Abstract
The Hardangerfjord, western Norway, is an area with a high concentration of salmon farms, high levels of infection of salmon lice in anadromous brown trout, and declining trout populations. This study assessed the marine survival rate of anadromous trout from the River Guddalselva, in the central part of the fjord, and tested the hypothesis that trout populations in this area are depressed by salmon lice infection. From 2001 to 2011, all descending smolts and trout returning from the fjord were captured in the traps at the field station of the Institute of Marine Research. In 2004 and 2005, parts of the smolt cohorts were treated with the Substance EX to prevent sea lice infection. From 2007 to 2010, all smolts (n=3557) were also tagged with individual tags. The results show a survival rate in the sea of only 0.58–3.41% for tagged smolts, which is extremely low. The highest survival rates appeared in the years with the lowest recordings of salmon lice in spring. The survival rate of Substance EX-treated smolts and controls was 3.41% and 1.76%, respectively. These findings suggest that salmon lice infection is an important contributor to the high mortality of anadromous trout populations in the Hardangerfjord.
Introduction
Severe infections by salmon lice Lepeophtheirus salmonis (Krøyer, 1837) on anadromous trout Salmo trutta Linnaeus, 1758 and Atlantic salmon Salmo salar Linnaeus, 1758 have been paid considerable attention over the past 20 years in Ireland, Scotland and Norway (Jakobsen et al. Citation1992; Tully et al. Citation1993, Citation1999; Birkeland Citation1996; Grimnes & Jakobsen Citation1996; Bjørn & Finstad Citation1997; Revie et al. Citation2009). The Hardangerfjord is one of the areas along the Norwegian west coast with particularly high infection levels in anadromous trout (Bjørn et al. Citation2011a). In Norway, a national monitoring programme for recording lice infection levels in fish farms and on wild Atlantic salmon, anadromous brown trout and Arctic charr Salvelinus alpinus (Linnaeus, 1758) has been developed (Bjørn et al. Citation2008, Citation2011b), and a comprehensive literature on the biology of the parasite and how it affects the migration, growth, physiology, reproduction and survival of its hosts has been published (Birkeland Citation1996; Birkeland & Jakobsen Citation1997; Finstad et al. Citation2000; Heuch et al. Citation2005; Bjørn et al. Citation2011a). However, although there is considerable documentation of infection levels of wild fish and of how the parasite affects individual fish, the direct relationship between infection level and mortality at the population level in anadromous brown trout is still poorly documented (Heuch et al. Citation2005; Anon. Citation2011). Such studies require accurate information about return rates of smolt cohorts, and preferably also about regional infection levels. In Atlantic salmon, comparisons of return rates of smolt groups protected against salmon lice by various chemicals with untreated controls have shown that treated groups often have higher survival rates, suggesting a population-regulating effect of salmon lice (Skilbrei & Wennevik Citation2006; Jackson et al. Citation2011; Gargan et al. Citation2012). For anadromous brown trout, however, this is less well documented. In contrast to Atlantic salmon, which migrate relatively quickly out of the fjords and coastal areas where the highest concentrations of fish farms and hosts for L. salmonis are located, the anadromous brown trout undertakes only shorter migrations, rarely over 40–100 km from its home river (Klemetsen et al. Citation2003), and may therefore be vulnerable to high salmon louse infection and reinfection throughout the whole marine phase. Accordingly, salmon lice may affect the two species differently, and to different degrees from one year to another, depending on annual variations in temperature, freshwater influence, infection pressure and the timing of smolt migration. The Hardangerfjord has one of the highest densities of salmon farms in Norway, with an increase in production from about 17,000 tonnes in 1997 to approximately 80,000 tonnes in 2011 (Otterå et al. Citation2004; www.fiskeridir.no) and a corresponding increase in hosts for salmon lice. During the 1990s, anglers claimed that the abundance of anadromous brown trout in the Hardangerfjord was declining, particularly in the middle section of the fjord, as was also revealed by a falling trend in the catch statistics during the last 10–15 years. This trend was strongly expressed in some rivers such as the Etneelva and Kinso, but more weakly in others (Statistics Norway 2012). The biomass of salmon lice hosts is at present at least 10,000 times as high as in the prefarming situation. For this reason, infection levels are monitored both in salmon farms and on wild anadromous fish (Bjørn et al. Citation2011b). With high infection levels being observed particularly in wild anadromous trout, it has been speculated whether there might be a causal link between the decline in anadromous brown trout populations and the salmon lice produced in fish farms (Heuch et al. Citation2005). However, as anadromous brown trout migrate between rivers and the marine environment, changes in abundance of ascending fish may be caused by a number of factors such as changes in smolt production and changes in abundance of predators, prey species, parasites and other disease organisms in the marine environment. The aim of the present study was first to assess the marine survival rate of anadromous trout in parts of the Hardangerfjord by detailed monitoring of downstream and upstream migrations and second, to test the hypothesis that salmon lice have contributed to the depression of anadromous trout populations in the Hardangerfjord.
