1956 and found an evident fjord effect on the species composition. He found the inner area to have lower biomass of plankton than the outer area during spring and summer, while in autumn the inner part of the fjord could maintain a higher biomass of particularly Skeletonema costatum (Greville) Cleve. He also found that the species composition could differ substantially between the inner and outer areas of the fjord. As phytoplankton communities were not studied in detail in this investigation and Braarud's study only lasted one year, it is difficult to draw any conclusions on the reasons for the lower differences between the phytoplankton communities in the inner and outer parts of Hardangerfjord.
Our study does not fully meet the requirements of the WFD for the biological quality element phytoplankton, as we merely measured chlorophyll-a values as fluorescence. The WFD requires actual measurements of phytoplankton abundance and studies of plankton composition. However, chlorophyll-a values are regarded as a useful expression of phytoplankton biomass and are a responsive indicator of nutrient enrichment in coastal water (Devlin et al. Citation2007; Harding Citation1994). The chlorophyll-a values measured in Hardangerfjord in this study give no indication of any ongoing eutrophication processes.
Macroalgae
The multimetric indices for the macroalgal communities showed high ecological conditions at all sites examined in the intermediate area of Hardangerfjord. In the inner area the macroalgal community at all sites showed a high ecological condition except for the innermost site (31). This site had the lowest species richness of the investigated sites, most likely explained by dense settlement of blue mussels in the intertidal zone or due to salinity stress in the innermost part of the fjord. The physical conditions in Hardangerfjord are known to be highly variable. The entire fjord is influenced by freshwater influx, but with the surface salinity being much lower (< 23) in the innermost fjord arms than in the middle and outer parts of the fjord, where surface salinity is generally>27 due to increasing mixing caused by the effect of winds (Asplin et al. Citation2014). A high freshwater influence, especially during summer, will exclude several freshwater-intolerant macroalgal species from the littoral community. A low intertidal species richness is therefore the rule rather that the exception when moving towards the inner parts of Hardangerfjord (Jorde & Klavestad Citation1963; Husa et al. Citation2014). The macroalgal index used in this study is developed for coastal areas and is still not approved for freshwater-influenced fjords; thus, it is not likely that the index is overestimating the ecological condition in the intertidal communities. A number of species on the reduced species list (Anon. Citation2009) were not present in the inner area of Hardangerfjorden.
Husa et al. (2013) compared macroalgal communities down to 30 m depth in Hardangerfjord with historical data from the 1950s and found that the abundance of habitat building species like kelp and fucoids had a high resilience in the fjord, despite 50 years of anthropogenic activity. They also found that the main drivers of the observed changes in community structure were higher sea temperatures and altered salinity in the fjord due to the hydro-electrical power plant industry. The high ecological condition in macroalgal communities in the intertidal zone is not surprising given the low nutrient level in the fjord. However, we cannot rule out that local impacts on macroalgal communities might occur in the vicinity of farms (< 1 km away). Such impacts have been shown for seagrass meadows and maerlbeds (Hall-Spencer et al. Citation2006; Diaz-Almela et al. Citation2008; Duarte et al. Citation2008; Sanz-Lazaro et al. Citation2011; Aquado-Gimènez & Ruiz-Fernàndez Citation2012).
