Abstract
A new goatfish, Upeneus torres sp. nov. (Mullidae), is described based on 27 specimens from Australia and Vanuatu using a diversified alpha-taxonomy approach that integrates species, population and size-related allometric differences. Based on large sets of comparative morphological and colour data, diagnoses and inter- and intraspecific comparisons are provided for similar and/or co-occurring species of the so-called japonicus species group, Upeneus torres sp. nov., U. australiae, U. guttatus, and U. japonicus. The new species can be distinguished from all congeneric species by the following combination of characters: seven spines in the first dorsal fin; 13–15 (typically 14) pectoral-fin rays, 22–25 total gill rakers, barbel length 24–28 %SL, and pectoral-fin length 24–26 %SL. Fish smaller than 65 mm SL (‘subadults’) of the new species differ from larger conspecifics (‘adults’) in shallower body, larger eyes, longer anal-fin base, and longer caudal fin. Similar size-related differences were also found for the three other species. Phenotypic population differences in the new species as well as in U. guttatus are reported that may reflect character displacement in the latter. The need to study size–depth–habitat relationships in more detail, the phylogeography of individual populations, and the overall diversity of the genus Upeneus from Australian waters is discussed.
http://zoobank.org/urn:lsid:zoobank.org:pub:6F5C9E39-08DE-4183-A98A-3E11B8993C97
Introduction
The goatfish genus Upeneus harbours considerable diversity that has only recently received enhanced attention in fish systematics. Since 2010, 12 of the currently known 35 species of this genus were described as new to science and two species were resurrected (Bos Citation2014; Uiblein & Gouws Citation2014 and citations therein). Because Upeneus spp. are often very similar and can easily be confused with each other, it has been necessary to study a large number of samples and characters. In addition, the species show considerable size- and population-related variation in morphology and colour, requiring enhanced attention in descriptions, diagnoses and comparisons.
In their taxonomic review of the genus from the Western Indian Ocean (WIO), Uiblein & Heemstra (Citation2010) used 40 morphometric, 10 meristic, and eight colour characters that were obtained from 252 specimens of 24 species. In so doing, they were able to distinguish four distinct species groups which were used and further refined in subsequent descriptions or new geographic records of Upeneus species (Uiblein & Heemstra Citation2011a, Citation2011b; Yamashita et al. Citation2011; Motomura et al. Citation2012; Uiblein & McGrouther Citation2012; Uiblein & Causse Citation2013; Uiblein & Lisher Citation2013; Uiblein & Gouws Citation2014). Currently, five phenotypically distinct species groups, the japonicus, moluccensis, stenopsis, tragula and vittatus groups, are distinguished (Uiblein & Causse Citation2013).
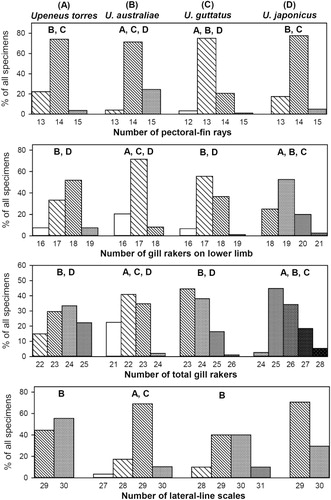
The japonicus group is characterized by seven dorsal-fin spines, 21–32 total gill rakers, 12–15 pectoral-fin rays and bars on the upper caudal-fin lobe of fresh fish (Uiblein & Lisher Citation2013). It includes the following nine species: Upeneus asymmetricus Lachner, 1954 (Philippines), U. australiae Kim & Nakaya, Citation2002 (Australia, New Caledonia), U. francisi Randall & Guézé, 1992 (New Zealand, Norfolk Island), U. guttatus (Day, 1868) (Indo-West Pacific), U. itoui Yamashita, Golani & Motomura, 2011 (Japan), U. japonicus (Houttuyn, 1782) (West Pacific), U. pori Ben-Tuvia & Golani, 1989 (Western Indian Ocean, Eastern Mediterranean), U. saiab Uiblein & Lisher, Citation2013 (northern Mozambique), and U. seychellensis Uiblein & Heemstra, Citation2011a (Seychelles Bank). This species group requires further taxonomic investigation, despite the very recent description of three new species, U. itoui, U. saiab, and U. seychellensis.
Recent exploration of goatfish material at five scientific fish collections, four in Australia (AMS, CSIRO, NMV, WAM) and one in France (MNHN), revealed the presence of one as yet undescribed species of the japonicus group occurring off northern Australia and the Vanuatu archipelago. Preliminary comparisons indicated considerable similarities with U. japonicus in body form and colour. Two identification guides featuring the fish fauna of northern Australia (Gloerfelt-Tarp & Kailola Citation1984; Sainsbury et al. Citation1985) referred to this form as U. bensasi (Temminck & Schlegel, 1844), a junior synonym of U. japonicus (Randall et al. Citation1993), based on a fresh colour photograph of a specimen deposited at CSIRO.
In the identification guides of the northern Australian fish fauna, two additional Upeneus species with seven spines in the first dorsal fin were reported: U. asymmetricus and an unidentified species listed as Upeneus sp. in Sainsbury et al. (Citation1985) and as Upeneus sp.1 in Gloerfelt-Tarp & Kailola (Citation1984). Our preliminary examinations of the photographs and CSIRO voucher cited in those two books showed that U. asymmetricus is a misidentification of the later-described U. australiae. The second, formerly unidentified species is U. guttatus according to Randall & Kulbicki (Citation2006) and Motomura et al. (Citation2012), respectively.
Here we adopt a diversified alpha-taxonomy approach to describe the new species and provide updated diagnoses for U. australiae, U. guttatus and U. japonicus, by extending the comparative approach of Uiblein & Heemstra (Citation2010) and subsequent authors to investigate in detail population differences and size-related allometric changes in body form. Distribution information including size–depth relationship is provided for each of the four species.