Material and methods
Study area
The River Guddalselva, is located in the middle section of the Hardangerfjord, among a number of rivers in which anadromous brown trout and Atlantic salmon () occur. The river's origin is the Folgefonna glacier, resulting in relatively low summer temperatures, with the average in July–August ranging between 11.0 and 11.9°C for the years 2009–2011. The total length of the river is about 13.5 km, of which the lowermost 2 km, up to the waterfall at Liarefossen, is accessible to anadromous salmonids. The total catchment area is 35.8 km2, and the mean water discharge is 3.94 m3 s−1. Both Atlantic salmon and anadromous brown trout inhabit the river, and there used to be a recreational fishery for both species. Since 2001, however, fishing has been strongly regulated, due to the low number of ascending fish. The catch statistics from the river are incomplete, but during the period 1978–1982 the average number of reported anadromous brown trout taken in the river was 156.
Capture of smolts and returning anadromous brown trout
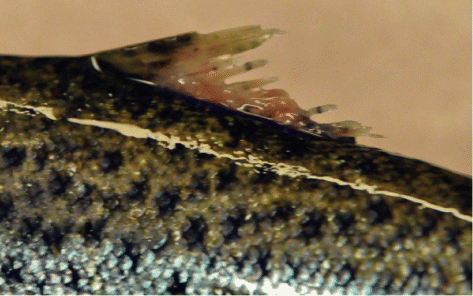
In order to capture all descending smolts, a Wolf trap was constructed in the lower part of the river, about 100 m from the tidal zone. Every year since 2000, the trap has been mounted in March, well before the start of the smolt run, and it is dismantled some weeks after the smolt run is over. A separate trap to capture ascending fish has been installed downstream of the smolt trap, just below the Seimsfossen waterfall. All captured smolts and ascending fish were anaestetized with benzocaine before length and weight measurements, markings and inspection. From 2002 onwards, dorsal fin damage related to salmon lice infections was recorded systematically in ascending fish by using a scale from 0 (no damage) to 3 (massive damage). Every year, the smolts were marked by cutting the adipose fin. After recovery, the smolts were released in the pool below the waterfall, while ascending fish were released about 100 m above the smolt trap. Most of the remaining fish not captured in the trap were captured by angling in the pool below the waterfall. These were first held in a tank at the river bank for data recording and then released upstream. During the spawning season, divers checked the remaining anadromous trout in the pool below the trap for adipose fin clips.
Growth and survival
The smolt year-class of each ascending, adipose clipped fish was determined from length data of previously recorded, adipose-clipped fish and length frequency distribution data of ascending fish instead of age determination by scale reading, in order to minimize stress and damage to ascending fish.
From 2007 to 2010, all trout smolts (n=3557) were tagged with individual passive implant transponders, or PIT tags (12×2 mm), in order to obtain individual data on growth and age during sea migration. The PIT tags were implanted in the posterior part of the body cavity by a single-shot injector (TRAC ID systems, Stavanger, Norway), and all returning, adipose-clipped individuals were checked for PIT tags.
In 2004 and 2005, the descending smolts, captured by the Wolf trap, were divided into two groups of which one (n=704) was dip-treated for 30 min with Substance EX (Alpharma, Oslo, Norway) to prevent sea lice infection. The prophylactic Substance EX inhibits chitin synthesis in salmon lice and is expected to protect the fish for up to 3–4 months in the sea (Hvidsten et al. Citation2007). This group was marked by cutting the adipose and left pelvic fins, while the untreated fish (control group, n=1306) were marked by cutting the adipose and right pelvic fins. Data on release and recapture for the two years were combined due to the generally low return rates and therefore small sample size of recaptured trout. Smolts descended from 17 April to 10 June in 2004 and from 3 May to 4 July in 2005. Recaptures were recorded in 2005 and 2006 by inspections of trout captured by fishermen, in addition to monitoring the fish ascending the fish trap.
