ABSTRACT
Shallow, near-shore water habitats on the continental shelf of the Northeast Atlantic have been productive fishing areas in the past. Here, we review the present knowledge about (i) recent trends in the abundance of plaice and cod in these habitats and (ii) hypotheses regarding the factors responsible for any trends. At present, only a few studies exist on the trends of abundance of plaice or cod, namely from the Bay of Biscay, the North Sea and the Skagerrak/Kattegat. They suggest a declining abundance in coastal, shallow areas and – at least for plaice – a latitudinal gradient with an erosion of the southern distribution boundary in the Bay of Biscay and deepening of stocks in the North Sea. In contrast, no trend in shallow water abundance of plaice similar to a decline in deep-water stocks during the 1970s and their slow recovery during the 2000s is apparent in the Skagerrak/Kattegat. Although shallow habitats fundamentally differ from deeper areas by the prevalence of juvenile stages, the declining trends coincide with decreasing abundance/landings and spatial stock relocations in the deeper areas. Whether this indicates a common trend pointing at connectivity between shallow and deep water remains open. Fundamental differences exist in the suggested causes of the trends in different geographical areas. High fishing pressure together with low local recruitment apparently prevents the recovery of overexploited plaice and cod stocks in the Skagerrak/Kattegat. In contrast, the responses of juveniles and adult fish to increasing seawater temperature are the main hypotheses for changes in distribution and abundance of both fish species in the North Sea/Bay of Biscay. However, temperature alone cannot explain the observed decline of fish in coastal areas, and the causes may be more complex, involving nutrient loading, primary productivity or food availability, although at present, knowledge of these factors is insufficient.
RESPONSIBLE EDITOR:
Introduction
Coastal marine ecosystems are ecologically and socioeconomically among the most valuable systems of the world, responsible for an important share of global goods and services. There is increasing concern about the sustainability of these resources in view of pressures originating from climate change and increasing human activity and demand (Worm et al. Citation2006; Brander Citation2010; McClanahan et al. Citation2015). Overexploitation and habitat destruction for centuries has led to severe degradation of coastal systems and a pronounced decline in invertebrate and vertebrate abundance and diversity (Jackson et al. Citation2001; Lotze et al. Citation2006; Harley et al. Citation2006).
In Denmark, coastal fishermen operating from small vessels or directly from beaches with gillnets, fish weirs or trawls have indicated declining fish abundance in coastal areas (Støttrup et al. Citation2014). Of 74 coastal fishermen, 68% and 54% reported that the abundance of cod (Gadus morhua Linnaeus, 1758) and plaice (Pleuronectes platessa Linnaeus, 1758) as major targeted species, respectively, has declined since the beginning of the last decade in coastal areas. This has reduced their fishing opportunities and forced them to move to more offshore fishing areas. A preliminary examination of the data from fishing surveys from the North Sea and Kattegat showed similar temporal changes in the spatial distribution of these two species (Støttrup et al. Citation2014). We found, therefore, a need for a more systematic overview about the available scientific information on the status of these two species in coastal areas of the Northeast Atlantic.
Large-scale changes in fish distribution, abundance and community composition occur in offshore areas at various geographic scales (Drinkwater Citation2005; Rose Citation2005; ter Hofstede et al. Citation2010; Nicolas et al. Citation2011). These include latitudinal expansion of warm-water fish (Quéro Citation1998; Stebbing et al. Citation2002; Beare et al. Citation2004; Poulard & Blanchard Citation2005), contractions or shifts of mean latitude or northern/southern distribution boundaries (Brander et al. Citation2003; Perry et al. Citation2005), and significant deepening of whole fish assemblages (Dulvy et al. Citation2008; Simpson et al. Citation2011). Analyses focusing on physical variables and fisheries effort found that fishing alone is insufficient to explain the observed trends (Perry et al. Citation2005; Dulvy et al. Citation2008; ter Hofstede et al. Citation2010). In contrast, significant relationships among sea water temperature or climate indices such as the North Atlantic Oscillation Index (NAO) and trends in fish stocks suggest that warming of the oceans affects the distribution of fish by exceeding the thermal limits of boreal species or by expanding the habitats of species preferring warm temperatures (Roessig et al. Citation2004; Harley et al. Citation2006; Simpson et al. Citation2011).
Less-comprehensive knowledge on changes of the fish fauna exists for coastal habitats, which are not regularly monitored by international surveys and are often studied locally. Coastal habitats are defined as areas ranging from coastal wetlands to estuaries, bays and open water less than 30 m deep (Seitz et al. Citation2014). Many of the commercially important fish species occurring on continental shelves like cod and plaice use them temporally as feeding, spawning, or nursery grounds and are connected to coastal areas by larval transport, immigration and emigration (Seitz et al. Citation2014). Because of the connectivity of coastal and offshore populations, coastal stocks, in turn, most likely depend on abundance changes in deeper areas. Thus, causes of decline in coastal fish fisheries cannot be examined without reference to the general trends in fish stocks established in offshore areas.
In this review, we assembled findings from a range of relevant studies about changes in the abundance and distribution of plaice and cod in the shallow continental coastal water of the Northeast Atlantic. We specifically address the questions of (i) whether there is evidence of a decline of plaice and cod in coastal water, (ii) how trends, if any, compare to those in well-monitored offshore waters and (iii) whether causes of changes in abundance and distribution have been identified in the studies observing these trends. Scientific literature was compiled from searches made using Google Scholar by combining the species name with attributes of the habitat (‘coastal’, ‘shallow’, ‘depth’) or variation (‘time-series’, ‘trend’). The review focuses on continental coastal waters of the NE Atlantic because relevant studies are limited to this area.
