ABSTRACT
The systematics of Coralliidae has been revised based on molecular phylogenetic analysis and detailed morphological studies. This revision has also revealed the existence of new species. In order to fully accomplish this revision, five new species including Hemicorallium aurantiacum sp. nov., Pleurocorallium bonsaiarborum sp. nov., P. clavatum sp. nov., and P. norfolkicum sp. nov. from New Caledonia, and H. guttatum sp. nov. from the Hawaiian Archipelago are described by integrating the phylogenetic inference and morphological comparisons. Moreover, the type specimens of Corallium tortuosum, H. reginae, H. halmaheirense, P. porcellanum and P. kishinouyei as well as non-type specimens of C. stylasteroides are redescribed. The sclerites of H. reginae, H. halmaheirense, P. porcellanum and C. stylasteroides were first depicted by scanning electron microcopy. A thorough comparison between P. porcellanum and P. kishinouyei indicated that they should be regarded as one species and the former was adopted as the senior synonym. The two new species of Hemicorallium can be separated by morphological features based on the result of multiple factor analysis. A key is proposed for the identification of all existing species of Coralliidae.
http://zoobank.org/urn:lsid:zoobank.org:pub:7B772D08-E3D6-4585-AE78-E5A31BE32ECC
RESPONSIBLE EDITOR:
Introduction
Coralliid corals (Anthozoa: Octocorallia) are distributed throughout all the oceans, from tropical to high latitudes, and from shallow to deep seas. Lamouroux (Citation1812) erected the family Coralliidae and since then, octocorals featuring a continuous stony axis of calcium carbonate and covered with a thin cortical layer have been classified as members of this family (Bayer Citation1956). Currently, there are 39 species of coralliid corals with a higher diversity in the Pacific Ocean (Nonaka et al. Citation2012; Tu et al. Citation2012, Citation2015a).
For centuries, due to the dense and colourful texture of their skeleton, coralliids have been intensively harvested for jewellery (Andaloro & Cicogna Citation1993; Grigg Citation1993). However, due to their slow growth rates and low fecundity, they are sensitive to fishing pressure (Linares et al. Citation2012; Nonaka et al. Citation2015). In recent years, more and more evidence has revealed the shift of the population structure of coralliids in major fishing grounds (Bramanti et al. Citation2014). In order to accurately identify the harvested species, several studies have reviewed the taxonomy of corallid corals by molecular information coupled with morphological characters. Based on the results, a three-genera scheme of classification proposed by Gray (Citation1867) was resurrected and redescribed (Ardila et al. Citation2012; Figueroa & Baco Citation2014; Tu et al. Citation2015b). Furthermore, the results revealed the existence of potential new species from New Caledonia. In the mitochondrial gene-based phylogenetic trees, three clades in Pleurocorallium were inferred as monophyletic or singletons, and independent from the valid species (Tu et al. Citation2015b, figure 1). Although the three clades have been regarded as independently evolved lineages by molecular information, their morphological characters have not been compared with described species. Therefore, the purpose of this study is to integrate morphological and molecular features for a thorough taxonomic revision of Coralliidae, focusing in particular on two Pacific archipelagos.
Current evidence has revealed that due to the recent diversification times and low mitochondrial DNA divergence, the widely used mitochondrial gene cannot delimit species in Hemicorallium (Ardila et al. Citation2012; Figueroa & Baco Citation2014; Tu et al. Citation2015b). The molecular resolution is inconsistent with morphological characters in Hemicorallium (Ardila et al. Citation2012). Therefore, morphological features of specimens in Hemicorallium were compared in detail. Two monophylies from New Caledonia and the Hawaiian Archipelago, respectively, are morphologically distinct from the other valid Hemicorallium. Thus, they were regarded as new species and are described here in order to fully accomplish the revision initiated by Tu et al. (Citation2015b). Furthermore, morphometrics will be introduced to statistically test for differences among Hemicorallium species. Both qualitative and quantitative features will be evaluated by multiple factor analysis (MFA; Blackith & Reyment Citation1971; Abdi et al. Citation2013).
Traditionally, the description of most species in Coralliidae mainly focused on the qualitative (presence/absence) depiction of morphological characters and lacked quantitative (size ranges) comparisons between morphologically closely related species. Additionally, the description of species distributed in the Indian Ocean was based on small fragments and lacked intraspecific comparisons (Ridley Citation1882; Thomson & Henderson Citation1906). This made the comparison and identification of newly encountered specimens very difficult. Therefore, the type specimens of five species and several non-type specimens are here redescribed. Five new species are described, one species is synonymized and an identification key is provided. This key includes also all other 37 valid coralliid species, of which 28 were examined and directly compared by the authors.
Material and methods
Material examined and its provenance
This study was based primarily on the material collected from the seamounts and ridges in the Coral Sea near New Caledonia, in the Central Pacific Ocean near the Hawaiian Archipelago and in the Indian Ocean near Reunion and Somalia by various cruises in recent decades and deposited in the invertebrate collection of the Muséum National d’Histoire Naturelle Paris, France (MNHN), the National Museum of Natural History, Smithsonian Institution, Washington, DC, USA (NMNH) and the Senckenberg Naturmuseum, Frankfurt, Germany (SMF). Type materials of described species deposited in MNHN, NMNH, ASIZ (Biodiversity Research Museum, Academia Sinica, Taipei, Taiwan), NMNS (National Museum of Natural Science, Taichung, Taiwan), NBC (Naturalis Biodiversity Center, Leiden, Netherlands), and IORAS (P. P. Shirshov Institute of Oceanology of the Russian Academy of Sciences, Moscow, Russia) were reviewed.
Specimen examination
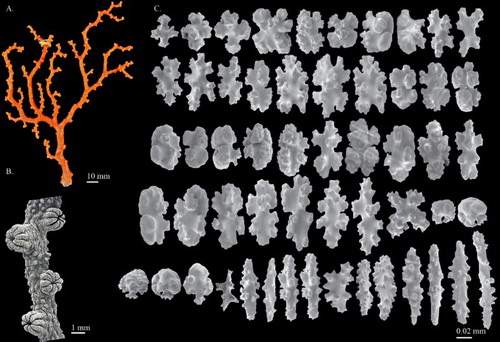
Coral fragments were treated using standard procedures, and images of sclerites and axis ultrastructure were obtained by scanning electron microscopy (SEM), using FEI Quanta 200 and Zeiss EVO 15LS SEM microscopes. The identification and description of Coralliidae is based on the 19 informative morphological characters listed in . Colonial, branch and autozooid characters are useful for genus-level identification. The differences in spiculation are essential for species-level identification. The types of sclerites found in Coralliidae are divided into four major categories, i.e. spindles and rods, multi-radiate forms (cross, 6-, 7- and 8-radiates), double clubs, as well as irregular forms. Each multi-radiate form is further divided into symmetric and asymmetric types (). For each specimen, 50 sclerites from branches were counted to examine the composition of the sclerites.
Table I. Morphological characters and states used for identification in Coralliidae.
Morphometric measurement and multiple factor analysis
Because double clubs as well as 6- and 8-radiates are the three major types of sclerites in Hemicorallium, their shapes were quantified by ImageJ (Rasband Citation2012). The quantitative parameters include the height, width, width/height ratio, circularity, aspect ratio, roundness and solidity, which is the area of selected features divided by the convex area, an indicator to show how ruffled the border of the selected features is (Ferreira & Rasb Citation2012). The measurement of each sclerite was performed on an SEM image and depended on the chromatic aberration between sclerites and background, automatically selected and measured by ImageJ. For each specimen, at least 10 sclerites of each type were measured and averaged. Morphological data of H. boshuense (Kishinouye, Citation1903), H. maderense (Johnson, Citation1898) and H. variabile (Thomson & Henderson, Citation1906) were not included because there are no appropriate specimens from which to take SEM photos of sclerites.
Multiple factor analysis (MFA) was carried out considering the species as the dependent variables (the groups) and the morphological characters, both qualitative and quantitative, as independent variables (the predictors). For all Hemicorallium species, the morphological characters that appear identical including branching pattern, the absence of axis pits, the contractile autozooids and the presence of long spindles, as well as colour features, were removed, and the remaining were regarded as qualitative features in MFA. Meanwhile, the quantitative features in MFA were composed of the mean values of the above seven measurements in each type of sclerite. Therefore, there were 36 variables engaged in the MFA. This analysis was run using FactoMine in R (Lê et al. Citation2008). The purpose was to examine the discriminatory capacity of the currently used morphological characters while trying to organize the observations.
Comparative material examined
Collection specimens of the following 28 species were studied and compared (see also the material listed in Tu et al. Citation2015a): Corallium japonicum Kishinouye, Citation1903, C. rubrum (Linnaeus, Citation1758), Hemicorallium sulcatum (Kishinouye, Citation1903), Pleurocorallium carusrubrum (Tu, Dai & Jeng, Citation2012), P. elatius (Ridley, Citation1882), P. inutile (Kishinouye, Citation1902) and P. konojoi (Kishinouye, Citation1903) from ASIZ; H. taiwanicum Tu et al., Citation2012 from NMNS; C. nix Bayer, Citation1996 and P. thrinax (Bayer and Stefani, in Bayer Citation1996) from MNHN; C. medea Bayer, Citation1964, H. abyssale (Bayer, Citation1956), H. bathyrubrum (Simpson & Watling, Citation2011), H. bayeri (Simpson & Watling, Citation2011), H. ducale (Bayer, Citation1955), H. imperiale (Bayer, Citation1955), H. laauense (Bayer, Citation1956), H. niobe (Bayer, Citation1964), H. regale (Bayer, Citation1956), P. borneense (Bayer, Citation1950), P. gotoense (Nonaka, Muzik & Iwasaki, Citation2012), P. kishinouyei (Bayer, Citation1996), P. niveum (Bayer, Citation1956), P. secundum (Dana, Citation1846) and P. uchidai (Nonaka, Muzik & Iwasaki, Citation2012) from NMNH; H. tricolor (Johnson, 1899) from SMF; H. variabile (Thomson and Henderson, Citation1906) from Indian Museum, Kolkata, India; C. johnsoni Gray, Citation1860 from Natural History Museum, London, UK (NHM); and P. occultum (Tu, Altuna & Jeng, 2015) from National Museum of Natural History, Madrid, Spain,
Results
Five new species are described and 38 worldwide distributed species are reviewed in this study, resulting in 42 species here recognized as valid (). Because non-type or type specimens of Hemicorallium boshuense, H. maderense and P. pusillum (Kishinouye, Citation1904) are not available, their characteristics in the following key are based on the original descriptions. The type specimens of H. reginae, H. halmaheirense and P. porcellanum, as well as a non-type specimen of C. stylasteroides Ridley, Citation1882, are redescribed in this study to provide complete morphological data for comparisons between species from the southern Pacific.
Table II. List of coralliid species as a result of the taxonomic revision.
Taxonomy
Class Anthozoa Ehrenberg, Citation1834
Order Alcyonacea Verrill, Citation1865
Family Coralliidae Lamouroux, Citation1812
Genus Corallium Cuvier, 1798
()
Type species: Madrepora rubra Linnaeus, Citation1758
Corallium Cuvier, Citation1798: 673; Lamarck 1801: 378; Gray Citation1867: 126; Ridley Citation1882: 222; Kishinouye Citation1904: 20; Hickson Citation1907: 2; Kükenthal Citation1924: 47; Bayer Citation1956: 73; Bayer Citation1964: 466; Pasternak Citation1981: 43; Bayer & Cairns Citation2003: 222; Castro et al. 2003: 1; Simpson & Watling Citation2011: 370; Ardila et al. Citation2012: 252; Nonaka et al. Citation2012: 3; Tu et al. Citation2012: 1; Tu et al. Citation2015a: 1; Tu et al. Citation2015b: 180.
Paracorallium Bayer & Cairns, Citation2003: 224; Uda et al. 2011: 36; Ardila et al. Citation2012: 252.
Diagnosis
Modified from Tu et al. Citation2015b. Coralliidae with pits ranging from slightly sunken (A,C) to deep pits on solid axis, pits usually raindrop-shaped (Figure S1B, supplementary material) especially near branch tips; axis has strong longitudinal grooves. Polyps are dimorphic and have sclerites. Autozooids retractile, can fully retract into cortical mounds (A,D). Siphonozooids fertile, normally between autozooids, and they may be hardly visible. Sclerites include crosses (Figure S1Ca), small rods (Figure S2Ea), 6- (Aa), 7- (Ba) and 8-radiates (Ab), which is the dominant type of sclerite in all species of Corallium; double clubs (Ac) only present in some species. However, long spindles (Ba) in tentacles have not been found in any species of Corallium.
Distribution
Atlantic, the Mediterranean Sea, Indian Ocean, Central Pacific, West Pacific. Except for C. rubrum, which is distributed from 20 to 600 m, distributed between 100 and 600 m.
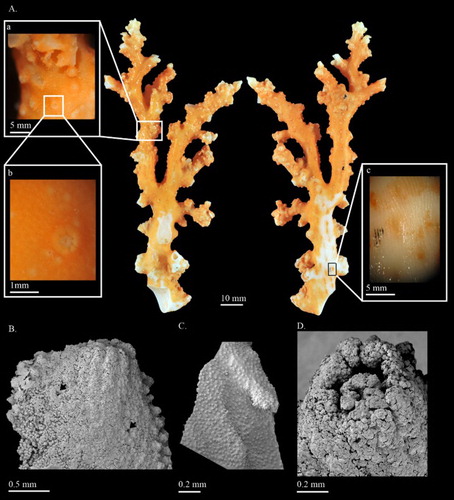
Corallium stylasteroides Ridley, 1882
(, )
Corallium stylasteroides Ridley, Citation1882: 225, pl. IX, figures 1–4; Bayer Citation1956: 74; Bayer Citation1993: 17, pl. 17; Tu et al. Citation2015b: 181.