Salmon lice infection of anadromous brown trout
As part of the national salmon louse monitoring programme (Bjørn et al. Citation2011b), prematurely returning anadromous brown trout are sampled annually during spring and summer by an electric shocker in freshwater pools, just above the tidal zone, in 40 rivers along the west coast of Norway. Data from four sites in the rivers Daleelva (UTM WGS84 32 V 321995 6657048), Mundheimselva (32 V 328473 6673817), Bondhuselva (32 V 348163 6667668) and Folkedalselva (32 V 370846 6708001), located in the middle part of the Hardangerfjord, were used to assess infection levels of salmon louse. In each sampling, 15 anadromous brown trout from each river were collected, total fish length measured, and the numbers and developmental stage (copepodites, chalimus larvae, preadults and adults) of lice were recorded while the fish were sedated with benzocaine. The time of first infection in each year was estimated on the basis of the stages of lice on the sampled fish, sea temperatures measured by the Institute of Marine Research (Sætre et al. Citation2003), and a correlation table that showed the developmental time of salmon lice at different temperatures.
Results
The smolt run
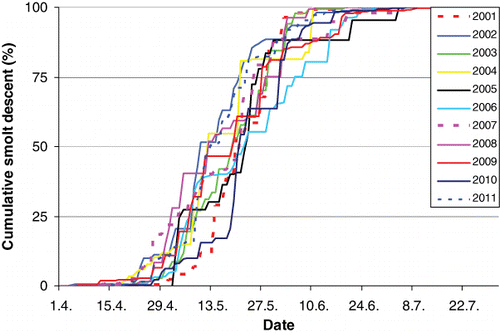
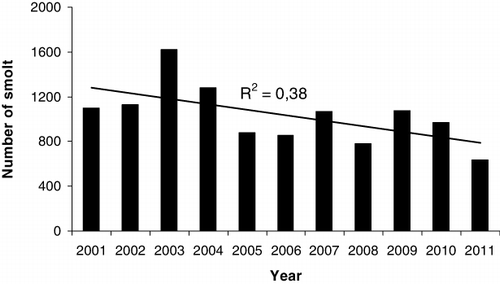
The annual smolt migration started in mid April and lasted until about mid June (). In all years, the 50% cumulative number of descending smolts was reached between 10 and 22 May (). A total of 11,388 smolts were recorded from 2001 to 2011, with annual numbers ranging between 633 and 1615. The annual numbers during 2001–2004 were significantly higher than during 2005–2011 (t-test, t=3.25, P=0.01; ). Mean smolt length ranged between 14.2 and 15.4 cm across years. There was no change in smolt length during the migration season, and no significant relationship between numbers of smolt and the smolt lengths (linear regression, F=1.41, P=0.27).
Number and size of ascending anadromous brown trout
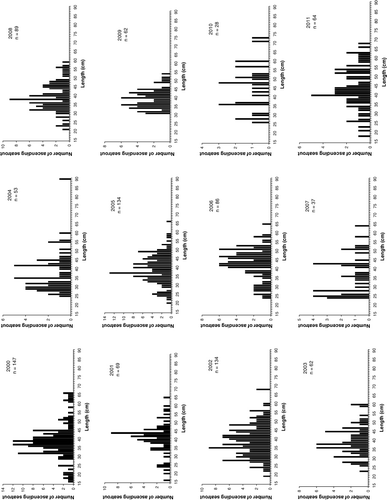
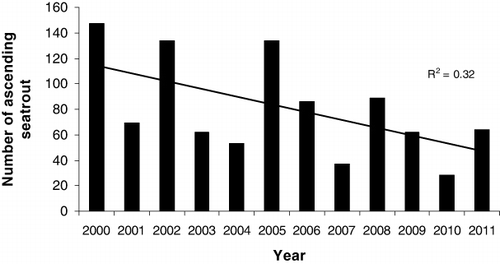
The ascending fish consisted of both adipose clipped and untagged individuals. The untagged individuals may be strays from neighbouring rivers or smolts from River Guddalselva which have passed the smolt trap undetected or originating from the pool below the smolt trap. The total numbers of ascending trout displayed a declining, although not significantly so, trend from 2000 to 2011 (linear regression, F=4.68, P=0.056; ). The highest numbers of ascending fish were observed in 2000, 2002 and 2005. The total number of ascending trout fell to a minimum in 2010, with only 28 individuals captured in the trap, while 147 fish were captured in 2000 (). The length of the trout at first return to the river ranged from 20 to 35 cm, and most of the fish in this size range were immature.