Material and methods
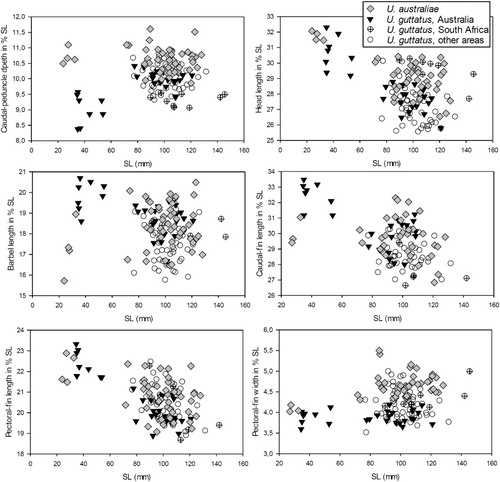
Standard length (SL) and a total of 40 morphometric and 10 meristic characters, as well as information on body and fin colour patterns from 27 specimens of the new species and 106 specimens of Upeneus australiae, U. guttatus, and U. japonicus were examined, using a large set of comparative data from earlier accounts (Uiblein & Heemstra Citation2010; Uiblein & Heemstra Citation2011a; Uiblein & Lisher Citation2013). Methods for measuring and counting as well as descriptions of colour based on fresh and preserved fish follow Uiblein & Heemstra (Citation2010, Citation2011a), Uiblein & Lisher (Citation2013), and Uiblein & Gouws (Citation2014).
To show allometric changes, morphometric characters were plotted against SL. For all species in this study, the most distinct allometric changes happened around approximately 65 mm SL, which coincides with the estimated onset of sexual maturity in smaller-sized Upeneus species (Dimitri Pavlov 2013, personal communication). Accordingly, the fish were split into two size-classes, with those of 65 mm SL and larger being referred to as adults, while those of 24–64 mm SL were referred to as subadults.
Species, size group, and population differences were accepted for overlapping ranges of quantitative characters, as long as the overlaps were minor compared to overall variation within compared samples, the sample means clearly differed from each other, and the number of specimens showing the overlap was clearly less than the sample size. All ranges and single specimen data (e.g. for holotype) of morphometric characters with values below 100 were expressed in two significant digits to facilitate comparisons. To differentiate species more clearly and because of the many overlaps occurring, statistical comparisons of meristic characters were performed using the Chi-square test for trend (GraphPad Prism Citation2007) with a significance level of p ≤ 0.01.
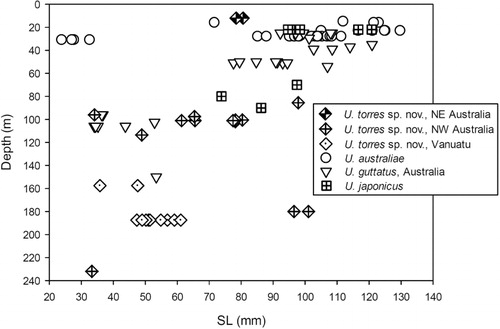
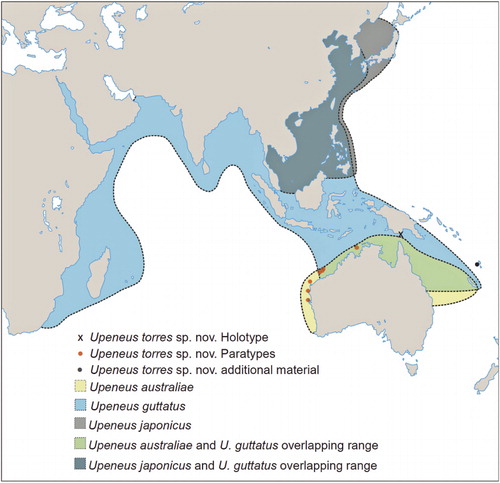
A distribution map was prepared using locality data of all material examined in the present study and that listed in Kim & Nakaya (Citation2002); Randall & Kulbicki (Citation2006); Motomura et al. (Citation2012) and Markevich & Balanov (Citation2012). The depth-range information of each species is based on the available depth data from all localities of the examined material and earlier published information. Depth data derived from individual trawls were averaged where plotted against size; for two lots of U. australiae from NW Australia (BMNH 1983 5.5.17–22; BMNH 1983 5.5.27; listed in Uiblein & Heemstra Citation2010), approximate depth data were determined from Google Earth using the coordinates.
Institutional abbreviations follow Eschmeyer (Citation2014). Other abbreviations are: HIFIRE = Fish collection of the Institute of Marine Research, Bergen, Norway; HT = holotype; PT = paratype.
Comparative material examined
Upeneus australiae (n = 49, 24–128 mm SL): Australia, Queensland: AMS I.22072-013, 4 (of 7), 24–33 mm, Queensland, Port Douglas, 16°29′58″S, 145°28′0″E, 29–33 m; CSIRO H 6894-04, 2, 122–123 mm, Queensland, W of Lizard Island, 14°36.81′S, 145°13.34′E, FRV Gwendoline May, 16 m; CSIRO H 6898-09, 72 mm, Queensland, Torres Strait, SW of Prince of Wales Island, 10°55.23′S, 141°49.15′E, FRV Gwendoline May, 16 m; CSIRO H 7477-01, 105 mm, Queensland, NE of Newcastle Bay, 10°27.72′S, 143°04.84′E, FRV Gwendoline May, 23 m; Western Australia: WAM P 25396.006, 18, 85–111 mm, Rowley Shoals, 17°26′S, 121°54′E, 28 m; furthermore, data from 23 specimens examined in earlier studies (Uiblein & Heemstra Citation2010, Citation2011a; Uiblein & Lisher Citation2013).