Plaice: trends in abundance and distribution
Shallow/coastal waters
Plaice is a temperate flatfish species found on sandy and muddy substrates in shelf waters of the eastern North Atlantic ranging from the Bay of Biscay to the Barents Sea (Rijnsdorp et al. Citation2010; ICES WKHVES Citation2012). Relatively few investigations have reported on long-term trends of plaice in shallow, coastal waters, namely from the Bay of Biscay, the continental coast of the North Sea and the Skagerrak/Kattegat (, ). In the Bay of Biscay, trawl surveys in the Bay of Vilaine and the eastern part of the shelf in the years 1987–2001 showed a significant decrease in the abundance of juvenile plaice over time and their complete disappearance from shallow water toward the end of the observation period (Désaunay et al. Citation2006). In the North Sea, a 37-year time series from the Dutch demersal fish survey in coastal continental waters, conducted since 1970, revealed a decline in demersal fish species biomass together with a change in their community composition (Tulp et al. Citation2008; Bolle et al. Citation2009a; Tulp & Bolle Citation2009). Following peak abundance in the mid-1980s, a continuous decline of plaice and other species such as eel, eelpout, whiting and dab occurred until the mid-2000s in most areas of the Dutch and German Wadden Sea. This decline was primarily observed in the shallow Wadden Sea, but not in the deeper coastal zone or the shallow Westerschelde (Tulp et al. Citation2008). The Wadden Sea is an important nursery area for plaice, especially in the intertidal and shallow subtidal zones (Zijlstra Citation1972; Zijlstra et al. Citation1982). Fyke net surveys showed that since the 1980s the abundance of plaice in age groups 1 and 2 and since the 1990s plaice in age group 0 also declined in these zones, which suggests a strong reduction of plaice in their nursery areas (van der Veer et al. Citation2011).
Figure 1. Locations of the studies on trends in abundance of plaice and cod on the northeastern continental shelf. Numbers refer to studies listed in and .
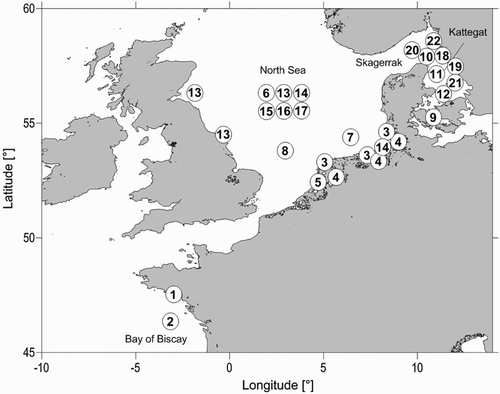
Table I. Trends and potential causes of changes observed in plaice abundance in various areas of the Bay of Biscay, North Sea and Skagerrak/Kattegat.
Table II. Trends and potential causes of changes observed in cod abundance in various areas of the North Sea and the Skagerrak/Kattegat.
Information on trends of plaice in shallower, coastal areas in the Skagerrak/Kattegat is scarce. Recently, surveys targeting young flatfish in inshore Danish waters in the Kattegat/Øresund (depth < 4.5 m) conducted from 1904 to 2006 were analysed for changes in the relative abundances of sole and plaice to understand potential shifts in flatfish community composition (Sparrevohn et al. Citation2013). Strong increases in the ratio of sole to plaice in these nursery grounds were recorded from the mid-1980s, which indicated a shift in community composition. However, this trend was largely determined by a sixfold increase in the catch of sole, while no difference in the catches of juvenile plaice was observed (Sparrevohn et al. Citation2013).
Deep/offshore waters
In recent decades, considerable changes in landings and abundance of plaice have also been recorded for the deeper offshore water of the Bay of Biscay, the North Sea and the Skagerrak/Kattegat (). In the Bay of Biscay, landings of plaice have moderately decreased since the 1990s (Rijnsdorp et al. Citation2010). This contrasts with a decrease in catches of plaice in 1995 observed in groundfish surveys during 1987–2006 (Hermant et al. Citation2010). Landings in the North Sea also decreased following a historical maximum in the mid-1980s, with some increase since 2008 (ICES Citation2015; Rijnsdorp et al. Citation2010). Spawning biomass in the North Sea has basically the same trend as the catches; however, the increase in spawning stock since 2008 has been significant, and the spawning stock estimate for 2013 has passed the historical maximum of the mid-1980s (; ICES Citation2015). Together with these long-term trends, changes in the geographical and water-depth distribution of the stock have occurred. These include a northward shift of the southern distribution boundary in the Bay of Biscay (Hermant et al. Citation2010) and a general increase in the mean depth of plaice in the North Sea with a deepening of ∼4 m per decade as deduced from the English groundfish surveys 1977–2006 (Perry et al. Citation2005; Dulvy et al. Citation2008). In addition, commercial fisheries data indicate a large-scale relocation of distribution centres of plaice with a westward shift and drop in plaice abundance along the east–central North Sea and in Danish waters in the 1990s to the 2000s (Engelhard et al. Citation2011; Poos et al. Citation2013).
Figure 2. Spawning stock biomass estimates from standard assessment (ICES Citation2015). (a) Estimates for plaice in the North Sea during 1963–2013. (b) Estimates for cod in the North Sea and Skagerrak/Kattegat for the years 1963–2014.
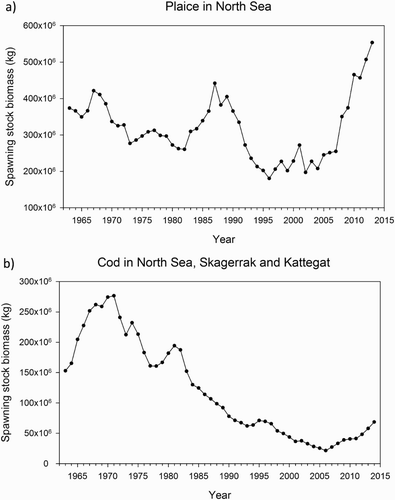
In the Kattegat/Skagerrak, landings of plaice continuously decreased from the mid-1970s until 2007 (Cardinale et al. Citation2010). The spawning stock biomass of plaice modelled from otter trawl surveys shows a decline from a peak in the mid-1960s until the mid-1970s (Cardinale et al. Citation2010). In contrast to the landings, however, a gradual increase in the modelled biomass was already discernible from the mid-1970s and particularly following strong recruitment events in 1998–1999. This led to a spawning stock biomass at the end of 2000s representing 38% of the maximum biomass observed during the twentieth century (Cardinale et al. Citation2010). The general decline of the plaice abundance observed in the surveys was accompanied by a strong contraction of four different aggregations of plaice, namely a western component on the Danish Skagerrak coast and in the southwestern Kattegat and an eastern component along the Swedish Skagerrak coast and in the Swedish eastern Kattegat (Cardinale et al. Citation2011). The recent increase in plaice biomass, however, shows spatiotemporal heterogeneity, and is mainly related to a recovery of the western component, whereas the eastern component remains at a historically low level. The importance of the western component in the Skagerrak and Kattegat as a productive fishery area for plaice was also stressed by the analysis of recent landings by Danish and Swedish fisheries from larger vessels (> 12 m) (Ulrich et al. Citation2013). The observed trends, however, differ from the plaice biomass modelled by Cardinale et al. (Citation2011). Although catches were reported to increase similarly in the Skagerrak, catches in the Kattegat have continuously decreased (Ulrich et al. Citation2013).