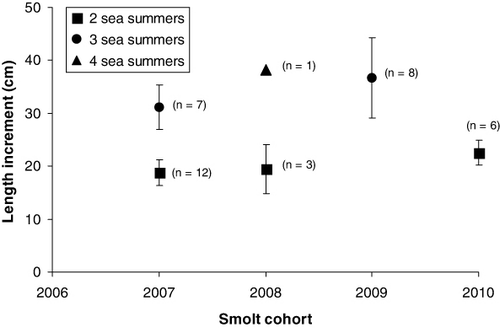
Most of the ascending fish were in the length-class 30–50 cm (), and the majority had spent two feeding seasons in the fjord. A few trout with lengths of between 60 and 75 cm also ascended. Based on recaptured, PIT-marked individuals, the growth during the first two summers in the fjord ranged from a mean of 18.8 cm for the 2007 cohort to 22.6 cm for the 2010 cohort, while after three summers at sea the growth in length ranged from a mean of 31.1 in 2007 to 36.6 cm in 2009 ().
Survival during sea migration
According to the recorded numbers and length at ascent, the return rates of adipose-clipped trout ranged from about 0.51% to 2.51%, with the highest returns represented by the 2004 and 2007 cohorts. Concurrently, the median salmon louse infections of anadromous trout in estuaries in the Hardangerfjord were at a minimum in 2004 and 2007 (). The length distribution of the ascending fish in 2000, with a high frequency of fish with two summer stays in the sea, suggests that the 1998 cohort had a high survival, although this cannot be calculated precisely due to lack of data on smolt numbers before the Wolf trap was installed.
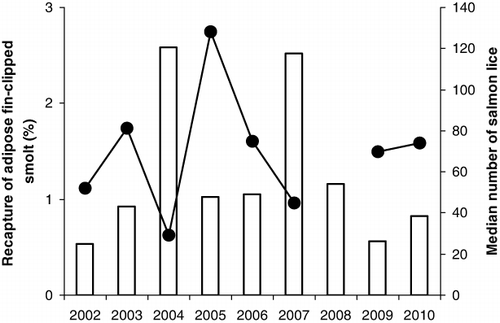
The survival of PIT-marked smolts from the smolt cohorts in 2007–2010 ranged from 0.58% to 1.51%, with an overall recapture rate of 1.0%, which is within the range for adipose fin-clipped trout in the study. The survival rate of the PIT-marked smolts increased with smolt length, but the relationship was not significant (Linear regression, R 2=0.80, P=0.104).
Survival of Substance EX-treated smolts and control groups
The mean lengths of smolts in the treated and control group were 14.8±1.4 cm and 14.7±1.2 cm in 2004, and 14.8±1.6 cm and 14.8±1.2 cm in 2005, respectively, with no significant differences in lengths between treated and untreated fish (Mann–Whitney U-test, P=0.75 in 2004, and P=0.63 in 2005). A total of 24 of the 704 smolts treated with Substance EX in 2004 and 2005 were recaptured in 2005 and 2006, while 23 of the 1306 smolts in the control group were recaptured, giving a significantly higher recapture rate of smolts treated with Substance EX than of the untreated group (Chi-square =4.49, df=1, P=0.034). According to the number of recaptures, which were 3.41% for treated smolt and 1.76% for the controls, the survival was nearly doubled for smolts treated with Substance EX.
Dorsal fin damage in ascending fish
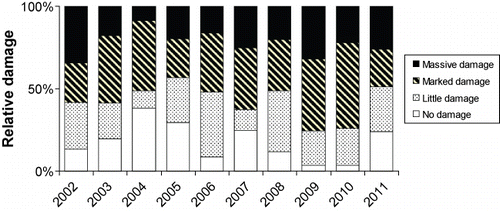
Between 2002 and 2011, more than 80–90% of the anadromous trout that ascended to the River Guddalselva had damaged dorsal fins (), although there were differences between years. In 2004, nearly 40% of the ascending fish had no damage, while in 2009 and 2010, fewer than 5% lacked damage ( and ).