U. guttatus (n = 92, 34–146 mm SL): Mozambique: SAIAB 82166, 95 mm, SW of Beira, Dr F. Nansen, M73, 20°53.80′S, 35°39.60′E, 61 m; South Africa, Kwa Zulu Natal: SAIAB 62725, 102 mm, St Lucia river mouth; SAIAB 186410, 2, 113–119 mm, KwaZulu-Natal, off Thukela, 29°21.82′S, 31°48.76′E, 70 m; SAIAB 188756, 107 mm, Park Rynie, 30°20.0′S, 30°75.00′E; SAIAB 188774, 3, 90–107 mm, St Lucia, 28°13.41′S, 32°32.05′E, 18 m; SAM MB-F016722, 114 mm, Delagoa Bay, 25°58.98′S, 32°47′E; SAM MB-F031418, 122 mm, 26°47.7′S, 32°54.6′E; SAM MB-F034155, 107 mm, 19°49.0′S, 36°05.0′E; SAM MB-F034163, 96 mm, 19°28.0′S, 36°37.0′E; SAM MB-F034164, 142 mm, 18°30′S, 37°15′E; NE Australia, Queensland: CSIRO H 6519-18, 2, 103–108 mm, NE of Cooktown, 15°02.24′S, 145°28.91′E, FRV Gwendoline May, 39 m; CSIRO H 7024-02, 114 mm, N of Cairns, 16°33.20′S, 145°52.69′E, FRV Gwendoline May, 37 m; CSIRO H 7206-01, 107 mm, Torres Strait, NE of Darnley Island, 9°28.85′S, 143°58.12′E, FRV Gwendoline May, 54 m; CSIRO H 7207-01, 108 mm, and CSIRO H 7207-02, 4, 92–108 mm, E of Cooktown, 15°29.88′S, 145°23.03′E, FRV Gwendoline May, 25 m; CSIRO H 7212-02, 121 mm, SE of Cairns, 17°08.47′S, 146°12.51′E, FRV Gwendoline May, 35 m; Western Australia: AMS I.22801-004, 3, 78–94 mm, off Port Hedland, 19°32′S, 118°09′E, RV Soela, 50–52 m; AMS I.22831-012, 4, 80–91 mm, NW Shelf, 140 km west of Port Hedland, 20°00′S, 117°16′E, FRV Soela, 50 m; CSIRO CA 283, 112 mm, N of Nickol Bay, 20°06′S, 117°06′E, FRV Courageous, 42–44 m; CSIRO CA 3044, 101 mm, NW of Port Hedland, 20°06′S, 118°00′E, FRV Soela, 29–30 m; NMV A 29657-012, 36–37 mm, NW of Montebello Islands, 20°12.30′S, 115°08.05′E, 95–97 m; NMV A 29661-005, 44 mm, NNE of Montebello Islands, 19°47.47′S, 115°28.58′E, 102–109 m; NMV A 29722-011, 54 mm, ESE Scott Reef, 14°33.68′S, 122°54.37′E, 135–165 m; NMV A 29725-011, 53 mm, SE of Cartier Island, 13°27.63′S, 124°01.20′E, 101–104 m; NMV A 29726-006, 3, 34–35 mm, NW Admiralty Gulf, 13°27.38′S, 124°00.67′E, 105–107 m; Malaysia, NE Pacific: KAUM 41717, 96 mm, Terengganu, 48 km off Cendering, Kuala Terengganu, 5°16′N, 103°11′E, 70–90 m; KAUM 41718, 101 mm, same locality; Vietnam: HIFIRE F 58 153, 108 mm, Nha Trang; HIFIRE F 58 154, 111 mm, Nha Trang; HIFIRE F 58 172, 132 mm, Nha Trang; Japan, Kagoshima: KAUM 13067, 121 mm, E of Sakinoyama, Kataura, Kasasa, Minamisatsuma, 31°25.44′N, 130°11.49′E, 27 m; KAUM 24423, 85 mm, same locality; furthermore, data from 45 specimens examined in earlier studies (Uiblein & Heemstra Citation2010, Citation2011a; Uiblein & Lisher Citation2013).
U. japonicus (n = 40, 51–123 mm SL): Philippines: SU 21063, 83 mm, Luzon, Buluan; SU 29445, 93 mm, Mindanao, Zamboanga Channel, near Santa Cruz Island; Vietnam, Nha Trang, Be Fish Market: HIFIRE F 58 151, 104 mm; HIFIRE F 58 152, 118 mm; HIFIRE F 58 167,123 mm; HIFIRE F 58 201, 4, 51–66 mm; China: AMS I.28094-007, 2, 90–92 mm, Xiamen, 24°26.77′N, 118°04.07′E; Hongkong: CSIRO H 7072-10, 86 mm, Sai Kung market; CSIRO H 7072-11, 81 mm, same locality; SU 61014, 5, 95–121 mm, Mirs Bay, off Waterwitch Rock, FRV Alister Hardy, 22°29′N, 114°23′E, 22 m; Taiwan: CAS 15240, 86 mm, Formosa Strait, 25°N, 120°E, ca. 90 m; CAS 224543, 93 mm, NE Taiwan, Tashi Fish Market; CAS 30017, 97 mm, North of NW Taiwan, ca. 50–90 m; South Korea: CAS 17572, 74 mm, southeast of Cheju Do Island, 80 m; Japan: Chiba Prefecture: CAS 232874, 112 mm, Heizaura, Aihohama Mura; CAS 232875, 69 mm, Nago Street, Awa County; CAS 232877, 2, 82–83 mm, Chiba Prefecture, Awa County; Sagami Bay: CAS 232876, 2, 83–85 mm, Manazuru; Honshu: WAM P 30055.032, 4, 98–118 mm, Western Wakasa Bay, 35°45′N, 135°50′E; WAM P 30260.019, 123 mm, Western Wakasa Bay, 35°30′N, 135°45′E; furthermore, data from seven specimens examined in earlier studies (Uiblein & Heemstra Citation2010; Uiblein & Lisher Citation2013).
Taxonomy
Genus Upeneus Cuvier, 1829
Upeneus torres sp. nov.
Torres goatfish
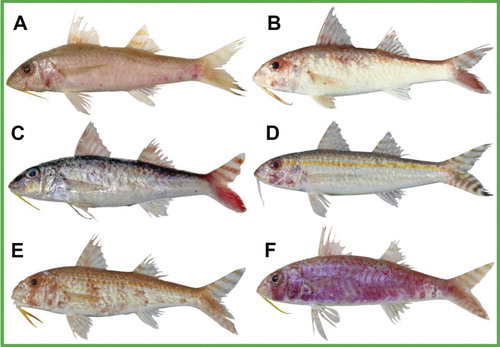
( and ; –)
Table I. Measurements and counts, for specimens of 65 mm SL and larger, of Upeneus torres sp. nov., U. australiae, U. guttatus, and U. japonicus.
Table II. Measurements and counts, for specimens smaller than 65 mm SL, of Upeneus torres sp. nov., U. australiae, U. guttatus, and U. japonicus.
U. bensasi non Temminck & Schlegel, 1843: Gloerfelt-Tarp & Kailola Citation1984; Sainsbury et al. Citation1985.
Holotype
CSIRO H 7202-02, 81 mm, NE Australia, Queensland, Torres Strait, north of Dalrymple Island, 9°19.70′S, 143°15.09′E, FRV Gwendoline May, 12 m, 25 January 2004, collected as part of the Torres Strait Ecosystem Survey (Pitcher et al. Citation2007).