Paracorallium stylasteroides. Bayer & Cairns Citation2003: 225.
Holotype
Deposited in NHM, association number not mentioned, Mauritius, 137 m, collected by Mr V. De Robillard (not seen).
Non-types
USNM 77450, RV Anton Bruun, IIOE, St. 463, Ras Binnah, Somalia, 11°24′00.00″N, 51°34′48.00″E, 75–175 m, 17 Dec 1964; SMF 11212, Marion Dufresne, MD32, St. FA40, off Saint-Pierre, Reunion, 21°21′06.00″S, 55°26′42.00″E, 150 m, 18 Aug 1982.
Diagnosis
Modified from Ridley (Citation1882) with additions. Colonies normally branch dichotomously in a uniplanar pattern. Cortex overlies the axis very thinly. Surface of axis decorated with longitudinal grooves, some modified to pits to accommodate autozooids. Retractile autozooids form cortical mounds that are slightly raised from the surface of the cortex and distributed singly on all sides of the colony. Siphonozooids inconspicuous and surrounding the cortical mounds. Predominant sclerite types are various radiate forms including 6-, 7- and 8-radiates. Double clubs and irregular forms also present.
Description
USNM 77450, 129.3 mm tall and 63.7 mm wide, is an almost complete colony which has only lost the holdfast. The colony branches in one plane up to the second degree of subdivision (A). An obvious broken edge at the base of the colony may have resulted from collection. The colony is composed of the main stem and the major branches without any tiny branchlets. The cross-sections of all branches are near-elliptic and gradually taper from 13.5 mm at base to 4.0 mm at tip. Around the distal points, there are some curled proliferous sheets protruded from branches (A).
The cortex is ca. 0.040–0.060 mm thick and abraded on one side of the main stem (A). Small, finely raised papillae evenly scattered over cortex on all sides of the colony (Aa,b,B). The surface of the axis is decorated with longitudinal grooves, which form narrow raised lips on each side to accommodate autozooids (Ac). Under SEM the surface of the axis exhibits regularly spaced structures resembling cone-like papillae (C). At the broken edge, growth rings can easily be observed.
Autozooids retractile, sitting singly in axial pits and distributed on all sides of the colony. When contracted, autozooids form cortical mounds that are slightly raised from the surface of the cortex (Aa,b,D) and measure 0.89–0.98 mm wide and ca. 0.46 mm tall. Siphonozooids appear relatively small, ca. 0.2 mm in diameter, rounded mounds with an apical pore (B indicated by black arrows). Some papillae on the surface of the cortex appear to have one or more pores that connect to a canal-like solenial system.
Sclerites
Eight-radiates represent 53% of sclerites at tip of the colony where they intermix with 6-radiates 28%, 7-radiates 5%, double clubs 11% and irregular form 3% (A). The composition and size of sclerites in the cortex at the base of colony (B) is similar to those at the tip and wall of autozooids (C). Long spindles are absent in this species. Both 6- and 8-radiates vary in symmetry and are similar in size, ca. 0.045 mm long for 6-radiates and 0.045–0.062 mm long for 8-radiates. Double clubs are modified from 8-radiates with rough heads, up to 0.053 mm wide.
Colour
The colony has an orange cortex with some yellowish-white parts at the cuspidate distal points. The colour of cortical mounds is orange similar to that of main stems and most branches. The axis is translucent to white; individual sclerites are transparent or light orange when examined with a microscope under transmitted light.
Commensals/epiphytes
Three commensal barnacles (Family: Pyrgomatidae) were found on branches.
Distribution
This species has been collected from the continental shelf near Mauritius, Somalia and Reunion in the Indian Ocean at depths between 75 and 150 m.
Remarks
The type specimen collected from Mauritius is at present not accessible for study. The identification of C. stylasteroides was based on the original description and the illustration of the original slide preparation of sclerites from the type specimen in Bayer (Citation1993). In the original description of C. stylasteroides, the colony and 8-radiates were well illustrated (Ridley Citation1882). However, the re-examination of the original slide preparation of sclerites from the holotype revealed four additional sclerites including 6-, 7-radiates, double clubs and irregular forms (Bayer Citation1993). The morphological characters of USNM 77450 and SMF 11212 closely resemble the colony described by Ridley (Citation1882) and sclerites by Bayer (Citation1993). Moreover, the two specimens were collected from Somalia and Reunion adjacent to the collection site of the holotype. Therefore, the two specimens were tentatively identified as C. stylasteroides and redescribed in this study.
Corallium tortuosum Bayer, 1956
(Figure S1, S2)
Corallium tortuosum Bayer, Citation1956: 82, figures 5b, 6c, 8e–g; Tu et al. Citation2015b: 181.
Corallium salomonense tortuosum. Bayer Citation1993: 16, pls. 10, 14–16.
Paracorallium tortuosum. Bayer & Cairns Citation2003: 225; Parrish et al. Citation2009: 87; Ardila et al. Citation2012: 248; Tu et al. Citation2012: 4 (in key); Nonaka et al. Citation2012: 16.
Holotype
USNM 49331 (Figure S1), RV Albatross, Hawaiian Explorations, St. 4100, Maui Island, Hawaii, USA, 21°05′30.12″N, 156°40′30.00″W, 238–276 m, 23 Jul 1902. (Examined.)
Paratypes
USNM 49332, RV Albatross, Hawaiian Explorations, St. 4045, Hawaii Island, Hawaii, USA, 20°01′45.12″N, 155°54′14.40″W, 269–362 m, 11 Jul 1902; USNM 49333, RV Albatross, Hawaiian Explorations, St. 3838, Molokai Island, Hawaii, USA, 21°04′05.16″N, 157°10′33.60″W, 168–388 m, 4 Apr 1902; USNM 49334, RV Albatross, Hawaiian Explorations, St. 3863, Maui Island, Hawaii, USA, 21°05′44.88″N, 156°40′51.60″W, 232–282 m, 10 Apr 1902; USNM 49469, RV Albatross, Hawaiian Explorations, St. 3885, Maui Island, Hawaii, USA, 21°05′09.96″N, 156°40′44.40″W, 249–271 m, 17 Apr 1902; USNM 52202, RV Albatross, Hawaiian Explorations, St. 4100, Maui Island, Hawaii, USA, 21°05′30.12″N, 156°40′30.00″W, 238–276 m, 23 Jul 1902.
Non-types
MNHN-IK-2011-1458, RV Alis, LITHIST, St. DW 13, Antigonia seamount, Norfolk Ridge, New Caledonia, 23°45′00.00″S, 167°42′00.57″E, 400 m, 12 Aug 1999; MNHN-IK-2011-1313, RV Alis, LITHIST, St. CP03, Antigonia seamount, Norfolk Ridge, New Caledonia, 23°37′00.01″S, 167°41′29.99″E, 447 m, 10 Aug 1999; MNHN-IK-2011-1462, RV Alis, TERRASSES, St. DW 3069, Antigonia seamount, Norfolk Ridge, New Caledonia, 23°18′06.01″S, 168°04′41.98″E, 300–320 m, 22 Oct 2008; USNM 1089600, RV Alis, Norfolk 2, St. 2049, Norfolk Ridge, New Caledonia, 23°42′51.84″S, 168°15′25.20″E, 470–621 m, 24 Oct 2003.
Diagnosis
Modified from Bayer (Citation1956). Colonies irregularly branching and most often in one plane but occasionally perpendicular to the dominant plane. Cortex overlies the axis, extremely thin with finely granular structures. Surface of axis decorated with longitudinal grooves and raindrop-shaped pits (Figure S1B). Retractile autozooids form inconspicuous cortical mounds that are distributed singly on all sides of the colony. Siphonozooids scattered on the cortex but most commonly near cortical mounds. Eight-radiates and crosses are the two major types of sclerites in both cortex and polyps. Small crosses and irregular rods in pharyngeal region. Cortex is pale pink or salmon pink in colour, and the region surrounding cortical mounds is darker.
Description
Modified from Bayer (Citation1956) with additions. MNHN-IK-2011-1458, 74.8 mm tall and 71.0 mm wide, is a complete colony with holdfast and intact branches (Figure S2). There are four branches vertically extending from the main stem and projecting in varying dimensions, forming a tridimensional and irregular-shaped colony. Both main stem and branches are robust, erratically curved, twisted and fixed at a similar width from base to tip. Branches and terminal branchlets usually ending in autozooid pits with slightly beaded margins similar to the shape of a raindrop. The cross-sections of the main stem and branches are irregular, ranging from 21.2 mm to 1.8 mm in diameter.
The cortex is ca. 0.05 mm thick and only slightly thickened above the autozooids. Close examination of the cortex surface reveals finely granular structures longitudinally arranged above the longitudinal grooves on the axis (Figure S2A). The autozooid pits on the skeleton are close to elliptic, ranging from 1.2 to 1.5 mm on the long axis. The surface of the skeleton possesses longitudinal grooves and is scattered with minute tubercles as well as thorny protuberances (Figure S2B).
Autozooids retractile, sit singly in axial pits (Figure S2C) and distributed on all sides of the colony, while on the terminal end of branchlets two to three polyps are grouped together within one pit. In contracted state, autozooids are fully hidden in the cortical mounds, measuring approximately 0.8 mm wide. Siphonozooids appear relatively small and faint as apical pores near cortical mounds.
Sclerites
In the cortex, crosses represent 36% of sclerites, 7-radiates 4% and 8-radiates 60% (Figure S2D). On the polyp wall, sclerites are similar to those present in the cortex (Figure S2E). Pharyngeal sclerites are short rods measuring from 0.042 to 0.047 mm long (Figure S2E). Each type of radiate and cross varies slightly in size and symmetry, ca. 0.053 mm long for 7-radiates, 0.051–0.062 mm long for 8-radiates and 0.040–0.049 mm long for crosses.
Colour
The colony has light yellowish-white cortex with tiny dark yellow granules and brown autozooids. The axis is pure white to slightly transparent; sclerites are transparent when examined under transmitted light.
Distribution
This species has been collected from the Hawaiian Islands, Taiwan and Norfolk Ridge from a depth of 200 to 500 m. This is the first record of C. tortuosum in the southern Pacific Ocean.
Comparisons
Intraspecifically, specimens of Corallium tortuosum from the Hawaiian Islands (Figure S1) and the Norfolk Ridge (Figure S2) are similar in morphology with only a few minor exceptions. The size of sclerites in colonies from the Hawaiian Islands is larger (0.062–0.095 mm for 8-radiates) than those in colonies from the Norfolk Ridge (0.051–0.06 mm for 8-radiates). Additionally, colonies from the Norfolk Ridge usually possess a higher ratio of asymmetrical 8-radiates.
The differences between C. stylasteroides and C. tortuosum include the growth form and sclerites. Corallium stylasteroides generally branches dichotomously in a uniplanar pattern. However, occasionally C. tortuosum may be abundantly and irregularly branched in one plane. The predominant sclerite types of C. stylasteroides are various radiate forms including 6-, 7- and 8-radiates, whereas crosses and 8-radiates are the dominant forms of sclerites in C. tortuosum. Furthermore, double clubs are only present in C. stylasteroides but not in C. tortuosum. Corallium stylasteroides has been recorded in the western Indian Ocean, while C. tortuosum is distributed in the Pacific Ocean near the Hawaiian Islands, Taiwan and the Norfolk Ridge.
Genus Hemicorallium Gray, 1867
()
Type species: Pleurocorallium tricolor Johnson, Citation1898
Hemicorallium Gray, Citation1867: 126; Ridley Citation1882: 222; Johnson 1899: 59; Kükenthal 1924: 47; Bayer Citation1956: 74; Bayer Citation1964: 467; Bayer & Cairns Citation2003: 222; Ardila et al. Citation2012: 254; Figueroa & Baco Citation2014: 83; Tu et al. Citation2015a: 302; Tu et al. Citation2015b: 173.
Diagnosis
Modified from Tu et al. (Citation2015b). Polyps are dimorphic and have sclerites. Autozooids are prominent, non-retractile and ovate–cylindrical () usually distributed on one side of the colony. When contracted, the autozooids cannot fully retract into the cortex. Siphonozooids are fertile, usually inconspicuous and distributed near the base of autozooids. Sclerites include small rods, crosses, 6-, 7- and 8-radiates; double clubs only present in some species. Long spindles present in tentacles.
