ABSTRACT
The first exploration of the kinorhynch meiofauna in Portuguese marine waters has revealed the existence of two undescribed species of the cyclorhagid genus Echinoderes. In the present contribution we describe Echinoderes lusitanicus sp. nov. and Echinoderes reicherti sp. nov., both collected from subtidal regions of the coast of Algarve in the southernmost region of Portugal. Echinoderes lusitanicus sp. nov. is recognized by the presence of tubes on segment 2 in subdorsal and ventrolateral positions, on segment 5 in lateroventral positions, on segment 8 in lateral accessory positions, and on segment 10 in laterodorsal positions. Spines are present in middorsal position on segments 4 to 8, and in lateroventral positions on segments 8 and 9. The females have minute lateral terminal accessory spines. The second species, E. reicherti sp. nov., is characterized by tubes on segment 2 in subdorsal and ventrolateral positions, on segment 5 in lateroventral positions, and segment 8 in sublateral positions. In addition, the species possesses acicular spines in the middorsal position on segment 4, and in lateroventral positions on segments 6 to 9. Morphological aspects such as tube/spine pattern of the trunk or sexually dimorphic traits are discussed and compared with other Echinoderes species showing close resemblance.
http://zoobank.org/urn:lsid:zoobank.org:pub:79C25EEE-9064-46F8-95E2-EE493DC82185
RESPONSIBLE EDITOR:
Introduction
The phylum Kinorhyncha is a group of meiobenthic animals of microscopic size (less than 1 mm in length) that is found exclusively in marine, brackish or estuarine realms (Higgins Citation1988; Sørensen & Pardos Citation2008; Neuhaus Citation2013). These animals live in sediments such as sand, shell gravel and mud, from coarse to fine granulometry. Although described already in the middle of the 19th century, by Dujardin (Citation1851), only a few more than 200 species have been described so far (Neuhaus Citation2013).
Our knowledge about the Portuguese kinorhynch fauna is extremely limited. The occurrence of this group along the Portuguese coast is hitherto only known from surveys on general meiofaunal abundance and total biomass (e.g. Bianchelli et al. Citation2010). More recently, Echinoderes dujardinii Claparède, Citation1863, Echinoderes hispanicus Pardos et al. Citation1998 and a third undescribed species of Echinoderes from the Algarve, the southernmost region of Portugal, were used in a comparative study on the myoanatomy of this genus (Herranz et al. Citation2014a). However, any attempt to identify or describe new species of kinorhynchs from Portugal has never been carried out. Hence, the Portuguese coast represents a huge gap in our knowledge about the distribution of Kinorhyncha along the Iberian Peninsula – a topic that has been studied over the last 24 years (Sánchez et al. Citation2012 and references therein). Comprehensive sampling in this region rendered a list of more than 11 genera and 29 species from Spanish territory only, with the most speciose genus being Echinoderes. Hence, given the high biodiversity found in this area, one could also expect a high diversity of kinorhynchs inhabiting the Portuguese coast.
Aiming at increasing knowledge about the biogeography and biodiversity of Kinorhyncha, we inspected the Portuguese coast for the first time in search of this marine group. In the present study, meiofaunal samples were taken in the area between Faro and Albufeira, in the Algarve. The samples were taken as part of several field samplings performed in Portugal between February 2012 and September 2014. Here, we focus on the description of two new species of Echinoderes, which together represent the first kinorhynch species described from Portugal.
Materials and methods
The study sites are situated in the Algarve, the southernmost region of Portugal, in the area between Faro and Albufeira (). Samples were taken with a Higgins meiobenthic dredge at various subtidal localities, and six of them yielded the specimens used in the present study. Stations 13.10.21.1 (36°54′24″N, 007°53′58″W), 13.10.21.2 (36°54′27″N, 007°52′13″W) and 13.10.21.4 (36°57′12″N, 007°52′39″W) are located at 96 m, 100 m and 44 m depth, respectively, off Faro. The sediment consists of mud at all stations, which were sampled on 21 October 2013. Stations 13.10.28.1 (36°57′40″N, 008°09′38″W), 13.10.28.2 (36°59′52″N, 008°09′19″W) and 13.10.28.4 (37°02′43″N, 008°06′49″W) are also subtidal but located at 45 m, 35 m and 15 m depth off Vale do Lobo, about 8 km west of Faro. The sediment consisted of mud in the first two samples and sand with mud in the last sample. All samples were collected on 28 October 2013.
The samples were brought to the Centro de Ciências do Mar (CCMAR) lab, at the University of Algarve, and processed alive. Kinorhynchs were extracted with the ‘bubbling and blot'-method (Higgins Citation1988), following the procedure described by Sørensen & Pardos (Citation2008). Kinorhynch specimens were picked up and observed live, and subsequently fixed in 4% paraformaldehyde buffered with phosphate buffer saline solution (PBS). Before fixation some specimens were exposed to fresh water to make them protrude their heads. Specimens for light microscopy (LM) were dehydrated through a graded series of glycerin, and mounted in Fluoromount G, either on a regular glass slide or between two cover slips attached to a plastic H-S slide. They were examined and photographed with an Olympus BX51 microscope, equipped with a drawing tube and an Olympus DP22 camera connected to a DP2-SAL camera controller. Line art illustrations were based on camera lucida drafts of mounted specimens that subsequently were scanned and drawn in Adobe® Illustrator CS6. Measurements were made with the software embedded in the DP2-SAL controller. All dimensions reported in the tables are based on LM measurements. If a dimension mentioned in the text is estimated from scanning electron microscopy (SEM) images, this will be indicated clearly. Specimens for scanning electron microscopy were dehydrated through a graded series of alcohol and subsequently transferred to acetone through a graded ethanol/acetone series, critical point dried, mounted on aluminium stubs, sputter coated with a platinum/palladium mix and examined with a JEOL JSM-6335F Field Emission scanning electron microscope.