Soft bottom infauna and oxygen
A relatively species poor and low abundance infauna community dominated by species commonly found in the silty deep basins of western Norwegian fjords, e.g. Nucula tumidula Malm, 1861 (Kutti et al. Citation2007a) and Paradiopathra fiordica, was found in the inner and deepest part of Hardangerfjord. The low species richness is in contrast to the general trend of increased infauna species richness with increasing depth (Holte et al. Citation2004) but in line with early observations of Fauchald (Citation1972, Citation1974) in that the basins of deep fjords (such as Hardangerfjord and Sognefjord) are often species-poor. Species richness of infauna is known to decrease with decreasing oxygen levels in the bottom water (Buhl-Mortensen et al. Citation2009). An extreme low infauna diversity (0.99 H′) and the dominance of the tolerant polychaete Spiochaetopterus typicus M. Sars, 1856 were found in this area in 1996 (Rygg & Skei Citation1997), indicating that periods of oxygen deficiency do occur and that the frequency of bottom water renewal is an important determinant for the ecological status of the deep benthic communities in the inner part of Hardangerfjord. At the time of our sampling, oxygen levels in the inner part of the fjord were within thresholds of good conditions and only slightly inferior to that of the outer part. The infauna species composition in our study also indicated that there had been no periods of oxygen deficiency in the deep waters of Hardangerfjord during the last couple of years. Infauna abundance is known at increase with both quality and quantity of organic matter arriving at the sea-bed (Dauer & Conner Citation1980; Rosenberg Citation1995; Flach & Heip Citation1996; Kutti et al. Citation2008). High siltation rates of mineral particles originating from run-off from the large Folgefonna glacier, in combination with the lower primary production in the inner part, could explain the lower total abundance of infauna (i.e. 70 ind. m−2) observed in the inner part of the fjord as compared to the outer part (i.e. 120 ind. m−2).
The examination targeting the accumulation area at 643 m depth in the intermediate part of the fjord showed no indications of enrichment, neither from the settling out of phytoplankton blooms nor from farm derived particulate waste. The sediment supported a highly diverse infauna community dominated by species characteristic for unaffected areas in western Norwegian fjords, e.g. Kelliella sp. and Amphilepis norvegica (Kutti et al. Citation2007a) and P. fiordica. The deep basin at 455 m depth, however, supported an infauna community dominated by opportunistic species, i.e. the cirratulids Aphelochaeta spp. and Chaetozone setosa, Paramphinome jeffreysii and the capitellid Heteromastus filiformis (Claparède, 1864). The infauna species composition at this site could be reflecting a beginning of enrichment, caused by the cumulative impact of the production and release of organic waste from several fish farms over many years to the accumulation basin. This is, however, likely to be a slow process, as below the highly dynamic surface layers of the fjord, current speeds are generally low and most waste settles and is processed by macrofauna and microorganisms in a restricted area close to the farms (Kutti et al. Citation2008). Another explanation for the dominance of opportunists could be the occurrence of large amounts of spicules derived from dead demosponges from surrounding areas. Opportunistic species are known to respond also to other types of disturbances besides organic enrichment, e.g. Bett & Rice (Citation1992) reported a considerable increase in the abundance of macrofauna in sediment samples containing sponge spicule mats relative to samples without them.
Conclusion
Overall, the benthic and pelagic communities beyond the immediate proximity of fish farms in Hardangerfjord seemed to be little affected by the effluents of nutrients and deposition of organic matter from the salmon farming industry. However, the innermost macroalgal site and the deep bottom fauna in the inner basin were only categorized as in ‘good condition’ according to the standards of The European Water Framework directive. The high assimilative capacity of nutrient emissions and organic waste is most likely due to the large water volume in the fjord and the dynamic physical environment. The European Water Framework directive requires a precautionary approach, meaning that when several sites are assessed in the same area, the site with the lowest Ecological Quality Ratio score provides the final ecological status for that specific quality element. Similarly, the overall ecological status of the water area should be determined by the quality element giving the lowest score of the quality elements included in the assessment (Anon. Citation2009). The inner part of Hardangerfjord has a low salmon production, with natural run-off being the main source for nitrogen emissions to the area (Anon. Citation2011). The fact that the scores of both littoral and deep infauna quality elements resulted in a classification of the ecological status as only ‘good’ indicates that several other stressors are acting on the communities. While freshwater run-off clearly structured the surface layer and intertidal communities, bottom water renewal may be the main structuring agent for the deep infauna communities.
Although there is little evidence of regional impact from fin-fish farming in Hardangerfjord, the cumulative effect of numerous impacted areas around the fish farms must be taken into consideration when further evaluating the total impact from fin-fish farming on ecosystem functioning in this area.
