ABSTRACT
Decreasing pH levels in the world’s oceans are widely recognized as a threat to marine life. Bryozoans are among several phyla that produce calcium carbonate skeletons potentially affected by ocean acidification (OA). Depending on species, bryozoan skeletons can consist of calcite, aragonite or have a bimineralic combination of these two minerals. Aragonite is generally more soluble in seawater than calcite, making aragonitic species more vulnerable to OA. Here, for the first time we use Raman spectroscopy to determine the mineral composition of a tropical bryozoan biota. Compared with bryozoan biotas from higher latitudes in which calcite predominates, aragonite was found to occur in a much higher proportion of the 22 cheilostome bryozoan species collected from the shorelines of Penang and Langkawi in Malaysia, where 46% of species are calcitic, 41% aragonitic and 13% bimineralic. All but one of the aragonitic or bimineralic species belong to the ascophorans, whereas calcitic skeletons characterized most of the anascans, many of which are primitive ‘weedy’ malacostegines. These results suggest a relatively high vulnerability of tropical bryozoan faunas to OA, with the weedier taxa likely to be least impacted.
RESPONSIBLE EDITOR:
Introduction
Ocean acidification (OA) is one of the results of anthropogenic CO2 emissions that is causing widespread concern because of its expected deleterious effects on marine organisms and ecosystems. A drop in ocean surface water pH of 0.3–0.4 units has been predicted by the year 2100 (Caldeira & Wickett Citation2005). Organisms secreting skeletons composed of CaCO3 may be especially vulnerable to OA (e.g. Orr et al. Citation2005; Fabry et al. Citation2008), requiring more energy both to biomineralize their skeletons and also to maintain them after formation as the oceans move towards being undersaturated with respect to CaCO3 minerals.
Responses of marine organisms with calcareous skeletons to low pH conditions are varied, although in experimental studies the majority show reduced levels of calcification (e.g. Ries et al. Citation2009). A range of factors may determine the extent of the burden of decreasing pH on marine organisms having calcareous skeletons, one of which is the mineralogy of the skeleton. Two skeletal minerals – aragonite and calcite – characterize most organisms with calcareous skeletons. Aragonite is relatively more soluble than calcite (except for calcite that contains appreciable substitution of Ca by Mg; Ries Citation2011), and species having aragonite in their skeletons may therefore be more vulnerable to OA than calcitic species.
Bryozoans are a diverse phylum of colonial metazoans in which the great majority of species biomineralize skeletons of calcium carbonate, including all species belonging to the Cheilostomata, the dominant order of living bryozoans. Cheilostome skeletons may be made of aragonite or calcite, or be bimineralic in which a framework of calcite is later covered by aragonite. The mineralogy of the bryozoan skeleton is under a high degree of organismal control (Smith Citation2014; Taylor et al. Citation2015), although bimineralic species may exhibit apparent ecophenotypic variability in the proportions of calcite and aragonite in their skeletons (e.g. Lombardi et al. Citation2008; Loxton et al. Citation2014).
Acid-bath immersion experiments on bryozoans of differing mineralogies failed to show the expected greater dissolution of the skeletons of bryozoan species containing high contents of aragonite (Smith & Garden Citation2012), the relative surface area of the complex skeletons being interpreted as a more important determinant. Nevertheless, the importance of mineral solubility is strongly suggested by the latitudinal pattern that is evident in bryozoan mineralogy: as in molluscs (Taylor & Reid Citation1990) and serpulids (Lowenstam Citation1954), the proportion of aragonitic bryozoan species declines significantly away from the equator into colder waters where the solubility of CaCO3 is greater (Kuklinski & Taylor Citation2009; Taylor et al. Citation2009; Taylor & Di Martino Citation2014).
Skeletal mineralogy is known for only a small proportion of living cheilostome species, the great majority coming from temperate or polar regions. Very few tropical cheilostomes have been analysed mineralogically (Taylor et al. Citation2009; Taylor & Di Martino Citation2014) and there are no publications dealing with the mineralogical composition of a bryozoan biota from the tropics (i.e. between 23.5°N and 23.5°S latitude). This paper presents the first mineralogical survey of a tropical bryozoan biota, based on shore collections made in Penang and Langkawi, Malaysia during 2013 (Taylor & Tan Citation2015). We employed Raman spectroscopy, a precise and non-destructive method of analysis, to determine the mineralogies of 22 species of cheilostomes. Our findings are discussed in the context of the latitudinal increase in aragonitic bryozoans towards the tropics and their enhanced vulnerability to OA, as well as the taxonomic correlation of cheilostome bryozoan mineralogy.
