ABSTRACT
We examined the isotopic signatures (δ13C, δ15N) of fauna living in association with the sponge Spongosorites coralliophaga colonizing coral rubble on cold-water coral reefs in the northeast Atlantic – the shallow inshore (122–131 m collection depth) Mingulay 01 area and the deep offshore (683–800 m) Logachev 02 mound. The δ15N signatures of suspended particulate organic matter and three primary consumers, i.e. Spongosorites coralliophaga, Reteporella beaniana and Parazoanthus anguicomus were used as trophic baselines and the resulting trophic structure was compared. In both regions four trophic levels were distinguished. However, the use of S. coralliophaga or R. beaniana as baselines resulted in a skewed trophic structure due to the enriched δ15Ν signatures of these two species on the Logachev 02 mound. Using suspended particulate organic matter and P. anguicomus as baselines, the Mingulay 01 area communities were characterized by elevated relative biomass of lower trophic levels compared to the Logachev 02 mound. Relative biomass of suspension/filter feeders was also higher at the Mingulay 01 area. The two regions differed significantly with regard to the prevailing environmental conditions: apart from the difference in depth and distance from shore, the Mingulay 01 area was characterized by higher primary production in surface waters, tight pelagic–benthic coupling and higher velocity of bottom currents, and it is hypothesized that these characteristics were the main drivers of the observed differences. This study highlighted that multiple trophic baselines can provide a better interpretation of food-web structure and that the use of sponges or bryozoans as baselines across bathymetric gradients should be avoided.
RESPONSIBLE EDITOR:
Introduction
Heterotrophic ecosystems in the deep sea (defined here as regions below 200 m water depth) ultimately depend on the flux of organic matter from the upper layers of the ocean (Gage Citation2003 and references therein), and the quality, quantity and timing of this flux affect community composition and biomass (e.g. Billett et al. Citation2001, Citation2010; Ruhl & Smith Citation2004; Wei et al. Citation2010; Tecchio et al. Citation2013). Over the last 30 years, studies on the trophic structure of deep-sea benthos have greatly benefited from the use of carbon and nitrogen isotopic signatures that can provide information on both the source(s) of organic carbon (measured through δ13C) and species trophic level (measured through δ15N) (Minagawa & Wada Citation1984; Peterson & Fry Citation1987; Post Citation2002). In addition, isotopic signatures present space- and time-integrated information and can constitute a reliable approach for those species where direct examination of diet via stomach-content analysis is not possible (e.g. small-sized invertebrates, organisms recovered from great depths) (Iken et al. Citation2001; Reid et al. Citation2012). Knowledge about trophic structure is necessary in order to understand important aspects of ecosystem functioning such as competition for food resources (e.g. Carlier et al. Citation2009; Iken et al. Citation2010; Lin et al. Citation2014) and elemental (re)cycling (Hoffmann et al. Citation2009; Maldonado et al. Citation2012; Perea-Blázquez et al. Citation2012; White et al. Citation2012; de Goeij et al. Citation2013).
Cold-water coral reefs are deep-sea heterotrophic ecosystems with high ecological and economical values (van Oevelen et al. Citation2009; Henry et al. Citation2013a, Citation2013b), but our knowledge of their trophic structure is limited even for reefs in the northeast Atlantic (Duineveld et al. Citation2007; van Oevelen et al. Citation2009), which are comparatively well studied (Roberts et al. Citation2006). Reef-forming cold-water coral species do not host symbiotic dinoflagellates (Roberts et al. Citation2006), but rely on organic matter produced in the euphotic zone (Kiriakoulakis et al. Citation2005; Dodds et al. Citation2009; Duineveld et al. Citation2012; Mueller et al. Citation2014) and as a result often occur in regions where processes such as down-welling and advection increase the supply of food particles to the seafloor (Duineveld et al. Citation2007; Davies et al. Citation2009).
This supply of organic matter also benefits other suspension- and filter-feeding organisms such as sponges (Duineveld et al. Citation2007; van Oevelen et al. Citation2009), which are among the most species-rich phyla on the northeast Atlantic cold-water coral reefs (van Soest & Lavaleye Citation2005; van Soest et al. Citation2007; Roberts et al. Citation2009). The sponges in turn often harbour species-rich epifaunal communities (e.g. Westinga & Hoetjes Citation1981; Çinar et al. Citation2002; Neves & Omena Citation2003; Schejter et al. Citation2012; Padua et al. Citation2013; Kazanidis et al. Citation2016), but to the best of our knowledge there are no studies examining the trophic structure of sponge epifaunal communities in deep-sea or shallow-water regions.
The Mingulay reef complex (outer Hebrides Sea) and the Logachev mounds (southeast Rockall Bank) are two reef settings in the northeast Atlantic which differ significantly with regard to food supply and hydrographic conditions. The Mingulay reef complex is an inshore and shallow reef setting where bottom currents are stronger (speed up to 60 cm s−1) (Davies et al. Citation2009) than those recorded in the offshore and deep Logachev mounds (up to 30 cm s−1) (Duineveld et al. Citation2007; Mohn et al. Citation2014). Both sea-surface chl-a concentrations (Fehling et al. Citation2012) and near-seabed polyunsaturated fatty acid concentrations (Kiriakoulakis et al. Citation2007 for the Logachev mounds; Duineveld et al. Citation2012 for the Mingulay reef complex) are indicative of higher productivity at the Mingulay reef complex than the Logachev mounds. In addition, down-welling at the Mingulay reef complex can transport food particles from the ocean surface to the benthos in less than an hour (Davies et al. Citation2009), whereas in the Logachev mounds such a rapid vertical transport has not been reported (Duineveld et al. Citation2007; Mienis et al. Citation2007; Mohn et al. Citation2014).
This paper presents an analysis of the trophic structure of the community colonizing the sponge Spongosorites coralliophaga (Stephens, 1915) mixed with coral rubble at the Mingulay 01 area (Mingulay reef complex) and the Logachev 02 mound (Logachev mounds) (Roberts & shipboard party Citation2013). Spongosorites coralliophaga is a massive sponge (van Soest et al. Citation2007; Roberts et al. Citation2009; Roberts & shipboard party Citation2013), found frequently in cold-water coral reef settings in the Logachev mounds (van Soest & Lavaleye Citation2005) and the Mingulay reef complex (Vad Citation2013). Recently it was shown that S. coralliophaga acts as a settlement surface for several species (Kazanidis et al. Citation2016). The average abundance of individuals living attached to S. coralliophaga in the Mingulay 01 area was ∼3 individuals cm−3 sponge while lower values were found for the epifauna living attached on coral rubble in that region or on sponge/coral rubble on the Logachev 02 mound (Kazanidis et al. Citation2016).
In the present study, (a) the number of trophic levels and (b) the relative distribution of biomass across trophic levels in the Mingulay 01 area and the Logachev 02 mound were investigated using isotopic signatures (δ13C, δ15Ν) of epifaunal species. The relative distribution of biomass across feeding types was also studied for each of the two regions. Multiple trophic baselines (i.e. suspended particulate organic matter and three primary consumers) were used in order to examine the role of trophic baseline in the trophic structure (Chouvelon et al. Citation2012; Lorrain et al. Citation2015). It is well known that differences in food supply and hydrographic conditions can affect the trophic structure of benthic communities (e.g. Bergmann et al. Citation2009; Iken et al. Citation2010; Feder et al. Citation2011; Tecchio et al. Citation2013; Lin et al. Citation2014). We hypothesize that higher food supply and higher bottom-current speeds at the Mingulay reef complex result in a relatively higher biomass of suspension/filter feeders and relatively higher biomass assembled in the lower trophic levels than observed at the Logachev mounds.
Materials and methods
Description of the study areas
During the ‘Changing Oceans’ expedition in May/June 2012 on board the Royal Research Ship (RRS) James Cook (Cruise JC073) (Roberts & shipboard party Citation2013), specimens of Spongosorites coralliophaga that had colonized coral rubble and their associated fauna were collected from two locations in the northeast Atlantic: the Mingulay 01 area (Mingulay reef complex; 122–131 m depth of sample collection) and the Logachev 02 mound (Logachev mounds; 683–800 m depth of sample collection) (, ). The Mingulay reef complex comprises live coral reef areas at 120–190 m depth in the Outer Hebrides Sea with the main coral species being the scleractinian Lophelia pertusa (Linnaeus, 1758) (Roberts et al. Citation2005, Citation2009). Studies on the hydrography of this area have shown a south-southwest to north-northeast direction in both surface and bottom flows as well as that rapid down-welling of surface water and advection of deep bottom water are major mechanisms supplying the reef benthos with food particles (Davies et al. Citation2009; Roberts et al. Citation2009; Duineveld et al. Citation2012; Findlay et al. Citation2014; Moreno Navas et al. Citation2014). The Logachev mounds are offshore carbonated mounds on the southeast Rockall Bank and they exist between 500 and 1200 m water depth (Van Weering et al. Citation2003; Mienis et al. Citation2006; Wheeler et al. Citation2007). They form a complex setting of mound clusters whose diameters range from hundreds of metres to a few kilometres (Wheeler et al. Citation2007). The Logachev 02 mound (Roberts & shipboard party Citation2013) is a large carbonated structure (6 km long) and it is separated from the main clusters of Logachev mounds by up to 4 km (Roberts & shipboard party Citation2013).