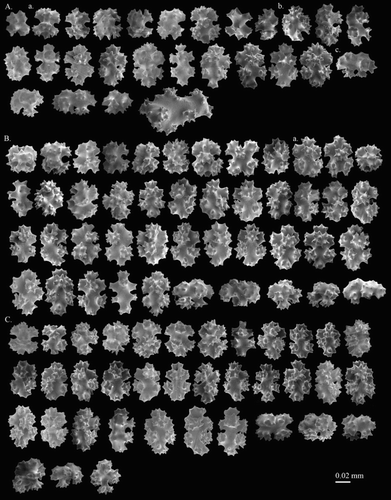
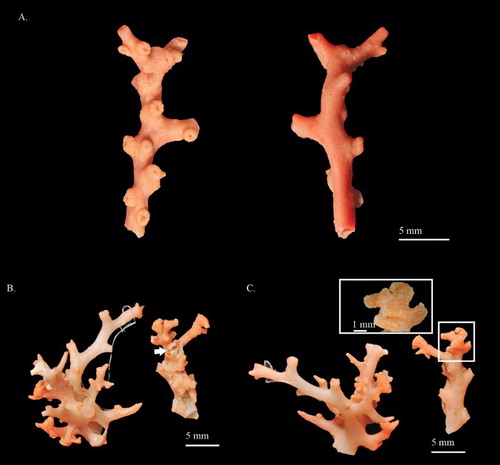
Figure 3. (A) Hemicorallium reginae. Syntype, NBC COEL_02395; (B) Hemicorallium halmaheirense. Syntype, NBC COEL_02393, large fragment, white arrow indicates polychaetes in the middle of the branch; (C) Hemicorallium halmaheirense. Syntype, NBC COEL_02393, small fragment and enlargement of autozooids.
Distribution
North Atlantic, Indian Ocean, Pacific between 100 and 2000 m.
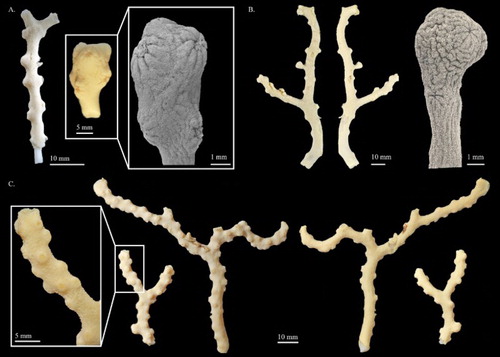
Hemicorallium reginae Hickson, 1907
(A, )
Corallium reginae Hickson Citation1905: 270; Hickson, Citation1907: 4, figures 1–4, 8, 10; Kükenthal Citation1924: 50; Bayer Citation1956: 76 (in key).
Hemicorallium reginae. Tu et al. Citation2015b: 181.
Syntype
NBC, COEL_02395, Siboga Expedition, label lost, fragment.
Diagnosis
Modified from Hickson (Citation1907). Colonies branch simply and irregularly, but principally in one plane. Cortex overlies the axis thickly. The surface of the axis is decorated with longitudinal shallow grooves. Except at the extremities of branches, the contractile autozooids are distributed on one side and surrounded by siphonozooids. The predominant type of sclerite is 8-radiate with 6-, 7- radiates and double clubs less common. Spiny spindles occur in tentacles. Both cortex and axis are dark pink.
Description
Colony COEL_2395 contains only a 38.0 mm tall and 17.8 mm wide fragment without holdfast and tiny branchlets (A). This fragment has two bifurcations, one in the middle of the branch, only 6.38 mm residual, and the other at the top that is almost perpendicular to the main branch. All branches slightly taper from 3.7 mm in diameter at base to 2.2 mm in diameter at tip.
The cortex is ca. 0.3 mm in thickness and increases in thickness in some regions forming irregular creases. Except for the cortical mounds, there are no prominent papillae protruding from the surface of the cortex. The axis is solid, round in cross-section, with longitudinal shallow grooves on its surface.
The autozooids are contractile and restricted to the front of the colony (A). Compared with other Coralliidae species, the number of autozooids is relatively low, containing only 12 polyps. Contracted autozooids form prominent cylinders that are 1.4–1.6 mm in height and 1.3–1.5 mm in diameter with eight prominent longitudinal grooves folding over the intersection of tentacles and extending halfway down the polyp body. Siphonozooids are located in the base of the contacted autozooids forming small round mounds with minute apical pores, approximately 0.28 mm in diameter.
Sclerites
In the cortex, the proportion of crosses, 6-, 7-, 8-radiates, double clubs and irregular forms is 5%, 18%, 6%, 60%, 2% and 9%, respectively (A). Autozooid sclerites include radiates, double clubs and irregular forms which appear similar to those in cortex (B). The outer polyp walls from the base to the aboral surface of the tentacles are composed of radiate forms, especially the asymmetrical 8-radiates ranging from 0.065 to 0.078 mm. On the oral surface of the tentacles, sclerites are substituted by abutting rows of spiny spindles up to 0.106 mm. Sclerites vary in size ranging between 0.050 and 0.056 mm long for 6-radiates, 0.052–0.071 mm for 7-radiates, 0.053–0.082 mm for 8-radiates and 0.074–0.116 mm for irregular forms. The large irregular forms are possibly formed from the fusion of sclerites.
Colour
The colony has a light orange cortex. The colour of the axis is between deep orange and red. The cross-section of the axis exhibits a white spot in the centre.
Distribution
This species is currently known only from the type locality.
Remarks
This species was named in Hickson (Citation1905), but a complete description was given in Hickson (Citation1907). The syntype, COEL_02395 described here, has also been portrayed by Hickson (Citation1907). The two specimens, COEL_02394 and COEL_02395, are the only two collection records of this species and the former has been lost. In the original description only 8-radiates were described, but in the re-examination, six different types of sclerites, including crosses (Aa), 6- (Ab), 7- (Ac), 8- (Ad) radiates, double clubs (Ae) and irregular (Af) forms were observed.
Hemicorallium halmaheirense Hickson, 1907
(B,C, )
Corallium halmaheirense Hickson, Citation1907: 6, figures 5, 6, 9; Kükenthal Citation1924: 51; Bayer Citation1956: 76 (in key).
Hemicorallium halmaheirense. Tu et al. Citation2015b: 181.
Syntype
NBC, COEL_02393, Siboga Expedition, St. 150, Pulau Gebe, Indonesia, 00°06′00.00″N, 129°27′11.88″E, 1089 m, 11 Aug 1899.
Diagnosis
Modified from Hickson (Citation1907). The ramification is mainly in one plane and the terminal branches exhibit secondary branchlets arising on one face only. The cortex overlying the axis is thin, with longitudinal grooves, but the surface of the axis is smooth. Autozooids are contractile and dimorphic, turning forwards on one face. Siphonozooids are distributed between autozooids. Sclerites mostly 8-radiates with fewer crosses and 6-radiates. Autozooid sclerites are similar in form and composition to those in the cortex except for the long spiny spindles lining the oral surface of the tentacles. Both cortex and autozooids are orange–red, and the axis is pale pink.
Description
Modified from Hickson (Citation1907) with additions. COEL_02393 includes two fragments, one 16.9 mm in height and 15.0 mm in width (B) and the other 15.6 mm in height and 8.5 mm in width (C). The former one, corresponding to specimen B in Hickson (Citation1907), is a small colony and it is therefore difficult to determine whether it is a complete colony or a terminal part of a large colony. This colony has the main axis with three branches ranging between 5.3 and 14.5 mm in one plane and two branches protruding at approximately 45 degrees from the main axis (B). Due to the fusion between protruding branches and the main axis, a fistula is formed between the main axis and the protruding branches, with the greatest diameter of 3.8 mm (B). The origin of the missing higher-order branches can be seen near the broken ends of the main branches. The small one is a fragment of a broken branch with a bifurcation at the top (C). Due to the small size and incomplete specimens, the pattern of bifurcation could not be determined.
The surface of the cortex is scraggy and there are longitudinal grooves on the small branches. Along the centre of the vertical section of the small fragment, the cortex is modified to form a tunnel-like structure which harbours commensal polychaetes (C). The main axis of the large fragment is half-moon shaped in cross-section and nearly round. The surface of the axis is smooth, without longitudinal grooves or pits.
Autozooids are contractile and conical in shape with eight deep longitudinal grooves folding over the intersection of tentacles and extending to the base of polyps (C). In the large fragments, the contracted autozooids are 2–3 mm tall near the tip of branches and the others between the protruding branches and the main axis are 0.8–0.9 mm tall. In the small fragments, only one size of autozooids approximately 2–3 mm tall are found and they are distributed over the extending cortex. Terminal branchlets end in a pair of contracted autozooids and are shaped as a capital T (B,C). Siphonozooids are distributed between the contracted autozooids as white specks.
Sclerites
Eight-radiate is the predominant type, representing 62% of sclerites in the cortex where they intermix with 6-radiates (35%) and crosses (3%) (A). Compared with the cortex, the sclerites in the autozooids include spindles 0.075–0.118 mm long which are only present in tentacles, while others are similar to those in the cortex in both composition and size (B). Eight-radiates vary in shape from spherical (Aa) to symmetrical in form, 0.035–0.081 mm long.
Colour
The colour of the cortex and the surface of contracted autozooids is orange–red. The axis is pale pink, almost white, with an eccentric darker pink core.
Commensal/epiphytes
The main branch is modified into tunnels of the commensal polychaete Gorgoniapolynoe sp. (B).
Distribution
This species is currently known only from the type locality.
Comparisons
A complete diagnosis of Hemicorallium halmaheirense was impeded by the fragmentation of the type specimens (Hickson Citation1907). Therefore, a comparison between H. halmaheirense with other similar Coralliidae species is listed below to interpret the taxonomic position of H. halmaheirense. Hickson (Citation1907) discussed the difficulty of separating H. halmaheirense from H. sulcatum, H. tricolor and H. maderense. The absence of double clubs in H. halmaheirense is the major distinction between it and the other three species.
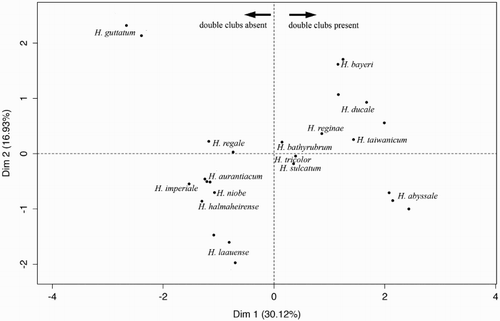
Because double clubs are also absent in H. bathyrubrum, H. imperiale (), H. laauense and H. niobe, H. halmaheirense was compared with them. Hemicorallium halmaheirense differs from H. bathyrubrum in possessing a higher portion of 6-radiates and the distinct shape of 8-radiates. Although both H. halmaheirense (B,C) and H. imperiale (A) possess an orange cortex and biserially attached autozooids, the elongated 8-radiates (Ba) present and abundant in H. imperiale, H. laauense and H. niobe is a critical feature to separate these two species. While Bayer (Citation1956) described the presence of double clubs in H. regale, in the examination of the holotype, USNM 49520, the double clubs modified from 6-radiates with two prominent heads are absent. Furthermore, the common shape of double clubs is also absent in Bayer (Citation1956) figure 7g and in Tu et al. (Citation2012) figure 13. Therefore, in the present study, double clubs are considered to be absent in H. regale. Therefore, H. halmaheirense was also compared with H. regale. Although both H. halmaheirense and H. regale have predominant 6- and 8-radiate sclerites, spherical 8-radiates (Aa) are only present in H. halmaheirense.
Hemicorallium aurantiacum sp. nov.
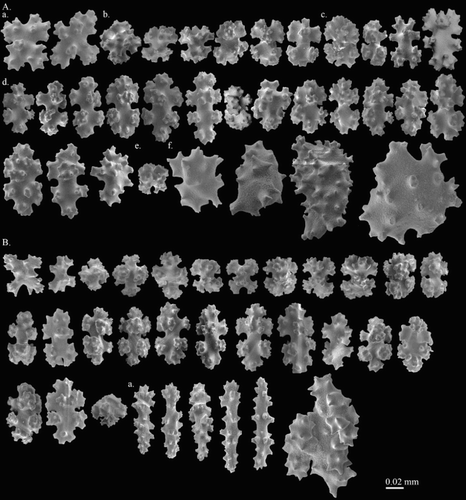
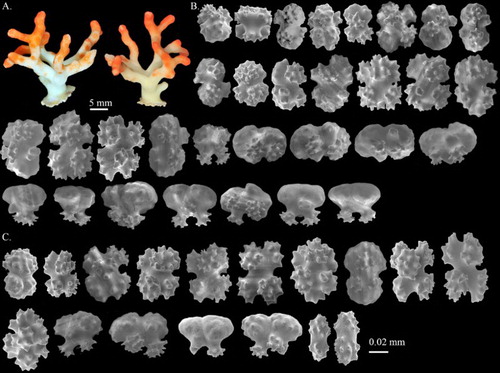
(, )
Figure 7. Hemicorallium aurantiacum sp. nov. (A)–(D) Holotype, MNHN-IK-2011-1580: (A) Front of colony; (B) enlargement of frontal colony, white arrows indicate the thickened cortex induced by polychaetes; (C) and (D) SEM of autozooids, white arrow indicates the apical pore of a siphonozooid; (E) paratype, USNM 1196454, front of colony.