Editorial responsibility: Ketil Hylland
smar_a_810754_sm1894.pdf
Download PDF (112.6 KB)Acknowledgements
This study is a part of the EPIGRAPH (Ecological Processes and Impacts Governing the Resilience and Alternations in the Porsangerfjord and the Hardangerfjord) and was funded by the Ministry of Fisheries and Coastal Affairs and the Research Council of Norway. We thank the project leader of EPIGRAPH-Hardanger Øystein Skaala (IMR) for good and enthusiastic management of the project and Vidar Wennevik (IMR) for creating maps. We thank Lars Asplin (IMR), Svein Nygård and the crew of ‘RV Hans Brattström’ for valuable assistance during field work. We also thank Are Pedersen (NIVA) for help with the calculation of EQR-values for macroalgal communities. We appreciate valuable comments on the manuscript by Geir Helge Johnsen, Stein Fredriksen and two anonymous reviewers.
References
- Anon. 2009. Klassifisering av miljøtilstand i vann. Direktoratsgruppa for gjennomføringen av vanndirektivet: Veileder 01:2009. 180 pages. (in Norwegian)
- Anon. 2011. Vurdering av eutrofieringssituasjonen i kystområder, med særlig fokus på Hardangerfjorden og Boknafjorden. Report. Ministry of fisheries and coastal affairs. 83 pages. (in Norwegian)
- Aquado-Giménez F, Ruiz-Fernández JM. 2012. Influence of an experimental fish farm on the spatio-temporal dynamic of a Mediterranean maerl algae community. Marine Environmental Research 74:47–55. 10.1016/j.marenvres.2011.12.003
- Asplin LC, Aure J, Sandvik AD, Albretsen J, Sundfjord V, Johnsen IA, et al. 2014. Fluctuations in the physical climate of the Hardangerfjord, and its influence on salmon lice distribution. Marine Biology Research 10:216–25.
- Aure J, Johannessen T. 1997. Næringssalter og Klorofyll-a fra Skagerrak til Vestlandet. Fisken og Havet 2. 45 pages. (in Norwegian)
- Aure J, Asplin L, Sætre R. 2007. Coast/fjord water exchange. In: Saetre R, editor. The Norwegian Coastal Current – Oceanography and Climate. Trondheim: Tapir Academic Press. 159 pages.
- Bermejo R, Vergara JJ, Hernández I. 2012. Application and reassessment of the reduced species list index for macroalgae to assess the ecological status under the Water Framework Directive in the Atlantic coast of Southern Spain. Ecological Indicators 12:46–57. 10.1016/j.ecolind.2011.04.008
- Best MA, Wither AW, Coates S. 2007. Dissolved oxygen as a physico-chemical supporting element in the Water Framework Directive. Marine Pollution Bulletin 55:53–64. 10.1016/j.marpolbul.2006.08.037
- Bett BJ, Rice AL. 1992. The influence of the hexactinellid sponge (Pheronema carpenteri) spicules on the patchy distribution of macrobenthos in the Porcupine Seabight (bathyal NE Atlantic). Ophelia 36:217–22. 10.1080/00785326.1992.10430372
- Bokn T, Murray SN, Moy FE, Magnusson JB. 1992. Changes in fucoid distribution and abundances in the inner Oslofjord, Norway: 1974–80 versus 1988–90. Acta Phytogeographica Suecia 78:117–24.
- Borja Á, Muxika I, Franco J. 2003. The application of a Marine Biotic Index to different impact sources affecting soft-bottom benthic communities along European coasts. Marine Pollution Bulletin 46:835–45.
- Borja Á, Franco J, Valencia V, Bald J, Muxika I, Belzunce MJ, Solaun O. 2004. Implementation of the European water framework directive from the Basque country (northern Spain): a methodological approach. Marine Pollution Bulletin 48:209–18.
- Boynton WR, Hagy JD, Murray L, Stokes C, Kemp WM. 1996. A comparative analysis of eutrophication patterns in a temperate coastal lagoon. Estuaries 19:408–21. 10.2307/1352459
- Braarud T. 1974a. The natural history of the Hardangerfjord. 10. The phytoplankton in 1955–1956. The quantitative phytoplankton cycle in the fjord waters and in the offshore coastal waters. Sarsia 55:63–98.