Figure 1. Sites of sample collection. Mingulay 01 area (Mingulay reef complex, MRC) and Logachev 02 mound (Logachev mounds, LM) in the northeast Atlantic.
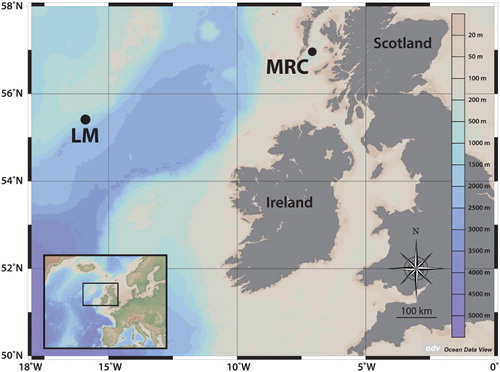
Table I. Coordinates and depth of locations where Spongosorites coralliophaga–coral rubble assemblages were collected in the Mingulay 01 area (Mingulay reef complex, MRC) and the Logachev 02 mound (Logachev mounds, LM). The ratio of dry sponge (S) volume/coral rubble (CR) volume is also given; the methodology followed for measuring these volumes is given in Kazanidis et al. (Citation2016).
The main coral species in the Logachev mounds are the scleractinians L. pertusa and Madrepora oculata Linnaeus, 1758 (van Weering et al. Citation2003; Duineveld et al. Citation2007). Studies on the hydrography of the region have shown the important role of advection in supplying the reef communities with food (Duineveld et al. Citation2007); in addition, recent modelling studies have revealed the important role that the carbonate mound structure plays in promoting local vertical mixing and the supply of organic matter to the benthic communities (Mohn et al. Citation2014).
Collection of samples
Being large in size and yellow in colour (see in Kazanidis et al. Citation2016), the specimens of Spongosorites coralliophaga were easily spotted during surveys using the remotely operated vehicle (ROV) Holland I. The collection of samples took place randomly and was carried out using the ROV manipulator arm. The scale of spacing between samples within each region ranged from a few metres up to a few hundreds of metres. Nine sponge–coral rubble assemblages were collected from the Mingulay 01 area and four from the Logachev 02 mound. After their collection, the S. coralliophaga–coral rubble assemblages and their epifauna were carefully transferred to the ROV biobox – a storage box that closes once withdrawn beneath the ROV – where they were kept until the return to the surface.
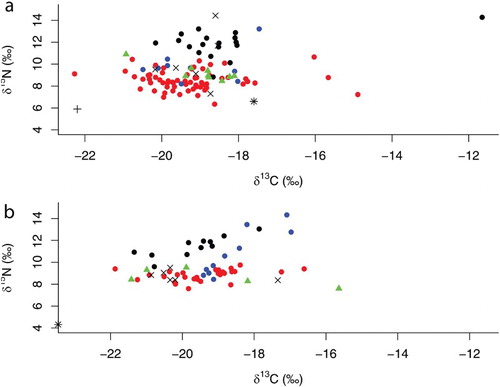
Figure 2. δ13C and δ15N values of suspension/filter feeders (![]()
Sample storage and assignment of feeding types
The tight sampling schedule did not allow for immediate taxonomic identification and the collected fauna was therefore preserved in 10% seawater formalin as in previous studies (Demopoulos et al. Citation2007; Mayor et al. Citation2012; Jeffreys et al. Citation2013). Formalin preservation was chosen because freezing often results in the break-off of specimens, thus preventing the classification at the lowest-possible taxonomic level required in this study (Fanelli et al. Citation2010). While we acknowledge the potential effect of formalin preservation on isotopic signatures, the outcome of systematic studies addressing this issue across various marine organisms is rather inconsistent (e.g. Sarakinos et al. Citation2002; Fanelli et al. Citation2010; Syvaranta et al. Citation2011). In addition, effects on faunal isotopic signatures have been reported even for freezing, i.e. the commonest sample preservation method before stable isotope analysis (Feuchtmayr & Grey Citation2003; Syvaranta et al. Citation2011; Liu et al. Citation2013). Clearly, species-specific studies are necessary in order to evaluate the exact effects of formalin preservation on faunal isotopic signatures (Kelly et al. Citation2006; Bicknell et al. Citation2011; Xu et al. Citation2011a; Gonzalez-Bergonzoni et al. Citation2015). Information on the possible effects of formalin preservation on the isotopic signatures of fauna from deep-sea regions is even more limited (Fanelli et al. Citation2010). In addition, studies about taxonomic groups common both in shallow-water and deep-sea regions (e.g. sponges, cnidarians, echinoderms, ascidians) are absent. Under these circumstances, we chose to address the problem through the correction of δ13C signatures (by adding 1‰ to each δ13C signature) as in previous studies (Demopoulos et al. Citation2007; Sweetman & Witte Citation2008; Gontikaki et al. Citation2011; Hunter et al. Citation2012). This correction factor for δ13C signatures is in agreement with previous studies mentioning a decrease up to 1‰ due to formalin preservation (Bosley & Wainright Citation1999; Edwards et al. Citation2002; Sarakinos et al. Citation2002; Syvaranta et al. Citation2008; Bicknell et al. Citation2011; de Lecea et al. Citation2011; Xu et al. Citation2011a; Lau et al. Citation2012; Rennie et al. Citation2012; Liu et al. Citation2013; Gonzalez-Bergonzoni et al. Citation2015). Furthermore, previous studies have shown that the effects of formalin preservation on δ15N signatures were minor compared to the generally accepted trophic shift between successive trophic levels (i.e. 3.4‰, Post Citation2002), enabling the accurate allocation of species preserved in formalin to trophic levels (Bosley & Wainright Citation1999; Sarakinos et al. Citation2002; Fanelli et al. Citation2010; Ruiz-Cooley et al. Citation2011; Rennie et al. Citation2012; Lau et al. Citation2012; Liu et al. Citation2013).
Following taxonomic identification, each species was categorized as belonging to a feeding type (i.e. suspension/filter feeders, omnivores, predators, deposit feeders/grazers) based mainly on information available in Henry et al. (Citation2013b). Additional information was collected from Tyler et al. (Citation1995), Boos et al. (Citation2010) (ophiuroids), Ericsson & Hansson (Citation1973) (asteroids), Carlier et al. (Citation2009) (echinoids), Vader (Citation1983) (amphipods), Fauchald & Jumars (Citation1979), Carrasco & Oyarzun (Citation1988), Nash & Keegan (Citation2003), Neves & Omena (Citation2003) (polychaetes), Hayward et al. (Citation1995) (gastropods), Nielsen & Riisgard (Citation1998) and Bader & Schafer (Citation2005) (bryozoans). For 15 specimens the characterization of their feeding type was not possible due to the lack of sufficient taxonomic resolution and/or the lack of information on their diet.
Processing of samples and stable isotope analysis
Fauna samples were dried at 60°C for 48 h and measurements of their dry weight were carried out (± 0.01 mg) (Kazanidis et al. Citation2016). After drying, samples were ground and subjected to a preliminary acidification test (using a few drops of 1 M hydrochloric acid) (Jaschinski et al. Citation2008; Vafeiadou et al. Citation2013) in order to identify the species with carbonate structures. In order to account for the optimum amount of dry matter (mg) to be placed in the silver/tin capsules for dual isotopic signatures analysis (δ13C and δ15N), acidified and non-acidified species were analysed for organic carbon (OC) and organic nitrogen (ON) content (as % of dry mass). Species with carbonate structures were subsequently divided into two groups of subsamples. The first group was not acidified and the second was acidified through the sequential addition of 15 μl of 1 M hydrochloric acid inside the silver capsules. The cessation of the effervescence was used as the criterion that carbonates had been removed (Vafeiadou et al. Citation2013 and references therein). All samples were then dried at 60°C overnight. No washing with distilled water was carried out (Mateo et al. Citation2008).
Samples were analysed for carbon and nitrogen isotopes at the University of California Davis Stable Isotopes Facility using an Elementar Micro Cube Elemental Analyzer (Elementar Analysensysteme GmbH, Hanau, Germany) interfaced to a PDZ Europa 20–20 isotope ratio mass spectrometer (Sercon Ltd, Cheshire, UK). Samples were combusted at 1000°C in a reactor packed with tungsten oxide. During analysis, samples were interspersed with several replicates of at least five laboratory standards that had been previously calibrated against international isotope standards. The long-term standard deviation is 0.2‰ for carbon and 0.3‰ for nitrogen. In the present study δ13C signatures of acidified samples and δ15N signatures of non-acidified samples were used; this was done in order to avoid the possible effects of acidification on δ15N signatures and thus on the trophic structure of the epifaunal community.