Synopsis
The literature survey shows that apart from studies in the Dutch coastal zone very limited data exist allowing insights into common trends of plaice in shallow and deep water over the species distribution range. In addition, a thorough comparison of common trends in habitats is hampered by the fact that some information about trends in deep water originates from landing of fisheries rather than surveys as in shallow water. This might lessen the identification of decreasing trends in the deeper areas. Apart from these restrictions, the available information suggests some latitudinal differences in trends. The survey data from the Bay of Biscay demonstrate the erosion of the southern distribution limit by the disappearance of plaice from both shallow and deep waters. The decline of plaice in shallow areas of the North Sea following the maximum abundance in the 1980s, in contrast, coincides with a general deepening and geographical relocation of plaice in the deeper North Sea indicated in groundfish surveys. This deepening, however, is not only a recent observation, but has occurred previously as a result of a gradual and age-dependent shift of juvenile plaice of age groups 0–2 to deeper waters along the Dutch, German and Danish continental coasts in the periods 1902–1909, 1983–1987 and 1999–2003 (van Keeken et al. Citation2007). In recent years, the deepening trend and the decline of plaice in the Wadden Sea apparently have persisted despite the stabilization of stocks in deep water (see ICES Citation2013, Citation2015). A recovery of plaice stocks after a decline during the 1970s occurred, at least in the western areas, in the Skagerrak/Kattegat region as well. Despite the limited information for this area, a decline in shallow, coastal areas is not yet apparent. The spatial heterogeneity in trends along the North Sea coast and the Skagerrak/Kattegat could indicate that the interaction of environmental factors and differential effects of the local habitat or interspecific interactions play an important role, as implied by Simpson et al. (Citation2011) for the entire North Sea.
Potential causes for changes in abundance and distribution of plaice
Temperature effects
Temperature is one of the primary factors determining the distribution of fish. In several of the reports from the North Sea, offshore displacement and decreasing abundance were correlated with increasing surface or bottom seawater temperatures and/or climate indices such as the North Atlantic Oscillation (NAO) in shallow (Désaunay et al. Citation2006; van Keeken et al. Citation2007; Tulp et al. Citation2008) as well as deep habitats (Hermant et al. Citation2010; Engelhard et al. Citation2011). The mechanisms underlying this temperature effect remain uncertain, but might reflect temperature exceeding metabolic thermal optima and fish behaviour (). At the boundary of their distribution, the sensitivity of plaice to increasing temperature might have caused their disappearance in the shallow Bay of Vilaine (Désaunay et al. Citation2006). Increases in the summer temperature beyond tolerance levels might also have forced the offshore movement of age groups 0–2 plaice in the shallow Wadden Sea (van Keeken et al. Citation2007). However, the continuous presence of juvenile flounder, which has a temperature tolerance similar to that of juvenile plaice (Fonds et al. Citation1992), probably excludes thermal sensitivity as the single cause of the disappearance of plaice in these shallower areas (van der Veer et al. Citation2011). Regarding long-term shifts, higher temperatures have been associated with a more northwestern and deeper distribution pattern of plaice in the North Sea, which suggests that fluctuations in the thermohaline circulation and temperature have affected adult plaice distribution in general (Engelhard et al. Citation2011).
Temperature sensitivity of population-dynamic processes may contribute to changes in abundance and distribution. Failure in recruitment because of higher temperatures in bottom waters of the shelf could have caused the decline of plaice in the shallow water of the Bay of the Biscay (Hermant et al. Citation2010). In the North Sea, year-class strength in plaice appears to be negatively correlated with sea temperature during the larval period, with strong classes arising during cold winters (Bolle et al. Citation2009b; van der Veer et al. Citation2009). This could be related to increasing mortality of eggs and larvae associated with increased food limitations imposed by increasing temperatures or temperature-mediated effects on predation pressure (van der Veer et al. Citation2009). In the shallow Wadden Sea, juvenile plaice growth was found to be maximal only shortly after settlement because otherwise food availability does not meet their increasing energy demands (van der Veer et al. Citation2010). Consequently, long-term increases in temperature will increase energy demands, cause an earlier offshore movement, and reduce the nursery function of the Wadden Sea (van Keeken et al. Citation2007; van der Veer et al. Citation2011).
Habitat changes
Several observations, however, question a prevailing role of temperature as the single determinant of the observed changes in plaice abundance and distribution, and point to an interplay with other factors such as food availability or competition. In the Dutch coastal area, general shifts in species composition and a decrease in demersal fish abundance were related to temperature in the coastal zone only and not in the Wadden Sea, in which the strongest decline in plaice was observed (Tulp et al. Citation2008; Bolle et al. Citation2009a). Moreover, in the shallow Westerschelde area, plaice stocks increased despite an increase in temperature. The decline of plaice in the Wadden Sea and the changes in the demersal fish composition were correlated with indicators of eutrophication and predators (Tulp et al. Citation2008). In the 1960s and 1970s, eutrophication likely resulted in an increase in primary and secondary production, and it may explain the observed increase in fish biomass. The growth rate of plaice was also positively correlated with eutrophication (Rijnsdorp & van Leeuwen, Citation1996; Teal et al. Citation2008). Consequently, the decline in abundance and growth in plaice stocks may be related to the more recent decline in productivity of the benthic system and associated changes in benthic biodiversity (Tulp et al. Citation2008). Changes in shrimp Crangon crangon (Linnaeus, 1758) abundance may also add to the decline (Tulp et al. Citation2008) because they are important predators of small juvenile plaice (Wennhage Citation2002; Gibson et al. Citation2002).