- Braarud T. 1974b. The natural history of the Hardangerfjord. 11. The fjord effect upon the phytoplankton in late autumn to early spring, 1955–56. Sarsia 55:99–114.
- Bricker SB, Ferreira JG, Simas T. 2003. An integrated methodology for assessment of estuarine trophic status. Ecological Modelling 169:39–60. 10.1016/S0304-3800(03)00199-6
- Buhl-Mortensen L, Oug E, Aure J. 2009. The response of hyperbenthos and infauna to hypoxia in fjords along the Skagerrak: Estimating loss of biodiversity due to eutrophication. In: Moksness E, Dahl E, Støttrup J, editors. Integrated Coastal Zone Management. UK: Wiley-Blackwell, p 79–96.
- Chen YS, Beveridge MCM, Telfer TC, Roy WJ. 2003. Nutrient leaching and settling rate characteristics of the faeces of Atlantic salmon (Salmo salar L) and the implications for modelling of solid waste dispersion. Journal of Applied Ichthyology 19:114–17. 10.1046/j.1439-0426.2003.00449.x
- Cromey CJ, Nickell TD, Black KD, Provost PG, Griffiths CR. 2002. Validation of a fish farm waste resuspension model by use of a particulate tracer discharged from a point source in a coastal environment. Estuaries 25:916–29. 10.1007/BF02691340
- Dauer DM, Conner W. 1980. Effects of moderate sewage input on benthic polychaete populations. Estuarine and Coastal Marine Science 10:335–46. 10.1016/S0302-3524(80)80106-X
- Devlin M, Best M, Coates D, Bresnan E, O'Boyle S, Park R, et al. 2007. Establishing boundary classes for the classification of UK marine waters using phytoplankton communities. Marine Pollution Bulletin 55:91–103. 10.1016/j.marpolbul.2006.09.018
- Diaz-Almela E, Marba N, Alvarez E, Santiago R, Holmer M, Grau A, et al. 2008. Benthic input rates predict seagrass (Posidonia oceanica) fish farm induced decline. Marine Pollution Bulletin 56:1332–42. 10.1016/j.marpolbul.2008.03.022
- Duarte CM, Frederiksen M, Grau A, Karakassis L, Marba N, Mirto S, et al. 2008. Effects of fish farm waste on Posidonia oceanica meadows: Synthesis and provision of monitoring and management tools. Marine Pollution Bulletin 56:1618–29. 10.1016/j.marpolbul.2008.05.020
- Ervik A, Hansen PK, Aure J, Stigebrandt A, Johannessen, Jahnsen T. 1997. Regulating the local environmental impact of intensive fish farming. I. The concept of the MOM system (Modelling–Ongrowing fish farms–Monitoring). Aquaculture 158:85–94. 10.1016/S0044-8486(97)00186-5
- Ervik A, Agnalt A-L, Asplin L, Aure J, Bekkvik TC, Døskeland I, et al. 2008. AkvaVis - dynamisk GIS-verktøy for lokalisering av oppdrettsanlegg for nye oppdrettsarter. Miljøkrav for nye oppdrettsarter og laks. Fisken og Havet 10/2008. 90 pages. (in Norwegian)
- Fauchald K. 1972. Some polychaetous annelids from the deep basins in Sognefjorden, western Norway. Sarsia 49:89–106.
- Fauchald K. 1974. Deep-water errant polychaetes from Hardangerfjorden, western Norway. Sarsia 57:1–31.
- Flach E, Heip C. 1996. Seasonal variations in faunal distribution and activity across the continental slope of the Goban Spur area (NE Atlantic). Journal of Sea Research 36:203–15. 10.1016/S1385-1101(96)90790-X
- Gowen RJ, Bradbury NB. 1987. The ecological impact of salmonid farming in coastal waters: A review. Oceanography and Marine Biology Annual Review 25:563–75.