Discussion
The return rates of both adipose fin-clipped and PIT-tagged smolts from the River Guddalselva were very low compared to previous reports from Ireland and Norway. In the River Burrishoole, on the west coast of Ireland, where there was full control of descending and ascending trout by means of traps, the marine survival from the smolt stage to the first return to fresh water ranged from 11.4% to 32.4%, with a mean survival value of 20% during the period 1971–1987, while survival dropped dramatically to a level ranging from 1.5% to 10% after 1989 (Poole et al. Citation1996). Similar findings on historically high return rates are reported from the River Vardnes on Senja, where the recapture of first-time migrants was 37% (Berg & Jonsson Citation1990). With a sea survival rate of only 0.58–3.41% for marked or tagged smolts from Guddalselva, the sea survival of trout from this river is extremely low compared with what is recognized as normal marine survival of anadromous brown trout, even when tagging-induced mortality is taken into account (Jonsson & Jonsson Citation2009). Since the highest return rates for the smolt cohorts from Guddalselva during 2002–2010 are concurrent with years in which the lowest infection levels of premature, returning trout were recorded in the same area, this suggests a connection between salmon louse infection and marine survival of trout, as has also been reported from the west coast of Scotland (Middlemas et al. Citation2010). However, assessing the infection pressure of salmon lice by monitoring the number of lice on prematurely returning trout suffers from several shortcomings, which may conceal a connection between infection level and marine survival. For example, the fraction of the trout population and the absolute number of trout which returns to delouse in a river outlet is not known, and in addition to salmon lice, other factors as for example temperature and salinity may influence this to a different extent from year to year. Other methods which allow for a more precise calculation of infection pressure were not available in this region until towards the last part of our observation period.
As Substance EX has a prophylactic function in preventing salmon louse infection for up to 16 weeks after treatment (Hvidsten et al. Citation2007), and because the survival rate of smolts from the River Guddalselva treated with Substance EX was twice as high as that of untreated smolts, this is a strong indication that the presence of salmon lice is an important direct or indirect mortality factor during the first marine period of the post-smolts. However, the efficacy of Substance EX has not been documented in detail and is probably less than 100%, with a diminishing effect as the fish grows and the concentration of the substance drops. Therefore, the observed differences in survival between treated and non-treated groups are probably minimal estimates of lice-induced mortality in trout. To the best of our knowledge, this is the first study to present evidence of induced mortality due to salmon lice infection at the population level in anadromous brown trout. Several studies have quantified mortality in Atlantic salmon caused by salmon lice by comparing the growth and survival of groups with prophylactic chemical treatment with untreated controls (Skilbrei & Wennevik Citation2006; Hvidsten et al. Citation2007; Gargan et al. Citation2012). Gargan et al. (Citation2012) compared treated and untreated groups of salmon in eight experimental releases from three locations in western Ireland and found that treated groups were 1.8 times as likely to return as controls. The authors concluded that salmon louse-induced mortality can significantly affect populations. These studies demonstrate that in some cases treated groups have significantly higher survival rates, as well as more rapid growth, than controls, while in other cases no differences were found, indicating that the impact of salmon lice on wild Atlantic salmon populations depends on a number of factors. In anadromous trout, however, less effort has been put into investigating the potential link between infection level and mortality at population level.
Several experiments have demonstrated that individual fish are negatively affected and stressed when infection levels exceed 0.1 lice per gram fish, especially when the lice become mobile (Finstad et al. Citation2000; Heuch et al. Citation2005). This suggests that 10 lice might be the limit for a post-smolt less than 100 g in weight, while 70–100 lice could be the limit for a trout weighing 700–1000 g (Bjørn & Finstad Citation1997; Finstad et al. Citation2000; Wagner et al. Citation2003, Citation2004; Wells et al. Citation2006; Citation2007; Tveiten et al. 2009). In late May 2011, the prevalence in the middle part of the Hardangerfjord was 100%, with an average intensity of about 50 lice per fish and with 46% of individuals having more than 0.1 lice per gram body weight. In late June, several individuals had over 100 lice and maximum infection was 328 lice. Prevalence was still 100%, with 77% of the individuals having more than 0.1 lice per gram fish weight, and 50% of the sampled individuals had more than 0.5 lice per gram fish weight. Although infection levels were lower during the previous years compared to the infection in 2011 (Bjørn et al. Citation2011b), salmon lice infection levels still seem to have caused a significant reduction in the survival rate of trout from the River Guddal in the years 2002–2010.