The terminology follows Neuhaus & Higgins (Citation2002) and Sørensen & Pardos (Citation2008). All examined material is deposited at the Zoological Museum, Natural History Museum of Denmark, University of Cophenhagen (ZMUC) or the Smithsonian Institution, National Museum of Natural History, Washington DC (USNM).
Comparative material examined
Comparison between the new species and congeners showing close resemblance was, when possible, based on the examination of type material.
For Echinoderes lusitanicus sp. nov.:
E. eximus Higgins & Kristensen, Citation1988: paratypes (ZMUC-KIN-27, ZMUC-KIN-28).
E. skipperae Sørensen & Landers, Citation2014: holotype (ZMUC-KIN-730), paratypes (ZMUC-KIN-742).
E. tchefouensis Lou, Citation1934: neotype (ZMUC-KIN-468), supplementary material (ZMUC-KIN-486).
E. caribiensis Kirsteuer, Citation1964: literature only; no type material or other specimens available for study.
For Echinoderes reicherti sp. nov.:
E. abbreviatus Higgins, Citation1983: holotype (USNM-69963), paratypes (USNM-69965); studied by MVS and MH.
E. adrianovi Herranz et al., Citation2014b: holotype (USNM-1196401); studied by MVS and MH.
E. cernunnos Sørensen et al., Citation2012: holotype (ZMUC-KIN-536), paratypes (ZMUC-KIN-537).
E. kanni Thormar & Sørensen, Citation2010: holotype (ZMUC-KIN-400), paratypes (ZMUC-KIN-408).
E. obtuspinosus Sørensen et al., Citation2012: holotype (ZMUC-KIN-549), paratypes (ZMUC-KIN-550).
Taxonomy
Class Cyclorhagida (Zelinka, Citation1896) Sørensen et al., Citation2015
Order Echinorhagata Sørensen et al., Citation2015
Family Echinoderidae Zelinka, Citation1894
Genus Echinoderes Claparède, Citation1863
Echinoderes lusitanicus sp. nov.
(–; –)
Figure 1. Map showing the approximate position of the sampling area off the Portuguese coast. Inset to the lower left shows a close-up of the sampling area between Faro and Albufeira. Two sampling localities (dots) yielded specimens of Echinoderes lusitanicus sp. nov., while Echinoderes reicherti sp. nov. was found at four localities (stars).
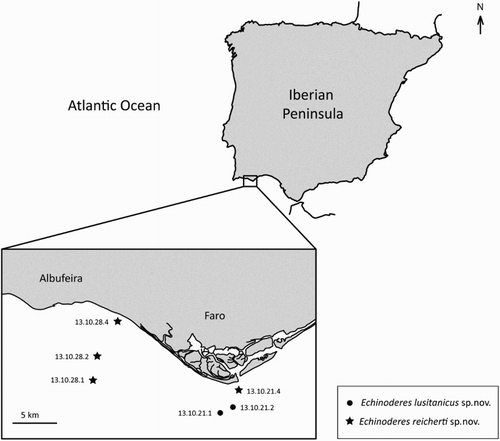
Figure 2. Line art illustrations of Echinoderes lusitanicus sp. nov. (A) Female, dorsal view; (B) Female, ventral view; (C) Male, segments 10 and 11, dorsal view; (D) Male, segments 10 and 11, ventral view. Abbreviations: gco1, glandular cell outlet type 1; lat, lateral accessory tube; ldt, laterodorsal tube; lvs, lateroventral spine; lvt, lateroventral tube; mds, middorsal spine; pe, penile spine; sdt, subdorsal tube; si, sieve plate; ss, sensory spot; vlt, ventrolateral tube.
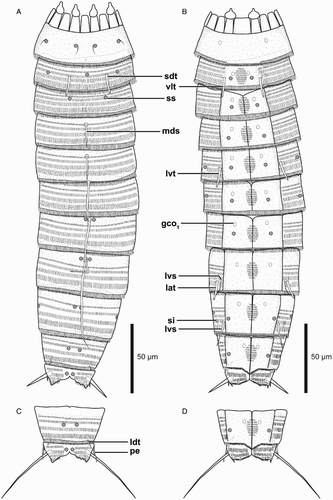
Figure 3. Light micrographs showing overviews and details of trunk morphology in Echinoderes lusitanicus sp. nov. A–I. Female, holotype (ZMUC-KIN-924). J. Male, paratype (ZMUC-KIN-925). (A) Ventral overview, anterior faces up; (B) Segments 1 to 3, dorsal view; (C) Segments 4 to 6, dorsal view; (D) Segments 7 and 8, dorsal view; (E) Segments 2 and 3, ventral view; (F) Segments 5 and 6, ventral view; (G) Segments 8 to 10, ventral view; (H) Segments 9 to 11, ventral view, showing female morphology; (I) Right half of segment 11, ventral view, showing female morphology; (J) Right half of segment 11, ventral view, showing male morphology. Abbreviations: gco1, glandular cell outlet type 1; lat, lateral accessory tube; ltas, lateral terminal accessory spine; lts, lateral terminal spine; lvs, lateroventral spine; lvt, lateroventral tube; mds (and arrowheads), middorsal spine; pe, penile spine; sdt, subdorsal tube; si, sieve plate; vlt, ventrolateral tube.
Figure 4. Scanning electron micrographs showing overviews and details in head and trunk morphology of Echinoderes lusitanicus sp. nov. paratypes (ZMUC-KIN-936). (A) Lateroventral overview of whole specimen, anterior faces up; (B) Introvert, sector 4; (C) Segments 1 to 3, subdorsal view; (D) Segments 2 and 3, ventral view; (E) Segments 5 and 6, lateroventral view; (F) Segments 8 to 10, lateroventral view; (G) Segment 9, ventral view; (H) Detail of right half of segment 11 in female showing lateral terminal accessory spine; (I) Partial view of segment 11 showing a penile spine, lateral view; (J) Detail of segment 11 in male showing the penile spines, laterodorsal view. Abbreviations: lat, lateral accessory tube; ltas, lateral terminal accessory spine; lts, lateral terminal spine; lvs, lateroventral spine; lvt, lateroventral tube; pe, penile spine; psp, primary spinoscalid; sdt, subdorsal tube; sp2–4 spinoscalid of ring 2 to 4; ss, sensory spot; vlt, ventrolateral tube.