Total organic carbon and total organic nitrogen was calculated for each species, as well as across feeding types and trophic levels in the Mingulay 01 area and the Logachev 02 mound. Whenever possible (e.g. for molluscs, brachiopods, the sea urchin Cidaris cidaris (Linnaeus, 1758)), the soft tissues were separated from the calcareous structure and dry mass weight was used in the calculation of organic matter.
Trophic baselines
The calculation of the trophic level of secondary consumers was carried out using multiple trophic baselines aiming to gain a better understanding of the trophic structure (Chouvelon et al. Citation2012; Lorrain et al. Citation2015). The calculation of trophic level (TL) was carried out using the average δ15N signatures of suspended particulate organic matter and three primary consumers (following Iken et al. Citation2010). The following equations were used:and
where 3.4‰ is the generally accepted trophic shift factor for aquatic consumers (Post Citation2002). TL(PC) was calculated using as baseline the average δ15Ν signatures of the primary consumers Spongosorites coralliophaga (Porifera), Reteporella beaniana (King, 1846) (Bryozoa) and Parazoanthus anguicomus (Norman, 1868) (Anthozoa) as each of these three primary consumers was collected both from the Mingulay 01 area and the Logachev 02 mound. TL(SPOM) was calculated using as a trophic baseline the average δ15Ν signature of suspended particulate organic matter collected close to the sea surface and/or a few metres above the seafloor. The average δ15Ν signatures of suspended particulate organic matter used in the present study (5.9‰ for surface and 6.6‰ for bottom suspended particulate organic matter from Mingulay; 4.5‰ for bottom suspended particulate organic matter on the southeast Rockall Bank) were found in table 5 in Duineveld et al. (Citation2012) and table 2 in Duineveld et al. (Citation2007).
Statistical analysis
Isotopic signatures (δ13C and δ15N) of four species common in the Mingulay 01 area and the Logachev 02 mound, i.e. Spongosorites coralliophaga, Parazoanthus anguicomus, Eunice dubitata Fauchald, 1974 and Syllidae sp., were compared between the two regions. The normality of the distributions was checked with the Shapiro–Wilk test. In the case of normal distributions and equal variances, the existence of significant differences was tested with the two-sample t test; in the case of normal distributions and unequal variances the Welch’s two-sample t test was used, while in the case of non-normal distributions a Wilcoxon rank sum test was carried out (following Reid et al. Citation2012; Kazanidis et al. Citation2016). Examination of differences was carried out in the statistical analysis environment R (R Core Team Citation2013).
Results
Stable isotopes
In the Mingulay 01 area the isotopic signatures of 45 species were examined. The δ13C signatures ranged from –22.27‰ in the sponge Haliclona (Haliclona) urceolus (Rathke & Vahl, 1806) up to –11.65‰ in the asteroid Porania (Porania) pulvillus (O.F. Müller, 1776). Most δ13C signatures were between –20 and –18‰ (, (a)). The δ15N signatures ranged from 6.35‰ in the bivalve Heteranomia squamula (Linnaeus, 1758) up to 14.49‰ in the polychaete Eunice pennata (Müller, 1776) (, (a)).
Table II. δ13C and δ15N isotopic signatures of fauna living in association with Spongosorites coralliophaga and underlying coral rubble in the Mingulay 01 area and the Logachev 02 mound in northeast Atlantic.
On the Logachev 02 mound the isotopic signatures of 36 species were investigated. The minimum δ13C signature (–23.04‰) was found in the bryozoan Reteporella beaniana and the maximum (–15.64‰) in the anthozoan Paraedwardsia sarsii (Dueben & Koren, 1847). Most of the δ13C signatures were between –20 and –18‰ (, (b)). The δ15N signatures ranged from 7.60‰ in the hydrozoan Zygophylax pinnata (Sars, 1874) up to 14.33‰ in the gastropod Diodora graeca (Linnaeus, 1758) (, (b)).
Intraspecific comparison of δ13C signatures between the Mingulay 01 area and the Logachev 02 mound revealed significant differences for Spongosorites coralliophaga (Wilcoxon rank sum test = 32, P = 0.033) and Syllidae sp. (two-sample t test = –3.3818, P = 0.019). In terms of δ15N signatures, S. coralliophaga was the only species that showed significant differences between the Mingulay 01 area and the Logachev 02 mound (two-sample t test = 5.4453, P < 0.001).
Relative distribution of biomass across feeding types
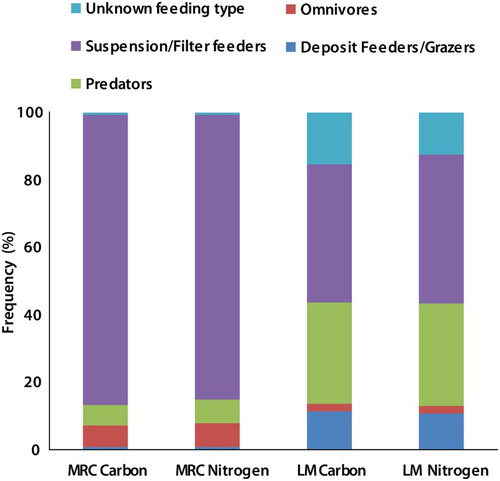
The relative distribution of biomass across feeding types differed between the two reef complexes, with the proportion of biomass of suspension/filter feeders at the Mingulay 01 area being almost double that at the Logachev 02 mound (). At the Mingulay 01 area, the relative biomass of predators and omnivores was similar, whereas predators were more important at the Logachev 02 mound ().
Relative distribution of species numbers and biomass across trophic levels
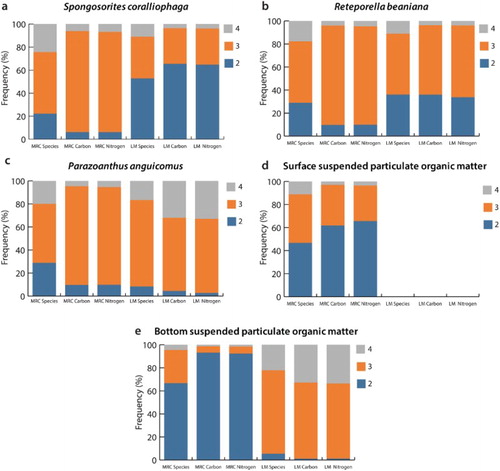
In both regions, four trophic levels were identified. However, as can be seen in , the distribution of species numbers and biomass across those levels (2, 3, 4) greatly depended on the baseline used. In the Mingulay 01 area two different patterns emerged, related to the use of suspended particulate organic matter vs. any of the three primary consumers. Interestingly, for the Logachev 02 mound there was a clear distinction between the use of either Reteporella beaniana or Spongosorites coralliophaga vs. Parazoanthus anguicomus or suspended particulate organic matter.
Figure 4. Distribution of epifaunal species and biomass across the second, third and fourth trophic level in the Mingulay 01 area (Mingulay reef complex, MRC) and the Logachev 02 mound (Logachev mounds, LM) in the northeast Atlantic using the average δ15Ν signatures of primary consumers and surface/bottom suspended particulate organic matter as trophic baselines. The average δ15Ν signatures of surface and bottom suspended particulate organic matter in the Mingulay were found in table 5 in Duineveld et al. (Citation2012) and the average δ15N signature of bottom suspended particulate organic matter in southeast Rockall Bank was found in table 2 in Duineveld et al. (Citation2007). Please note that the δ13C signatures of either surface or bottom suspended particulate organic matter at the southeast Rockall Bank are not available.
Discussion
The matter of the trophic baseline
The δ15Ν signatures of suspended or sedimented particulate organic matter have traditionally been used as a baseline for the calculation of species trophic level (e.g. Grall et al. Citation2006; Iken et al. Citation2010; Divine et al. Citation2015); however, the high nitrogen turnover rates of primary producers have led to high spatial and temporal variability in their δ15Ν signatures, which has complicated trophic level calculations and comparisons between regions; as a response to this, the use of long-lived primary consumers as trophic baselines has been suggested due to the smaller spatial/temporal variability in their δ15N signatures compared to those of primary producers (Cabana & Rasmussen Citation1996; Vander Zanden & Rasmussen Citation1999; Post Citation2002; Vander Zanden & Fetzer Citation2007). However, studies published more recently than those mentioning the usefulness of primary consumers as trophic baselines revealed substantial variability in δ15Ν signatures of primary consumers both at intraspecific (Howard et al. Citation2005; McIntyre & Flecker Citation2006; Guzzo et al. Citation2011; Magni et al. Citation2013) and interspecific levels (Kohzu et al. Citation2009; Xu et al. Citation2011b). As a way forward, the simultaneous use of multiple trophic baselines (i.e. the use of more than one primary consumer, primary consumers and suspended or sedimented particulate organic matter) has been suggested as an approach to facilitate a better assessment of species trophic level (Iken et al. Citation2010; Chouvelon et al. Citation2012; Mancinelli et al. Citation2013; Lorrain et al. Citation2015).