Materials and methods
Localities and collection
The bryozoans used in this study were collected during a reconnaissance survey of the shorelines of Penang and Langkawi during October 2013. Situated off the west coast of the Malaysian Peninsula, Penang is an island in the Strait of Malacca, at latitude 5°N. Langkawi is an archipelago of islands 80 km north of Penang in the Andaman Sea, at latitude 6°N. The four sites sampled at Penang and the eight sites at Langkawi yielded a total of 23 species of cheilostome bryozoans (site details, species descriptions and specimen repository information can be found in Taylor & Tan Citation2015); one species, Antropora minor (Hincks, 1880), was represented by a single colony deep within a gastropod shell and could not be analysed. Some of the bryozoans fouled man-made structures, such as pier wharves, fishing floats and plastic objects, whereas others encrusted mollusc shells or rocks. All occurrences are from shallow water. Only relatively new parts of the skeletons were analysed, which were probably formed no more than six months prior to collection. Environmental records for Langkawi and Penang show water temperatures from April to October ranging from 29.7 to 32.3°C, and salinities from 27 to 33 ppt. Specimens were washed and air-dried after collection. Scanning electron microscopy was employed for imaging and to confirm species' identities after bleaching (see Taylor & Tan Citation2015).
Mineralogical determinations
The mineralogy of 22 cheilostome species was determined using Raman spectroscopy, a non-destructive, non-invasive and confocal method that permits precise analysis at submicron (∼0.8 μm) resolution (Taylor et al. Citation2008). Duplicate analyses were made from all specimens, and mineralogical constancy was also checked by analysing more than one colony for some of the species. Unequivocal identification of calcite and aragonite is possible because the Raman spectra for these two minerals are readily distinguishable. These mineralogical analyses were undertaken using a T64000 triple-stage laser-Raman system equipped with a Coherent Innova 90 argon ion laser projected through an Olympus 154 BX41 microscope in the Schopf lab (UCLA). Point spectra were obtained from the frontal walls of the bryozoan zooids. When these walls proved to be aragonitic, additional analyses were made of orificial rims, basal walls and/or avicularian crossbars to test for a bimineralic composition as one or more of these structures will be calcitic in bimineralic species (Taylor et al. Citation2008).
Results
As is shown in , 10 (46%) of the 22 species analysed were found to be calcitic, nine (41%) aragonitic and three bimineralic (13%). The 11 anascan-grade cheilostomes were predominantly calcitic; only one species – Cranosina coronata (Hincks, 1881) – was aragonitic (). In contrast, none of the 11 ascophoran-grade cheilostomes had a calcite skeleton: eight were entirely aragonitic and three species were bimineralic (Hippopodina feegeensis (Busk, 1884), Schizoporella japonica Ortmann, 1890 and Microporella sp.).
Figure 1. Mineralogical compositions of cheilostome bryozoan biotas from different latitudes. Note the increasing proportion from the poles to the tropics of species biomineralizing aragonite, either monomineralically (a, pale grey) or bimineralically with calcite (b, mid grey), and the corresponding decrease in calcitic species (c, dark grey). As noted by Kuklinski & Taylor (Citation2009), the one record (Borisenko & Gontar Citation1991) of an aragonitic bryozoan species in the Antarctic is questionable (see also Krzeminska et al. Citation2016 who found no aragonitic bryozoans in their samples from King George Island, Antarctica).
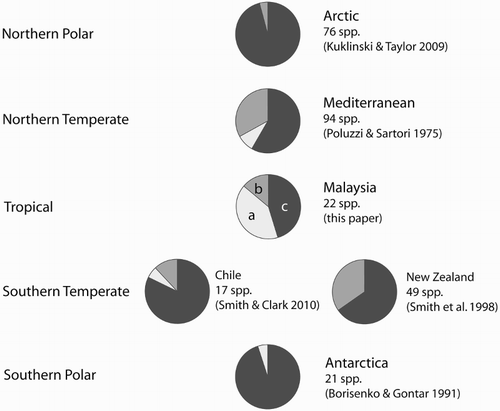
Table I. Results of the Raman spectroscopic mineralogical analyses of Malaysian cheilostome bryozoans.
Discussion
Judging from the limited previously available data, the proportion (54%) of cheilostome species biomineralizing aragonite, either alone or in combination with calcite, matches expectations for a tropical bryozoan biota. Taylor et al. (Citation2009) compiled published analyses of the mineralogy of 37 cheilostome species between latitudes 30°N and 30°S and added new analyses, finding 24% of the species to be aragonitic, 38% to be calcitic and 38% bimineralic. Taylor & Di Martino (Citation2014) used Raman spectroscopy to determine the mineralogy of 23 cheilostome species encrusting the undersides of plate-like scleractinian corals from reefs in Puerto Rico and Peninsular Malaysia, finding 10 (43%) of these species to be calcitic, six (27%) aragonitic and seven (30%) bimineralic.