With only a few per cent of trout surviving to maturity in the River Guddalselva, compared to much higher survival rates in areas with little or no sea pen salmonid farming, the high mortality appears to be directly linked to salmon farming and production of salmon lice on farmed fish. The positive relationship between length and survival rate of the PIT-marked smolts may be attributed to the higher tolerance of larger smolts to treatment. However, it may also be a result of higher predation pressure on smaller smolts, which may be substantially amplified when smolts are infected by salmon lice, making them more easily captured by fish predators (Krkošek et al. Citation2011). The same study also concluded that predation of smolts is an important component of salmon louse dynamics.
The large increase in sea pen production of Atlantic salmon is considered to be a serious threat to wild Atlantic salmon, due both to genetic changes in wild populations as a result of hybridization with escapees and to increased infections with salmon lice produced in sea pens (Hutchinson Citation1997; Krkošek et al. Citation2007; Ford & Myers Citation2008). Likewise, the marked declining trend in trout populations may be explicable in terms of the increased burden imposed by salmon lice, especially in regions with high densities of sea pen salmon farms such as the Hardangerfjord, as indicated by the observations on return rates and on skin and fin damage due to sea lice infections of anadromous brown trout from Guddalselva. According to the fisheries management regulations, aquaculture must be conducted in an environmentally friendly manner, i.e. diseases and parasites from the fish farms should not have negative effects on wild populations (www.fkd.dep.no). Removal or relocation of salmon farms may significantly reduce the production of salmon lice copepodites at the vacated site, but in a study by Penston et al. (Citation2011), the infection pressure at the farm site was still not reduced, probably because the infectious planktonic stages of salmon lice can be transported over large distances. Similarly, in modelling infections of salmon lice on Atlantic salmon in 44 farms in the Hardangerfjord, Gettingby et al. (Citation2011) obtained a better fit when the mobility of lice was included. Salmon farms over a large area therefore need to be removed or relocated, or effective methods to remove salmon lice from the farmed fish have to be implemented, such as extensive fallowing of salmon farms during the anadromous brown trout smolt run, in order to achieve environmentally friendly fish farming, i.e. forms of production that will enable anadromous trout populations to be restored and maintained.
Editorial responsibility: Geir Ottersen
Acknowledgements
Technical assistance by Helen Petersen and Britt Iren Tjelle Østebø and collaboration with the River Guddal owners association is highly appreciated. This project was funded by the Ministry of Fisheries and Coastal Affairs and the Norwegian Research Council (Ecological Processes and Impacts Governing the Resilience and Alternations in the Porsangerfjord and the Hardangerfjord, EPIGRAPH, project no. 188955/130).
References
- Anon. 2011. Risk assessment environmental impact of Norwegian aquaculture. In Taranger GL, Boxaspen KK, Abdullah SM, Svåsand T, editors. Extracts from Fisken og havet særnummer 3–2010. Institute of Marine Research: Bergen. 52 pages.
- Berg OK, Jonsson B. 1990. Growth and survival rates of the anadromous trout, Salmo trutta, from the Vardnes River, northern Norway. Biology of Fishes 29:145–54. 10.1007/BF00005031
- Birkeland K. 1996. Consequences of premature return by sea trout (Salmo trutta) infested with the salmon louse (Lepeophtheirus salmonis Krøyer): Migration, growth, and mortality. Canadian Journal of Fisheries and Aquatic Sciences 53:2808–13. 10.1139/f96-231
- Birkeland K, Jakobsen PJ. 1997. Salmon lice, Lepeophtheirus salmonis, infestation as a causal agent of premature return to rivers and estuaries by sea trout, Salmo trutta, juveniles. Environmental Biology of Fishes 49:129–37. 10.1023/A:1007354632039
- Bjørn PA, Finstad B. 1997. The physiological effects of salmon lice infection on sea trout post-smolts. Nordic Journal of Freshwater Research 73:60–72.
- Bjørn PA, Finstad B, Nilsen R, Asplin L, Uglem I, Skaala Ø, et al. 2008. Norwegian national surveillance of salmon lice epidemics on wild Atlantic salmon, sea trout and Arctic char in connection with Norwegian national salmon rivers and fjords. NINA Report 377. Trondheim, Norway: NINA. 33 pages. ( in Norwegian)
- Bjørn PA, Sivertsgard R, Finstad B, Nilsen R, Serra-Llinares RM, Kristoffersen R. 2011a. Area protection may reduce salmon louse infection risk to wild salmonids. Aquaculture Environment Interactions 1:233–44. 10.3354/aei00023
- Bjørn PA, Nilsen R, Serra Llinares RM, Asplin L, Boxaspen KK, Finstad B, et al. 2011b. Rapport fra Havforskningen Nr 19-2011. Institute of Marine Research: Bergen. 33 pages.