In both reef complexes investigated here, the indicated trophic structure was dependent on the baseline used. In the Mingulay 01 area the use of primary consumers resulted in most of the species/biomass being allocated to the third trophic level while the use of surface or bottom suspended particulate organic matter resulted in most of them being found in the second trophic level. This was probably a response to trophic-shift factor(s) lower than 3.4‰ (McCutchan et al. Citation2003; Alp et al. Citation2013; Dodds et al. Citation2014; Hussey et al. Citation2014) or relatively enriched (i.e. higher) δ15Ν signatures of suspended particulate organic matter due to regional patterns of nitrogen biogeochemistry (Peipoch et al. Citation2012; Wang et al. Citation2013) and/or terrestrial run-offs (Kohzu et al. Citation2009; Magni et al. Citation2013). In contrast to the Mingulay 01 area, species/biomass found in the second trophic level of the Logachev 02 mound when primary consumers were used as a baseline were higher than those measured using suspended particulate organic matter. This finding was probably due to the relatively low δ15N signature of suspended particulate organic matter for the Logachev 02 mound (Duineveld et al. Citation2007), trophic-shift factor(s) (see above) and/or enriched δ15N signatures of Spongosorites coralliophaga and Reteporella beaniana. Furthermore, the use of S. coralliophaga or R. beaniana as baselines did not show any differences between the Mingulay 01 area and the Logachev 02 mound. On the contrary, when using suspended particulate organic matter or Parazoanthus anguicomus as a baseline, a clear difference was recorded, i.e. more biomass was found on the fourth trophic level of the Logachev 02 mound than at the Mingulay 01 area. This difference was attributed to the transfer of the predator Eunice dubitata from the fourth (using suspended particulate organic matter or P. anguicomus as a trophic baseline) to the third trophic level (using S. coralliophaga or R. beaniana as a baseline). This transfer from the fourth to the third trophic level was a response to the higher δ15Ν signatures of S. coralliophaga and R. beaniana at the Logachev 02 mound than at the Mingulay 01 area. The enriched δ15Ν signatures of these two species were probably the result of a combination of (a) enriched δ15Ν signatures of small-sized food particles (i.e. pico- to nanoplankton) in deeper regions due to extended resuspension and microbial degradation (Saino & Hattori Citation1980; Altabet Citation1988; Mintenbeck et al. Citation2007; Bergmann et al. Citation2009), and (b) selective feeding of S. coralliophaga and R. beaniana on small-sized particles (Winston Citation1977; Witte et al. Citation1997). The findings on nitrogen stable isotope values (δ15Ν) of suspension/filter feeders across a bathymetric gradient presented here were in good agreement with previous studies (Mintenbeck et al. Citation2007; Bergmann et al. Citation2009). In contrast to selective suspension feeders, deposit feeders have not shown a bathymetric enrichment in their δ15N values, probably because they relied on a wide spectrum of food particles settled on the seabed (Mintenbeck et al. Citation2007; Bergmann et al. Citation2009). A depth-stratified approach should be followed in food-web studies across a wide bathymetric range in order to avoid the misinterpretation of findings arising from the enrichment of δ15N values (Mintenbeck et al. Citation2007; Bergmann et al. Citation2009). Using suspended particulate organic matter or P. anguicomus as a baseline, a clear difference between the Mingulay 01 area and the Logachev 02 mound was found with regard to the distribution of biomass across the trophic levels. A different pattern was also found with regard to the distribution of biomass across feeding types. Specifically, the relative biomass (a) of suspension/filter feeders and (b) representatives of the lower trophic levels was indicated to be higher at the Mingulay 01 area than the Logachev 02 mound. These results support the hypothesis that environmental conditions would play an important role in the trophic structure of benthic communities. However, it should be mentioned that the use of suspended particulate organic matter as a baseline revealed more species and higher biomass at the second trophic level for the Mingulay 01 area than the second trophic level at the Logachev 02 mound. This finding was likely due to the higher δ15Ν signatures of suspended particulate organic matter at the Mingulay 01 area than the Logachev 02 mound and reinforced previous suggestions that spatial/temporal variability in the δ15N signatures of primary producers can complicate the comparison of trophic structure between regions (Iken et al. Citation2010; see also above). Based on present findings we recommend that multiple trophic baselines should be used in studies focusing on the trophic structure of ecosystems (Iken et al. Citation2010; Chouvelon et al. Citation2012; Mancinelli et al. Citation2013; Lorrain et al. Citation2015) and that sponges, bryozoans and suspended particulate organic matter as baselines across bathymetric gradients should be avoided. On the other hand, we suggest that cnidarians can probably serve as a more reliable trophic baseline compared to sponges and bryozoans. Present findings have also shown that equal food chain length between two regions may be accompanied by differences in the distribution of biomass across the tropic levels. Based on this observation we recommend that studies on food-web structure should incorporate both the length of the food chain (e.g. Vander Zanden & Fetzer Citation2007) and the distribution of biomass across the trophic levels (Grall et al. Citation2006; Iken et al. Citation2010).
Environmental factors and the trophic structure of benthos
The present findings highlighted the important role of differences in the input of organic matter in the ecology of cold-water coral reefs in the northeast Atlantic. Previous studies have shown that the quantity of organic matter supplied to deep-sea ecosystems may have an influence on aspects such as the abundance, biomass, community structure (Ruhl Citation2008; Wolff et al. Citation2011), species nutritional status (Cummings et al. Citation2013) and community trophic structure (Iken et al. Citation2010). In this study, higher levels of surface primary production over the Mingulay reef complex (Fehling et al. Citation2012), in combination with the rapid down-welling (Davies et al. Citation2009), indicated that more and fresher organic matter was supplied to the shallow inshore Mingulay reef complex than the deep offshore Logachev mounds. This in turn probably fuelled higher biomass of suspension/filter feeders at the Mingulay reef complex.
The quality of organic matter settling on the deep-sea floor is an important parameter for ecosystem functionality. Changes in the biochemical composition of the organic matter input have been hypothesized as the main driving force behind radical shifts in benthic community composition, structure and functionality in the Porcupine Abyssal Plain in the northeast Atlantic (Billett et al. Citation2001, Citation2010). In the Mingulay 01 area and the Logachev 02 mound the δ13C signatures in most suspension/filter feeders and grazers were depleted (i.e. smaller) compared to the δ13C values of bottom suspended particulate organic matter in the Mingulay reef complex. In contrast, the δ13C signatures of suspension/filter feeders and grazers were in good agreement with the δ13C values of surface suspended particulate organic matter. Thus, it may be suggested that the Mingulay 01 area and the Logachev 02 mound benthos relied mainly on surface suspended particulate organic matter as a carbon source, at least for the time period of our study. Previous studies on the biochemical composition of suspended particulate organic matter in these two regions have revealed a higher concentration of polyunsaturated fatty acids in the Mingulay reef complex (Duineveld et al. Citation2012) than the Logachev mounds (Kiriakoulakis et al. Citation2007). Polyunsaturated fatty acids are essential components for species maintenance, growth and reproduction in a number of species (Brett & Müller-Navarra Citation1997; Hudson et al. Citation2003; Barras et al. Citation2009) and thus their assimilation may have supported higher fecundity and successful recruitment in the Mingulay reef complex (Fuiman & Ojanguren Citation2011; Callan et al. Citation2012; Toupoint et al. Citation2012). The composition of polyunsaturated fatty acids in Spongosorites coralliophaga–coral rubble epifauna at the Mingulay 01 area and the Logachev 02 mound was not available; however, taking into account the close relationship between diet and reproduction (e.g. Wigham et al. Citation2003a, Citation2003b; Fitz-George Balfour et al. Citation2010; Kazanidis et al. Citation2014), it seems that the assimilation of polyunsaturated fatty acids by Mingulay 01 area suspension/filter feeders has facilitated their proliferation. This was most likely for the species Parazoanthus anguicomus, which was dominant at the Mingulay 01 area in terms of abundance and biomass but had low abundance and biomass at the Logachev 02 mound (Kazanidis et al. Citation2016).
Bottom currents can play an important role in the distribution of suspension feeders in the deep sea, since they suspend food particles, enhancing particle-encounter rates (Gage Citation2003 and references therein; Carlier et al. Citation2009; Purser et al. Citation2010; Duineveld et al. Citation2012), and they prevent detrimental effects arising from the accumulation of non-edible particles on organisms (Purser & Thomsen Citation2012; Larsson et al. Citation2013). The higher velocity of bottom currents at the Mingulay reef complex than those at the Logachev mounds has likely contributed to these aspects. The suspension of food particles at the Mingulay reef complex and the Logachev mounds reef settings probably inhibit the presence of deposit-feeding megafauna (e.g. holothurians) that thrive in deeper regions with lower suspension and a higher accumulation of particulate organic matter on the sediments (e.g. abyssal plains, Billett et al. Citation1983, Citation2001; Wolff et al. Citation2011).