Table I. Measurements (µm) from light microscopy of adult Echinoderes lusitanicus sp. nov., including number of measured specimens (n) and standard deviation (SD).
Table II. Summary of nature and location of sensory spots, glandular cell outlets and spines arranged by series in Echinoderes lusitanicus sp. nov.
Material examined
Adult female, S coast of Portugal, off Faro, 36°54′27″N, 007°52′13″W, st. 13.10.21.2, mud, 100 m, 21 Oct. 2013, mounted in Fluoromount G (ZMUC-KIN-924).
Four adults (2 males, 2 females), from type locality, mounted in Fluoromount G (ZMUC-KIN-925 to ZMUC-KIN-928); 4 adult females, from type locality, mounted on SEM stub (ZMUC-KIN-929); 1 juvenile, 36°54′24″N, 007°53′58″W, st. 13.10.21.1, mud, 96 m, 21 Oct. 2013, mounted in Fluoromount G (ZMUC-KIN-930); 5 adults (3 males, 2 females), same locality as preceding, mounted in Fluoromount G (ZMUC-KIN-931 to ZMUC-KIN-935); 5 adults (2 males, 3 undetermined), same locality as preceding, mounted on SEM stub (ZMUC-KIN-936).
Diagnosis
Echinoderes with tubes on segment 2 in subdorsal and ventrolateral positions, on segment 5 in lateroventral positions, on segment 8 in lateral accessory positions, and on segment 10 in laterodorsal positions; spines are present in middorsal position on segments 4 to 8, and in lateroventral positions on segments 8 and 9. Males with three penile spines, females with minute lateral terminal accessory spines.
Description
Adults with head, neck and 11 trunk segments (a,b, a and a). Trunk with rectangular overall appearance; dorsal and lateral body regions with dense, uniform covering of cuticular hairs. Pachycycli are well developed in all segments, interrupted middorsally on segments 2–11. Lateral terminal accessory spines in females relatively short (i and h). For complete overview of measures and dimensions, see . Distribution of cuticular structures, i.e. sensory spots, glandular cell outlets, sieve plates, spines and tubes, is summarized in .
The head consists of a retractable mouth cone and an introvert. In specimens mounted for SEM, the introvert was either fully or partly retracted, or alternatively too dirty to allow complete examination of scalid arrangements. Hence, the following description of the head appendages is only partial. The mouth cone is equipped with nine outer oral styles, each consisting of two joined units, arranged as one style anterior to each introvert sector, except the middorsal sector 6. The styles alternate slightly in lengths, between longer and shorter ones. A pair of lateral spikes is located at the base of each style, and a fringe consisting of four long spikes is located even more posteriorly.
Introvert Ring 01 has 10 primary spinoscalids that each consists of a basal sheath and an end piece (b). The basal sheath carries a proximal fringe with four fringe tips, and distally, along its margin, another fringe also with four tips. Introvert Rings 02, 03 and 04, with 10, 20 and 10 scalids, respectively. Scalids in these rings consist of a basal sheath with fringed posterior margin and a pointed end piece; the scalids become gradually shorter from Ring 02 towards Ring 04 (b). It was not possible to examine scalid arrangements or morphology posterior to Ring 04.
The neck has 16 placids, measuring 11 µm in length (a,b). Midventral placid broadest, measuring 10 µm in width, whereas all others are narrower, measuring 6 µm in width. A total of six trichoscalids are present, located in introvert sectors 2, 4, 5, 7, 8 and 10. All trichoscalids attach to trichoscalid plates (a,b).
Segment 1 consists of a complete cuticular ring. Sensory spots are located medially on the segment in subdorsal and laterodorsal positions (a and c). The sensory spots are small and rounded, with a single long cuticular hair extending from the lateral margin of each sensory spot. Glandular cell outlets type 1 are present in lateroventral positions (b). Cuticular hairs absent. Posterior segment margin is straight. The segment terminates into a primary pectinate fringe (c). Fringe with relatively long and thin, sometimes undulating fringe tips along the dorsal, lateral and lateroventral parts of the segment margin; ventral parts of the margin with considerably shorter fringe tips (b and d).
Segment 2 consists of a complete cuticular ring, with tubes (c. 15 µm long based on SEM) located in subdorsal and ventrolateral positions (a,b, b,e and c,d). Sensory spots are located in middorsal, laterodorsal and ventromedial positions, and glandular cell outlets type 1 in ventromedial positions (a,b and e). Sensory spots on this and all following segments are small and rounded. Secondary pectinate fringe present on anterior part of segment, consisting of short, thin, flexible fringe tips. On this and all following segments, cuticular hairs are densely distributed dorsally and laterally, forming a uniform covering between the secondary pectinate fringe and the primary pectinate fringe. The cuticular hairs emerge from a short elevation of the cuticle and their length increases from anterior to posterior on the segment, i.e. posteriormost cuticular hairs are slightly longer than the anteriormost. The hair covering is interrupted on the ventral side of the segment, where only a ventromedial round patch is present. Primary pectinate fringe of posterior margin with relatively long and thin, sometimes undulating fringe tips along the whole segment margin.
Segment 3, and remaining segments, consist of one tergal and two sternal plates (b and a). Segment 3 with sensory spots in subdorsal and ventromedial positions, and glandular cell outlets type 1 in ventromedial positions (a,b). Cuticular hairs densely distributed over tergal plate, between secondary pectinate fringe and intersegmental joint line. Sternal plates completely covered with hairs, except in one hairless patch on each plate, on the anterior half of the segment, near the transition between the ventromedial and paraventral areas. Posterior segment margin with primary pectinate fringe as on preceding segment.