Influence of fishing and recruitment
Fishing mortality was investigated as an additional factor potentially complicating the assessment of the environmental impact on fish populations, because spatial differences in fishing effort can explain spatial changes in abundance (e.g. Perry et al. Citation2005; Dulvy et al. Citation2008). However, in shallow areas of the North Sea no effect of beam trawl fishery on the abundance of plaice in the Wadden Sea was found (Tulp et al. Citation2008). The shift of juvenile plaice to deeper water observed in coastal areas further contrasts with increasing offshore fishing intensity (van Keeken et al. Citation2007). Also, in the deeper areas of the North Sea fishing mortality was not correlated with changes in latitudinal, longitudinal, or depth distribution, and therefore cannot predict plaice distribution (Engelhard et al. Citation2011). Similarly, although a reduction in stocks of commercially targeted species was recorded in the Bay of Biscay, the analysis of trends in abundance of fish communities revealed no impact of exploitation (Hermant et al. Citation2010). Nevertheless, overfishing could amplify the effects of climate change (Perry et al. Citation2005; Hermant et al. Citation2010).
In the Kattegat/Skagerrak, analyses on the effects of fisheries focus exclusively on the data from the deeper groundfish surveys. Here, historical declines in plaice stocks have been attributed to high fishing pressure in the region (Cardinale et al. Citation2010, Citation2011). The recent increase in plaice biomass on the northwestern Danish Skagerrak coast, also recorded by Ulrich et al. (Citation2013), occurs, however, in spite of an increasing trend in fishing effort, whereas the eastern stocks apparently have been eradicated by overexploitation. This increase may be due to large year-classes and strong recruitment in the last decade, which might reflect increased water temperature, reduced predation mortality because of declining cod stocks, or an increased productivity of benthic prey stimulated by trawling (Cardinale et al. Citation2010). This contrasts conclusions about a negative influence of increasing temperatures and trawling effects on food abundance in the shallow Wadden Sea area (Tulp & Bolle Citation2009).
Cod: trends in abundance and distribution
Shallow/coastal waters
Reports about trends in abundance of cod in shallow and coastal areas along the continental coast of the Northeast Atlantic are scarce. Few studies exist from the North Sea and Skagerrak/Kattegat (, ). Estimates of the stock spawning biomass from the Scottish groundfish survey or the International Bottom Trawl survey in the North Sea indicate a decline of cod in coastal areas along the British east coast from the mid-1980s (Holmes et al. Citation2008) or the Danish, German and Dutch coasts from the early 1990s (Hjermann et al. Citation2013). Dutch surveys covering the continental coast and Wadden Sea area have confirmed the decrease in stocks in shallow areas (Tulp et al. Citation2008; Bolle et al. Citation2009a). Here, cod declined following increased abundance during the 1970s–1980s in most areas ranging from the western Dutch Wadden Sea to the North Frisian Wadden Sea (Tulp et al. Citation2008). The decline of cod, however, was spatially heterogeneous and was particularly strong in the eastern Wadden Sea and northern Frisian waters compared with the western Dutch Wadden Sea (Bolle et al. Citation2009a).
In the Skagerrak and Kattegat, knowledge about coastal cod stocks is restricted to the eastern, Swedish coast. A severe reduction in abundance was previously recorded in landings by fishermen, sport fishermen and the mixed Nephrops fishery in coastal waters during the 1980s (Svedäng Citation2003; Svedäng & Bardon, Citation2003).
Deep/offshore waters
Cod is one of the most important commercial fish species, but in recent decades stock size estimated from groundfish surveys and landings have declined almost throughout the species range, following the gadoid outburst from the late 1960s to the mid-1980s (Brander Citation2007; Holmes et al. Citation2008; Lescrauwaet et al. Citation2010; Rijnsdorp et al. Citation2010). This tendency is more prominent for North Sea cod, in which the spawning stock biomass has declined from a maximum of 277 × 106 kg in 1971 to a minimum of 22 × 106 kg in 2006. Since this low, there has been some increase in the biomass (; ICES Citation2015). Similar to that seen in plaice, the decline of the cod stock was accompanied by distributional changes identified from international bottom trawl surveys in deeper waters of the North Sea (Hedger et al. Citation2004). The analysis of trends in the spatial distribution in the years 1980–1999 revealed that the bulk of mature cod was distributed in the shallower waters of the German Bight during 1980–1989, but shifted toward the deeper northern areas in the following decade (Hedger et al. Citation2004). This generally agrees with a northward shift in the mean latitude of cod distribution and increase of mean depth in the North Sea (Perry et al. Citation2005; Dulvy et al. Citation2008).
The analysis of otter trawl surveys revealed that cod stocks in the Skagerrak and Kattegat have also declined rapidly since the early 1980s (Cardinale & Svedäng Citation2004; Bartolino et al. Citation2012). During the last decade, however, spawning stock biomass showed a moderate increase in the western Skagerrak (Bartolino et al. Citation2012). In the eastern Kattegat, in contrast, biomass continuously declined and reached historically low levels in several subpopulations (Svedäng et al. Citation2010; Bartolino et al. Citation2012). Older age groups in particular declined and disappeared in the northern and western Kattegat and along the Swedish west coast, in contrast to increased age diversity found in the Öresund, where trawling is prohibited (Svedäng & Bardon, Citation2003; Svedäng et al. Citation2010).
Synopsis
The few known trends in the abundance of cod in shallow, coastal areas of the North Sea and the Skagerrak/Kattegat identified in various surveys largely follow those of landings and surveys of cod in deeper water. However, the observed decline in coastal waters appears to be much more pronounced than offshore. This is apparent from a greater decline of cod in inshore areas compared with offshore areas along the British east coast (Holmes et al. Citation2008) or the Danish and German coasts (Hjermann et al. Citation2013) based on survey data. In the Skagerrak/Kattegat, large fish virtually disappeared in coastal waters compared to offshore areas (Svedäng Citation2003; Svedäng & Bardon Citation2003). However, this decline preceded the decline in offshore areas by 5–10 years. Because these data are based on landings of sport fishermen, this needs to be interpreted with caution.