Acknowledgements
Special thanks to Bill Richardson (Master) and the crew of the RRS James Cook during the JC073 Changing Oceans Expedition, Will Handley and the Holland-I ROV team. Also thanks to Dr Evina Gontikaki and Dr Solveig Bourgeois (University of Aberdeen) for their guidance on sample preparation for isotope analysis, Kenneth Cruickshank (University of Aberdeen) for analysis of sample elemental composition, and Dr Joy Matthews, Sylvia Duncan and Emily Schick at UC Davis Stable Isotope Facility for their cooperation on sample stable isotope analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alp M, Peckarsky BL, Bernasconi SM, Robinson CT. 2013. Shifts in isotopic signatures of animals with complex life-cycles can complicate conclusions on cross-boundary trophic links. Aquatic Sciences 75:595–606. doi:10.1007/s00027-013-0303-x
- Altabet MA. 1988. Variations in nitrogen isotopic composition between sinking and suspended particles: Implications for nitrogen cycling and particle transformation in the open ocean. Deep Sea Research A 35:535–54. doi:10.1016/0198-0149(88)90130-6
- Bader B, Schafer P. 2005. Impact of environmental seasonality on stable isotope composition of skeletons of the temperate bryozoan Cellaria sinuosa. Palaeogeography, Palaeoclimatology, Palaeoecology 226:58–71. doi:10.1016/j.palaeo.2005.05.007
- Barras C, Geslin E, Duplessy J, Jorissen FJ. 2009. Reproduction and growth of the deep-sea benthic foraminifer Bulimina marginata under different laboratory conditions. The Journal of Foraminiferal Research 39:155–65. doi:10.2113/gsjfr.39.3.155
- Bergmann M, Dannheim J, Bauerfeind E, Klages M. 2009. Trophic relationships along a bathymetric gradient at the deep-sea observatory HAUSGARTEN. Deep Sea Research I 56:408–24. doi:10.1016/j.dsr.2008.10.004
- Bicknell AW, Campbell M, Knight ME, Bilton DT, Newton J, Votier SC. 2011. Effects of formalin preservation on stable carbon and nitrogen isotope signatures in calanoid copepods: implications for the use of Continuous Plankton Recorder Survey samples in stable isotope analyses. Rapid Communications in Mass Spectrometry 25:1794–800. doi:10.1002/rcm.5049
- Billett D, Lampitt R, Rice A, Mantoura R. 1983. Seasonal sedimentation of phytoplankton to the deep-sea benthos. Nature 302:520–22. doi:10.1038/302520a0
- Billett DSM, Bett BJ, Rice AL, Thurston MH, Galeron J, Sibuet M, Wolff GA. 2001. Long-term change in the megabenthos of the Porcupine Abyssal Plain (NE Atlantic). Progress in Oceanography 50:325–48. doi:10.1016/S0079-6611(01)00060-X
- Billett DSM, Bett BJ, Reid WDK, Boorman B, Priede IG. 2010. Long-term change in the abyssal NE Atlantic: The ‘Amperima Event’ revisited. Deep Sea Research II 57:1406–17. doi:10.1016/j.dsr2.2009.02.001
- Boos K, Gutow L, Mundry R, Franke H. 2010. Sediment preference and burrowing behaviour in the sympatric brittlestars Ophiura albida Forbes, 1839 and Ophiura ophiura (Linnaeus, 1758) (Ophiuroidea, Echinodermata). Journal of Experimental Marine Biology and Ecology 393:176–81. doi:10.1016/j.jembe.2010.07.021
- Bosley KL, Wainright SC. 1999. Effects of preservatives and acidification on the stable isotope ratios (15N:14N, 13C: 12C) of two species of marine animals. Canadian Journal of Fisheries and Aquatic Sciences 56:2181–85. doi:10.1139/f99-153
- Brett MT, Müller-Navarra DC. 1997. The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshwater Biology 38:483–99. doi:10.1046/j.1365-2427.1997.00220.x
- Cabana G, Rasmussen JB. 1996. Comparison of aquatic food chains using nitrogen isotopes. Proceedings of the National Academy of Sciences 93:10844–47. doi:10.1073/pnas.93.20.10844
- Callan CK, Laidley CW, Forster IP, Liu KM, Kling LJ, Place AR. 2012. Examination of broodstock diet effects on egg production and egg quality in flame angelfish (Centropyge loriculus). Aquaculture Research 43:696–705. doi:10.1111/j.1365-2109.2011.02877.x
- Carlier A, Le Guilloux E, Olu K, Sarrazin J, Mastrototaro F, Taviani M, Clavier J. 2009. Trophic relationships in a deep Mediterranean cold-water coral bank (Santa Maria di Leuca, Ionian Sea). Marine Ecology Progress Series 397:125–37. doi:10.3354/meps08361
- Carrasco FD, Oyarzun O. 1988. Diet of the polychaete Lumbrineris tetraura (Schmarda) (Lumbrineridae) in a polluted soft-bottom environment. Bulletin of Marine Science 42:358–65.
- Chouvelon T, Spitz J, Caurant F, Mèndez-Fernandez P, Chappuis A, Laugier F, et al. 2012. Revisiting the use of δ15N in meso-scale studies of marine food webs by considering spatio-temporal variations in stable isotopic signatures – the case of an open ecosystem: the Bay of Biscay (North-East Atlantic). Progress in Oceanography 101:92–105. doi:10.1016/j.pocean.2012.01.004
- Çinar ME, Katagan T, Ergen Z, Sezgin M. 2002. Zoobenthos-inhabiting Sarcotragus muscarum (Porifera: Demospongiae) from the Aegean Sea. Hydrobiologia 482:107–17. doi:10.1023/A:1021260314414
- Cummings DO, Lee RW, Nodder SD, Simpson SJ, Holmes SP. 2013. Trophic status and condition of Hyalinoecia longibranchiata from two regions of contrasting oceanic productivity. Marine Ecology Progress Series 477:147–59. doi:10.3354/meps10160
- Davies AJ, Duineveld GCA, Lavaleye MSS, Bergman MJN, van Haren H, Roberts JM. 2009. Downwelling and deep-water bottom currents as food supply mechanisms to the cold-water coral Lophelia pertusa (Scleractinia) at the Mingulay Reef complex. Limnology and Oceanography 54:620–29. doi:10.4319/lo.2009.54.2.0620
- de Goeij JM, van Oevelen D, Vermeij MJA, Osinga R, Middelburg JJ, de Goeij AFPM, Admiraal W. 2013. Surviving in a marine desert: the sponge loop retains resources within coral reefs. Science 342:108–10. doi:10.1126/science.1241981
- de Lecea AM, Cooper R, Omarjee A, Smit AJ. 2011. The effects of preservation methods, dyes and acidification on the isotopic values (δ15N and δ13C) of two zooplankton species from the KwaZulu-Natal Bight, South Africa. Rapid Communications in Mass Spectrometry 25:1853–61. doi:10.1002/rcm.5051
- Demopoulos AW, Fry B, Smith CR. 2007. Food web structure in exotic and native mangroves: a Hawaii–Puerto Rico comparison. Oecologia 153:675–86. doi:10.1007/s00442-007-0751-x
- Divine LM, Iken K, Bluhm BA. 2015. Regional benthic food web structure on the Alaska Beaufort Sea shelf. Marine Ecology Progress Series 531:15–32. doi:10.3354/meps11340
- Dodds LA, Black KD, Orr H, Roberts JM. 2009. Lipid biomarkers reveal geographical differences in food supply to the cold-water coral Lophelia pertusa (Scleractinia). Marine Ecology Progress Series 397:113–24. doi:10.3354/meps08143
- Dodds WK, Collins SM, Hamilton SK, Tank JL, Johnson S, Webster JR, et al. 2014. You are not always what we think you eat: selective assimilation across multiple whole-stream isotopic tracer studies. Ecology 95:2757–67. doi:10.1890/13-2276.1
- Duineveld GCA, Lavaleye MSS, Bergman MJN, de Stigter H, Mienis F. 2007. Trophic structure of a cold-water coral mound community (Rockall Bank, NE Atlantic) in relation to the near-bottom particle supply and current regime. Bulletin of Marine Science 81:449–67.