Segment 4 with short acicular spine in middorsal position (a and c). Sensory spots and glandular cell outlets type 1 present in ventromedial positions only (b). Secondary pectinate fringe, primary pectinate fringe of posterior margin and cuticular hairs as on preceding segment.
Segment 5 with short acicular spine in middorsal position and tubes in lateroventral positions (a,b, c,f and e). Sensory spots present in sublateral and ventromedial positions. Glandular cell outlets, secondary pectinate fringe, primary pectinate fringe and cuticular hairs as on preceding segment.
Segment 6 with acicular spine in middorsal position (a and c). This acicular spine is slightly longer than that of the preceding segment. Sensory spots (e), glandular cell outlets (f), secondary pectinate fringe, primary pectinate fringe and cuticular hairs as on preceding segment.
Segment 7 with long acicular spine in middorsal position (a and d). This acicular spine is approximately twice as long as those of segments 4 and 5. Sensory spots are located in paradorsal and ventromedial positions, and glandular cell outlets type 1 are located in ventromedial positions. Secondary pectinate fringe, primary pectinate fringe and cuticular hairs as on preceding segment.
Segment 8 with long acicular spine in middorsal position, tubes in lateral accessory positions, and short spines in lateroventral positions (a,b, d,g, and f). Sensory spots are located in paradorsal positions only, and glandular cell outlets type 1 in ventromedial positions. Secondary pectinate fringe, primary pectinate fringe and cuticular hairs as on preceding segment.
Segment 9 with small short spines in lateroventral positions (b, g,h and f,g). Sensory spots present in paradorsal, midlateral and ventrolateral positions. Glandular cell outlets type 1 located in ventromedial positions. A pair of small sieve plates is located in lateral accessory positions (b and g,h). Hairs, secondary pectinate fringe, posterior segment margin and its primary pectinate fringe as on preceding segment.
Segment 10 with minute laterodorsal tubes located at the posterior segment margin (a,c). These tubes are slightly longer in males than in females. Sensory spots present in subdorsal and ventrolateral positions. Glandular cell outlets type 1 located in paraventral positions. Cuticular hair covering as on preceding segments. Posterior segment margin of tergal plate straight; sternal plates extend posteriorly into a midventral triangular projection (b). Fringe tips in primary pectinate fringe of posterior margin as on preceding segment.
Segment 11 with lateral terminal spines (a–d, h–j and h–j); lateral terminal spines in males conspicuously longer than in females (male average LTS length = 50 µm; female average LTS length = 20 µm). Males with three pairs of penile spines: dorsal and ventral penile spines are long, flexible and slender, medial one is much shorter and thicker (c,d, j, and i,j). Females with rather minute lateral terminal accessory spines (a,b, i and h). Sensory spots present in paradorsal positions. Cuticular hair covering dense on both tergal and sternal plates. Tergal plate projects slightly beyond sternal plates, and terminates in two short tergal extensions with interrupted medial margins.
Etymology
The species name ‘lusitanicus’ is derived from Lusitania – the Latin name for the ancient Iberian Roman province that includes approximately all of modern Portugal south of the Douro river, as well as a part of Spain.
Differential diagnosis
Echinoderes lusitanicus sp. nov. can be distinguished from all other kinorhynchs by its possession of lateroventral tubes/spines on segments 5, 8 and 9 only, combined with the presence of subdorsal tubes on segment 2. No other kinorhynch shows this particular tube/spine pattern. The presence of lateroventral tubes and/or spines on only segments 5, 8 and 9 is shared with four other species only: Echinoderes caribiensis Kirsteuer, Citation1964, E. eximus Higgins & Kristensen, Citation1988, E. skipperae Sørensen & Landers, Citation2014 and E. tchefouensis Lou, Citation1934 (see Kirsteuer Citation1964; Higgins & Kristensen Citation1988; Sørensen et al. Citation2012; Sørensen & Landers Citation2014). Echinoderes caribiensis is a shallow-water species from mangroves in Venezuela (Kirsteuer Citation1964), and it possesses lateroventral tubes or spines on segments 5, 8 and 9, but since it otherwise does not have any spines on segments 1 to 10, it cannot in any way be confused with E. lusitanicus sp. nov. Echinoderes tchefouensis is widely distributed in the West Pacific, from Korea to Malaysia (Sørensen et al. Citation2012, Citation2016.), while E. eximus is known from West Greenland (Higgins & Kristensen Citation1988). Besides their similarity in lateroventral spine pattern, E. tchefouensis and E. eximus do not resemble E. lusitanicus sp. nov. in any other way. First, the lateral spine patterns are actually not identical, because in E. tchefouensis and E. eximus the lateral spine on segment 8 is displaced to the lateral accessory position (Sørensen et al. Citation2012). Furthermore, these species have much longer lateral terminal accessory spines, no tubes on segment 2, and in addition, E. tchefouensis has a pair of very large and conspicuous glandular cell outlets type 2 on segment 8 (Sørensen et al. Citation2012). The species that shows most resemblance to E. lusitanicus sp. nov. is E. skipperae. The two species share the same tube/spine distribution in the lateral series, and E. lusitanicus sp. nov. also shows, at least to some extent, the same rectangular overall trunk shape that is highly characteristic for E. skipperae (Sørensen & Landers Citation2014). However, the new species does not have the conspicuously strong pachycycli that are found in E. skipperae, and the dorsal spine patterns are also different, since E. lusitanicus sp. nov. has middorsal spines on segments 4 to 8, whereas E. skipperae has them on segments 4, 6 and 8 only. Furthermore, E. lusitanicus sp. nov. has subdorsal tubes on segment 2, which are lacking in E. skipperae. Instead, the latter has sublateral tubes on this segment, which are absent in E. lusitanicus sp. nov.