The new data presented here on the skeletal mineralogy of the cheilostome biota from Penang and Langkawi make it possible for the first time to contrast the mineralogy of a tropical biota with biotas from higher latitudes (summarized by Kuklinski & Taylor Citation2009, ).
compares the mineralogical compositions of polar (Arctic and Antarctic), northern temperate (Mediterranean), southern temperate (Chile and New Zealand) and tropical (Malaysia) cheilostome bryozoan biotas. There is a strong and consistent increase in the proportion of aragonitic species towards the equator, with a corresponding decrease in calcitic species. This pattern is partly due to latitudinal changes in the mineralogies of widely distributed genera, and partly to the differences in genera present in the tropics compared with higher latitudes. For example, the four species of the cosmopolitan genus Parasmittina analysed from Malaysia were found to have aragonitic skeletons, whereas previous analyses of the skeletal composition of other species of this genus from higher latitudes showed them to be calcitic or bimineralic (see Taylor et al. Citation2009: supplementary material S 3). On the other hand, the specimen of Cranosina analysed in this study, a genus known to occur only in the tropics of the Indo-Pacific, Hawaii and Caribbean (Chimonides & Cook Citation1994) and not at higher latitudes, was found to be aragonitic.
Sampling depth is a potential confounding factor in the latitudinal comparisons shown in . All of the bryozoans from Langkawi and Penang are shore samples of colonies from very shallow water (<10 m), whereas many of the bryozoans reported in previous publications came from mid- to outer shelf depths. Analyses of bryozoan assemblage mineralogy relative to depth on the continental shelf are lacking, although Loxton et al. (Citation2014) found a positive correlation between depth and the proportion of aragonite in skeletons of the bimineralic cheilostome Microporella ciliata (Pallas, 1766) in NE Scotland. The generality of this trend within and among species is unknown but if applied to the latitudinal comparisons made here more aragonite would be expected in bryozoans from deeper parts of the Malaysian shelf and the contrast with regions of higher latitude would be even greater.
A relatively high proportion (26%) of the sampled bryozoan biota from Penang and Langkawi in Malaysia consists of malacostegines. Typically, malacostegines account for less than 10% of cheilostomes in higher latitude biotas; for example, of the 262 cheilostome species listed from the Italian biota by Rosso et al. (Citation2010), only 12 (4.6%) are malacostegines. Malacostegines are primitive cheilostomes characterized by planktotrophic larvae that are not brooded. This contrasts with the brooded lecithotrophic larvae of the neocheilostomes occurring in the great majority of cheilostome bryozoan species. All of the Malaysian malacostegines were found to have calcitic skeletons. Indeed, calcitic skeletons are almost ubiquitous among malacostegines (Taylor et al. Citation2009), a notable exception being the algal epiphyte Membranipora. Smith & Clark (Citation2010: 231) commented on the mineralogical variability within putative Membranipora membranacea (Linnaeus, 1767) from several localities around the globe, noting that some analyses found it to be aragonitic, others calcitic, and yet others bimineralic. The related M. isabelleana (d’Orbigny, 1847) from Chile has an aragonitic skeleton (Smith & Clark Citation2010). These two species of Membranipora apart, malacostegines have retained the calcitic skeleton that is primitive for cheilostomes (Taylor et al. Citation2009). Malacostegines seldom have defensive polymorphs (Taylor Citation1987) and may be characterized as ‘weedy’ bryozoans that grow particularly rapidly: in a recent compilation of bryozoan growth rates (Smith Citation2014: supplemental data table 1), the two malacostegine species M. membranacea and Einhornia crustulenta (Pallas, 1766) showed the highest growth rates.
Assessment of the relative vulnerabilities of bryozoans to ocean acidification is complicated (see Fortunato Citation2015 and references therein) and the skeletal mineralogies of different species must certainly be taken into account. The first analysis of a tropical cheilostome biota presented here show that it is particularly rich in species that biomineralize aragonite, the more soluble of the two polymorphs of CaCO3 occurring in skeletonized marine organisms. All of the cheilostome species from Penang and Langkawi in Malaysia that have entirely calcitic skeletons are of anascan-grade and include many primitive malacostegines known or inferred to possess planktotrophic larvae. On the basis of biomineralogy alone, ocean acidification can be expected to impact tropical bryozoan biotas to a greater extent than those of higher latitudes, affecting ascophoran-grade species most and ‘weedy’ malacostegine anascans least.