- Duineveld GCA, Jeffreys RM, Lavaleye MSS, Davies AJ, Bergman MJN, Watmough T, Witbaard R. 2012. Spatial and tidal variation in food supply to shallow cold-water coral reefs of the Mingulay Reef complex (Outer Hebrides, Scotland). Marine Ecology Progress Series 444:97–115. doi:10.3354/meps09430
- Edwards MS, Turner TF, Sharp ZD. 2002. Short-and long-term effects of fixation and preservation on stable isotope values (δ13C, δ15N, δ34S) of fluid-preserved museum specimens. Copeia 2002:1106–12. doi:10.1643/0045-8511(2002)002[1106:SALTEO]2.0.CO;2
- Ericsson S, Hansson HG. 1973. Observations on the feeding biology of Porania pulvillus (O. F. Müller), (Asteroidea), from the Swedish west coast. Ophelia 12:53–58. doi:10.1080/00785326.1973.10430119
- Fanelli E, Cartes JE, Papiol V, Rumolo P, Sprovieri M. 2010. Effects of preservation on the δ13C and δ15N values of deep sea macrofauna. Journal of Experimental Marine Biology and Ecology 395:93–97. doi:10.1016/j.jembe.2010.08.020
- Fauchald K, Jumars PA. 1979. The diet of worms: a study of polychaete feeding guilds. Oceanography and Marine Biology – An Annual Review 17:193–284.
- Feder HM, Iken K, Blanchard AL, Jewett SC, Schonberg S. 2011. Benthic food web structure in the southeastern Chukchi Sea: an assessment using δ13C and δ15N analyses. Polar Biology 34:521–32. doi:10.1007/s00300-010-0906-9
- Fehling J, Davidson K, Bolch CJS, Brand TD, Narayanaswamy BE. 2012. The relationship between phytoplankton distribution and water column characteristics in north west European shelf sea waters. PLoS One 7(3):e34098. 16 pages. doi:10.1371/journal.pone.0034098
- Feuchtmayr H, Grey J. 2003. Effect of preparation and preservation procedures on carbon and nitrogen stable isotope determinations from zooplankton. Rapid Communications in Mass Spectrometry 17:2605–10. doi:10.1002/rcm.1227
- Findlay H, Hennige SJ, Wicks LC, Moreno Navas J, Woodward EMS, Roberts JM. 2014. Fine-scale nutrient and carbonate system dynamics around cold-water coral reefs in the northeast Atlantic. Scientific Reports 4:3671. 10 pages. doi:10.1038/srep03671
- FitzGeorge-Balfour T, Billett DSM, Wolff GA, Thompson A, Tyler PA. 2010. Phytopigments as biomarkers of selectivity in abyssal holothurians; interspecific differences in response to a changing food supply. Deep Sea Research II 57:1418–28. doi:10.1016/j.dsr2.2010.01.013
- Fuiman LA, Ojanguren AF. 2011. Fatty acid content of eggs determines antipredator performance of fish larvae. Journal of Experimental Marine Biology and Ecology 407:155–65. doi:10.1016/j.jembe.2011.06.004
- Gage JD. 2003. Food inputs, utilization, carbon flow and energetics. In: Tyler PA, editor. Ecosystems of the World. Volume 28: Ecosystems of the Deep Oceans. Amsterdam: Elsevier, p 315–82.
- Gontikaki E, Mayor DJ, Narayanaswamy BE, Witte UFM. 2011. Feeding strategies of deep-sea sub-Arctic macrofauna of the Faroe-Shetland Channel: combining natural stable isotopes and enrichment techniques. Deep Sea Research I 58:160–72. doi:10.1016/j.dsr.2010.11.011
- González-Bergonzoni I, Vidal N, Wang B, Ning D, Liu Z, Jeppesen E, Meerhoff M. 2015. General validation of formalin-preserved fish samples in food web studies using stable isotopes. Methods in Ecology and Evolution 6:307–14. doi:10.1111/2041-210X.12313
- Grall J, Le Loc'h F, Guyonnet B, Riera P. 2006. Community structure and food web based on stable isotopes (δ15N and δ13C) analysis of a North Eastern Atlantic maerl bed. Journal of Experimental Marine Biology and Ecology 338:1–15. doi:10.1016/j.jembe.2006.06.013
- Guzzo MM, Douglas Haffner G, Sorge S, Rush SA, Fisk AT. 2011. Spatial and temporal variabilities of δ13C and δ15N within lower trophic levels of a large lake: implications for estimating trophic relationships of consumers. Hydrobiologia 675:41–53. doi:10.1007/s10750-011-0794-1
- Hayward PJ, Wigham GD, Yonow N. 1995. Molluscs (Phylum Mollusca). In: Hayward PJ, Ryland JS, editors. Handbook of the Marine Fauna of North-West Europe. Oxford: Oxford University Press, p 484–628.
- Henry L, Moreno Navas M, Hennige SJ, Wicks LC, Vad J, Roberts JM. 2013a. Cold-water coral reef habitats benefit recreationally valuable sharks. Biological Conservation 161:67–70. doi:10.1016/j.biocon.2013.03.002
- Henry L, Moreno Navas J, Roberts JM. 2013b. Multi-scale interactions between local hydrography, seabed topography, and community assembly on cold-water coral reefs. Biogeosciences 10:2737–46. doi:10.5194/bg-10-2737-2013
- Hoffmann F, Radax R, Woebken D, Holtappels M, Lavik G, Rapp HT, et al. 2009. Complex nitrogen cycling in the sponge Geodia barretti. Environmental Microbiology 11:2228–43. doi:10.1111/j.1462-2920.2009.01944.x
- Howard JK, Cuffey KM, Solomon M. 2005. Toward using Margaritifera falcata as an indicator of base level nitrogen and carbon isotope ratios: insights from two California Coast Range rivers. Hydrobiologia 541:229–36. doi:10.1007/s10750-004-5711-4
- Hudson IR, Wigham BD, Billett DS, Tyler PA. 2003. Seasonality and selectivity in the feeding ecology and reproductive biology of deep-sea bathyal holothurians. Progress in Oceanography 59:381–407. doi:10.1016/j.pocean.2003.11.002
- Hunter WR, Levin LA, Kitazato H, Witte U. 2012. Macrobenthic assemblage structure and organismal stoichiometry control faunal processing of particulate organic carbon and nitrogen in oxygen minimum zone sediments. Biogeosciences 9:993–1006. doi:10.5194/bg-9-993-2012
- Hussey NE, MacNeil MA, McMeans BC, Olin JA, Dudley SFJ, Cliff G, et al. 2014. Rescaling the trophic structure of marine food webs. Ecology Letters 17:239–50. doi:10.1111/ele.12226
- Iken K, Brey T, Wand U, Voigt J, Junghans P. 2001. Food web structure of the benthic community at the Porcupine Abyssal Plain (NE Atlantic): a stable isotope analysis. Progress in Oceanography 50:383–405. doi:10.1016/S0079-6611(01)00062-3
- Iken K, Bluhm B, Dunton K. 2010. Benthic food-web structure under differing water mass properties in the southern Chukchi Sea. Deep Sea Research II 57:71–85. doi:10.1016/j.dsr2.2009.08.007
- Jaschinski S, Hansen T, Sommer U. 2008. Effects of acidification in multiple stable isotope analyses. Limnology and Oceanography: Methods 6:12–15. doi:10.4319/lom.2008.6.12
- Jeffreys RM, Burke C, Jamieson AJ, Narayanaswamy BE, Ruhl HA, Smith Jr KL, Witte U. 2013. Feeding preferences of abyssal macrofauna inferred from in situ pulse chase experiments. PLoS One 8(11):e80510. 15 pages. doi:10.1371/journal.pone.0080510
- Kazanidis G, Tyler PA, Billett DSM. 2014. On the reproduction of the simultaneous hermaphrodite Paroriza prouhoi (Holothuroidea: Synallactidae) in the Porcupine Abyssal Plain, north-east Atlantic. Journal of the Marine Biological Association of the United Kingdom 94:847–56. doi:10.1017/S0025315413001537
- Kazanidis G, Henry L-A, Roberts JM, Witte UFM. 2016. Biodiversity of Spongosorites coralliophaga (Stephens, 1915) on coral rubble at two contrasting cold-water coral reef settings. Coral Reefs 35:193–208. doi:10.1007/s00338-015-1355-2
- Kelly B, Dempson J, Power M. 2006. The effects of preservation on fish tissue stable isotope signatures. Journal of Fish Biology 69:1595–611. doi:10.1111/j.1095-8649.2006.01226.x
- Kiriakoulakis K, Fisher E, Wolff GA, Freiwald A, Grehan A, Roberts JM. 2005. Lipids and nitrogen isotopes of two deep-water corals from the North-East Atlantic: initial results and implications for their nutrition. In: Freiwald A, Roberts JM, editors. Cold-Water Corals and Ecosystems. Berlin, Heidelberg: Springer, p 715–29.