Potential causes for changes in abundance and distribution of cod
Temperature effects
Few studies have so far explicitly analysed the causes of the decline of cod in near-shore or coastal areas, so reasons for the decline remain largely unclear. Therefore, very little is known about the effects of temperature on cod abundance or distribution in coastal, shallow areas and most information originates from analyses of stocks in deeper areas, even in those studies including coastal areas (Holmes et al. Citation2008, Hjermann et al. Citation2013). A decreasing abundance and spatial shifts in the North Sea cod stock have been linked primarily to increasing seawater temperature and, therefore, climate warming (Hedger et al. Citation2004; Perry et al. Citation2005; Dulvy et al. Citation2008). The exact mechanisms, however, are not clear. The southern North Sea might have become too warm to support a resident population. However, the effect of temperature is not evident in the distributional patterns of adult cod. Righton et al. (Citation2010) made direct observations of habitat occupation of adult cod, showing that they will be able to tolerate warming seas within a wide range, but they suggest that climate change will affect cod populations at earlier life-history stages, as well as cod prey species. Direct effects of temperature on development, growth and survival could have reduced the recruitment (Hedger et al. Citation2004; Rijnsdorp et al. Citation2010). This effect was shown by Planque & Frédou (Citation1999) when they combined analyses of recruitment variability of several cod stocks in a single meta-analysis. They demonstrated that recruitment of Atlantic cod is linked to interannual fluctuations in temperature in such a way that for stocks located in warm water, as also seen in the North Sea and Skagerrak/Kattegat, the relationship is negative, but for stocks located in cold water the relationship is positive.
Habitat changes
In the study of the long-term dynamics of demersal fish in the coastal zone and the Wadden Sea, the decline of cod in coastal waters was negatively correlated with the abundance of predators (seals) and beam trawl effort and positively correlated with run-off and nutrient load, but these correlations were not significant (Tulp et al. Citation2008; Bolle et al. Citation2009a). The causes of the particularly strong decline of coastal cod detected in the Scottish and International Bottom Trawl surveys also remain unknown (Holmes et al. Citation2008; Hjermann et al. Citation2013). The simultaneous decline of cod in the Wadden Sea and deeper areas during the 1980s, however, could indicate that the dynamics of cod stocks in shallow water are strongly influenced by processes acting on North Sea stocks in general (Tulp et al. Citation2008; Bolle et al. Citation2009a).
Influence of fishing and recruitment
Fisheries exploitation together with effects of climate change are the major but controversial causes of the decline in North Sea cod populations (Horwood et al. Citation2006; Brander, Citation2007; Rijnsdorp et al. Citation2010; Engelhard et al. Citation2014). Again, knowledge is largely restricted to deeper, offshore areas. North Sea cod stocks have been subjected to extensive fishing pressures. A latitudinal gradient in fishery effort with greater trawling in the southern and the central North Sea than in the Northern North Sea could cause overall distribution shifts and deepening of the mean depth of cod stocks, if the species has several subpopulations of which only some are highly exploited (Dulvy et al. Citation2008; Rijnsdorp et al. Citation2010; Engelhard et al. Citation2014).
In the Skagerrak and Kattegat, fishing pressure was identified as the most important variable explaining adult fish abundance in shallow and deep areas, but there was no correlation with environmental factors and climate indices (Cardinale & Svedäng Citation2004; Svedäng et al. Citation2010; Bartolino et al. Citation2012). Very high fishing pressure in the Skagerrak and Kattegat, as well as along the eastern Swedish coast, has likely caused the eradication of local spawning aggregations and has largely increased the dependence on transport of recruits from the North Sea (Svedäng et al. Citation2010; Bartolino et al. Citation2012). Therefore, recruitment depends strongly on the reproduction of the North Sea stock and favourable wind and temperature conditions (Cardinale & Svedäng Citation2004). Such intermittent and variable transport was demonstrated by cod larvae surveys and genetic studies (Knutsen et al. Citation2004; Svedäng & Svenson Citation2006). The recent increase in adult cod abundances in the western area of the Skagerrak, therefore, may result from a major contribution from the neighbouring North Sea cod population to the Skagerrak rather than recovery of the local aggregations (Bartolino et al. Citation2012). The decline recorded in landings from inshore areas at the Swedish east coast of the Skagerrak/Kattegat might be similarly caused by a lacking recruitment of overfished stocks from offshore areas (Svedäng Citation2003; Svedäng & Bardon Citation2003).
Conclusions
In comparison to an increasing knowledge about long-term changes in the fish fauna in offshore waters of the northeastern Atlantic continental shelf, near-shore studies systematically investigating trends in abundance, distribution, or community composition in relation to water depth or distance from shore are limited to a few case studies in areas such as the Dutch Coast/Wadden Sea (Tulp et al. Citation2008; Bolle et al. Citation2009a) or the western Swedish coast of the Skagerrak/Kattegat (Svedäng et al. Citation2010; Bartolino et al. Citation2012). In addition, few studies at present address potential drivers of changes in coastal ecosystems (e.g. Collie et al. Citation2008; Tulp et al. Citation2008; van der Veer et al. Citation2011). This essentially reflects limited data availability as a result of restricted spatial overlap of shallower coastal areas with regular, large-scale bottom-trawl monitoring surveys or a lack of records of fish caught by the smaller fishing vessels exploiting coastal areas during short fishing trips (1–3 days).
The few observations available for shallow, near-shore areas generally appear to integrate broadly into the large-scale changes recently described for North Atlantic fish fauna. However, common trends need to be interpreted with caution because shallow areas often harbour the early year-classes of fish, which use the areas as nursery grounds and, therefore, trends and the effects of environmental factors or fisheries influencing the abundance in both habitats might principally differ. In the North Sea, the observed decline in some coastal areas matches the long-term trends in the erosion of southern distribution boundaries, in north- or westward shifts in distribution centres, or in the contraction of demersal fish stocks extracted from bottom-trawl surveys. Notably, the considerable increase in the mean depth of demersal fish in the North Sea and in the Wadden Sea would provide a fundamental explanation for decreasing catches in near-shore fishing grounds, particularly in the case of plaice. The shallow-water emigration and spatial stock redistribution might represent a general latitudinal trend along the continental shelf that restricts the presently dense coastal populations of cod and plaice largely to the north and into western Skagerrak. This process needs to be investigated further, especially with regard to the potential role of a spatial relocation of spawning stocks for the recruitment of juvenile stages in shallow nursery areas, which is the least-understood process.