Paratypes
(n = 14, 33–101 mm SL): NE Australia, Queensland: CSIRO H 7202-01, 78 mm, same locality and collection data as for holotype; Western Australia: AMS I.22804-014, 78 mm, North West Shelf, 130 km north of Port Hedland, 18°57′S, 118°12′E, FRV Soela, 100–104 m; CSIRO CA 413, 101 mm, N of North West Cape, 21°24′S, 114°19′E, FRV Courageous, 175–185 m; CSIRO CA 414, 97 mm, same locality; CSIRO CA 2067, 98 mm, N of Joseph Bonaparte Gulf, 13°18′S, 128°21′E, FRV Soela, 84–87 m; CSIRO H 6405-05, 5, 61–80 mm, NW of Shark Bay, 24°01′S, 113°03′E, FRV Southern Surveyor, 100–101 m; CSIRO H 6452-04, 65 mm, W of Shark Bay, 25°54′S, 112°49′E, FRV Southern Surveyor, 95–100 m; NMV A 29657-013, 34 mm, NW of Montebello Islands, 20°12.30′S, 115°08.05′E, 95–97 m; NMV A 29665-003, 49 mm, NW of Port Hedland, 19°23.13′S, 117°06.68′E, 113–114 m; NMV A 29667-002, 33 mm, NW of Port Hedland, 19°08.32′S, 117°47.02′E, 113–351 m.
Non-types (n = 12, 36–61 mm SL): Vanuatu: MNHN 2002-0037, 9, 47–61 mm, 15°37.98′S, 167°30′E, 174–210 m; MNHN 2002-0070, 3, 36–48 mm, 15°37.98′S, 167°03′E, 140–175 m.
Diagnosis
Dorsal fins VII + 9; pectoral fins 13–15; gill rakers 5–7 + 16–19 = 22–25; adults, measurements in %SL: body depth at first dorsal-fin origin 23–27; body depth at anus 19–22; caudal-peduncle depth 8.9–11; maximum head depth 19–23; head depth through eye 15–19; head length 28–31; orbit length 7.4–8.9; upper jaw length 10–12; barbel length 24–26; caudal-fin length 28–30; anal-fin height 16–20; pelvic-fin length 20–23; pectoral-fin length 24–26; first dorsal-fin height 21–24; second dorsal-fin height 16–19; 4 pale brown or red oblique bars on upper caudal-fin lobe in fresh fish; lower caudal-fin lobe almost entirely covered by a broad, distally darkening orange or red stripe, bordered by white pelvic-fin margin, white lobe tip, and dark fin margin dorsally; in preserved specimens only weak indications of upper caudal-fin lobe bars and a dark pigmented zone remaining on the lower lobe close to tip; barbels yellow; no or only weak indications of lateral body stripe; head and body rose or white ventrally and laterally, and orange or red dorsally; preserved fish uniformly pale brown or brown.
Diagnosis of subadults
Measurements as %SL: body depth at first dorsal-fin origin 21–24; body depth at anus 17–20; caudal-peduncle depth 8.2–9.1; maximum head depth 18–22; head depth through eye 16–19; head length 28–34; orbit length 8.3–9.7; upper jaw length 10–13; barbel length 24–28; caudal-fin length 29–34; anal-fin height 17–20; pelvic-fin length 21–24; pectoral-fin length 24–26; first dorsal-fin height 20–24; second dorsal-fin height 16–20.
Description
Measurements as %SL and counts are given in ; morphometric data as ratios of SL for holotype, data for paratypes in brackets: body moderately deep, its depth at first dorsal-fin origin 4.1 [3.7–4.2]; body depth at anal-fin origin 4.6 [4.7–5.4]; head depth through eye 6.5 [5.4–6.0]; head length 3.6 [3.2–3.4], larger than maximum depth of body and subequal to caudal-fin length (3.6 [3.4–3.6]); height of second dorsal fin 6.3 [5.3–6.1], clearly smaller than barbel length (4.2 [3.9–4.2]); pectoral-fin length 4.1 [3.8–4.2], longer than pelvic-fin length (4.9 [4.3–4.8]); orbit length 13.5 [11.3–13.3], smaller than caudal-peduncle depth (9.2 [9.5–11.2]).
Fresh colour
Holotype (): head and body pale rose, ventrally lighter and dorsally darker, rose–orange near bases of dorsal fins and from behind second dorsal fin to caudal-fin base; a weakly indicated lateral stripe formed by pale orange-coloured scales on posterior part of body, most conspicuous in area below second dorsal-fin base; a short vertical red bar behind base of maxilla and below eye, in height of vertical orbit diameter; at approximately the same horizontal level a red patch at lower operculum margin; three red blotches on ventral side of body behind anal-fin base; eye background reddish; barbels yellow; dorsal fins hyaline with two rose–orange stripes, one stripe close to fin bases, the other stripe close to unpigmented fin tips; pectoral fins hyaline, pelvic and anal fins whitish, rays pale rose on pelvic and white on anal fin; upper caudal-fin lobe with 4 oblique pale brown bars (the proximal-most close to fin base); lower caudal-fin lobe almost entirely covered by a broad orange stripe that ends distally in a dark, brown to black patch of similar width as horizontal orbit diameter, bordered by a white ventral fin margin below, a white lobe tip behind, and a brownish dorsal fin margin above.
Paratype (): head and body white ventrally to eye level, orange–reddish dorsally, with two orange–reddish patches extending towards mid body below bases of dorsal fins; no indication of lateral stripe; a rectangular red blotch of pupil size below eye and behind base of maxilla and one slightly larger, rounded red blotch behind eye; eye background reddish; barbels yellow; dorsal fins hyaline with two pale red stripes positioned similarly as in holotype, colour of other fins similar to holotype apart from a more vivid red colouration of caudal-fin bars and stripe, with dark pigmentation on the lower caudal-fin lobe distally of stripe and along dorsal fin margin.
Preserved colour
Head and body uniformly pale brown or brown; fins hyaline; in some specimens weakly retained oblique bars on upper caudal-fin lobe; area close to tip of lower caudal-fin lobe usually pigmented, becoming darker distally.
Distribution and size
From off Shark Bay, northwestern Australia, to Torres Strait, northeastern Australia (), 12–351 m depth; Vanuatu: 140–210 m depth. Attains at least 10 cm SL.