- Kiriakoulakis K, Freiwald A, Fisher E, Wolff GA. 2007. Organic matter quality and supply to deep-water coral/mound systems of the NW European Continental Margin. International Journal of Earth Sciences 96:159–70. doi:10.1007/s00531-006-0078-6
- Kohzu A, Tayasu I, Yoshimizu C, Maruyama A, Kohmatsu Y, Hyodo F, et al. 2009. Nitrogen-stable isotopic signatures of basal food items, primary consumers and omnivores in rivers with different levels of human impact. Ecological Research 24:127–36. doi:10.1007/s11284-008-0489-x
- Larsson AI, van Oevelen D, Purser A, Thomsen L. 2013. Tolerance to long-term exposure of suspended benthic sediments and drill cuttings in the cold-water coral Lophelia pertusa. Marine Pollution Bulletin 70:176–88. doi:10.1016/j.marpolbul.2013.02.033
- Lau DC, Leung KM, Dudgeon D. 2012. Preservation effects on C/N ratios and stable isotope signatures of freshwater fishes and benthic macroinvertebrates. Limnology and Oceanography: Methods 10:75–89. doi:10.4319/lom.2012.10.75
- Lin HY, Lin PY, Chang NN, Shiao JC, Kao SJ. 2014. Trophic structure of megabenthic food webs along depth gradients in the South China Sea and off northeastern Taiwan. Marine Ecology Progress Series 501:53–66. doi:10.3354/meps10681
- Liu B, Liu Y, Li Y, Wang H, Xu J. 2013. An assessment of sample preservation methods for the determination of stable carbon and nitrogen isotope ratios in mollusks. Analytical Letters 46:2620–34. doi:10.1080/00032719.2013.805415
- Lorrain A, Graham BS, Popp BN, Allain V, Olson RJ, Hunt BPV, et al. 2015. Nitrogen isotopic baselines and implications for estimating foraging habitat and trophic position of yellowfin tuna in the Indian and Pacific Oceans. Deep Sea Research II 113:188–98. doi:10.1016/j.dsr2.2014.02.003
- Magni P, Rajagopal S, Como S, Jansen JM, van der Velde G, Hummel H. 2013. δ13C and δ15N variations in organic matter pools, Mytilus spp. and Macoma balthica along the European Atlantic coast. Marine Biology 160:541–52.
- Maldonado M, Ribes M, van Duyl FC. 2012. Nutrient fluxes through sponges: Biology, budgets, and ecological implications. Ιn: Becerro MA, Uriz MJ, Maldonado M, Turon X, editors. Advances in Sponge Science: Physiology, Chemical and Microbial Diversity, Biotechnology. Oxford: Academic Press, p 113–82.
- Mancinelli G, Vizzini S, Mazzola A, Maci S, Basset A. 2013. Cross-validation of δ15N and FishBase estimates of fish trophic position in a Mediterranean lagoon: the importance of the isotopic baseline. Estuarine, Coastal and Shelf Science 135:77–85. doi:10.1016/j.ecss.2013.04.004
- Mateo MA, Serrano Ο, Serrano L, Michener RH. 2008. Effects of sample preparation on stable isotope ratios of carbon and nitrogen in marine invertebrates: implications for food web studies using stable isotopes. Oecologia 157:105–15. doi:10.1007/s00442-008-1052-8
- Mayor DJ, Thornton B, Hay S, Zuur AF, Nicol GW, McWilliam JM, Witte UFM. 2012. Resource quality affects carbon cycling in deep-sea sediments. ISME Journal 6:1740–48. doi:10.1038/ismej.2012.14
- McCutchan Jr JH, Lewis Jr WM, Kendall C, McGrath CC. 2003. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102:378–90. doi:10.1034/j.1600-0706.2003.12098.x
- McIntyre PB, Flecker AS. 2006. Rapid turnover of tissue nitrogen of primary consumers in tropical freshwaters. Oecologia 148:12–21. doi:10.1007/s00442-005-0354-3
- Mienis F, van Weering T, de Haas H, de Stigter H, Huvenne V, Wheeler A. 2006. Carbonate mound development at the SW Rockall Trough margin based on high resolution TOBI and seismic recording. Marine Geology 233:1–19. doi:10.1016/j.margeo.2006.08.003
- Mienis F, de Stigter HC, White M, Duineveld GCA, de Haas H, van Weering TCE. 2007. Hydrodynamic controls on cold-water coral growth and carbonate-mound development at the SW and SE Rockall Trough Margin, NE Atlantic Ocean. Deep Sea Research I 54:1655–74. doi:10.1016/j.dsr.2007.05.013
- Minagawa M, Wada E. 1984. Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochimica et Cosmochimica Acta 48:1135–40. doi:10.1016/0016-7037(84)90204-7
- Mintenbeck K, Jacob U, Knust R, Arntz WE, Brey T. 2007. Depth-dependence in stable isotope ratio δ15N of benthic POM consumers: the role of particle dynamics and organism trophic guild. Deep Sea Research I 54:1015–23. doi:10.1016/j.dsr.2007.03.005
- Mohn C, Rengstorf A, White M, Duineveld G, Mienis F, Soetaert K, Grehan A. 2014. Linking benthic hydrodynamics and cold-water coral occurrences: a high-resolution model study at three cold-water coral provinces in the NE Atlantic. Progress in Oceanography 122:92–104. doi:10.1016/j.pocean.2013.12.003
- Moreno Navas J, Miller PI, Henry L-A, Hennige SJ, Roberts JM. 2014. Ecohydrodynamics of cold-water coral reefs: a case study of the Mingulay Reef Complex (Western Scotland). PLoS One 9(5):e98218. 12 pages. doi:10.1371/journal.pone.0098218
- Mueller CE, Larsson AI, Veuger B, Middelburg JJ, van Oevelen D. 2014. Opportunistic feeding on various organic food sources by the cold-water coral Lophelia pertusa. Biogeosciences 11:123–33. doi:10.5194/bg-11-123-2014
- Nash R, Keegan BF. 2003. Aspects of the feeding biology of the fanworm Bispira volutacornis (Polychaeta: Sabellidae). Journal of the Marine Biological Association of the United Kingdom 83:453–56. doi:10.1017/S002531540300732Xh
- Neves G, Omena E. 2003. Influence of sponge morphology on the composition of the polychaete associated fauna from Rocas Atoll, northeast Brazil. Coral Reefs 22:123–29. doi:10.1007/s00338-003-0295-4
- Nielsen C, Riisgard HU. 1998. Tentacle structure and filter-feeding in Crisia eburnea and other cyclostomatous bryozoans, with a review of upstream-collecting mechanisms. Marine Ecology Progress Series 168:163–86. doi:10.3354/meps168163
- Padua A, Lanna E, Klautau M. 2013. Macrofauna inhabiting the sponge Paraleucilla magna (Porifera: Calcarea) in Rio de Janeiro, Brazil. Journal of the Marine Biological Association of the United Kingdom 93:889–98. doi:10.1017/S0025315412001804
- Peipoch M, Marti E, Gacia E. 2012. Variability in δ15N natural abundance of basal resources in fluvial ecosystems: a meta-analysis. Freshwater Science 31:1003–15. doi:10.1899/11-157.1
- Perea-Blázquez A, Davy SK, Bell JJ. 2012. Estimates of particulate organic carbon flowing from the pelagic environment to the benthos through sponge assemblages. PLoS One 7(1):e29569. 11 pages. doi:10.1371/journal.pone.0029569
- Peterson BJ, Fry B. 1987. Stable isotopes in ecosystem studies. Annual Review of Ecology and Systematics 18:293–320. doi:10.1146/annurev.es.18.110187.001453
- Post DM. 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–18. doi:10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
- Purser A, Thomsen L. 2012. Monitoring strategies for drill cutting discharge in the vicinity of cold-water coral ecosystems. Marine Pollution Bulletin 64:2309–16. doi:10.1016/j.marpolbul.2012.08.003
- Purser A, Larsson AI, Thomsen L, van Oevelen D. 2010. The influence of flow velocity and food concentration on Lophelia pertusa (Scleractinia) zooplankton capture rates. Journal of Experimental Marine Biology and Ecology 395:55–62. doi:10.1016/j.jembe.2010.08.013
- R Core Team. 2013. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. http://www.R-project.org. Computer program.
- Reid WDK, Wigham BD, McGill RAR, Polunin NVC. 2012. Elucidating trophic pathways in benthic deep-sea assemblages of the Mid-Atlantic Ridge north and south of the Charlie-Gibbs Fracture Zone. Marine Ecology Progress Series 463:89–103. doi:10.3354/meps09863
- Rennie MD, Ozersky T, Evans DO. 2012. Effects of formalin preservation on invertebrate stable isotope values over decadal time scales. Canadian Journal of Zoology 90:1320–27. doi:10.1139/z2012-101
- Roberts JM, shipboard party. 2013. Changing Oceans Expedition 2012. RRS James Cook 073 Cruise Report. Edinburgh: Heriot-Watt University. 224 pages.