Two other morphological features attract special attention in E. lusitanicus sp. nov. Both regard sexually dimorphic characters, namely the extraordinary short lateral terminal accessory spines in females, and the significant difference in length of lateral terminal spines between males and females. The easy way to discriminate the sexes among species of Echinoderes is to look for the presence of either penile spines, present in males only, or for lateral terminal accessory spines that are restricted to females (see, e.g., Sørensen & Pardos Citation2008; Neuhaus Citation2013). In E. lusitanicus sp. nov. though, the sexes can also be discriminated by the lengths of the lateral terminal spines. In females, the lateral terminal spines are short and measure between 17 and 22 µm, which is less than 10% of the trunk length. Conversely, the lateral terminal spines in males are more than twice as long, measuring 45–57 µm, which is around 20% of the trunk length. To our knowledge, a similar sexual dimorphism is not known for any other species of Echinoderidae.
The other sexually dimorphic specialty regards the lateral terminal accessory spines that usually are present exclusively in females. There are exceptions though, since lateral terminal accessory spines actually are missing in females of several species belonging to the E. coulli-group (see Sørensen Citation2014), but also of species such as E. capitatus Zelinka, Citation1928 and E. isabelae GaOrdoñez et al., Citation2008 (see Zelinka Citation1928; GaOrdonez et al. Citation2008). A recently described species from the E. coulli-group, E. komatsui Yamasaki & Fujimoto, Citation2014, shows an interesting intermediate condition in regard to the presence of lateral terminal accessory spines. Here, the new species actually has lateral terminal accessory spines, and they are restricted to females only, but the spines are highly reduced (Yamasaki & Fujimoto Citation2014). In females of E. lusitanicus sp. nov. the lateral terminal accessory spines are even more reduced and appear only as a pair of short spikes. This apparent reduction of a female secondary sexual character is interesting, and suggests that E. lusitanicus sp. nov. belongs to a lineage of Echinoderes that moves towards a reduction or complete loss of lateral terminal accessory spines. This could either indicate that E. lusitanicus sp. nov. is closely related with species of the E. coulli-group, or support the fact that species of Echinoderes went through a gradual reduction and subsequent loss of lateral terminal accessory spines, at least twice. Additionally, the presence of a slightly larger sieve plate, compared with the regular small and round ones present in most Echinoderes, might support a closer relationship between E. lusitanicus and species of the E. coulli group.
Echinoderes reicherti sp. nov.
(, –; –)
Figure 5. Line art illustrations of Echinoderes reicherti sp. nov. (A) Female, dorsal view; (B) Female, ventral view; (C) Male, segments 10 and 11, dorsal view; (D) Male, segments 10 and 11, ventral view. Abbreviations: gco1, glandular cell outlet type 1; gco2, glandular cell outlet type 2; ldt, laterodorsal tube; lvs, lateroventral spine; lvt, lateroventral tube; mds, middorsal spine; pe, penile spine; sdt, subdorsal tube; si, sieve plate; slt, sublateral tube; ss, sensory spot; vlt, ventrolateral tube.
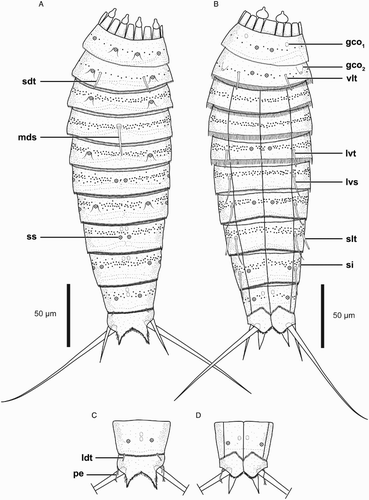
Figure 6. Light micrographs showing overviews and details of trunk morphology in Echinoderes reicherti sp. nov. A–H. Female, holotype (ZMUC-KIN-937). I. Male, paratype (ZMUC-KIN-943). (A) Ventral overview, anterior faces up; (B) Segments 2 and 3, dorsal view; (C) Segments 4 and 5, dorsal view; (D) Segments 1 to 3, ventral view; (E) Segments 4 to 6, ventral view; (F) Segments 6 and 7, ventral view; (G) Segments 8 to 10, ventral view; (H) Segments 10 and 11 showing female morphology, ventral view; (I) Segment 11, ventral view, showing male morphology. Abbreviations: gco2, glandular cell outlet type 2; ltas, lateral terminal accessory spine; lts, lateral terminal spine; lvs, lateroventral spine; lvt, lateroventral tube; mds, middorsal spine; pe, penile spine; sdt, subdorsal tube; slt, sublateral tube; vlt, ventrolateral tube.
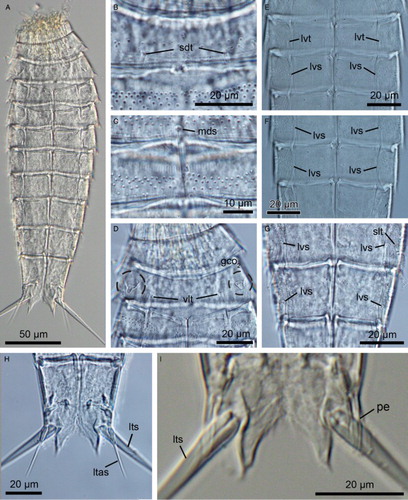
Figure 7. Scanning electron micrographs showing overviews and details in trunk morphology of Echinoderes reicherti sp. nov. paratypes (ZMUC-KIN-954). (A) Ventrolateral overview of whole specimen, anterior faces up; (B) Segment 1, ventral view. The square outlines the region where a glandular cell outlet type 1 is located, as shown in B’; (B’) Detail of a glandular cell outlet type 1 located in segment 1; (C) Segments 2 and 3, ventral view; (D) Segments 4 to 6, lateroventral view; (E) Segments 5 to 7, ventral view; (F) Segments 7 and 8, lateroventral view; (G) Segments 8 and 9, ventral view; (H) Close-up showing subdorsal tube on segment 2; (I) Close-up showing middorsal spine on segment 4; (J) Close-up showing laterodorsal tube on segment 10 in female; (K) Close-up showing laterodorsal tube on segment 10 in male; (L) Segment 11 in female, dorsal view. Abbreviations: ldt, laterodorsal tube; lts, lateral terminal spine; lvs, lateroventral spine; lvt, lateroventral tube; mds, middorsal spine; pe, penile spine; sdt, subdorsal tube; slt, sublateral tube; vlt, ventrolateral tube.