Etymology
The name ‘torres’ is used as a noun in apposition based on the type locality in the Torres Strait which was named after Luis Váez de Torres, a sixteenth–seventeenth century maritime explorer serving the Spanish Crown, noted for the first recorded navigation of the strait which separates Australia from the island of New Guinea.
Comparisons
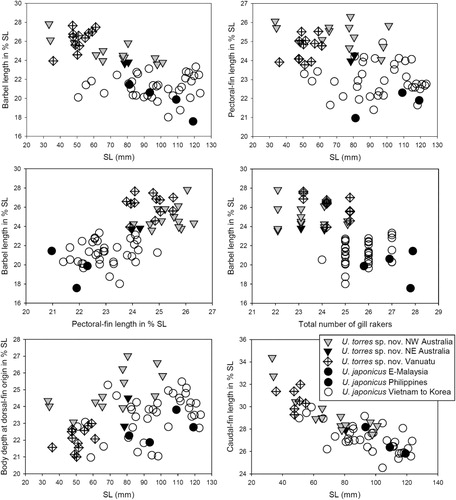
Upeneus torres sp. nov. differ from all other congeneric species in the combination of the following characters: first dorsal fin VII; 13–15 (mostly 14) pectoral-fin rays; 22–25 total gill rakers; barbel and pectoral-fin length 24–26 %SL in adults, and a broad orange or red stripe on the lower caudal-fin lobe in fresh fish; from the three most similar and/or co-occurring adults of the japonicus group they differ as follows: from U. australiae by longer barbels and pectoral fins, larger interdorsal distance, no conspicuous lateral body stripe and no bars on lower caudal-fin lobe; from U. guttatus by larger half-body depth at dorsal-fin origin, longer barbels and pectoral fins, and presence of a broad orange or red stripe on lower caudal-fin without bars; and from U. japonicus they differ in longer barbels and pectoral fins, longer caudal fin, and fewer gill rakers. For statistical differences between U. torres sp. nov. and these three species see .
Adult Upeneus torres sp. nov. differ from the other six species of the japonicus group (U. asymmetricus, U. francisi, U. itoui, U. pori, U. saiab, U. seychellensis; cf. Uiblein & Lisher Citation2013) in having a deeper body at dorsal-fin origin (23–27 vs. 20–24 %SL) and longer barbels (24–26 vs. 16–22 %SL), from all except for U. francisi in longer pectoral fins (24–25 vs. 19–22 %SL), and from all except for U. itoui in lower number of total gill rakers (22–25 vs. 25–32); furthermore, they differ from U. francisi in shorter pelvic fins (20–23 vs. 24 %SL) and from U. itoui in higher first dorsal fin (21–24 vs. 19–20 %SL).
Subadults
Upeneus torres sp. nov. subadults differ from adults in shallower body, larger eyes, longer caudal fin, and longer anal-fin base; they differ from the other three species in longer barbels and pectoral fins and, in addition, from U. australiae in shallower body and head, shorter interorbital length, higher anal fin, and longer pelvic fin, and from U. guttatus in lesser maximum head depth.
Upeneus torres sp. nov. of Vanuatu differ from the similar-sized subadults of NW Australia in lesser maximum body and head depth, shorter jaws, longer caudal peduncle, and a higher ratio of developed versus undeveloped gill rakers on both limbs, i.e. ceratobranchial and epibranchial.
Remarks
Upeneus torres sp. nov. resembles considerably its allopatric sister species U. japonicus in colour patterns and body structure, which may explain why it has not been described earlier. The present study is the first to examine specimens of U. japonicus from Malaysia and Philippines in a comparative context, which contributed to the identification of the new species. The southwestern populations differ in diagnostic characters such as barbel length, pectoral-fin length, and number of gill rakers more clearly from U. torres sp. nov. than U. japonicus from more distant, northeastern areas of the West Pacific ().
Upeneus torres sp. nov. specimens from Vanuatu differ considerably in body form from similar-sized conspecifics of northern Australia and were not designated as types. The slight variation in fresh body colour between the holotype and one paratype () is not matched by any morphological differences ().
Upeneus torres sp. nov. occurs over a wide depth range from the shallow littoral to slightly below the shelf margin (200 m). Subadults have only been found at depths greater than 100 m ().
Upeneus australiae Kim & Nakaya, Citation2002
Australian goatfish
( and ; , , –)
U. asymmetricus non Lachner, 1954: Gloerfelt-Tarp & Kailola Citation1984; Sainsbury et al. Citation1985.
U. australiae Kim & Nakaya, Citation2002: Randall & Kulbicki Citation2006; Uiblein & Heemstra Citation2010 (in part); Uiblein & Heemstra Citation2011a; Uiblein & Lisher Citation2013.
Diagnosis
Dorsal fins VII + 9; pectoral fins 13–15; gill rakers 5–7 + 16–18 = 22–25; adults, measurements as %SL: body depth at first dorsal-fin origin 23–27; body depth at anus 20–23; caudal-peduncle depth 9.9–12; maximum head depth 20–22; head depth through eye 15–18; head length 27–30; orbit length 6.0–8.0; upper jaw length 9.3–12; barbel length 16–20; caudal-fin length 27–32; anal-fin height 15–18; pelvic-fin length 20–23; pectoral-fin length 19–22; first dorsal-fin height 18–23; second dorsal-fin height 14–18; total bars on caudal fin 7–13, upper caudal-fin lobe with 5–6 brown or black bars with usually five bars distally from fork and one bar close to fin base, of similar width or narrower than the pale interspaces between bars; lower lobe with 6–8 bars of similar width as those on upper lobe, pale interspaces narrower and the 2–3 distal-most bars (one on tip) darker and wider; caudal-fin lobe bars mostly well-retained in preserved fish, especially on dorsal side of lower lobe; barbels white; body and head white–silvery or pale beige, dark beige above lateral line, a yellow–orange lateral body stripe from behind eye to caudal fin base in fresh fish; body pale brown and dorsally darkened in preserved fish.
Diagnosis of subadults
Measurements as %SL: body depth at first dorsal-fin origin 23–25; body depth at anus 19–22; caudal-peduncle depth 10–11; maximum head depth 22–23; head depth through eye 19–20; head length 31–32; orbit length 9.4–9.8; upper jaw length 11–12; barbel length 16–19; caudal-fin length 29–31; anal-fin height 16–18; pelvic-fin length 20–21; pectoral-fin length 21–23; first dorsal-fin height 21–22; second dorsal-fin height 17.