Acknowledgements
We are grateful to Ivan Chinon Yaman, Woo Sau Pin and Poi Khoy Yen for assistance during fieldwork.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Borisenko YA, Gontar VI. 1991. Skeletal composition of cold-water bryozoans. Biologiya Morya 1:80–90. (in Russian )
- Caldeira K, Wickett ME. 2005. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. Journal of Geophysical Research 110:C09S04. 12 pages. doi:10.1029/2004JC002671
- Chimonides PJ, Cook PL. 1994. Notes on the genus Cranosina (Bryozoa, Cheilostomida). Zoologica Scripta 23:43–49. doi:10.1111/j.1463-6409.1994.tb00372.x
- Fabry VJ, Seibel BA, Feely RA, Orr JC. 2008. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES Journal of Marine Science 65:414–32. doi:10.1093/icesjms/fsn048
- Fortunato H. 2015. Bryozoans in climate and ocean acidification research: a reappraisal of an under-used tool. Regional Studies in Marine Science 2:32–44. doi:10.1016/j.rsma.2015.08.010
- Krzeminska M, Kuklinski P, Najorka J, Iglikowska A. 2016. Skeletal mineralogy patterns of Antarctic Bryozoa. Journal of Geology 124(3):411–22. doi:10.1086/685507
- Kuklinski P, Taylor PD. 2009. Mineralogy of Arctic bryozoan skeletons in a global context. Facies 55:489–500. doi:10.1007/s10347-009-0179-3
- Lombardi C, Cocito S, Hiscock K, Occhipinti-Ambrogi A, Setti M, Taylor PD. 2008. Influence of seawater temperature on growth bands, mineralogy and carbonate production in a bioconstructional bryozoan. Facies 54:333–42. doi:10.1007/s10347-008-0143-7
- Lowenstam HA. 1954. Factors affecting the aragonite:calcite ratios in carbonate secreting marine organisms. Journal of Geology 62:284–322. doi:10.1086/626163
- Loxton J, Kuklinski, P, Najorka J, Spencer Jones M, Porter JS. 2014. Variability in the skeletal mineralogy of temperate bryozoans: the relative influence of environmental and biological factors. Marine Ecology Progress Series 510:45–57. doi:10.3354/meps10889
- Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, et al. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437:681–86. doi:10.1038/nature04095
- Poluzzi A, Sartori R. 1975. Report on the carbonate mineralogy of Bryozoa. Documents des Laboratoires de Géologie de la Faculté des Sciences de Lyon, Hors Serie 3:193–210.
- Ries JB. 2011. Skeletal mineralogy in a high-CO2 world. Journal of Experimental Marine Biology and Ecology 403:54–64. doi:10.1016/j.jembe.2011.04.006
- Ries JB, Cohen AL, McCorkle DC. 2009. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37:1131–34. doi:10.1130/G30210A.1
- Rosso A, Chimenz C, Balduzzi A. 2010. Bryozoa. Biologia Marina Mediterranea 17(Supplement 1):589–615.
- Smith AM. 2014. Growth and calcification of marine bryozoans in a changing ocean. Biological Bulletin 226:203–10.
- Smith AM, Clark DE. 2010. Skeletal carbonate mineralogy of bryozoans from Chile: an independent check of phylogenetic patterns. Palaios 25:229–33. doi:10.2110/palo.2009.p09-106r
- Smith AM, Garden CJ. 2012. Being a bimineralic bryozoan in an acidifying ocean. In: Ernst A, Schäfer P, Scholz J, editors. Bryozoan Studies 2010. Berlin and Heidelberg: Springer, p 327–37.
- Smith AM, Nelson CS, Spencer GH. 1998. Skeletal carbonate mineralogy of New Zealand bryozoans. Marine Geology 151:27–46. doi:10.1016/S0025-3227(98)00055-3
- Taylor JD, Reid DG. 1990. Shell microstructure and mineralogy of the Littorinidae: Ecological and evolutionary significance. Hydrobiologia 193:199–215. doi:10.1007/BF00028077
- Taylor PD. 1987. Skeletal morphology of malacostegan grade cheilostome Bryozoa. In: Ross JRP, editor. Bryozoa: Present and Past. Bellingham: Western Washington University, p 269–76.
- Taylor PD, Di Martino E. 2014. Why is the tropical Cenozoic fossil record so poor for bryozoans? Studi Trentini di Scienze Naturali 94:249–57.
- Taylor PD, Tan S-HA. 2015. Cheilostome Bryozoa from Penang and Langkawi, Malaysia. European Journal of Taxonomy 149:1–34.
- Taylor PD, Kudryavtsev AB, Schopf JW. 2008. Calcite and aragonite distributions in the skeletons of bimineralic bryozoans as revealed by Raman spectroscopy. Invertebrate Biology 127:87–97. doi:10.1111/j.1744-7410.2007.00106.x
- Taylor PD, James NP, Bone Y, Kuklinski P, Kyser TK. 2009. Evolving mineralogy of cheilostome bryozoans. Palaios 24:440–52. doi:10.2110/palo.2008.p08-124r
- Taylor PD, Lombardi C, Cocito S. 2015. Biomineralization in bryozoans: present, past and future. Biological Reviews 90:1118–50. doi:10.1111/brv.12148