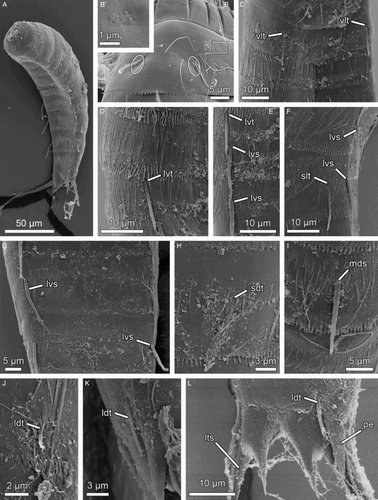
Table III. Measurements (µm) from light microscopy of adult Echinoderes reicherti sp. nov., including number of measured specimens (n) and standard deviation (SD).
Table IV. Summary of nature and location of sensory spots, glandular cell outlets and spines arranged by series in Echinoderes reicherti sp. nov.
Material examined
Adult female, S coast of Portugal, off Vale do Lobo, about 8 km W of Faro, 36°59′52″N, 008°09′19″W, st. 13.10.28.2, mud, 35 m, 28 Oct. 2013, mounted in Fluoromount G (ZMUC-KIN-937).
16 adults (9 males, 7 females), from type locality, mounted in Fluoromount G (ZMUC-KIN-938–ZMUC-KIN-953); 11 adults (4 males, 5 females, 2 undetermined), from type locality, mounted on SEM stubs (ZMUC-KIN-954, ZMUC-KIN-955).
Other material
Three specimens, S coast of Portugal, off Vale do Lobo, 36°57′40″N, 008°09′38″W, st. 13.10.28.1, 45 m, 28 Oct. 2013, mounted on SEM stub (ZMUC); several specimens, S coast of Portugal, off Vale do Lobo, 36°57′40′′N, 008°09′38′′W, st. 13.10.28.1, 45 m, 28 Oct. 2013, mounted in Fluoromount G (ZMUC); several specimens, S coast of Portugal, off Vale do Lobo, 37°02′43″N, 008°06′49″W, st. 13.10.28.4, 15 m, 28 Oct. 2013, mounted in Fluoromount G (ZMUC); several specimens, S coast of Portugal, off Faro, 36°57′12″N, 007°52′39″W, st. 13.10.21.4, 44 m, 21 Oct. 2013, mounted in Fluoromount G (ZMUC).
Diagnosis
Echinoderes with tubes on segment 2 in subdorsal and ventrolateral positions, on segment 5 in lateroventral positions, and segment 8 in sublateral positions; spines are present in middorsal position on segment 4, and in lateroventral positions on segments 6 to 9. Glandular cell outlets type 2 present in sublateral positions of segment 2. Males with three penile spines, females with slender lateral terminal accessory spines.
Description
Adult with head, neck and 11 trunk segments (a,b, 6a and 7a). For complete overview of measures and dimensions, see . Distribution of cuticular structures, i.e. sensory spots, glandular cell outlets, sieve plates, spines and tubes, is summarized in .
All specimens mounted for SEM had their heads retracted, which made detailed examination of mouth cone and introvert structures impossible.
The neck has 16 placids (a,b), measuring 11 µm in length. Midventral placid broadest, measuring 13 µm in width, whereas all others are narrower, measuring 7 µm in width. A total of six trichoscalids are present, and located in introvert sectors 2, 4, 5, 7, 8 and 10. The trichoscalids attach to trichoscalid plates.
Segment 1 consists of a complete cuticular ring. Sensory spots are located in subdorsal, laterodorsal and ventromedial positions (a,b and b). All sensory spots are rounded, with a central pore. A cuticular hair emerges on each side of the subdorsal sensory spots. Glandular cell outlets type 1 are present in ventrolateral positions (b and b, b’). A few cuticular hairs are present, scattered around the segment, located along a medial band. Each hair emerges through a rounded perforation site. The segment terminates into a primary pectinate fringe. Fringe with very short fringe tips along the whole segment margin.
Segment 2 consists of a complete cuticular ring with subdorsal (c. 11 µm long based on SEM) and ventrolateral tubes (c. 11 µm long based on SEM) (a,b, b,d and c,h). Sensory spots are located in subdorsal – which are flanked by a cuticular hair on each side – and ventromedial positions (a,b). Glandular cell outlets type 2 are present in sublateral position (b and d). Cuticular hairs are bracteate, present in a median band around the segment. Posterior segment margin is straight along the dorsal to lateral edges. Secondary pectinate fringe present on anterior part of segment, consisting of short, thin, flexible fringe tips. On this and all following segments, primary pectinate fringe with relatively long and thin, sometimes undulating fringe tips along the lateral and lateroventral parts of the segment margin; ventral parts of the margin with slightly shorter fringe tips.
Segment 3, and remaining segments, consist of one tergal and two sternal plates (a,b and a). Segment with sensory spots in subdorsal positions, which are flanked by a cuticular hair on each side. Glandular cell outlets not observed. Cuticular hairs as on preceding segment, but covering more densely the median area of the segment. Secondary pectinate fringe and primary pectinate fringe as on preceding segment.
Segment 4 with small acicular spine in middorsal position (a, c and i). Sensory spots and glandular cell outlets not observed. Secondary pectinate fringe, primary pectinate fringe and cuticular hairs as on preceding segment.
Segment 5 with lateroventral tubes (c. 12 µm long based on SEM) (b, e and d,e). Sensory spots present in subdorsal – which are flanked by a cuticular hair on each side – and ventromedial positions (a,b). Glandular cell outlets are not observed. Secondary pectinate fringe, primary pectinate fringe and cuticular hairs as on preceding segment.