Distribution and size
Occurs in northern Australia from Fremantle, Western Australia, to Deception Bay, Queensland (E Australia), and off New Caledonia; 6–80 m depth (mostly shallower than 40 m). Attains 16 cm SL.
Comparisons
Upeneus australiae differ from all other congeneric species in the combination of the following characters: dorsal fins VII; 13–15 (mostly 14) pectoral-fin rays; 22–25 total gill rakers; caudal-peduncle depth 9.9–12 %SL and maximum head depth 20–22 %SL in adults, a yellow-orange lateral body stripe in fresh fish, white barbels, and dark oblique bars on lower caudal-fin lobe in fresh fish, the latter also present in preserved fish; from the rather similar and co-occurring U. guttatus they differ in slightly more pectoral-fin rays, presence of a lateral body stripe in fresh fish, and dark oblique bars on the lower caudal-fin lobe in both fresh and preserved fish; and from U. japonicus they differ in shorter barbels and pectoral fins, fewer gill rakers, dark oblique bars versus a broad reddish stripe on the lower caudal-fin lobe in fresh specimens, and in preserved fish the caudal-fin bars mostly retained vs. none and the body dorsally darkened vs. uniformly pale brown.
Subadults
Upeneus australiae subadults differ from adults in having deeper and longer heads, shorter snouts, larger eyes, and longer pectoral fins; they differ from U. guttatus in deeper caudal peduncle, deeper head through eye, larger eyes, shorter barbels, longer caudal peduncle, and shorter caudal fin; and from U. japonicus they differ in deeper caudal peduncle, deeper and longer head, larger eyes, shorter barbels, and shallower dorsal fins.
Remarks
Both subadults and adults occur at relatively shallow depths of the upper shelf and usually around 20–30 m (; Randall & Kulbicki Citation2006).
Upeneus guttatus (Day, 1868)
Two-tone goatfish
( and ; Table SI; , , –)
Upeneus sp.: Sainsbury et al. Citation1985.
Upeneus sp.1: Gloerfelt-Tarp & Kailola Citation1984.
U. guttatus (Day, 1868): Randall & Kulbicki Citation2006; Uiblein & Heemstra Citation2010, Citation2011a; Motomura et al. Citation2012; Uiblein & Lisher Citation2013.
Diagnosis
Dorsal fins VII + 9; pectoral fins 12–15; gill rakers 5–8 + 16–19 = 23–26; adults, measurements as %SL: body depth at first dorsal-fin origin 22–26; body depth at anus 18–22; caudal-peduncle depth 9.1–11; maximum head depth 18–22; head depth through eye 15–18; head length 26–30; orbit length 5.9–8.8; upper jaw length 9.5–12; barbel length 16–20; caudal-fin length 27–31; anal-fin height 15–19; pelvic-fin length 19–22; pectoral-fin length 19–22; first dorsal-fin height 18–24; second dorsal-fin height 14–18; total bars on caudal fin 7–13, upper caudal-fin lobe with five (rarely four) reddish bars with four (rarely three) bars distally from fork and one bar close to fin base, of similar width or narrower than the pale interspaces between bars; 2–8 faint, irregular red bars on ventral margin of lower caudal-fin lobe, sometimes extending to dorsal half of lobe, connecting to a red stripe which covers the lobe dorsally to two-thirds of its width at maximum; caudal-fin lobe bars and stripe fade away post mortem, only traces retained in preserved fish; barbels yellow or white in fresh fish; body colour variable, often white or rose below lateral line, covered by red pigmentation above lateral line which may also reach down ventrally and to head, sometimes forming red patches or blotches; belly white; body pale brown and not dorsally darkened in preserved fish.
Diagnosis of subadults
Measurements as %SL: body depth at first dorsal-fin origin 22–26; body depth at anus 18–21; caudal-peduncle depth 8.4–9.6; maximum head depth 21–23; head depth through eye 18–19; head length 29–32; orbit length 7.8–9.0; upper jaw length 11–13; barbel length 19–21; caudal-fin length 31–33; anal-fin height 17–20; pelvic-fin length 21–23; pectoral-fin length 22–23; first dorsal-fin height 19–24; second dorsal-fin height 17–19.
Distribution and size
Occurs from South Africa (KwaZulu-Natal) to Red Sea, northern Australia, New Caledonia, and Japan; 8–165 m depth. Attains 16 cm SL.
Comparisons
Adult Upeneus guttatus differ from all other congeneric species in the combination of the following characters: dorsal fins VII; 12–15 (mostly 13) pectoral-fin rays; 23–26 total gill rakers; in fresh specimens usually five oblique red bars on upper caudal-fin lobe and 2–8 short red bars on ventral margin of lower lobe which is covered by a red stripe to maximally two-thirds of lobe width; no lateral body stripe; preserved specimens uniformly pale brown coloured; from U. japonicus examined here, they differ in the combination of shorter barbels and pectoral fins, lower number of gill rakers, and short oblique bars on ventral margin of lower caudal-fin lobe present in fresh fish (for distinctions with other species of the japonicus group see the species accounts above and Uiblein & Heemstra Citation2011a; Uiblein & Lisher Citation2013).
At the population level (Table SI), adult U. guttatus of Australia can be distinguished from conspecifics of the South African coast (Western Indian Ocean), in having shorter heads, longer caudal fin, higher first dorsal fin, and slightly fewer pectoral-fin rays; and from conspecifics of the NW Pacific region (Malaysia to Japan) they differ in narrower caudal peduncle, larger head depth through eye, and longer barbels. No such marked differences occur when the Australian population is compared with other areas in the WIO and the NE Indian Ocean.
When interspecific comparisons are restricted to sympatric populations of U. guttatus only, additional characters can be found that cannot be used for distinction when combining data from the entire species' range. Australian U. guttatus differ from U. torres in longer head and from U. australiae they differ in deeper body at anal-fin origin, wider pectoral fins, and usually yellow vs. white barbels. Upeneus guttatus from the area between Malaysia to Japan differ from U. japonicus in shorter head and dorsal-fin base, apart from all the differences indicated in the comparison with U. guttatus from the entire species' range further above.