The various factors causing the changing trends in abundance and distribution in near-shore and also offshore shelf waters are not fully understood at present. Although common factors such as temperature or fisheries are often invoked as major factors in both coastal and offshore areas, changes will affect the different age groups in the habitats differently. Fundamental differences apparently exist in the significant variables identified for changes in plaice and cod abundance in the North Sea and the Skagerrak/Kattegat. In the latter, a continuously high fishing pressure, together with a strong dependence of local recruitment on transport from the North Sea, seemingly prevents the recovery of overexploited plaice and cod stocks (e.g. Cardinale et al. Citation2011; Bartolino et al. Citation2012). At present, however, it is unclear whether this affects inshore and offshore abundance of juveniles similarly. In contrast, changes in distribution and abundance in the North Sea are largely attributed to changing environmental factors (Tulp et al. Citation2008; Dulvy et al. Citation2008; Simpson et al. Citation2011). In particular, direct and indirect responses of juvenile and adult fish to increasing seawater temperature dominate the present discussion on the causative effects. However, temperature alone cannot explain the observed decline of fish in coastal areas, and the causes may be more complex, involving long-term trends not only in climate but also in nutrient loading, food availability, and changed larval transport or immigration/emigration patterns. In addition, the impact of anthropogenic activity, such as eutrophication, pollution, or habitat destruction, has to be considered when evaluating changes in local fish stocks.
Acknowledgements
We are grateful to the anonymous reviewers for valuable comments on earlier versions of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bartolino V, Cardinale M, Svedang H, Linderholm HW, Casini M, Grimwall A. 2012. Historical spatiotemporal dynamics of eastern North Sea cod. Canadian Journal of Fisheries and Aquatic Sciences 69:833–41. doi:10.1139/f2012-028
- Beare DJ, Burns F, Greig A, Jones EG, Peach K, Kienzle M, et al. 2004. Long-term increases in prevalence of North Sea fishes having southern biogeographic affinities. Marine Ecology Progress Series 284:269–78. doi:10.3354/meps284269
- Bolle LJ, Neudecker T, Vorberg R, Damm U, Diedrichs B, Jager Z, et al. 2009a. Trends in Wadden Sea Fish Fauna. Part I: Trilateral Cooperation. IMARES Report no. C108/08. 69 pages.
- Bolle LJ, Dickey-Collas M, van Beek JKL, Erftemeijer PLA, Witte JIJ, van der Veer HW, Rijnsdorp AD. 2009b. Variability in transport of fish eggs and larvae. III. Effects of hydrodynamics and larval behaviour on recruitment in plaice. Marine Ecology Progress Series 390:195–211. doi:10.3354/meps08177
- Brander KM. 2007. The role of growth changes in the decline and recovery of North Atlantic cod stocks since 1970. ICES Journal of Marine Science 64:211–17. doi:10.1093/icesjms/fsl021
- Brander KM. 2010. Impacts of climate change on fisheries. Journal of Marine Systems 79:389–402. doi:10.1016/j.jmarsys.2008.12.015
- Brander KM, Blom G, Borges MF, Erzini K, Henderson G, MacKenzie BR, et al. 2003. Changes in fish distribution in the eastern North Atlantic: are we seeing a coherent response to changing temperature? ICES Marine Science Symposia 219:261–70.
- Cardinale M, Svedäng HS. 2004. Modelling recruitment and abundance of Atlantic cod, Gadus morhua, in the eastern Skagerrak–Kattegat (North Sea): evidence of severe depletion due to a prolonged period of high fishing pressure. Fisheries Research 69:263–82. doi:10.1016/j.fishres.2004.04.001
- Cardinale M, Hagberg J, Svedang H, Bartolino V, Gedamke T, Hjelm J, et al. 2010. Fishing through time: population dynamics of plaice (Pleuronectes platessa) in the Kattegat-Skagerrak over a century. Population Ecology 52:251–62. doi:10.1007/s10144-009-0177-x
- Cardinale M, Bartolino V, Llope M, Maiorano L, Sköld M, Hagberg J. 2011. Historical spatial baselines in conservation and management of marine resources. Fish and Fisheries 12:289–98. doi:10.1111/j.1467-2979.2010.00393.x
- Collie JS, Wood AD, Jeffries HP. 2008. Long-term shifts in the species composition of a coastal fish community. Canadian Journal of Fisheries and Aquatic Sciences 65:1352–65. doi:10.1139/F08-048
- Désaunay Y, Guérault D, Le Pape O, Poulard JC. 2006. Changes in occurrence and abundance of northern/southern flatfishes over a 20-year period in a coastal nursery area (Bay of Vilaine) and on the eastern continental shelf of the Bay of Biscay. Scientia Marina 70S1:193–200. doi:10.3989/scimar.2006.70s1193
- Drinkwater KF. 2005. The response of Atlantic cod (Gadus morhua) to future climate change. ICES Journal of Marine Science 62:1327–37. doi:10.1016/j.icesjms.2005.05.015
- Dulvy NK, Rogers SI, Jennings S, Stelzenmüller V, Dye SR, Skjoldal HR. 2008. Climate change and deepening of the North Sea fish assemblage: a biotic indicator of warming seas. Journal of Applied Ecology 45:1029–39. doi:10.1111/j.1365-2664.2008.01488.x
- Engelhard GH, Pinnegar JK, Kell LY, Rijnsdorp AD. 2011. Nine decades of North Sea sole and plaice distribution. ICES Journal of Marine Science 68:1090–104. doi:10.1093/icesjms/fsr031