Segment 6 with small acicular spines in lateroventral positions (b, f and e). Sensory spots present in paradorsal and midlateral positions. Secondary pectinate fringe, primary pectinate fringe and cuticular hairs as on preceding segment.
Segment 7 with small acicular spines in lateroventral positions (b, f and e,f) and with sensory spots in subdorsal, midlateral and ventromedial positions (a,b). Secondary pectinate fringe, primary pectinate fringe and cuticular hairs as on preceding segment.
Segment 8 with sublateral tubes (b, g and f) and small acicular spines in lateroventral positions (b, g and f,g). Sensory spots present in paradorsal position (a), and glandular cell outlets type 1 in paradorsal positions (a). Secondary pectinate fringe, primary pectinate fringe and cuticular hairs as on preceding segment.
Segment 9 with small acicular spines in lateroventral positions (b, g and g). Sensory spots present in laterodorsal, midlateral and ventrolateral position (a,b). Glandular cell outlets as on preceding segment. A pair of small sieve plates is located in the lateral accessory positions (b). Secondary pectinate fringe, primary pectinate fringe and cuticular hairs as on preceding segment.
Segment 10 with minute laterodorsal tubes located at the posterior segment margin (a,c and j,k); tubes are longer in males (c. 9 µm long based on SEM) than in females (ca. 3 µm long based on SEM). Sensory spots present in subdorsal and ventrolateral positions (a,b). Two glandular cell outlets type 1 located in middorsal position, and one additional pair in paraventral positions (a,b). Cuticular hair covering as on preceding segments but less dense. Posterior segment margin of tergal plate straight; sternal plates with curved posterior margin, which extend posteriorly into a midventral triangular projection. Fringe tips in primary pectinate fringe as on preceding segment.
Segment 11 with lateral terminal spines (, h,i and l). Males with three pairs of elongate and thin penile spines (c,d, i and l). Females with lateral terminal accessory spines (a,b and h). Sensory spots and glandular cell outlets not observed. Cuticular hairs present only at the most posterior part of the segment. Tergal plate projects beyond sternal plates, and terminates into two pointed tergal extensions with interrupted medial margins (h,i).
Etymology
This species is named after Prof. Dr Heinrich Reichert, an enthusiastic supporter of the study of meiofauna and lesser-known invertebrates.
Differential diagnosis
Echinoderes reicherti sp. nov. is distinguished from all other congeners by the unique combination of a number of specific characters. Indeed, the presence of subdorsal and ventrolateral tubes, as well as sublateral glandular cell outlets type 2 on segment 2 combined with the presence of a middorsal spine only on segment 4, is a pattern not present in any other kinorhynch.
The presence of lateroventral and subdorsal tubes on segment 2 is shared with four other species only: Echinoderes abbreviatus Higgins, Citation1983, E. adrianovi Herranz et al., 2014, Echinoderes kanni Thormar & Sørensen, Citation2010 and Echinoderes lusitanicus sp. nov. Both E. adrianovi and E. kanni, from the Floridian west coast and the Solomon Islands, respectively, possess a pattern of lateral tubes/spines that is almost identical to that of E. reicherti sp. nov. (cf. Thormar & Sørensen Citation2010; Herranz et al. Citation2014b). However, in E. adrianovi and E. kanni the tubes in segment 8 are located in lateral accessory positions rather than in sublateral as observed in E. reicherti (in fact, the latter condition is known only from Echinoderes astridae Sørensen Citation2014). In addition, E. adrianovi and E. kanni possess middorsal spines on segments 4–8 and thus, they cannot be confused with E. reicherti sp. nov., which possesses a middorsal spine only on segment 4. The other new species described here, E. lusitanicus sp. nov. is easily distinguished from E. reicherti because it possesses tubes and/or spines on only segments 5, 8 and 9. The presence of ventrolateral tubes in E. abbreviatus was not mentioned in the species’ original description (Higgins Citation1983), but the presence of such tubes was confirmed after a recent reexamination of the type material (Sørensen & Herranz, unpubl. obs. 3 April 2014). However, with its short and stout lateral terminal accessory spines, and middorsal spines on segments 4, 6 and 8 only, E. abbreviatus cannot be confused with E. reicherti sp. nov.
Another feature on segment 2 that attracts attention is the presence of glandular cell outlets type 2 in sublateral position. This characteristic is for now shared with E. abbreviatus (Sørensen & Herranz, unpubl. obs. 3 April 2014) from Belize, as well as E. cernunnos Sørensen et al., Citation2012 and E. obtuspinosus Sørensen et al., Citation2012, two Korean species (see Sørensen et al. Citation2012). However, all three species possess glandular cell outlets type 2 also in subdorsal, laterodorsal and ventrolateral positions, which makes E. reicherti sp. nov. easily distinguishable (Higgins Citation1983; Sørensen et al. Citation2012). A fifth species with glandular cell outlets type 2 in sublateral positions on segment 2 is currently being described from the Gulf of Mexico. Interestingly, this species also possesses only a single middorsal spine (on segment 4), but it also differs from E. reicherti sp. nov. by having one additional pair of glandular cell outlets type 2 in subdorsal positions on segment 2, only tubes in lateroventral positions on segment 2 (opposite to subdorsal and ventrolateral in E. reicherti sp. nov.), and tubes in lateral accessory positions on segment 8 (opposite to sublateral in E. reicherti sp. nov.).