Subadults
Upeneus guttatus subadults from Australia differ from adults in having a deeper and longer head, larger eyes, longer jaws, longer barbels, longer caudal fin, longer anal-fin base, and longer pectoral fins; and they differ from subadult U. japonicus in deeper head, longer barbels, longer dorsal-fin base, longer caudal fin, longer anal-fin base, and narrower pectoral fin.
Remarks
Several updated diagnoses and/or redescriptions of U. guttatus have been published recently (e.g., Randall & Kulbicki Citation2006; Uiblein & Heemstra Citation2010, Citation2011a; Motomura et al. Citation2012). Nevertheless, the overall phenotypic variability within this species' large range still requires further attention. This article takes a further step by examining a large number of specimens from Australia and from many other areas of the species' range. Additional evidence of population differentiation is provided, but – as in earlier studies (e.g. Uiblein & Heemstra Citation2011a) – this does not support a splitting into different species.
Off NW Australia the depth distribution of U. guttatus indicates an upwards directed vertical migration during late ontogeny (). Two small subadults (36–37 mm SL) were found together with a similar-sized U. torres sp. nov. (34 mm SL) in a single trawl haul at 20°12.3′S, 115°08.52′E at 95–97 m depth.
Upeneus japonicus (Houttuyn, 1782)
Japanese goatfish
( and ; –)
U. japonicus (Houttuyn, 1782): Randall et al. Citation1993; Kim & Nakaya Citation2002; Uiblein & Heemstra Citation2010; Yamashita et al. Citation2011; Uiblein & Lisher Citation2013.
Diagnosis
Dorsal fins VII + 9; pectoral fins 13–15; gill rakers 6–8 + 18–21 = 24–28; adults, measurements as %SL: body depth at first dorsal-fin origin 21–25; body depth at anus 18–22; caudal-peduncle depth 8.0–11; maximum head depth 18–21; head depth through eye 15–17; head length 27–31; orbit length 6.1–8.2; upper jaw length 9.7–12; barbel length 18–23; caudal-fin length 25–29; anal-fin height 15–19; pelvic-fin length 19–23; pectoral-fin length 21–25; first dorsal-fin height 20–24; second dorsal-fin height 15–19; 3–4 red oblique bars on upper caudal-fin lobe in fresh fish; lower caudal-fin lobe almost entirely covered by a broad red stripe that becomes darker distally and is partially surrounded by a dark grey line and by a contrasting white fin margin and white lobe tip exteriorly; only weak indications of upper caudal-fin lobe bars in preserved specimens with a dark pigmented zone remaining distally at the lower lobe; barbels yellow; no lateral body stripes; head and body white or silvery ventrally and laterally, sometimes covered with red blotches, and uniformly red dorsally of lateral line; preserved fish pale brown or brown.
Diagnosis of subadults
Measurements as %SL: body depth at first dorsal-fin origin 21–23; body depth at anus 18–20; caudal-peduncle depth 9.3–9.6; maximum head depth 18–20; head depth through eye 16; head length 29–31; orbit length 8.2–9.3; upper jaw length 11–12; barbel length 20–22; caudal-fin length 29–30; anal-fin height 17; pelvic-fin length 21–22; pectoral-fin length 23; first dorsal-fin height 23–24; second dorsal-fin height 18–19.
Distribution and size
W Malaysia and Philippines to South Korea and Peter the Great Bay, Russia; 3.7–90 m depth. Attains 16 cm SL.
Comparisons
For comparisons with the three other species examined here, see the species accounts above. Upeneus japonicus differ from other co-occurring species of the japonicus group from the northern West Pacific (cf. Uiblein & Heemstra Citation2011a; Uiblein & Lisher Citation2013) as follows: from U. asymmetricus in longer pectoral fins SL (21–25 vs. 19–21 %SL), more pectoral-fin rays (13–15 vs. 13), absence of dark oblique bars on lower caudal-fin lobe, bars on upper lobe not retained in preserved fish, and uniformly pale brown vs. dorsally darker body in preserved fish; and from U. itoui they differ in deeper head across eye (15–17 vs. 13–14 %SL), longer barbels (18–23 vs. 17 %SL), longer pectoral-fin rays (21–25 vs. 19–20 %SL), fewer gill rakers on lower limb (18–21 vs. 16–17), absence of dark oblique bars on lower caudal-fin lobe, and uniformly pale brown vs. dorsally darker body in preserved fish.
Subadults
U. japonicus subadults differ from conspecific adults in larger eyes and longer caudal fin.
Remarks
This species seems to occur mostly in the waters of the upper continental shelf (< 100 m depth), but may reach greater depths: a 108 mm SL specimen was reported from a catch off Kochi, Japan, at 100–200 m depth (Yamashita et al. Citation2011).
Discussion
This diversified alpha-taxonomy approach has facilitated the discovery and description of Upeneus torres sp. nov. by the ability to integrate a large number of phenotypic characters of four similar species. To our knowledge, this study is the first one to provide diagnoses and comparisons for separate size-/age-classes of goatfish species, while also accounting for population differences in body form.
The morphological differentiation of the population of U. guttatus from Australia would require a genetically supported phylogeographic approach to better understand if the distinction with the two co-occurring, rather similar species U. australiae and U. torres sp. nov. reflects character displacement or has rather evolved independently. Phenotypic divergence in areas of overlapping distribution with possible involvement of character displacement was recently reported for populations of the two closely related species, U. heemstra Uiblein & Gouws, Citation2014 and U. tragula Richardson, 1846 (Uiblein & Gouws Citation2014).
The depth distributions and size–depth relationships of the four species indicate a diversity of vertical habitat preferences. Upeneus torres sp. nov. occurs from the upper shelf to below the shelf margin, while U. australiae occurs more shallowly. Upeneus guttatus may migrate into shallower water with growth, while subadults and adults of U. japonicus seem to be rather widely distributed, but remain mostly at less than 100 m depth. However, for a better understanding of species-specific and size-/age-specific habitat preferences, more data on depth distribution and biology of species, populations, and size-classes need to be gathered.
The ongoing identification and description of new goatfish species from Australian waters (Uiblein & Heemstra Citation2010; Uiblein & McGrouther Citation2012; present study) highlights the need for further exploration and taxonomic work in that region. Diversity at numerous levels remains to be fully discovered, described, and interpreted, so as to better understand local and regional species composition and the distribution patterns of populations and life-history stages.