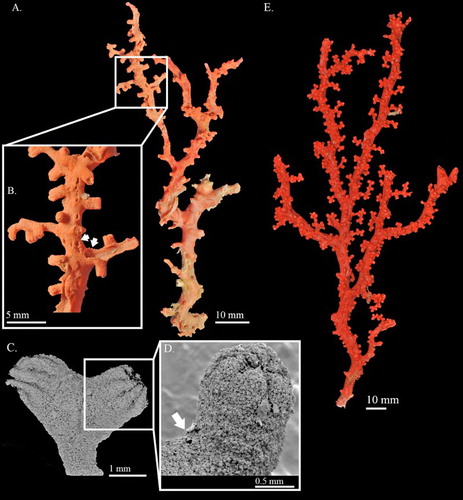
Figure 8. Hemicorallium aurantiacum sp. nov. Holotype, MNHN-IK-2011-1580: (A) Cortical sclerites, (a) 6-radiates, (b) criss-cross, (c) 7-radiates, (d) spherical 8-radiates; (B) sclerites from autozooid, (a) spherical 8-radiates, (b) 7-radiates, (c) spiny spindles, (d) long spindles.

Corallium sp. 6. Tu et al. Citation2015b: 177, 178, figures 1, 2.
Holotype
MNHN-IK-2011-1580, RV Alis, NORFOLK1, St. DW1695, Introuvable Bank, Norfolk Ridge, New Caledonia, 24°40′08.27″S, 168°39′43.31″E, 562–587 m, 24 Jun 2001.
Paratype
USNM 1196454, RV Alis, NORFOLK2, St. DW2060, Introuvable Bank, Norfolk Ridge, New Caledonia, 24°40′12.00″S, 168°39′E, 582–600 m, 25 Oct 2003.
Non-type
USNM 1195220, RV Alis, NORFOLK2, St. DW2056, Norfolk Ridge, New Caledonia, 24°40′S, 168°39′E, 573–600 m, 25 Oct 2003.
Diagnosis
Colonies are dichotomously and symmetrically branched. Cortex is thin and scattered over irregular creases. The surface of the axis is smooth. Contractile autozooids featured by 8-longitudinal grooves are distributed on one side of the colony. Siphonozooids scattered between autozooids as apical pores. Sclerites are mostly 8-radiates with 6- and 7-radiates being less common. Spiny spindles and long spindles are only present in pharynx and tentacles, respectively. Both cortex and autozooids are dark pinkish-orange. The axis is pale pinkish-orange.
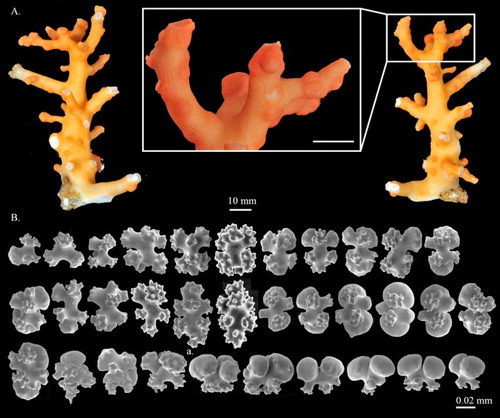
Description
The holotype is 98.9 mm long and 39.6 mm wide in an approximately dichotomous open manner with complete holdfast, but some branches have been damaged and only broken ends remain (A). The colony branches three times almost in one plane. The main stem is 5.2 mm in diameter, bearing tiny branchlets ca. 10 mm tall. The cross-section of the main stem is elliptic and the higher-order branches are close to round. Due to the impact of commensal polychaetes, there are cannular structures in the centre of autozooids on side and higher branches (B). All branches gradually taper from base to tip, which is about 1.2 mm in diameter.
The cortex is thin and severely abraded on the first-order branches (A). There are irregular creases scattered over the surface of the cortex between autozooids, with no papillae (A). Due to the impact of polychaetes, the axis of the second and third branches has been eroded and the remaining cortex was thickened at the edges and decorated with stripes (B, indicated by arrows). Similar to other coralliid species, regularly spaced structures that resemble cone-like papillae were observed on the surface of the axis under SEM.
Autozooids are contractile and distributed singly on the front of the colony and extend slightly toward the flanks, whereas near the end of the terminal branchlets they usually group together in a Y shape (). In the contracted state, autozooids form prominent cylinders that measure 1.3–1.7 mm in height and 0.8–1.0 mm in diameter, with eight longitudinal grooves folding over the intersection of tentacles and extending down to one-third of the polyp (C,D). Siphonozooids are scattered between autozooids and appear merely as apical pores with 0.04–0.06 mm diameter (C,D; indicated by arrow).
Sclerites
Eight-radiates, the commonest type, represent 75% of sclerites in the cortex where they are intermixed with 6-radiates (10%) and 7-radiates (15%, A). The 6-radiates vary in symmetry and size, including a symmetrical form 0.059 mm long (Aa) and a criss-cross 0.050–0.059 mm long (Ab). Most 7-radiates are symmetrical and 0.040–0.060 mm long (Ac, Bb). The 8-radiates include both spherical (Ad, Ba) and symmetrical forms which are 0.045 mm and 0.056–0.080 mm long, respectively. The relative abundance and shape of sclerites in autozooids are similar to those in the cortex (B). Spiny spindles (Bc) and long spindles (Bd) occur in the pharynx and tentacles, respectively.
Colour
The cortex of the colony in dry state is dark pinkish-orange and the axis is paler. Individual sclerites are transparent or light yellow when examined with a microscope under transmitted light.
Commensal/epiphytes
The main branch is modified by tunnels of the commensal polychaete Gorgoniapolynoe sp. (B).
Distribution
This species is currently only known from Norfolk Ridge off New Caledonia near Introuvable Bank, at depths of 562–587 m.
Etymology
The specific name aurantiacum is derived from the Latin word aurantiacum = orange, which describes the axis and cortex colour of this species.
Comparisons
All specimens of Hemicorallium aurantiacum sp. nov. examined in the present study were collected from Introuvable Bank at depths between 500 and 600 m on different expeditions. Among the examined specimens, the paratype, USNM 1196454 (E), is the most complete one, with holdfast and the cortex near the holdfast was not abraded. The colonies of USNM 1195220 were fragments lacking a holdfast. These specimens dichotomously and symmetrically branched. The size of colonies varied between 57 and 195.5 mm in height and 52 and 92 mm in width. Regardless of the size of colonies, the axis of all specimens was eroded by commensal polychaetes. However, the paratype was less affected by commensal polychaetes. Therefore, the irregular creases and small protuberances on the cortex are more obvious in the paratype, and additionally, autozooids were more abundant than in other specimens. The sclerites of the examined specimens agreed well with those of the holotype.
The absence of double clubs in H. aurantiacum sp. nov. can be used to distinguish this new species from H. abyssale, H. variabile, H. reginae (A, ), H. taiwanicum, H. ducale (), H. boshuense, H. sulcatum, H. tricolor and H. bayeri. Although double clubs were depicted in the original description of H. imperiale (pl. 2d in Bayer Citation1955), the shape of the illustrated double clubs is closer to 6-radiates, not the highly modified double clubs described in figure 3 in Bayer (Citation1956). Furthermore, the examination of the holotype of H. imperiale (USNM 50111) also did not reveal the existence of the highly modified double clubs (). Therefore, H. imperiale was compared with the new species and with others that also do not possess double clubs. Compared with H. laauense, H. niobe and H. imperiale (), the new species does not possess elongated 8-radiates (Ba) reaching 0.1 mm long among its sclerites. Although 8-radiates is the predominant type in both H. aurantiacum sp. nov. and H. bathyrubrum, the shape and size of the sclerites are different between them. In H. bathyrubrum, the rays of sclerites are asymmetrical with protuberances of different sizes (figure 4 in Simpson et al. 2011), while the rays are nearly symmetrical and of equal size in H. aurantiacum sp. nov. (). The ratio and shape of 7-radiates are the major differences between H. aurantiacum sp. nov. and H. halmaheirense. Seven-radiates are rare and asymmetrical in H. halmaheirense (), but they are abundant and symmetrical in H aurantiacum sp. nov. The presence of symmetrical 7-radiates (Ac, Bb) and spherical 8-radiates (Ad,Ba) in H. aurantiacum sp. nov. can be used to distinguish it from H. regale.
Remarks
The phylogenetic trees constructed on the mitochondrial locus in Tu et al. (Citation2015b) revealed that the currently used markers could not well delimit species in Hemicorallium despite the existence of obvious morphological differences. Based on the morphological features differing from other valid species in Hemicorallium, the specimens labelled as sp6_NC_1, sp6_NC_3, sp6_NC_4 and sp6_NC_5 are named as H. aurantiacum sp. nov. and described herein (Figure S5).
Hemicorallium guttatum sp. nov.
(, )
Figure 10. Hemicorallium guttatum sp. nov. Holotype, USNM 1072452 (A)–(C): (A) Front and back of colony; (B) SEM of branch and enlargement of autozooid; (C) SEM of the inner structure of autozooid and enlargement of partial cross-section of tentacle; (D) paratype, USNM 1116818; white arrows indicate the thickened cortex induced by polychaetes.
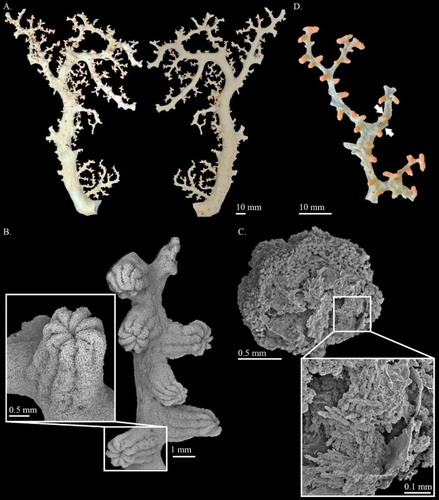
Figure 11. Hemicorallium guttatum sp. nov. Holotype, USNM 1072452: (A) Sclerites from cortex, (a) spherical 8-radiates; (B) sclerites from autozooid, (a) spherical 8-radiates.

Corallium sp. 8. Tu et al. Citation2015b: 177, 178, figures 1, 2.
Holotype
USNM 1072452, Pisces V DSR, St. P5-532, seamount east of Laysan Island, the Hawaiian Islands, USA, 25°42′06.84″N, 171°26′42.00″W, 1509 m, 18 Oct 2003.
Paratypes
USNM 1175422, Pisces V DSR, Necker Ridge Cruise, Gardner Pinnacles, the Hawaiian Islands, USA, 25°39′28.08″N, 168°49′30.00″W, 1339 m, 24 Nov 2011; USNM 1116818, Pisces V DSR, St. 703-5, Twin Banks, the Hawaiian Islands, USA, 23°07′31.80″N, 163°07′55.20″W, 942 m, 17 Nov 2007.
Diagnosis
Colonies dichotomously and asymmetrically branch in roughly one plane. The cortex is thin, with numerous protuberances forming meshed decoration on the surface. The surface of the axis is smooth. Autozooids, possessing 8 longitudinal grooves extending from the distal end to the base of polyps, are contractile and concentrated on one side of the colony. Siphonozooids forming small mounds with apical pore are scattered over the entire surface of the colony. Predominant sclerites in both cortex and autozooids are 8-radiates, with 6- and 7-radiates less common. Long spindles are only present in the tentacles of polyps. The cortex of the colony is yellowish-white and autozooids are pale vermilion red. The colour of the axis is milk white.
Description
The holotype, which is 212.2 mm tall and 140.4 mm wide, is an almost complete colony although it lacks the holdfast (A). The colony dichotomously and asymmetrically branches four times in roughly a single plane except for the terminal branchlets, which are mostly attached perpendicularly to higher-order branches. The main axis and other branches are highly flexuous, curving at angles greater than 90 degrees. The cross-section of most branches is circular and gradually tapers from a diameter of 14.8 mm at the base to 1.0 mm at the terminal branchlets.
The cortex is ca. 0.09 mm in thickness and evenly covers the skeleton. Numerous protuberances scattered over the colony are varied in shape, including small raised siphonozooids, and numerous papillae ranging from elongated, conical or hair-like projections to short, raised ‘bumps’. On the main axis, the papillae connect to form several prominent longitudinal veins (A). Tunnel structures formed by commensal polychaetes are present on the first- and third-order branches. Some edges of the tunnels are adorned with hair-like projections. Under examination with a dissecting microscope, the axis surface is smooth, without longitudinal grooves.
Autozooids are contractile and restricted to the front of the colony and predominantly aggregate on the terminal branchlets. Depending on the length of branchlets, three to four autozooids may be attached along the axis in an alternating manner and usually form a Y shape on the terminal end (). On some branchlets, the four autozooids may be concentrated on the terminal ends, forming a star-like shape. When contracted, autozooids form prominent cylinders with eight grooves corresponding to the eight tentacles extending from the distal end to the base of polyps (B), measuring 1.7–2.2 mm in height and 1.4–1.6 mm in diameter. Siphonozooids form small, rounded mounds with an apical pore. These are scattered between autozooids and are more concentrated near the base of autozooids. Some papillae may appear different and have an apical pore.
Sclerites
There are four types of sclerites in the cortex, 6-radiates representing 29% of sclerites, 7-radiates (10%), 8-radiates (55%) and irregular form (6%) (A). In general, the relative abundance and shapes of sclerites forming the outer and inner walls are similar to those of the cortex (). On the oral surface of the tentacles, long spindles (C; B), which are up to 0.117 mm long, are the predominate type of sclerites, intermingled with some elongated radiates (C).
There is much variation in the size of sclerites: 0.042–0.065 mm long for 6-radiates, 0.058–0.078 mm long for 7-radiates, 0.062–0.090 mm long for 8-radiates and up to 0.1 mm for irregular form. Asymmetry and the unequal size tubercles are the major features of radiates (). Some 8-radiates are modified to a spherical shape (Aa, Ba).