- Engelhard GH, Righton DA, Pinnegar JK. 2014. Climate change and fishing: a century of shifting distribution in North Sea cod. Global Change Biology 20:2473–83. doi:10.1111/gcb.12513
- Fonds M, Cronie R, Vethaak AD, van der Puyl P. 1992. Metabolism, food consumption and growth of plaice (Pleuronectes platessa) and flounder (Platichthys flesus) in relation to fish size and temperature. Netherlands Journal of Sea Research 29:127–43. doi:10.1016/0077-7579(92)90014-6
- Gibson RN, Robb L, Wennhage H, Burrows MT. 2002. Ontogenetic changes in depth distribution of juvenile flatfishes in relation to predation risk and temperature on a shallow-water nursery ground. Marine Ecology Progress Series 229:233–44. doi:10.3354/meps229233
- Harley CDG, Hughes AF, Hultgren KM, Miner BG, Sorte CJB, Thornber CS, et al. 2006. The impacts of climate change in coastal marine systems. Ecology Letters 9:228–41. doi:10.1111/j.1461-0248.2005.00871.x
- Hedger RE, McKenzie E, Heath M, Wright P, Scott B, Gallego A, Andrews J. 2004. Analysis of the spatial distribution of mature cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) abundance in the North Sea (1980-1999) using generalized additive models. Fisheries Research 70:17–25. doi:10.1016/j.fishres.2004.07.002
- Hermant M, Lobry J, Bonhommeau S, Poulard JC, Le Pape O. 2010. Impact of warming on abundance and occurrence of flatfish populations in the Bay of Biscay (France). Journal of Sea Research 64:45–53. doi:10.1016/j.seares.2009.07.001
- Hjerman DØ, Fisher JAD, Rouyer T, Frank KT, Stenseth NC. 2013. Spatial analysis of North Sea cod recruitment: concurrent effects of changes in spawning stock biomass, temperature and herring abundance. Marine Ecology Progress Series 480:263–75. doi:10.3354/meps10315
- Holmes SJ, Wright PJ, Fryer RJ. 2008. Evidence from survey data for regional variability in cod dynamics in the North Sea and West of Scotland. ICES Journal of Marine Science 65:206–15. doi:10.1093/icesjms/fsm192
- Horwood J, O’Brien C, Darby C. 2006. North Sea cod recovery? ICES Journal of Marine Science 63:961–68. doi:10.1016/j.icesjms.2006.05.001
- ICES. 2013. Mixed-fisheries Advice North Sea. Report of the ICES Advisory Committee, 2013. ICES Advice 2013, Book 6, Section 6.4.18. 256 pages.
- ICES. 2015. Report of the Working Group on the Assessment of the Demersal Stocks in the North Sea and Skagerrak. ICES Document CM 2014/ACOM:13. 1493 pages.
- ICES WKVHES. 2012. Report of the Workshop on the Value of Coastal Habitats for Exploited Species (WKVHES). ICES Document CM/SSGSUE:05. 66 pages.
- Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, et al. 2001. Historical overfishing and the recent collapse of coastal ecosystems. Science 293:629–38. doi:10.1126/science.1059199
- Knutsen H, André C, Jorde PE, Skogen MD, Thuróczy E, Stenseth NC. 2004. Transport of North Sea cod larvae into the Skagerrak coastal populations. Proceedings of the Royal Society B 271:1337–44. doi:10.1098/rspb.2004.2721
- Lescrauwaet AK, Debergh H, Vincx H, Mees J. 2010. Fishing in the past: historical data on sea fisheries landings in Belgium. Marine Policy 34:1279–89. doi:10.1016/j.marpol.2010.05.006
- Lotze HK, Lenihan HS, Bourque J, Bradbury RH, Cooke RG, Kay MC, et al. 2006. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312:1806–09. doi:10.1126/science.1128035
- McClanahan TE, Allison EH, Cinner JE. 2015. Managing fisheries for human and food security. Fish and Fisheries 16:78–103. doi:10.1111/faf.12045
- Nicolas D, Chaalali A, Drouineau H, Lobry J, Uriarte A, Borja A, et al. 2011. Impact of global warming on European tidal estuaries: some evidence of northward migration of estuarine fish species. Regional Environmental Change 11:639–49. doi:10.1007/s10113-010-0196-3
- Perry AL, Low PJ, Ellis JR, Reynolds JD. 2005. Climate change and distribution shifts in marine fishes. Science 308:1912–15. doi:10.1126/science.1111322
- Planque B, Frédou T. 1999. Temperature and the recruitment of Atlantic cod (Gadus morhua). Canadian Journal of Fisheries and Aquatic Sciences 56:2069–77. doi:10.1139/f99-114
- Poos JJ, Aarts G, Vandemaele S, Willems W, Bolle LJ, van Helmond ATM. 2013. Estimating spatial and temporal variability of juvenile North Sea plaice from opportunistic data. Journal of Sea Research 75:118–128. doi:10.1016/j.seares.2012.05.014
- Poulard JC, Blanchard F. 2005. The impact of climate change on the fish community structure of the eastern continental shelf of the Bay of Biscay. Journal of Marine Science 62:1436–43.
- Quéro JC. 1998. Changes in the Euro-Atlantic fish species composition resulting from fishing and ocean warming. Italian Journal of Zoology 65:493–99. doi:10.1080/11250009809386873
- Righton DA, Andersen KH, Neat F, Thorsteinsson V, Steingrund P, Svedäng H, et al. 2010. Thermal niche of Atlantic cod Gadus morhua: limits, tolerance and optima. Marine Ecology Progress Series 420:1–13. doi:10.3354/meps08889
- Rijnsdorp AD, van Leeuwen PI. 1996. Changes in growth of North Sea plaice since 1950 in relation to density, eutrophication, beam-trawl effort, and temperature. ICES Journal of Marine Science 53:1199–213. doi:10.1006/jmsc.1996.0145
- Rijnsdorp AD, Peck MA, Engelhard GH, Möllmann C, Pinnegar JK. 2010. Resolving Climate Impacts on Fish Stocks. ICES Cooperative Research Report 301. 372 pages.