- Finstad B, Bjorn PA, Grimnes A, Hvidsten NA. 2000. Laboratory and field investigations of salmon lice [Lepeophtheirus salmonis (Kroyer)] infestation on Atlantic salmon (Salmo salar L.) post-smolts. Aquaculture Research 31:795–803. 10.1046/j.1365-2109.2000.00511.x
- Ford JS, Myers RA. 2008. A global assessment of salmon aquaculture impacts on wild salmonids. PLOS Biology 6:411–17. 10.1371/journal.pbio.0060033
- Gargan PG, Forde G, Hazon N, Russell DJF, Todd CD. 2012. Evidence for sea lice-induced marine mortality of Atlantic salmon (Salmo salar) in western Ireland from experimental releases of ranched smolts treated with emamectin benzoate. Canadian Journal of Fisheries and Aquatic Sciences 69:343–53. 10.1139/f2011-155
- Gettinby G, Robbins C, Lees F, Heuch PA, Finstad B, Malkenes R, et al. 2011. Use of a mathematical model to describe the epidemiology of Lepeophtheirus salmonis of farmed Atlantic salmon Salmo salar in the Hardangerfjord, Norway. Aquaculture 320:164–70. 10.1016/j.aquaculture.2011.03.017
- Grimnes A, Jakobsen PJ. 1996. The physiological effects of salmon lice infection on post-smolt of Atlantic salmon. Journal of Fish Biology 48:1179–94. 10.1111/j.1095-8649.1996.tb01813.x
- Heuch PA, Bjørn PA, Finstad B, Holst JC, Asplin L, Nilsen F. 2005. A review of the Norwegian ‘National action plan against salmon lice on salmonids’: The effect on wild salmonids. Aquaculture 246:79–92. 10.1016/j.aquaculture.2004.12.027
- Hutchinson P, editor. 1997. Interactions between salmon culture and wild stocks of Atlantic salmon: The scientific and management issues. ICES Marine Science Symposia 205. London: Academic Press. 264 pages.
- Hvidsten NA, Finstad B, Kroglund F, Johnsen BO, Strand R, Arnekleiv JV, et al. 2007. Does increased abundance of sea lice influence survival of wild Atlantic salmon post-smolt? Journal of Fish Biology 71:1639–48. 10.1111/j.1095-8649.2007.01622.x
- Jackson D, Cotter D, O'Maoiléidigh N, O'Donohoe P, White J, Kane F, et al. 2011. An evaluation of the impact of early infestation with the salmon louse Lepeophtheirus salmonis on the subsequent survival of outwardly migrating Atlantic salmon, Salmo salar L., smolts. Aquaculture 320:159–63. 10.1016/j.aquaculture.2011.03.029
- Jakobsen PJ, Birkeland K, Grimnes A, Nylund A, Ordal K. 1992. Investigations of salmon lice infections on sea trout and salmon smolt in 1992. Report from Zoologisk Museum, Økologisk avdeling, Universitetet i Bergen, Bergen. 38 pages. ( in Norwegian)
- Jonsson B, Jonsson N. 2009. Migratory timing, marine survival and growth of anadromous brown trout Salmo trutta in the River Imsa, Norway. Journal of Fish Biology 74: 621–38. 10.1111/j.1095-8649.2008.02152.x
- Klemetsen A, Amundsen P-A, Dempson JB, Jonsson B, Jonsson N, O'Connell MF, et al. 2003. Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): A review of aspects of their life histories. Ecology of Freshwater Fish 12:1–40. 10.1034/j.1600-0633.2003.00010.x
- Krkošek M, Ford JS, Morton A, Lele S, Myers RA, Lewis MA. 2007. Declining wild salmon populations in relation to parasites from farm salmon. Science 318:1772–75. 10.1126/science.1148744
- Krkošek M, Connors BM, Ford H, Peacock S, Mages P, Ford JS, et al. 2011. Fish farms, parasites, and predators: Implications for salmon population dynamics. Ecological Applications 21:897–914. 10.1890/09-1861.1