Colour
The cortex of the colony is yellowish-white with pale vermilion red autozooids. The axis is milk white, lighter than the colour of the cortex, which has a slightly pink centre. Individual sclerites range from slightly pink to transparent when examined under transmitted light.
Commensal/epiphytes
Some branches are modified by tunnels of the commensal polychaete Gorgoniapolynoe sp. (D).
Distribution
This species is currently only known from the area between Twin Banks and Laysan Island in the Hawaiian Islands in the North Pacific Ocean, at depths between 942 and 1509 m.
Etymology
The name guttatum is derived from the Latin word guttatus meaning spotted, which describes the pink colour of autozooids on the colony that resemble spots.
Comparisons
All specimens of Hemicorallium guttatum sp. nov. examined were collected from the surrounding region of the Hawaiian Islands. Although the collection depth of the paratype, USNM 1116818 (D), was shallower (942 m) than for the other specimens (1300–1500 m), they shared similar morphological characters. All specimens branched in an asymmetrical pattern in one plane, but due to the loss of the holdfast in some specimens, it was difficult to determine the original size of each specimen. The surface of the axis in all specimens was eroded by commensal polychaetes to different degrees. Branches near the terminal end were more obviously bored by the commensal polychaetes. Autozooids in all specimens were pale vermilion red with 8 longitudinal grooves extending to the base. The sclerites of the examined specimens agreed well with those of the holotype.
The absence of double clubs can be used to distinguish H. guttatum sp. nov. from H. abyssale, H. variabile, H. reginae (A, ), H. taiwanicum, H. ducale (), H. boshuense, H. sulcatum, H. tricolor and H. bayeri. The absence of elongated 8-radiates (Ba) reaching 0.1 mm in length differentiates H. guttatum sp. nov from H. laauense, H. niobe and H. imperiale (). Hemicorallium guttatum sp. nov. is distinct from H. regale and H. aurantiacum sp. nov. by the 8 longitudinal grooves extending from the distal end to the base of polyps in H. guttatum sp. nov. (B). In contrast, the 8 grooves only extended to half the length of the autozooids in H. regale and H. aurantiacum sp. nov. (C). Additionally, spherical 8-radiates (Aa, Ba) are present in H. guttatum sp. nov. and not in H. regale. The presence of spherical 8-radiates can also be used to distinguish H. guttatum sp. nov. from H. bathyrubrum. The two species are also distinct from each other in distribution region and colony colour. The records of Hemicorallium bathyrubrum currently only occur in the North Atlantic, but H. guttatum sp. nov. was found in the Hawaiian Islands. The composition of sclerites is distinct in H. halmaheirense () and H. guttatum sp. nov. (). The predominant type in H. halmaheirense are 8-radiates with 6-radiates common and 7-radiates and irregular radiates absent. However, in H. guttatum sp. nov. both 6- and 8-radiates are predominant and 7-radiates and irregular radiates are present.
Remarks
Based on the phylogenetic trees constructed on the mitochondrial locus, specimens of H. guttatum sp. nov. form a monophyletic clade with high supported value (Figure S5, bootstrap value = 75 in the tree constructed by maximum likelihood, and posterior probability = 1.0 in the tree constructed by Bayesian inference). Additionally, the examination of morphological characters also indicates its uniqueness. Thus, specimens labelled as sp. 8 in Figure S5 are described here as a new species.
Genus Pleurocorallium Gray, 1867
()
Type species: Corallium secundum (Dana, Citation1846)
Pleurocorallium Gray, Citation1867: 126; Ridley Citation1882: 222; Johnson 1899: 59; Kükenthal 1924: 47; Bayer Citation1956: 74; Bayer Citation1964: 467; Bayer & Cairns Citation2003: 222; Figueroa & Baco Citation2014: 83; Tu et al. Citation2015a: 302; Tu et al. Citation2015b: 173.
Diagnosis
Modified from Tu et al. (Citation2015b). Coralliidae with retractile autozooids that can be fully retracted into prominent hemispherical cortical mounds (). Siphonozooids are fertile, inconspicuous and normally surrounding the base of the cortical mounds. The colony may branch in one plane or in different directions forming a three-dimensional structure. Axial pits are not present in all species. For those species without axial pits, their sclerites include small rods, crosses, 6-, 7-, 8-radiates, and double clubs. For those species with round axial pits (C), their sclerites include 6-radiates and double clubs, but no 8-radiates. Long spindles in the tentacles and raindrop-shaped pits do not exist in species of Pleurocorallium.
Distribution
North Atlantic, Pacific at depths between 100 and 1200 m.
Pleurocorallium porcellanum (Pasternak, 1981)
(, , , S3)
Corallium porcellanum Pasternak, Citation1981: 43, figure 2; Simpson & Watling Citation2011: 370.
Pleurocorallium porcellanum. Tu et al. Citation2015b: 181.
Corallium kishinouyei Bayer, Citation1996: 218, figures 11–19; Ardila et al. Citation2012: 248; Tu et al. Citation2012: 4 (in key).
Pleurocorallium kishinouyei. Tu et al. Citation2015b: 181.
Holotype
IORAS IV-9-Alc-11-009, RV Vityaz, St. 3789, Mid-Pacific Seamounts, Pacific Ocean, 13°27′24.01″N, 173°26′53.98″W, 1213–1292 m, 24 Nov 1957.
Diagnosis
Modified from Pasternak (Citation1981). Colonies sparsely branched in one plane. The cortex overlying the axis is thick, with longitudinal grooves. The surface of the axis is rough, with longitudinal grooves, while pits are absent. Autozooids are retractile, forming cortical mounds biserially situated along the flank sides of the main stem. Siphonozooids form small blunt lumps with an apical pore. Eight-radiate is the most common type of sclerite in both cortex and autozooids. The proportions of crosses, 6-, and 7-radiates are similar.
Description
Modified from Pasternak (Citation1981) with additions. The holotype is represented by several fragments possibly damaged during dredging. Although the configuration and the complete branching pattern of this colony is not recoverable, these fragments exhibit a uniplanar branching pattern. The fragment examined is a 44.3 mm tall and 10.5 mm wide Y-shaped twig branching in one plane (A). The diameter at the base is approximately 3.6 mm, and gradually tapering to 1.0 mm at the terminal end.
The cortex is ca. 0.49 mm in thickness, especially on the lateral sides, where it slightly exceeds 1 mm in thickness. The surface of the cortex displays longitudinal grooves, which extend along the axis and downward from the base of cortical mounds (A). The axis is solid, with round cross-section in the basal end and the two ends of the bifurcation. The surface of the axis is rough, with longitudinal grooves. Deep pits beneath the autozooids are absent.
Autozooids are retractile and most are solitary, situated along the front and flanks of the main stem; however, near the terminal end of branches, three to four polyps are usually grouped together. In contracted state, autozooids form low and mound-like cortical mounds up to 2 mm in diameter at the base and 0.9–1.1 mm in height, with a central orifice surrounded by eight marginal lobes (A). Siphonozooids also fully retract to form small blunt lumps which are approximately 0.33 mm in diameter with an apical pore (A), distributed at the base of cortical mounds or in the space between them.
Sclerites
Sclerites in the cortex are composed of 70% 8-radiates, 14% 6-radiates, 6% 7-radiates and 10% crosses (; A). In general, the relative abundance and size of sclerites are similar in cortex and autozooids (B). They are relatively large in size, up to 0.073 mm long for crosses, 0.08 mm long for 6-radiates, 0.07 mm long for 7-radiates and 0.1 mm long for 8-radiates. The rods in tentacles are slender, bluntly pointed, up to 0.100 mm in length (B).
Figure 13. Pleurocorallium porcellanum. Holotype, IORAS IV-9-Alc-11-009: (A) Cortical sclerites; (B) sclerites from autozooid.
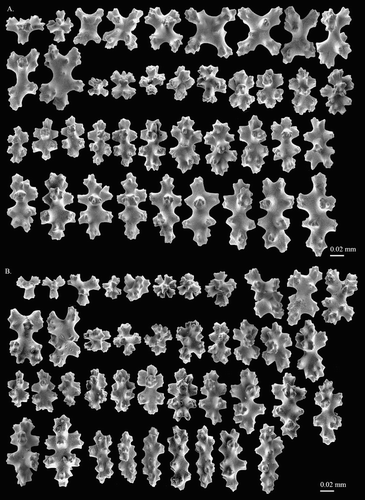
Table III. Main characters of Pleurocorallium porcellanum (Pasternak, Citation1981) and Pleurocorallium kishinouyei (Bayer, Citation1996).
Colour
The colony has yellowish-white cortex with cortical mounds in uniform colour. The axis is white and slightly transparent. Sclerites are colourless and transparent.
Distribution
Pleurocorallium porcellanum is currently known only from the type locality. P. kishinouyei was found in the central Pacific Ocean near the Hawaiian Islands, from 18°N, 155°W to 25°N, 171°W at depths between 1100 and 1800 m.
Remarks
In the original description, Pleurocorallium porcellanum was regarded as closely related to C. japonicum (Kishinouye, Citation1903) from Japan based on sclerite comparisons. Also, it was compared with Hemicorallium laauense and P. niveum from the Hawaiian Islands. Pleurocorallium porcellanum differs significantly from C. japonicum by having no pits under the autozooids on the axis, from H. laauense by the retractile autozooids and the absence of long spindles in tentacles, and from P. niveum by the arrangement of autozooids and the composition of sclerites.
However, this species is highly similar to the Hawaiian species P. kishinouyei. According to the original description of P. kishinouyei, the open, branched uniplanar colonial form and the sclerites reaching a larger size were considered as two unique characters of this species which were not present in other Coralliidae species (Bayer Citation1996). The two mentioned unique characters are also shared by P. porcellanum. Comparing their morphological features and region of distribution revealed that they should be regarded as one species and the senior synonym is Pleurocorallium porcellanum (Pasternak, Citation1981). A detailed comparison between these two species is provided below.
Comparisons
The comparison between Pleurocorallium porcellanum (A) and P. kishinouyei (B,C) given in encompasses the colonial form, the shape and the arrangement of autozooids, the types and size of sclerites, the colour, as well as the distribution. Although the complete configuration and branching pattern of P. porcellanum is not recoverable, the remaining branches still display an openly planar bifurcation pattern which is identical to the branching pattern of P. kishinouyei. Besides, both of them do not have tiny brush branchlets with at least 1 mm wide terminal branchlets. The autozooids of both species are biserially situated along the two sides of the main stem and form low and mound-like cortical mounds up to 2 mm in diameter (). The large size of the low mound-like cortical mound is only observed in P. konojoi Kishinouye, Citation1903 and P. niveum. However, the cortical mounds of P. konojoi and P. niveum are not biserially distributed, but grouped together on one side of the colony (Tu et al. Citation2012).
The predominant type of sclerites of both species are 8-radiates varying in size from 0.044 to 0.119 mm in length in P. porcellanum (), and 0.051 to 0.127 mm in length in P. kishinouyei (Figure S3). Although the 8-radiates are smaller in P. porcellanum, the shapes are almost identical, including both asymmetrical and symmetrical 8-radiates with width to height ratio from 0.43 to 0.53 in P. porcellanum and from 0.43 to 0.55 in P. kishinouyei, and rods with width to height ratio from 0.30 to 0.39 in P. porcellanum and from 0.33 to 0.36 in P. kishinouyei. In addition, 8-radiates represent almost 70% of the sclerites of the two species. Among the valid Pleurocorallium species, only P. porcellanum and P. kishinouyei have the unique symmetrical 8-radiates which can reach more than 0.1 mm in length (, S3). P. porcellanum has a yellowish-white cortex and white axis, and P. kishinouyei has the same colour pattern. The similarity between them is not only restricted to morphological features, but also in the type localities, which are about 1700 km apart at a similar depth, ca. 1200 m for P. porcellanum and 1145 m for P. kishinouyei. Even though the complete colonial form of P. porcellanum was not recoverable, all other features exhibit high similarities between P. porcellanum and P. kishinouyei.
Pleurocorallium bonsaiarborum sp. nov.
()
Figure 14. Pleurocorallium bonsaiarborum sp. nov. Holotype, MNHN-IK-2009-942: (A) Different sides of colony; (B) cortical sclerites; (C) sclerites from autozooid.
Corallium sp. 2. Tu et al. Citation2015b: 177, 178, figures 1, 2.
Holotype
MNHN-IK-2009-942, RV Alis, EXBODI, St. DW3860, Loyalty Ridge, New Caledonia, 22°17′17.99″S, 169°01′12.00″E, 412–436 m, 15 Sep 2011.
Diagnosis
Colony branches dichotomously into different planes. Cortex is thin and smooth. Autozooid pits decorated with beaded margin are present on the axis. There are no striations on the axis. Autozooids are retractile and form cortical mounds distributed on all sides of the colony. Siphonozooids are inconspicuous and scattered between cortical mounds. Sclerites are composed of double clubs and 6-radiates in almost equal proportions. Small rods are present in the pharyngeal region.
Description
The holotype, which is 25.9 mm tall and 24.5 mm wide, is a complete colony with holdfast and all branches (A). The main stem is divided into two pairs of first-order branches on different planes and then dichotomously divided twice to the third order. The transverse section of the main stem is oval, 5.9 mm in diameter with similar width from base to the branching site. Branches are similar in width with clavate ends.