Supplementary material
(Table SI)
The supplementary material for this article is available via the Supplemental tab of the article's online page at http://dx.doi.org/10.1080/17451000.2014.958088
Editorial responsibility: Christopher Kenaley
Table SI
Download MS Word (149.5 KB)Acknowledgements
We thank the following colleagues for hospitality and assistance during collections visits or for providing other collection-related favours: Mark McGrouther, Amanda Hay, and Sally Reader (AMS); Dave Catania, Mysi Hoang, and Tomio Iwamoto (CAS); Alastair Graham, Will White, Peter Last, John Pogonoski, and Robert Ward (CSIRO); Rupert Wienerroither (HIFIRE); Hiroyuki Motomura (KAUM); Romain Causse and Patrice Pruvost (MNHN); Martin Gomon and Dianne Bray (NMV); Roger Bills and the SAIAB fish collection staff, Gavin Gouws, and Alan Whitfield (SAIAB); Michael Bougaardt (SAM); Sue Morrison (WAM); and Dimitri Pavlov, Moscow State University. For photographs of fresh fish and/or additional information we thank Alastair Graham (CSIRO) and Hiroyuki Motomura (KAUM). The holotype of the new species was obtained during an extensive scientific project undertaken in the Torres Strait (Pitcher et al. Citation2007) and hence we thank the crew of the FRV Gwendoline May for assistance and support. Carlie Devine (CSIRO) assisted in the preparation of the colour plate (). We thank John Pogonoski, an anonymous referee, Danny Eibye-Jacobsen, and Chris Kenaley for many valuable comments which significantly improved the manuscript. The first author thanks CSIRO Hobart, SAIAB, the Nansen Programme of the Center for Developmental Fisheries at the Institute of Marine Research, Bergen, Norway, and the Russian-Vietnamese Tropical Research and Technological Center for travel and/or logistic support. The second author was supported by the Australian Government's National Environmental Research Program (NERP) Marine Biodiversity Hub.
References
- Bos AR. 2014. Upeneus nigromarginatus, a new species of goatfish (Perciformes: Mullidae) from the Philippines. Raffles Bulletin of Zoology 62:745–753.
- Eschmeyer WN. (editor) 2014. Catalog of Fishes: Genera, Species, References. http://research.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed 20 February 2014).
- Gloerfelt-Tarp T, Kailola PJ. 1984. Trawled Fishes of Southern Indonesia and Northwestern Australia. The German Agency for Technical Cooperation. Singapore: Tien Wah Press. 406 pages.
- GraphPad Prism. 2007. Graph Pad Prism Version 5.00 for Windows. San Diego: GraphPad Software. www.graphpad.com (accessed 21 March 2014). Computer program.
- Kim BJ, Nakaya K. 2002. Upeneus australiae, a new goatfish (Mullidae: Perciformes) from Australia. Ichthyological Research 49:128–32. 10.1007/s102280200016
- Markevich AI, Balanov AA. 2012. Description of Upeneus japonicus (Mullidae), Japanese goatfish, a rare species in the Russian waters. Journal of Ichthyology 52:656–60. 10.1134/S0032945212060045
- Motomura H, Yamashita M, Itou M, Haraguchi Y, Iwatsuki Y. 2012. First records of the two-tone goatfish, Upeneus guttatus, from Japan, and comparisons with U. japonicus (Perciformes: Mullidae). Species Diversity 17:7–14. 10.12782/sd.17.1.007
- Pitcher CR, Haywood M, Hooper J, Coles R, Bartlett C, Browne M, et al. 2007. Mapping and Characterisation of Key Biotic & Physical Attributes of the Torres Strait Ecosystem. CSIRO/QM/QDPI CRC Torres Strait Task Final Report. Brisbane, Australia: CSIRO Marine & Atmospheric Research. 145 pages.
- Randall JE, Kulbicki M. 2006. A review of the goatfishes of the genus Upeneus (Perciformes: Mullidae) from New Caledonia and the Chesterfield Bank, with a new species, and four new records. Zoological Studies 45:298–307.
- Randall JE, Bauchot ML, Guézé P. 1993. Upeneus japonicus (Houttyn), a senior synonym of the Japanese goatfish U. bensasi (Temnick et Schlegel). Japan Journal of Ichthyology 40:301–05.
- Sainsbury KJ, Kailola PJ, Leyland GG. 1985. Continental Shelf Fishes of Northern and Northwestern Australia. CSIRO Division of Fisheries Research. Canberra, Australia: Clouston & Hall and Peter Pownall Fisheries Information Service. 375 pages.
- Uiblein F, Causse R. 2013. A new deep-water goatfish of the genus Upeneus (Mullidae) from Vanuatu, South Pacific. Zootaxa 3666:337–44. 10.11646/zootaxa.3666.3.4
- Uiblein F, Gouws G. 2014. A new goatfish species of the genus Upeneus (Mullidae) based on molecular and morphological screening and subsequent taxonomic analysis. Marine Biology Research 10:655–81. 10.1080/17451000.2013.850515
- Uiblein F, Heemstra PC. 2010. A taxonomic review of the Western Indian Ocean goatfishes of the genus Upeneus (Family Mullidae) with descriptions of four new species. Smithiana Bulletin 11:35–71.
- Uiblein F, Heemstra PC. 2011a. A new goatfish species, Upeneus seychellensis sp. nov. (Mullidae), from the Seychelles Bank, with remarks on Upeneus guttatus and a key to Western Indian Ocean Upeneus species. Marine Biology Research 7:637–50. 10.1080/17451000.2010.547202
- Uiblein F, Heemstra PC. 2011b. Description of a new goatfish species, Upeneus randalli sp. nov. (Mullidae), from the Persian Gulf, with remarks and identification keys for the genus Upeneus. Scientia Marina 75:585–94. 10.3989/scimar.2011.75n3585
- Uiblein F, Lisher M. 2013. A new goatfish of the genus Upeneus (Mullidae) from Angoche, northern Mozambique. Zootaxa 3717:85–95. 10.11646/zootaxa.3717.1.7
- Uiblein F, McGrouther M. 2012. A new deep-water goatfish of the genus Upeneus (Mullidae) from northern Australia and the Philippines, with a taxonomic account of U. subvittatus and remarks on U. mascareinsis. Zootaxa 3550:61–70.
- Yamashita Y, Golani D, Motomura H. 2011. A new species of Upeneus (Perciformes: Mullidae) from southern Japan. Zootaxa 3107:47–58.