- Gowen RJ, Ezzi IA. 1994. Assessment and Prediction of the Potential for Hypernutrification and Eutrophication Associated with Cageculture of Salmonids in Scottish Coastal Waters. Oban, Scotland: Dunstaffnage Marine Laboratory. 137 pages.
- Gowen RJ, Tett P, Jones KJ. 1992. Predicting marine eutrophication: the yield of chlorophyll from nitrogen in Scottish coastal waters. Marine Ecology Progress Series 85:153–61. 10.3354/meps085153
- Hall-Spencer J, White N, Gillespie E, Katie G, Foggo A. 2006. Impact of fish farms on maerl beds in strongly tidal areas. Marine Ecology Progress Series 326:1–9. 10.3354/meps326001
- Hansen PK, Ervik A, Schaanning M, Johannessen P, Aure J, Jahnsen T, et al. 2001. Regulating the local environmental impact of intensive, marine fish farming. II. The monitoring programme of the MOM system (Modelling–Ongrowing fish farms–Monitoring). Aquaculture 194:75–92. 10.1016/S0044-8486(00)00520-2
- Harding L. 1994. Long term trends in the distribution of phytoplankton in Chesapeake Bay: Roles of light, nutrients and streamflow. Marine Ecology Progress Series 104:267–91. 10.3354/meps104267
- Holte B, Oug E, Cochrane S. 2004. Depth related benthic macrofaunal diversity patterns in three undisturbed north Norwegian fjords. Sarsia 89:91–101. 10.1080/00364820410003496
- Holtedahl, H. 1975. The geology of the Hardangerfjord, West Norway. Norges geologiske undersøkelse 323:1–87.
- Husa V, Steen H, Sjøtun K. 2014. Historical changes in the macroalgal communities in Hardangerfjord. Marine Biology Research 10:226–40.
- Jorde I, Klavestad N. 1963. The natural history of the Hardangerfjord. 4. The benthonic algal vegetation. Sarsia 9:1–100.
- Kaymakci Basaran A, Aksu M, Egemen O. 2010. Impacts of the fish farms on the water column nutrient concentrations and accumulation of heavy metals in the sediments in the eastern Aegean Sea (Turkey). Environmental Monitoring and Assessment 162:439–51. 10.1007/s10661-009-0808-x
- Krause-Jensen D, Middelboe AL, Carstensen J, Dahl K. 2007. Spatial patterns of macroalgal abundance in relation to eutrophication. Marine Biology 152:25–36. 10.1007/s00227-007-0676-2
- Kutti T. 2008. Regional Impact of Organic Loading from a Salmonid Farm – Dispersal, Sedimentation Rates and Benthic Fauna Response. PhD Thesis. University of Bergen. 54 pages.
- Kutti T, Ervik A, Hansen PK, Høisæter T, Johannessen P. 2007a. Effects of organic effluents on a fjord system. II. Temporal and spatial patterns in infauna community composition. Aquaculture 262:355–66. 10.1016/j.aquaculture.2006.10.008
- Kutti T, Ervik A, Hansen PK. 2007b. Effects of organic effluents on a fjord system. I. Vertical export and dispersal processes. Aquaculture 262:367–81. 10.1016/j.aquaculture.2006.10.010
- Kutti T, Ervik A, Høisæter T. 2008. Effects of organic effluents on a fjord system. III. Linking deposition rates of organic matter and benthic productivity. Aquaculture 282:47–53. 10.1016/j.aquaculture.2008.06.032
- Molvær J, Knutzen J, Magnusson J, Rugg B, Skei J, Sørensen J. 1997. Klassifisering av miljøkvalitet i fjorder og kystfarvann. [Classification of environmental quality in fjords and coastal waters] SFT: Veileder 1997:3. 35 pages. (in Norwegian)
- Munda IM. 1996. The northern Adriatic Sea. In: Scramm E & Nienhaus PH, editors. Marine Benthic Vegetation: Recent Changes and the Effects of Eutrophication. Ecological Studies, volume 123. Berlin: Springer-Verlag. 470 pages.