- Middlemas SJ, Raffell JA, Hay DW, Hatton-Ellis M, Armstrong JD. 2010. Temporal and spatial patterns of sea lice levels on sea trout in western Scotland in relation to fish farm production cycles. Biology Letters 6:548–51. 10.1098/rsbl.2009.0872
- Otterå H, Skilbrei O, Skaala Ø, Boxaspen K, Aure J, Taranger GL, et al. 2004. Hardangerfjorden, produksjon av laksefisk og effekter på de ville bestandene av laksefisk. Fisken og havet, nr 3. 36 pages. ( in Norwegian)
- Penston MJ, McBeath AJA, Millar CP. 2011. Densities of planktonic Lepeophtheirus salmonis before and after an Atlantic salmon farm relocation. Aquaculture Environment 1: 225–32. 10.3354/aei00022
- Poole WR, Wheelan KF, Dillane MG, Cooke DJ, Matthews M. 1996. The performance of sea trout, Salmo trutta L., stocks from the Burrishoole system western Ireland, 1970–1994. Fisheries Management and Ecology 3:73–92. 10.1111/j.1365-2400.1996.tb00131.x
- Revie C, Dill L, Finstad B, Todd CD. 2009. Salmon Aquaculture Dialogue Working Group Report on Sea Lice, commissioned by the Salmon Aquaculture Dialogue. http://wwf.worldwildlife.org/site/PageNavigator/SalmonSOIForm (accessed 22 October 2012)
- Skilbrei OT, Wennevik V. 2006. Survival and growth of searanched Atlantic salmon Salmo salar L., treated against sea lice before release. ICES Journal of Marine Science 63:1317–25. 10.1016/j.icesjms.2006.04.012
- Statistics Norway. 2010. http://www.ssb.no/english (accessed 21 October 2012)
- Sætre R, Aure J, Danielssen DS. 2003. Long-term hydrographic variability patterns off the Norwegian coast and in the Skagerrak. ICES Marine Science Symposia 219:150–59.
- Tully O, Poole WR, Whelan KF, Merigoux S. 1993. Parameters and possible causes of epizootics of Lepeophtheirus salmonis Krøyer infesting sea trout (Salmo trutta L.) off the west of Ireland. In: Boxshall GA, Defaye D, editors. Pathogens of Wild Farmed Fish: Sea Lice. Chichester: Ellis Horwood, p 202–13.
- Tully O, Gargan P, Poole WR, Whelan KF. 1999. Spatial and temporal variation in the infestation of sea trout (Salmo trutta L.) by the caligid copepod Lepeophtheirus salmonis (Krøyer) in relation to sources of infection in Ireland. Parasitology 119: 41–51. 10.1017/S003118209900445X
- Tveiten H, Bjørn PA, Johnsen HK, Finstad, B, McKinley RS. 2010.Effects of the sea louse Lepeophtheirus salmonis on temporal changes in cortisol, sex steroids, growth and reproductive investment in Arctic charr Salvelinus alpinus. Journal of Fish Biology 76:2318–41. 10.1111/j.1095-8649.2010.02636.x
- Wagner GN, McKinley RS, Bjørn PA, Finstad B. 2003. Physiolocical impact of sea lice on swimming performance of Atlantic salmon. Journal of Fish Biology 62:1000–09. 10.1046/j.1095-8649.2003.00091.x
- Wagner GN, McKinley RS, Bjørn PA, Finstad B. 2004. Short-term freshwater exposure benefits sea lice infected Atlantic salmon. Journal of Fish Biology 64:1593–604. 10.1111/j.0022-1112.2004.00414.x
- Wells A, Grierson CE, MacKenzie M, Russon IJ, Reinardy H, Middlemiss C, et al. 2006. The physiological effects of simultaneous, abrupt seawater entry and sea lice (Lepeophtheirus salmonis) infestation of wild, sea-run brown trout (Salmo trutta) smolts. Canadian Journal of Fisheries and Aquatic Sciences 63:2809–21. 10.1139/f06-160
- Wells A, Grierson CE, Marshall L, MacKenzie M, Russon IJ, Reinardy H, et al. 2007. Physiological consequences of ‘premature freshwater return’ for wild sea-run brown trout (Salmo trutta) post-smolts infested with sea lice (Lepeophtheirus salmonis). Canadian Journal of Fisheries and Aquatic Sciences 64:1360–69. 10.1139/f07-107