- Roessig JM, Woodley CM, Cech JJ, Hansen LJ. 2004. Effects of global climate change on marine and estuarine fishes and fisheries. Reviews in Fish Biology and Fisheries 14:251–75. doi:10.1007/s11160-004-6749-0
- Rose GA. 2005. On distributional responses of North Atlantic fish to climate change. ICES Journal of Marine Science 62:1360–74. doi:10.1016/j.icesjms.2005.05.007
- Seitz RD, Wennhage H, Bergström U, Lipcius RN, Ysebaert T. 2014. Ecological value of coastal habitats for commercially and ecologically important species. ICES Journal of Marine Science 71:648–65. doi:10.1093/icesjms/fst152
- Simpson SD, Jennings S, Johnson MP, Blanchard JL, Schön PJ, Sims DW, et al. 2011. Continental shelf-wide response of a fish assemblage to rapid warming of the sea. Current Biology 21:1565–70. doi:10.1016/j.cub.2011.08.016
- Sparrevohn CR, Lindegreen M, MacKenzie BR. 2013. Climate-induced response of commercially important flatfish species during the 20th century. Fisheries Oceanography 22:400–08. doi:10.1111/fog.12030
- Stebbing ARD, Turk SMT, Wheeler A, Clarke KR. 2002. Immigration of southern fish species to south-west England linked to warming of the North Atlantic (1960–2001). Journal of the Marine Biological Association of the United Kingdom 82:177–80. doi:10.1017/S0025315402005325
- Støttrup JH, Lund S, Munk P, Dutz J, Kindt-Larsen L, Egekvist J, et al. 2014. KYSTFISK I. Udviklingen i kystnære fiskebestande. DTU-Report 278, National Institute of Aquatic Resources. 45 pages. (in Danish)
- Svedäng H. 2003. The inshore demersal fish community on the Swedish Skagerrak coast: regulation by recruitment from offshore sources. ICES Journal of Marine Science 60:23–31. doi:10.1006/jmsc.2002.1329
- Svedäng H, Bardon G. 2003. Spatial and temporal aspects of the decline in cod (Gadus morhua L.) abundance in the Kattegat and eastern Skagerrak. ICES Journal of Marine Science 60:32–37. doi:10.1006/jmsc.2002.1330
- Svedäng H, Svenson S. 2006. Cod Gadus morhua L. populations as behavioural units: inference from time series on juvenile abundance in the eastern Skagerrak. Journal of Fish Biology 69(Suppl C):151–64. doi:10.1111/j.1095-8649.2006.01272.x
- Svedäng H, Stål J, Sterner T, Cardinale M. 2010. Consequences of subpopulation structure on fisheries management: cod (Gadus morhua) in the Kattegat and the Öresund (North Sea). Reviews in Fisheries Science 18:139–50. doi:10.1080/10641260903511420
- Teal LR, van Keeken OA. 2011. The Importance of the Surf Zone for Fish and Brown Shrimp in the Netherlands. IMARES Report C045/11. 80 pages.
- Teal LR, de Leeuw JJ, van der Veer HW, Rijnsdorp AD. 2008. Effects of climate change on growth of 0-group sole and plaice. Marine Ecology Progress Series 358:219–30. doi:10.3354/meps07367
- ter Hofstede R, Hiddink JG, Rijndorp AD. 2010. Regional warming changes fish species richness in the eastern North Atlantic Ocean. Marine Ecology Progress Series 414:1–9. doi:10.3354/meps08753
- Tulp I, Bolle LJ, Rijnsdorp AD. 2008. Signals from the shallows: In search of common patterns in long-term trends in Dutch estuarine and coastal fish. Journal of Sea Research 60:54–73. doi:10.1016/j.seares.2008.04.004
- Tulp I, Bolle LJ. 2009. Trends in Wadden Sea Fish Fauna, Part II: Dutch Demersal Fish Survey (DFS). IMARES Report C109/08. 33 pages.
- Ulrich C, Boje J, Cardinale M, Gatti P, LeBras Q, Andersen M, et al. 2013. Variability and connectivity of plaice populations from the Eastern North Sea to the Western Baltic Sea, and implications for assessment and management. Journal of Sea Research 84:40–48. doi:10.1016/j.seares.2013.04.007
- van der Veer HW, Bolle LJ, Geffen AJ, Witte JIJ. 2009. Variability in transport of fish eggs and larvae. IV. Interannual variability in larval stage duration of immigrating plaice in the Dutch Wadden Sea. Marine Ecology Progress Series 390:213–23. doi:10.3354/meps08176
- van der Veer HW, Freitas V, Koot J, Witte JIJ, Zuur AF. 2010. Food limitation in epibenthic species in temperate intertidal systems in summer: analysis of 0-group plaice Pleuronectes platessa. Marine Ecology Progress Series 416:215–27. doi:10.3354/meps08786
- van der Veer HW, Koot J, Aarts G, Dekker R, Diderich W, Freitas V, Witte JIJ. 2011. Long-term trends in juvenile flatfish indicate a dramatic reduction in nursery function of the Balgzand intertidal, Dutch Wadden Sea. Marine Ecology Progress Series 434:143–54. doi:10.3354/meps09209
- van Keeken OA, van Hoppe M, Grift GE, Rijnsdorp AD. 2007. Changes in the spatial distribution of North Sea plaice (Pleuronectes platessa) and implications for fisheries management. Journal of Sea Research 57:187–97. doi:10.1016/j.seares.2006.09.002
- Wennhage H. 2002. Vulnerability of newly settled plaice (Pleuronectes platessa L.) to predation: effects of habitat structure and predator functional response. Journal of Experimental Marine Biology and Ecology 269:129–45. doi:10.1016/S0022-0981(02)00005-9
- Worm B, Barbier EB, Beaumont N, Duffy JE, Folke C, Halpern BS, et al. 2006. Impacts of biodiversity loss on ocean ecosystem services. Science 314:789–90.
- Zijlstra JJ. 1972. On the importance of the Wadden Sea as a nursery area in relation to the conservation of the southern North Sea fishery resources. Symposia of the Zoological Society of London 29:233–58.
- Zijlstra JJ, Dapper R, Witte JIJ. 1982. Settlement, growth and mortality of post-larval plaice (Pleuronectes platessa) in the western Wadden Sea. Netherlands Journal of Sea Research 15:250–72. doi:10.1016/0077-7579(82)90007-2