Acknowledgements
We acknowledge technical and field staff at CCMAR (Portugal). Field collection in Faro (Portugal) was partially funded by ASSEMBLE Grant No. 227799 to RCN and MH. We are greatly indebted to Prof. Dr Heinrich Reichert for his financial support during our field trips. Funding from EU research exchange programme SYNTHESYS (DK-TAF-4892) was provided to RCN to study the type specimens described here.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bianchelli S, Gambi C, Zeppilli D, Danovaro R. 2010. Metazoan meiofauna in deep-sea canyons and adjacent open slopes: a large scale comparison with focus on the rare taxa. Deep Sea Research I 57:420–33. doi:10.1016/j.dsr.2009.12.001
- Claparède ARE. 1863. Zur Kenntnis der Gattung Echinoderes Duj. Beobachtungen über Anatomie und Entwicklungsgeschichte wirbelloser Thiere an der Küste von Normandie angestellt. Leipzig: Verlag von Wilhelm Engelmann, pls XVI–XVII, p 90–92, 119.
- Dujardin F. 1851. Sur un petit animal marin, l'Echinodère, formant un type intermédiare entre les crustacés et les vers. Annales des Sciences naturelles (Zoologie sér. 3) 15:158–60.
- GaOrdóñez D, Pardos F, Benito J. 2008. Three new Echinoderes (Kinorhyncha, Cyclorhagida) from North Spain, with new evolutionary aspects in the genus. Zoologischer Anzeiger 247:95–111. doi:10.1016/j.jcz.2007.07.001
- Herranz M, Boyle M, Pardos F, Neves R. 2014a. Comparative myoanatomy of Echinoderes (Kinorhyncha): a comprehensive investigation by CLSM and 3D reconstruction. Frontiers in Zoology 11:31. 26 pages. doi:10.1186/1742-9994-11-31
- Herranz M, Sánchez N, Pardos F, Higgins RP. 2014b. New Kinorhyncha from Florida coastal waters. Helgoland Marine Research 68:59–87. doi:10.1007/s10152-013-0369-9
- Higgins RP. 1983. The Atlantic barrier reef ecosystem at Carrie Bow Cay, Belize, II: Kinorhyncha. Smithsonian Contributions to the Marine Sciences 18:1–131. doi:10.5479/si.01960768.18.1
- Higgins RP. 1988. Kinorhyncha. In: Higgins RP, Thiel H, editors. Introduction to the Study of Meiofauna. Washington, DC: Smithsonian Institution Press, p 328–31.
- Higgins RP, Kristensen RM. 1988. Kinorhyncha from Disko Island, West Greenland. Smithsonian Contributions to Zoology 458:1–56. doi:10.5479/si.00810282.458
- Kirsteuer E. 1964. Zur Kenntnis der Kinorhynchen Venezuelas. Zoologischer Anzeiger 173:388–93.
- Lou T-H. 1934. Sur la presence d'un nouveau kinorhynque à Tchefou: Echinoderes tchefouensis sp. nov. Contributions from the Institute of Zoology, National Academy of Peiping 1:1–9. (in Chinese with French translation)
- Neuhaus B. 2013. Kinorhyncha (=Echinodera). In: Schmidt-Rhaesa A, editor. Handbook of Zoology. Gastrotricha, Cycloneuralia and Gnathifera. Volume 1: Nematomorpha, Priapulida, Kinorhyncha, Loricifera. Berlin/Boston: De Gruyter, p 181–348.
- Neuhaus B, Higgins RP. 2002. Ultrastructure, biology, and phylogenetic relationships of Kinorhyncha. Integrative and Comparative Biology 42:619–32. doi:10.1093/icb/42.3.619
- Pardos F, Higgins RP, Benito J. 1998. Two new Echinoderes (Kinorhyncha, Cyclorhagida) from Spain, including a reevaluation of kinorhynch taxonomic characters. Zoologischer Anzeiger 237:195–208.
- Sánchez N, Herranz M, Benito J, Pardos F. 2012. Kinorhyncha from the Iberian Peninsula: new data from the first intensive sampling campaigns. Zootaxa 3402:24–44.
- Sørensen MV. 2014. First account of echinoderid kinorhynchs from Brazil, with the description of three new species. Marine Biodiversity 44:251–74. doi:10.1007/s12526-013-0181-4
- Sørensen MV, Landers SC. 2014. Two new species of Echinoderes (Kinorhyncha: Cyclorhagida) from the Gulf of Mexico. Frontiers in Marine Science 1:8. 18 pages.
- Sørensen MV, Pardos F. 2008. Kinorhynch systematics and biology – an introduction to the study of kinorhynchs, inclusive identification keys to the genera. Meiofauna Marina 16:21–73.
- Sørensen MV, Rho HS, Min W, Kim D, Chang CY. 2012. An exploration of Echinoderes (Kinorhyncha: Cyclorhagida) in Korean and neighboring waters, with the description of four new species and a redescription of E. tchefouensis Lou, 1934. Zootaxa 3368:161–96.
- Sørensen MV, Dal Zotto M, Rho HS, Herranz M, Sánchez N, Pardos F, Yamasaki H. 2015. Phylogeny of Kinorhyncha based on morphology and two molecular loci. PLoS ONE 10(7):e0133440. 33 pages. doi:10.1371/journal.pone.0133440
- Sørensen MV, Gąsiorowski L, Randsø PV, Sánchez N, Neves RC. 2016. First report of kinorhynchs from Singapore, with the description of three new species. Raffles Bulletin of Zoology 64:3–27.
- Thormar J, Sørensen MV. 2010. Two new species of Echinoderes (Kinorhyncha: Cyclorhagida) from the Solomon Islands. Meiofauna Marina 18:67–96.
- Yamasaki H, Fujimoto S. 2014. Two new species in the Echinoderes coulli group (Echinoderidae, Cyclorhagida, Kinorhyncha) from the Ryukyu Islands, Japan. ZooKeys 382:27–52. doi:10.3897/zookeys.382.6761
- Zelinka K. 1894. Über die Organisation von Echinoderes. Verhandlungen der Deutschen Zoologischen Gesellschaft 4:46–49.
- Zelinka K. 1896. Demonstration von Tafeln der Echinoderes-Monographie. Verhandlungen der Deutschen Zoolog-ischen Gesellschaft 6:197–99.
- Zelinka K. 1928. Monographie der Echinodera. Leipzig: Verlag Wilhelm Engelmann. 396 pages.