- Roberts JM, Brown CJ, Long D, Bates CR. 2005. Acoustic mapping using a multibeam echosounder reveals cold-water coral reefs and surrounding habitats. Coral Reefs 24:654–69. doi:10.1007/s00338-005-0049-6
- Roberts JM, Wheeler AJ, Freiwald A. 2006. Reefs of the deep: the biology and geology of cold-water coral ecosystems. Science 312:543–47. doi:10.1126/science.1119861
- Roberts JM, Davies AJ, Henry L, Dodds LA, Duineveld GCA, Lavaleye MSS, et al. 2009. Mingulay reef complex: an interdisciplinary study of cold-water coral habitat, hydrography and biodiversity. Marine Ecology Progress Series 397:139–51. doi:10.3354/meps08112
- Ruhl HA. 2008. Community change in the variable resource habitat of the abyssal northeast Pacific. Ecology 89:991–1000. doi:10.1890/06-2025.1
- Ruhl HA, Smith Jr KL. 2004. Shifts in deep-sea community structure linked to climate and food supply. Science 305:513–15. doi:10.1126/science.1099759
- Ruiz-Cooley R, Garcia K, Hetherington E. 2011. Effects of lipid removal and preservatives on carbon and nitrogen stable isotope ratios of squid tissues: implications for ecological studies. Journal of Experimental Marine Biology and Ecology 407:101–07. doi:10.1016/j.jembe.2011.07.002
- Saino T, Hattori A. 1980. 15N natural abundance in oceanic suspended particulate matter. Nature 283:752–54. doi:10.1038/283752a0
- Sarakinos HC, Johnson ML, Zanden MJV. 2002. A synthesis of tissue-preservation effects on carbon and nitrogen stable isotope signatures. Canadian Journal of Zoology 80:381–87. doi:10.1139/z02-007
- Schejter L, Chiesa IL, Doti BL, Bremec C. 2012. Mycale (Aegogropila) magellanica (Porifera: Demospongiae) in the southwestern Atlantic Ocean: Endobiotic fauna and new distributional information. Scientia Marina 76:753–61.
- Sweetman AK, Witte U. 2008. Response of an abyssal macrofaunal community to a phytodetrital pulse. Marine Ecology Progress Series 355:73–84. doi:10.3354/meps07240
- Syväranta J, Vesala S, Rask M, Ruuhijärvi J, Jones RI. 2008. Evaluating the utility of stable isotope analyses of archived freshwater sample materials. Hydrobiologia 600:121–30. doi:10.1007/s10750-007-9181-3
- Syväranta J, Martino A, Kopp D, Céréghino R, Santoul F. 2011. Freezing and chemical preservatives alter the stable isotope values of carbon and nitrogen of the Asiatic clam (Corbicula fluminea). Hydrobiologia 658:383–88. doi:10.1007/s10750-010-0512-4
- Tecchio S, van Oevelen D, Soetaert K, Navarro J, Ramirez-Llodra E. 2013. Trophic dynamics of deep-sea megabenthos are mediated by surface productivity. PLoS One 8(5):e63796. 8 pages. doi:10.1371/journal.pone.0063796
- Toupoint N, Gilmore-Solomon L, Bourque F, Myrand B, Pernet F, Olivier F, Tremblay R. 2012. Match/mismatch between the Mytilus edulis larval supply and seston quality: effect on recruitment. Ecology 93:1922–34. doi:10.1890/11-1292.1
- Tyler PA, Paterson GJL, Sibuet M, Guille A, Murton BJ, Segonzac M. 1995. A new genus of ophiuroid (Echinodermata: Ophiuroidea) from hydrothermal mounds along the Mid-Atlantic Ridge. Journal of the Marine Biological Association of the United Kingdom 75:977–86. doi:10.1017/S0025315400038303
- Vad J. 2013. Lophelia pertusa and Associated Species Spatial Distribution Patterns and Density at Mingulay Reef Complex. Internship Report. Paris: Ecole Normale Supérieure. 21 pages.
- Vader W. 1983. Associations between amphipods (Crustacea: Amphipoda) and sea anemones (Anthozoa, Actiniaria). In: Lowry JK, editor. Papers from the Conference on the Biology and Evolution of Crustacea. Australian Museum Memoir 18. Sydney: Trustees of the Australian Museum, p 141–53.
- Vafeiadou A, Adão H, De Troch M, Moens T. 2013. Sample acidification effects on carbon and nitrogen stable isotope ratios of macrofauna from a Zostera noltii bed. Marine and Freshwater Research 64:741–45. doi:10.1071/MF12169
- van Oevelen D, Duineveld G, Lavaleye M, Mienis F, Soetaert K, Heip CHR. 2009. The cold-water coral community as a hotspot for carbon cycling on continental margins: a food-web analysis from Rockall Bank (northeast Atlantic). Limnology and Oceanography 54:1829–44. doi:10.4319/lo.2009.54.6.1829
- van Soest RWM, Lavaleye MSS. 2005. Diversity and abundance of sponges in bathyal coral reefs of Rockall Bank, NE Atlantic, from boxcore samples. Marine Biology Research 1:338–49. doi:10.1080/17451000500380322
- van Soest RWM, Cleary DFR, de Kluijver MJ, Lavaleye MSS, Maier C, van Duyl FC. 2007. Sponge diversity and community composition in Irish bathyal coral reefs. Contributions to Zoology 76:121–42.
- van Weering TCE, de Haas H, de Stigter HC, Lykke-Andersen H, Kouvaev I. 2003. Structure and development of giant carbonate mounds at the SW and SE Rockall Trough margins, NE Atlantic Ocean. Marine Geology 198:67–81. doi:10.1016/S0025-3227(03)00095-1
- Vander Zanden MJ, Fetzer WW. 2007. Global patterns of aquatic food chain length. Oikos 116:1378–88. doi:10.1111/j.0030-1299.2007.16036.x
- Vander Zanden MJ, Rasmussen JB. 1999. Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology 80:1395–404. doi:10.1890/0012-9658(1999)080[1395:PCCANA]2.0.CO;2
- Wang Y, Yu X, Zhang L, Lei G. 2013. Seasonal variability in baseline δ15N and usage as a nutrient indicator in Lake Poyang, China. Journal of Freshwater Ecology 28:365–73. doi:10.1080/02705060.2013.763296
- Wei C, Rowe GT, Escobar-Briones E, Boetius A, Soltwedel T, Caley MJ, et al. 2010. Global patterns and predictions of seafloor biomass using Random Forests. PLoS One 5(12):e15323. 15 pages. doi:10.1371/journal.pone.0015323
- Westinga E, Hoetjes PC. 1981. The intrasponge fauna of Spheciospongia vesparia (Porifera, Demospongiae) at Curaçao and Bonaire. Marine Biology 62:139–50. doi:10.1007/BF00388176
- Wheeler AJ, Beyer A, Freiwald A, de Haas H, Huvenne VAI, Kozachenko M, et al. 2007. Morphology and environment of cold-water coral carbonate mounds on the NW European margin. International Journal of Earth Sciences 96:37–56. doi:10.1007/s00531-006-0130-6
- White M, Wolff GA, Lundälv T, Guihen D, Kiriakoulakis K, Lavaleye M, Duineveld G. 2012. Cold-water coral ecosystem (Tisler Reef, Norwegian Shelf) may be a hotspot for carbon cycling. Marine Ecology Progress Series 465:11–23. doi:10.3354/meps09888
- Wigham BD, Hudson IR, Billett DSM, Wolff GA. 2003a. Is long-term change in the abyssal Northeast Atlantic driven by qualitative changes in export flux? Evidence from selective feeding in deep-sea holothurians. Progress in Oceanography 59:409–41. doi:10.1016/j.pocean.2003.11.003
- Wigham BD, Tyler PA, Billett DSM. 2003b. Reproductive biology of the abyssal holothurian Amperima rosea: an opportunistic response to variable flux of surface derived organic matter? Journal of the Marine Biological Association of the United Kingdom 83:175–88. doi:10.1017/S0025315403006957h
- Winston JE. 1977. Feeding in marine bryozoans. In: Woollacott RM, Zimmer RL, editors. Biology of Bryozoans. New York: Academic Press, p 233–71.
- Witte U, Brattegard T, Graf G, Springer B. 1997. Particle capture and deposition by deep-sea sponges from the Norwegian-Greenland Sea. Marine Ecology Progress Series 154:241–52. doi:10.3354/meps154241
- Wolff GA, Billett DSM, Bett BJ, Holtvoeth J, FitzGeorge-Balfour T, Fisher EH, et al. 2011. The effects of natural iron fertilisation on deep-sea ecology: the Crozet Plateau, southern Indian Ocean. PLoS One 6(6):e20697. 9 pages. doi:10.1371/journal.pone.0020697
- Xu J, Yang Q, Zhang M, Zhang M, Xie P, Hansson L. 2011a. Preservation effects on stable isotope ratios and consequences for the reconstruction of energetic pathways. Aquatic Ecology 45:483–92. doi:10.1007/s10452-011-9369-5
- Xu J, Zhang M, Xie P. 2011b. Sympatric variability of isotopic baselines influences modeling of fish trophic patterns. Limnology 12:107–15. doi:10.1007/s10201-010-0327-z