The cortex is ca. 0.08 mm in thickness. Except for the cortical mounds, there are no prominent papillae protruding from the surface of the cortex. Near the terminal ends of the branches, the axial pits beneath autozooids are decorated with wave-shaped margins. Other pits on the centre of branches are decorated with a beaded margin. The axial surface is generally smooth without obvious longitudinal grooves, except for the pits and their surrounding regions.
Autozooids are retractile, solitary and distributed on all sides of the colony with higher density near the terminal ends of branches (A). In contracted state, they form hemispherical cortical mounds that measure 0.9 mm in diameter and slightly protrude from the surface. Siphonozooids retract in small round mounds which are distributed between cortical mounds and also on all sides of the colony.
Sclerites
Sclerites in the cortex are composed of 6-radiates representing 48% of sclerites and double clubs (52%). In autozooids, the sclerites are similar in size and composition to those in the cortex. Small rods up to 0.058 mm long are only present in the pharyngeal region (C). Sclerites vary in symmetry and size, 0.052–0.072 mm long for 6-radiates and 0.049–0.065 mm wide for double clubs. The heads of double clubs include the predominant scraggy surface which is occasionally present on the smooth surface (B,C).
Colour
The colony has a light yellowish-white cortex on the main stem and the lower regions of branches. The upper regions of branches are orange. Cortical mounds are also orange. The axis is pure white to slightly transparent.
Distribution
This species is currently known only from the type locality.
Etymology
The specific name bonsaiarborum is a combination of bonsai (a Japanese art form) with the Latin word arbor = tree describing the colony branching in three dimensions, similar to a bonsai tree.
Comparisons
In Pleurocorallium, the round pits decorated with a beaded margin only occur in P. inutile, P. thrinax (Figure S4), P. norfolkicum sp. nov. (, ) and P. bonsaiarborum sp. nov. A comparison between Pleurocorallium norfolkicum sp. nov. and P. bonsaiarborum sp. nov. is given in the description of P. norfolkicum sp. nov. Among P. inutile, P. thrinax and P. bonsaiarborum sp. nov., P. inutile is distinct from the remaining two species in the colonial form, pale pink axis and reddish cortex. Pleurocorallium bonsaiarborum sp. nov. and P. thrinax are distributed in the South Pacific and are similar in colonial form (A, S4A). However, the three species can be distinguished by the obvious longitudinal grooves and the shape of double clubs. The axial surface of P. inutile and P. thrinax is decorated with obvious longitudinal grooves which are absent in P. bonsaiarborum sp. nov. The heads of double clubs are flatter and wider, with a greater height ratio in P. bonsaiarborum sp. nov. than those in P. inutile and P. thrinax. Moreover, the surface of double clubs in both P. inutile and P. thrinax (Figure S4B) is smooth instead of rough in P. bonsaiarborum sp. nov. (C).
Remarks
The taxonomic status of Pleurocorallium bonsaiarborum sp. nov. was revealed by a molecular-based method and subsequently confirmed by examination of morphological characteristics. In the phylogenetic tree, P. bonsaiarborum sp. nov. (sp. 2) was basal to the group composed of Pleurocorallium norfolkicum sp. nov. (sp. 4), and it is regarded as a singleton by GMYC analyses (Figure S5).
Pleurocorallium clavatum sp. nov.
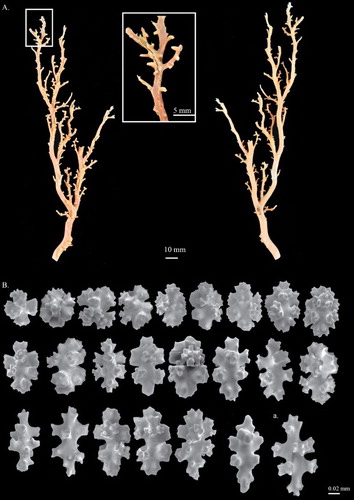
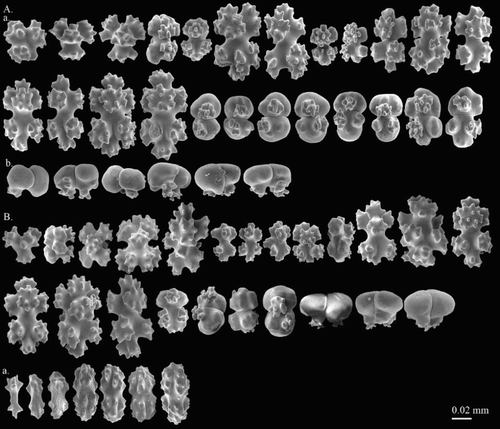
(, )
Figure 15. Pleurocorallium clavatum sp. nov. Holotype, USNM 1195223: (A) Front and back sides of colony, and enlargement of branch; (B) SEM image of branch and cross-section.
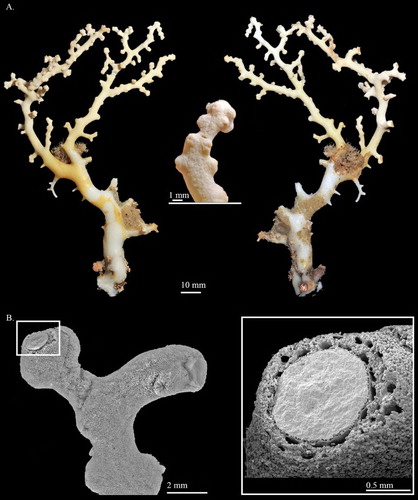
Figure 16. Pleurocorallium clavatum sp. nov. Holotype, USNM 1195223: (A) Cortical sclerites, (a) 6-radiates similar to the radiation warning symbol, (b) double clubs with smooth surface; (B) sclerites from autozooid, (a) rods in pharyngeal region.
Corallium sp. 9. Tu et al. Citation2015b 177, 178, figures 1, 2.
Holotype
USNM 1195223, RV Alis, NORFOLK 2, St. DW 2088, Banc Stylaster, Norfolk Ridge, New Caledonia, 24°57′00.00″S, 168°22′12.00″E, 469–860 m, 20 Jun 2001.
Diagnosis
Colony branches asymmetrically and dichotomously. The cortex covering the axis is thick with inconspicuous warts. The surface of the axis is decorated with faint, longitudinal grooves without pits. Autozooids are retractile and form cortical mounds biserially attached on branches. Siphonozooids construct small, round mounds scattered between cortical mounds. Eight-radiates represent the most common type, with other radiates and double clubs less common. In the pharyngeal region, small rods are present. Both the cortex and cortical mounds are yellowish-white and the axis is white.
Description
The holotype, which is 136.5 mm tall and 84.5 mm wide, is an almost complete colony, although it lacks some basal branches. The colony is a skewed Y-shape, branching three times in roughly a single plane and possessing somewhat claviform ends due to the accumulation of polyps (A). Except for the irregularly shaped main stem, the cross-section of most branches is circular and slightly tapers from a diameter of 7.2 mm at the major bifurcation to 3.0 mm at the terminal ends ().
The cortex is ca. 0.23 mm in thickness and evenly covers the skeleton, although it is abraded on the main stem (A). There are small and inconspicuous warts on the surface of the specimen including siphonozooids and papillae. Solenial channels are abundant in the cortex surrounding the axis. The axial surface with longitudinal grooves appears faint (A).
Autozooids are retractile and biserially attached to the flanks of the branches and form small clusters of 3–4 polyps near the end of the branches (). When contracted, autozooids form cortical mounds that possess an 8-rayed orifice at the summit and are wider than high (1.08 × 0.92–1.10 × 0.99 mm). Siphonozooids appear as small, rounded mounds (∼0.32 mm in diameter) with an apical pore, scattered between autozooids (B).
Sclerites
There are four types of sclerites in the cortex, with double clubs representing 15%, 6-radiates 13%, 7-radiates 7% and 8-radiates 65% (A). Six-radiates are similar in size and vary in shape, 0.046–0.058 mm long for the symmetrical form, and 0.050 mm for the highly modified form, which is similar to the radiation-warning symbol (Aa). The remaining sclerites are symmetrical and vary in size from up to 0.083 mm long for 7-radiates and 0.040–0.099 mm long for 8-radiates. Double clubs are asymmetrical and similar in size, 0.041–0.052 mm wide, with smooth surface (Ab). The shape and relative abundance of autozooid sclerites resemble those in the cortex (B). Small rods (Ba), restricted to the pharyngeal region of the polyp, are 0.045–0.064 mm long.
Colour
The cortex and cortical mounds of the colony in dry state are yellowish-white. The skeletal axis is also lighter than the cortex; sclerites are yellowish and colourless.
Distribution
This species is currently known only from the type locality.
Etymology
The specific name clavatum is derived from the Latin word clavatum = clavate describing the club-shaped ends of branches.
Comparisons
Pleurocorallium borneense () and P. clavatum sp. nov. are the only two species in Pleurocorallium characterized by double clubs with a smooth surface (Ab, Ba) and no axial pits on the axis. The differences between these two species include the pattern of branching, the surface of the cortex, and the composition of the sclerites. Pleurocorallium borneense branches on all sides (A), but P. clavatum sp. nov. (A) branches roughly in a single plane. In P. borneense, papillae and small warts are conspicuous on the surface of the cortex (A), but the surface of the cortex is not decorated with protuberances (A). Furthermore, the predominant form of sclerites in P. borneense is double clubs (B; Bayer Citation1950), but in P. clavatum sp. nov. it is 8-radiates ().
Remarks
The result of phylogenetic analyses indicates that P. clavatum sp. nov. is closely related to P. occultum (here reassigned from the genus Corallium) from the northeastern Atlantic Ocean (Figure S5). In the phylogenetic trees, P. clavatum sp. nov. is basal to the clade formed by the specimens of P. occultum. Despite the similarity in colour and configuration of cortical mounds, the two species are distinct in the composition and shape of sclerites. Therefore, the specimen labelled as sp. 9 is described here as a new species (Figure S5).
Pleurocorallium norfolkicum sp. nov.
(, )
Figure 18. Pleurocorallium norfolkicum sp. nov. Holotype, MNHN-IK-2011-1467 (A)–(C): (A) Front and back sides of colony; (B) close-up of cortical mounds and autozooids, white and black arrows indicate cortical mounds and openings of siphonozooids, respectively; (C) deep autozooid pits with beaded margins; (D) paratype, MNHN-IK-2011-1706.
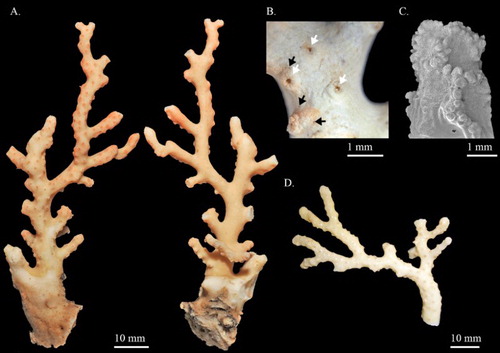
Figure 19. Pleurocorallium norfolkicum sp. nov. Holotype, MNHN-IK-2011-1467: (A) Cortical sclerites from the tip of the colony, (a) symmetrical 6-radiates, (b) asymmetrical 6-radiates, (c) 7-radiate, (d) mushroom-like double clubs, (e) regular double clubs; (B) cortical sclerites from the base of the colony, (a) 7-radiates; (C) sclerites from cortical mounds and autozooid, (a) spherical 6-radiates, (b) small rods in pharyngeal region.
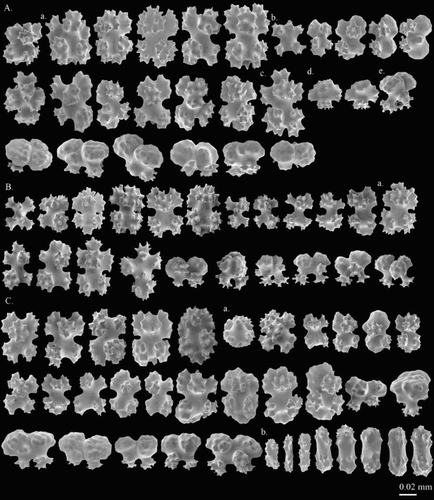
Corallium sp. 4. Tu et al. 2015: 177, 178, figures 1, 2.
Holotype
MNHN-IK-2011-1467, RV Alis, TERRASSES, St. DW3069, Antigonia seamount, Norfolk Ridge, New Caledonia, 23°18′06.01″S, 168°04′41.98″E, 300–320 m, 22 Oct 2008.
Paratype
MNHN-IK-2011-1706, RV Alis, NORFOLK1, St. CP1671, Jumeau Bank, Norfolk Ridge, New Caledonia, 23°42′29.15″S, 168°00′39.27″E, 320–397 m, 21 Jun 2001.
Non-type
MNHN-IK-2011-1474, RV Alis, NORFOLK 1, St. DW1704, Jumeau Bank, Norfolk Ridge, New Caledonia, 23°46′56.39″S, 168°17′02.16″E, 400–420 m, 25 Jun 2001.
Diagnosis
Colonies asymmetrically and dichotomously branch in almost one plane. The cortex overlying the axis is thick and smooth. The surface of the axis is decorated with longitudinal grooves and round pits with obviously beaded margin. Autozooids are retractile, forming cortical mounds evenly scattered on the front and flank sides of the colony. Siphonozooids, forming small round mounds, are distributed near the base of cortical mounds. Double clubs and 6-radiates represent the predominant types of sclerites, with few 7-radiates and irregular forms in both cortex and cortical mounds. In the pharyngeal region, there are minute and irregular rods.