- Parsons TR, Maita Y, Lalli CM. 1992. A manual of chemical and biological methods for sea water analysis. New York: Pergamon Press. 173 pages.
- Pedersen MF, Borum J. 1996. Nutrient control of algal growth in estuarine waters. Nutrient limitation and the importance of nitrogen requirements and nitrogen storage among phytoplankton and species of macroalgae. Marine Ecology Progress Series 142:261–72. 10.3354/meps142261
- Pihl L, Svenson A, Moksnes PO, Wennehage H. 1999. Distribution of green algal mats throughout shallow soft bottoms of the Swedish Skagerrak archipelago in relation to nutrient sources and wave exposure. Journal of Sea Research 41:281–95. 10.1016/S1385-1101(99)00004-0
- Pitta P, Apostolaki ET, Tsagaraki T, Tsapakis M, Karakassis I. 2006. Fish farming effects on the chemical and microbiological variables of the water column: A spatio-temporal study along the Mediterranean Sea. Hydrobiologia 563:99–108. 10.1007/s10750-005-1593-3
- Read P, Fernandes T. 2003. Management of environmental impacts of marine aquaculture in Europe. Aquaculture 226:139–63. 10.1016/S0044-8486(03)00474-5
- Rosenberg R. 1995. Benthic marine fauna structured by hydrodynamic processes and food availability. Netherlands Journal of Sea Research 34:303–17. 10.1016/0077-7579(95)90040-3
- Rueness J, Fredriksen S. 1991. An assessment of possible pollution effects on the benthic algae of the outer Oslofjord, Norway. Oebalia 17:223–35.
- Rygg B, Skei J. 1997. Tiltaksorienterte miljøundersøkelser i Sørfjorden og Hardangerfjorden 1996, Delrapport 2. Sedimenter og Bløtbunnsfauna. NIVA report TA-1483/1997. 74 pages. (in Norwegian)
- Sanz-Lazaro C, Belando MD, Marin-Guirao L, Navarrete-Mier F, Marin A. 2011. Relationship between sedimentation rates and benthic impact on Maërl beds derived from fish farming in the Mediterranean. Marine Environmental Research 71:22–30. 10.1016/j.marenvres.2010.09.005
- Skejić S, Marasović I, Vidjak O, Kušpilić G, Ninčević, Gladan Z. 2011. Effects of cage fish farming on phytoplankton community structure, biomass and primary production in an aquaculture area in the middle Adriatic Sea. Aquaculture Research 42:1393–405. 10.1111/j.1365-2109.2010.02733.x
- Skogen M, Eknes M, Asplin LC, Sandvik AD. 2009. Modelling the environmental effects of fish farming in a Norwegian fjord. Aquaculture 298:70–75. 10.1016/j.aquaculture.2009.10.018
- Soto D, Norambuena F. 2004. Evaluation of salmon farming effects on marine systems in the inner seas of southern Chile: A large-scale mensurative experiment. Journal of Applied Ichthyology 20:493–501. 10.1111/j.1439-0426.2004.00602.x
- Stigebrandt A, Aure J, Ervik A, Hansen PK. 2004. Regulating the local environmental impact of intensive marine fish farming. III: A model for estimation of the holding capacity in the MOM system (Modelling–Ongrowing fish farm–Monitoring). Aquaculture 234:239–61. 10.1016/j.aquaculture.2003.11.029
- Taranger GL, Boxaspen KK, Madhun AS, Svåsand T 2011. Risk assessment – environmental impacts of Norwegian aquaculture. Extracts from: Fisken og Havet, Særnummer 3, 2010. 99 pages.
- Thomsen L, Gust G. 2000. Sediment erosion thresholds and characteristics of resuspended aggregates on the western European continental margin. Deep Sea Research I 47:1881–97.
- Worm B, Sommer U. 2000. Rapid direct and indirect effects of a single nutrient pulse in a seaweed–epiphyte grazer system. Marine Ecology Progress Series 2002:283–88. 10.3354/meps202283