Description
The holotype, which is 94.7 mm tall and 38.2 mm wide, branches laterally three times, in an asymmetrically dichotomous manner in one plane, consisting only of the main stem and the main branches without tiny branchlets (A). All branches taper from base to tip. The main colony stem is irregular in shape and measures 15.3 mm in diameter near its base. The stem extends for 24.8 mm and then bisects into two primary (first-order) branches that are approximately circular in cross-section and measure 6.3 mm and 8.1 mm in diameter, respectively, at their origin. A 12.9 mm segment originating from the back of the first-order branch near the dichotomous point extends in parallel with the main plane (A).
The cortex is ca. 0.1 mm in thickness and evenly covers the axis except for the base of the main stem, which has been mineralized without living tissue on it (A). Cortical mounds and the small round mounds formed by retracted siphonozooids are the only protuberances on the cortex (B). The surface of the axis is longitudinally grooved and is covered with minute tubercles, decorated with thorny protuberances. There are round pits occupied by autozooids with beaded margins and a raised rim (C) dispersed on the front and the flanks, approximately 0.9–1.0 mm in diameter.
Autozooids are retractile and form hemispherical cortical mounds, which are evenly distributed on the front and flanks and completely absent on the back (A,B). On the blunt end of the branches, 2–3 cortical mounds group together to form small clusters (A). The cortical mounds appear as only slightly elevated, 0.4–0.7 mm in height and 0.9–1.2 mm in diameter, with an indistinct 8-lobed orifice (A).
Siphonozooids also form small round mounds with minute apical pores, ca. 0.2 mm in diameter, grouped and scattered over the base of cortical mounds (A), but some of them are also present in the cortex interspersed between cortical mounds. The openings of solenial canals are scattered on the back of the colony, appearing as small pores approximately 0.038 mm in diameter.
Sclerites
The cortex on the tip of the colony consists of four types of sclerites, 6-, 7-radiates, double clubs and irregular forms, representing 37%, 6%, 54% and 3% of sclerites, respectively (A). The composition and size of sclerites in the cortex at the base of colony (B) and autozooid wall (C) do not appear significantly different from those at the tip of colony. Six-radiates include symmetrical (Aa), asymmetrical (Ab) and spherical forms (Ca). Seven-radiates are roughly symmetrical with distinct tubercular projections (Ac,Ba). Some of the double clubs are highly modified to form a mushroom-shape (Ad) and others are asymmetrical with scraggy heads (Ae). Sclerites vary in size from up to 0.058 mm wide for double clubs, 0.076 mm long for 6-radiates and 0.080 mm for 7-radiates. Additionally, in the pharyngeal region and oral disk of the anthocodiae, there are minute and irregular rods up to 0.058 mm in length (Cb).
Colour
The colony has an ivory white cortex ornamented with deep pink cortical mounds. On the back side, the ivory white cortex is decorated with brown spots indicating the openings of solenial canals. The axis colour is white; individual sclerites are transparent when examined with a microscope under transmitted light.
Distribution
This species is currently only known from off New Caledonia Norfolk Ridge, at depths of 300–397 m.
Etymology
The specific name norfolkicum alludes to the name of the submarine ridge, Norfolk Ridge, where the specimens were collected.
Comparisons
Specimens of Pleurocorallium norfolkicum sp. nov. examined in the present study were collected from Norfolk Ridge on different expeditions from similar water depths. Except for the holotype, the other specimens are fragments without a holdfast. With the loss of holdfasts, the complete colonial form of other specimens could not be determined; their higher-order branches are asymmetrical and dichotomous in almost one plane. Autozooids in these specimens are distributed on the front and flanks of colonies. The major difference between holotype (A) and paratype (D) is the colour of the autozooids, which are deep pink in the holotype but ivory white in the paratype. The sclerites of the examined specimens agreed well with those of holotype.
Species in Pleurocorallium possessing axial pits include P. bonsaiarborum sp. nov., P. inutile, P. thrinax and P. norfolkicum sp. nov. The presence of double clubs with a smooth surface can be used to distinguish P. norfolkicum sp. nov. () from P. inutile and P. thrinax (Figure S4B). The differences between P. bonsaiarborum sp. nov. and P. norfolkicum sp. nov. include the pattern of branching, the axial surface and the composition of sclerites. Pleurocorallium bonsaiarborum sp. nov. branches in different planes (A), but P. norfolkicum sp. nov. branches dichotomously in almost one plane (A,D). The axial surface is generally smooth without obvious longitudinal grooves in P. bonsaiarborum sp. nov. However, the axial surface in P. norfolkicum is decorated with obvious longitudinal grooves. Moreover, the sclerites in P. bonsaiarborum sp. nov. are composed of double clubs and 6-radiates, with 7-radiates being rare (), but sclerites as P. norfolkicum sp. nov. include 6-radiates, double clubs and 7-radiates as common (c).
Remarks
Pleurocorallium norfolkicum is labelled as sp. 4 in the phylogenetic trees (Figure S5). Specimens labelled as sp. 4 shared similar morphological characters which are distinct from other valid species in Pleurocorallium. Therefore, they are regarded as a new species.
Multiple factors analysis (MFA)
Results from MFA performed on morphological features from 26 Hemicorallium specimens are shown in . For some species, such as H. aurantiacum sp. nov., H. abyssale, H. bayeri, H. guttatum sp. nov., H. laauense and H. regale, at least two specimens were included in the analysis. Together, the first two dimensions account for 47.05% of the variance within the sample. The first dimension accounts for 30.12% of variance and separates the two specimens of H. guttatum sp. nov. from the others. Additionally, except for H. bathyrubrum, this dimension separates species with double clubs in their sclerites on the right side with positive scores. The classification of H. bathyrubrum with positive scores might result from the similarity between highly modified 8-radiates and double clubs. The second dimension accounts for 16.93% of the variance and separates the three specimens of H. aurantiacum sp. nov. from the other valid species. For species with two or more specimens in the analysis, the intraspecific difference in the second dimension is smaller than interspecific variation ().
Discussion and conclusion
In this study, we use molecular-based phylogenetic trees (Tu et al. Citation2015b; Figure S5) to support the delimitation of new species along with morphological comparisons which are commonly applied in the taxonomy of Coralliidae and most Octocorallia. The three new species in Pleurocorallium described here are supported by both morphological and molecular evidence. While previous studies indicated that species within Hemicorallium could not be delimited by mitochondrial markers (Ardila et al. Citation2012; Figueroa & Baco Citation2014; Tu et al. Citation2015b), morphometric measurements coupled with MFA can be used to separate most Hemicorallium species. Thus, by applying statistical comparisons of morphological features, the current study has succeeded in delimiting coralliids. This has allowed us to fully accomplish the taxonomic revision initiated by Tu et al. (Citation2015b) using a truly integrative approach.
Based on the results of the current study, there are 43 species within the Coralliidae, with 7, 18 and 18 species in Corallium, Hemicorallium and Pleurocorallium, respectively. Several studies have revealed that the diversity hotspots of coralliid species are located in the west and central Pacific including the surrounding seas of New Caledonia, Taiwan, Japan and the Hawaiian Archipelago (Bayer Citation1956; Tu et al. Citation2012; Nonaka et al. Citation2012). In contrast, the species diversity of the Coralliidae is relatively low in the Atlantic, with only a few records (Sampaio et al. Citation2009; Simpson & Watling Citation2011; Tu et al. Citation2015a). However, this distribution pattern may not reflect the natural distribution of Coralliidae because most members of this family are distributed in the deep ocean, increasing the difficulty of collection. More than half of the Coralliidae species are distributed in the west Pacific, and the populations there are declining dramatically due to intensive commercial harvesting (Chen Citation2012; Iwasaki et al. Citation2012; Bruckner Citation2014). Control or restriction of commercial harvesting is needed to prevent the further decline of population size of most Coralliidae species.
Key to species of Coralliidae (modified from Tu et al. 2012, 2015a)
1. Pits on the axis absent …………… 2
– Pits on the axis present …………… 3
2. Autozooids contractile, forming prominent cylinders when contracted; long spindles in the tentacles present …………… 4 (Genus Hemicorallium)
– Autozooids retractile within short, hemispherical cortical mounds; long spindles in the tentacles absent …………… 27 (Genus Pleurocorallium)
3. Slightly sunken to deep raindrop-shaped pits without obviously beaded margin (Figure S1B, supplementary material); 8-radiates present …………… ……………………………… . 21 (Genus Corallium)
– Deep round pits decorated with prominent beaded margin (C); 8-radiates absent …………… ………………………… . 39 (Genus Pleurocorallium)
4. Double clubs present …………… 5
– Double clubs not present …………… 14
5. Surface of double clubs smooth …………… 6
– Surface of double clubs rough …………… 7
6. Autozooids widely separated (8–10 mm apart), biserial; branching sparse, the main stem zig-zag …………… H. abyssale
– Autozooids very numerous and all seated on the anterior aspect of the branches; branching luxuriantly in one plane to the seventh or eighth degree of subdivision, branches irregularly flexuose, not anastomosing ……………… H. maderense (see pl. VII, figures 1, 4 in Johnson 1899)
7. Six-radiates not present or rare …………… 8
– Six-radiates present …………… 11
8. Crosses and irregular radiates present …………… 9
– Crosses and irregular radiates not present …………… 10
9. Main branches laterally compressed; terminal twigs sharply pointed; yellow cortex, milk white axis …………… H. boshuense (see pl. III, figure 3, pl. VII, figure 2 in Kishinouye Citation1904)
– Colony branched in one plane, tiny branchlets on the frontal side and some of them anastomose with each other; pink cortex, pink axis with white centre ……………… H. sulcatum (see pl. IV, figures 1, 2, pl. VII, figure 3, pl. VIII, figure 19 in Kishinouye Citation1904; figure 14 in Tu et al. Citation2012)
10. Colony irregularly branching in one plane; grooves formed by tentacles not extending to the base of autozooids; axis superficially smooth …………… H. bayeri (see figures 5–7 in Simpson & Watling Citation2011)
– Colony profusely branching in one plane; grooves formed by tentacles extending to base of autozooids; axis marked by very fine striations, often very faint …………… H. variabile (see pl. I, figure 9 and pl. IX, figure 13 in Thomson & Henderson Citation1906)
11. Autozooids sparsely distributed in the front of the stem, not arranged biserially …………… 12
– Autozooids densely distributed biserially along the stem or branches …………… 13
12. Autozooids larger than 1 mm in height; cortex light orange, axis deep orange or red with a white spot in centre; sclerites including slender symmetrical 8-radiates in abundance and other symmetrical radiates are common …………… H. reginae (A, )
– Autozooids less than 1 mm in height; cortex and axis dark pink; sclerites including oval asymmetrical 8-radiates in abundance and other asymmetrical radiates are common …………… H. taiwanicum (see figures 11, 12 in Tu et al. Citation2012)
13. Surface of axis faintly striated; grooves formed by tentacles extending to the base of autozooids; siphonozooids forming apparent hemispherical calyces distributed on all sides of colony ………………… . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . H. ducale ()
– Surface of axis smooth; grooves formed by tentacles extending only on the oral part of autozooids; siphonozooids small, with an obvious orifice surrounding the base of autozooids …………… H. tricolor (see figure 10 in Tu et al. Citation2015a)
14. Spheroidal 6-radiates absent …………… 15
– Spheroidal 6-radiates present …………… H. regale (see figures 5c, 7e–g in Bayer Citation1956; figure 13 in Tu et al. Citation2012)
15. Elongated 8-radiates reaching 0.1 mm in length present …………… 16
– Elongated 8-radiates not present …………… 18
16. Eight prominent longitudinal grooves extending along the length of the polyp …………… 17
– Eight prominent longitudinal grooves extending only on the distal part…………… … H. laauense (see figures 5e,f, 7h–j in Bayer Citation1956; figure 9 in Tu et al. Citation2012)
17. Autozooids distributed predominantly on secondary and higher-order branches; siphonozooids occurring in clusters near autozooids; pale yellow cortex, white axis; elongated 8-radiates similar to rods …………… H. niobe (see figures 8, 9 in Simpson & Watling Citation2011)
– Autozooids distributed evenly on one side of the colony; siphonozooids occurring between autozooids; pink cortex and axis; elongated 8-radiates with enlarged and pointed terminal tubercles …………… H. imperiale ()
18. Eight prominent longitudinal grooves extending along the length of the autozooids …………… 19
– Eight prominent longitudinal grooves extending only on the distal part …………… H. aurantiacum sp. nov. (, )
19. Spheroid 8-radiates present (Aa) …………… 20
– Spheroid 8-radiates not present …………… H. bathyrubrum (see figures 1–4 in Simpson & Watling Citation2011)
20. Autozooids conical, 2–3 mm in height; orange red cortex and autozooid, pale pink axis; 8-radiates predominant, 6-radiates common, 7-radiates and irregular radiates absent … H. halmaheirense(B,C, )
– Autozooids tall and cylindrical, 1.72–2.0 mm in height; yellowish-white with pale vermilion red autozooids; 6- and 8-radiates predominant, 7-radiates and irregular radiates present … H. guttatum sp. nov. (, )
21. Double clubs present …………… 22
– Double clubs not present …………… 23
22. Cortical mounds conspicuous, 2–3 mm in diameter at base, usually clustered together on one side of colony; cortex and axis white …………… C. medea (see figures 1–3 in Bayer Citation1964; figure 5 in Tu et al. Citation2015a)
– Cortical mounds inconspicuous, 0.89–0.98 mm in diameter at base, evenly distributed on all sides of colony . . . C. stylasteroides (see pl. IX, figures 1–4 in Ridley Citation1882; –)
23. Six-radiates present and common …………… 24
– Six-radiates not present or rare …………… 25
24. Cortical mounds inconspicuous and the axial pit ca. 0.75 mm wide; colony irregularly branched; cortex and axis pure white; short 8-radiates, with 6- and 7-radiates common, crosses very rare …………… C. nix Bayer, Citation1996 (see figures 7–10 in Bayer Citation1996)
– Cortical mounds conspicuous, 1.20–1.55 mm wide at base; colony dichotomously branched; cortex and axis deep red; 8-radiates, 6-radiates and crosses common …………… C. rubrum
25. Colony dichotomously branched almost in one plane, with small prickle-like twigs on front and sides of branches; pits on axis without beaded margin; cortex and axis deep red …………… ……………C. japonicum (see figure 2 in Tu et al. Citation2012)
– Colony irregularly branched in one plane, without small prickle-like twigs; pits on axis decorated with slightly beaded margin; cortex and axis white to pale pink …………… 26
26. Eight-radiates ca. 0.07 mm in length and not decorated with intensive protuberance ……………. C. tortuosum (see pl. 10, 14–16 in Bayer Citation1993)
– Eight-radiates ca. 0.1 mm or more in length and decorated with intensive protuberance …………… . . . . . . . . . . . . . . . . . . C. salomonense (see pl. 10–13 in Bayer Citation1993)
27. Eight-radiates present and not rare …………… 28
– Eight-radiates not present or rare …………… 34
28. Double clubs present …………… 29
– Double clubs absent . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . P. porcellanum (, )
29. Double clubs with smooth heads …………… …………… P. clavatum sp. nov. (, )
– Double clubs with rough heads …………… 30
30. Six-radiates present and common …………… 31
– Six-radiates not present or rare …………… …………… P. pusillum (see pl. 5, figures 3, 4, pl. 7, figure 4 in Kishinouye Citation1904)
31. Colony branches sparsely; prickle-twigs absent …………… 32
– Colony branches densely; prickle-twigs present …………… …………… P. secundum (see figures 5, 6, 8 in Bayer Citation1956; figure 4 in Tu et al. Citation2012)
32. Most cortical mounds cluster together; 6- and 8-radiates predominant, double clubs equipped with asymmetrical shorter heads and decorated with sharply pointed protuberance …………… P. niveum (see figure 8 in Tu et al. Citation2012)
– Cortical mounds separately distributed; sclerites including predominant 8-radiates, double clubs equipped with longer and smoother heads …………… 33
33. Elongated 8-radiates and spherical heads of double clubs present in both cortex and cortical mounds …………… P. johnsoni(see figure 8 in Tu et al. Citation2015a)
– Elongated 8-radiates and spherical heads of double clubs absent in both cortex and cortical mounds …………… P. occultum (see figures 2–4 in Tu et al. Citation2015a)
34. Double clubs with smooth heads …………… P. borneense ()
– Double clubs with rough heads …………… 35
35. Cortical mounds predominantly distributed on one side of the colony …………… 36
– Cortical mounds distributed around the colony …………… P. uchidai (see figures 10–17 in Nonaka et al. Citation2012)
36. Cortical mounds evenly distributed on one side of the colony …………… 37
– Cortical mounds clustered in groups on one side of the colony …………… 38
37. Minute warts on cortex not visible to the naked eye; cortex and axis crimson red to salmon pink; 6-radiates predominant …………… P. elatius (see figures 2–9 in Nonaka et al. Citation2012; figure 3 in Tu et al. Citation2012)
– Minute warts on cortex apparent; cortex and axis whitish; double clubs predominant …………… P. gotoense (see figures 18–24 in Nonaka et al. Citation2012)
38. Cortical mounds short, cylindrical, 0.4 mm wide at base; cortex with fine granular surface; cortex and cortical mounds orange with white spots, axis orange …………… P. carusrubrum (see figures 5, 6 in Tu et al. Citation2012)
– Cortical mounds large, hemispherical, 1.3 mm wide at base; cortex smooth; cortex yellowish white to orange with orange cortical mounds, axis white …………… P. konojoi (see figures 28–34 in Nonaka et al. Citation2012; figure 7 in Tu et al. Citation2012)
39. Double clubs with smooth heads …………… 40
– Double clubs with rough heads …………… 41
40. Cortical mounds predominant on one side of colony; cortex and axis white; distributed in southern Pacific …………… P. thrinax (see figures 1–6 in Bayer Citation1996; Figure S4, supplementary material)
– Cortical mounds on all sides of colony; cortex orange, axis white; distributed in northern west Pacific …………… P. inutile (see figures 35–42 in Nonaka et al. Citation2012; figure 1 in Tu et al. Citation2012)
41. Colony branched in one plane; conspicuous longitudinal grooves on axial surface; cortex ivory white and axis white; double clubs predominant, 6- and 7-radiates common …………… …………… P. norfolkicum sp. nov. (, )
– Colony branched in three dimensions; axial surface smooth; cortex yellowish white on the main stem and the lower branches, cortex on the terminal ends of branches orange, axis white; 6-radiates and double clubs predominant …………… …………… P. bonsaiarborum sp. nov. ()
Figure S1. Corallium tortuosum Bayer, 1956 (A) holotype, USNM 49331, different sides of colony; (B) Paraty, USNM 49334, rain-drop shaped pits (C) holotype, USNM 49331, sclerites from autozooid, a. crosses
Download JPEG Image (1.2 MB)Figure S2. Corallium tortuosum Bayer, 1956, MNHN-IK-2011-1458: (A) Different sides of colony; (B) SEM of branch; (C) SEM of axial pit and cortical mound (D) Sclerites from cortex; (E) Sclerites from autozooid, a. small rods
Download JPEG Image (2.4 MB)Figure S3. Pleurocorallium kishinouyei (Bayer, 1996), paratype, USNM 94463: (A) Cortical sclerites; (B) Sclerites from autozooid
Download JPEG Image (3.5 MB)Figure S4. Pleurocorallium thrinax (Bayer, 1996), paratype, USNM 96511: (A) Colony; (B) SEM image of sclerites
Download JPEG Image (1 MB)Figure S5. The phylogenetic trees modified from figure 1 in Tu et al. (2015b) to indicate the phylogenetic relationships of the four of the five new species described in the present study
Download JPEG Image (4.5 MB)Acknowledgements
We are grateful to Stephen Cairns (NMNH), Kun-Hsuan Lee (NMNS), Tina Molodtsova (IORAS), Leen P. van Ofwegen (NBC) and Sarah Samadi (MNHN) for their assistance in accessing museum collections and examining the type specimens. Thanks are also due to Tai-Lang Lin (ASIZ) and Scott Whittaker (NMNH) for their help in SEM photography.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abdi H, Williams LJ, Valentin D. 2013. Multiple factor analysis: principal component analysis for multitable and multiblock data sets. Wiley Interdisciplinary Reviews: Computational Statistics 5:149–79. doi:10.1002/wics.1246
- Andaloro F, Cicogna F. 1993. Fishing red coral: problems and management. In: Cicogna F & Cattaneo-Vietto R, editors. Red Coral in the Mediterranean Sea: Art, History and Science. Rome: Ministero delle Risorse di Agricole e Alimentari e Forestali, p. 131–57.
- Ardila NE, Giribet G, Sánchez JA. 2012. A time-calibrated molecular phylogeny of the precious corals: reconciling discrepancies in the taxonomic classification and insights into their evolutionary history. BMC Evolutionary Biology 12:246. 11 pages. doi:10.1186/1471-2148-12-246
- Bayer FM. 1950. A new precious coral from North Borneo. Journal of the Washington Academy of Sciences 40:59–61.
- Bayer FM. 1955. Contributions to the nomenclature, systematics, and morphology of the Octocorallia. Proceedings of the United States National Museum 105:207–20. doi:10.5479/si.00963801.105-3357.207
- Bayer FM. 1956. Descriptions and redescriptions of the Hawaiian octocorals collected by the US Fish Commission steamer “Albatross” (2. Gorgonacea: Scleraxonia). Pacific Science 10:67–95.
- Bayer FM. 1964. The genus Corallium (Gorgonacea: Scleraxonia) in the western North Atlantic Ocean. Bulletin of Marine Science of the Gulf and Caribbean 14:465–78.
- Bayer FM. 1993. Generic reassignment and affinities of Sympodium salomonense Thomson & Mackinnon (Coelenterata: Octocorallia). Precious Corals & Octocoral Research 1:14–19.
- Bayer FM. 1996. Three new species of precious coral (Anthozoa: Gorgonacea, genus Corallium) from Pacific waters. Proceedings of the Biological Society of Washington 109:205–28.
- Bayer FM, Cairns SD. 2003. A new genus of the scleraxonian family Coralliidae (Octocorallia: Gorgonacea). Proceedings of the Biological Society of Washington 116:222–28.
- Blackith R, Reyment RA. 1971. Multivariate Morphometrics. New York: Academic Press. 412 pages.
- Bramanti L, Vielmini I, Rossi S, Tsounis G, Iannelli M, Cattaneo-Vietti R, et al. 2014. Demographic parameters of two populations of red coral (Corallium rubrum L. 1758) in the North Western Mediterranean. Marine Biology 161:1015–26. doi:10.1007/s00227-013-2383-5
- Bruckner AW. 2014. Advances in management of precious corals in the family Corallidae: are new measures adequate? Current Opinion in Environmental Sustainability 7:1–8. doi:10.1016/j.cosust.2013.11.024
- Chen CS. 2012. Management of the precious coral fishery in Taiwan: progress and perspectives. Marine Policy 36:623–29. doi:10.1016/j.marpol.2011.10.016
- Cuvier G. 1798. Tableau Elementaire de l'Histoire Naturelle des Animaux. Paris: Baudouin imprimeur Press. 774 pages.
- Dana JW. 1846. Zoophytes. United States Exploring Expedition during the years 1838, 1839, 1840, 1841, 1842, under the command of Charles Wilkes. Vol. 7. Philadelphia: Lea and Blanchard. 740 pages.
- Ehrenberg CG. 1834. Die Corallenthiere des Rothen Meeres physiologisch untersucht und systematisch verzeichnet. Beiträge zur physiologischen Kenntniss der Corallenthiere im allgemeinen und besonders des Rothen Meeres, nebst einem Versuche zur physiologischen Systematik derselben. Abhandlungen der Königlichen Akademie der Wissenschaften zu Berlin 1832:225–380.
- Ferreira T, Rasb W. 2012. ImageJ user guide. 187 pages.
- Figueroa DF, Baco AR. 2014. Complete mitochondrial genomes elucidate phylogenetic relationships of the deep-sea octocoral families Coralliidae and Paragorgiidae. Deep Sea Research II 99:83–91. doi:10.1016/j.dsr2.2013.06.001
- Gray JE. 1860. Description of a new coral (Corallium johnsoni) from Madeira. Proceedings of the Zoological Society of London 1860:393–94.
- Gray JE. 1867. Additional note on Corallium johnsoni. Proceedings of the Zoological Society of London 1867:125–27.
- Grigg RW. 1993. Precious coral fisheries of Hawaii and the US Pacific Islands. Marine Fisheries Review 55:50–60.
- Hickson SJ. 1905. On a new species of Corallium from Timor. Proceedings of the Meeting of Saturday September 30: 268–71.
- Hickson SJ. 1907. Die Alcyoniden der Siboga-expedition: I. Coralliidae. Leiden, the Netherlands: EJ Brill. 134 pages.
- Iwasaki N, Fujita T, Bavestrello G, Cattaneo-Vietti R. 2012. Morphometry and population structure of non-harvested and harvested populations of the Japanese red coral (Paracorallium japonicum) off Amami Island, southern Japan. Marine and Freshwater Research 63:468–74. doi:10.1071/MF11254
- Johnson JY. 1898. Notes on the Coralliidae of Madeira, with descriptions of two new species. Proceedings of the Zoological Society of London 1899:57–63.
- Kishinouye K. 1902. Honpousansangonoichi shinshu. Doubutsu Gaku Zasshi 14:623–26. (in Japanese)
- Kishinouye K. 1903. Preliminary note on the Coralliidae of Japan. Zoologischer Anzeiger 26:623–26.
- Kishinouye K. 1904. Notes on the natural history of corals. Journal of the Imperial Fishery Bureau 14:1–32. (in Japanese)
- Kükenthal W. 1924. Gorgonaria. Das Tierreich 47:1–478.
- Lamouroux JVF. 1812. Extrait d’un mémoire sur la classification des polypiers coralligènes non entièrement pierreux. Nouveau Bulletin des Sciences, par la Société Philomatique 3:181–88.
- Lê S, Josse J, Husson F. 2008. FactoMineR: an R package for multivariate analysis. Journal of Statistical Software 25:1–18. doi: 10.18637/jss.v025.i01
- Linares C, Garrabou J, Hereu B, Diaz D, Marschal C, Sala E, Zabala M. 2012. Assessing the effectiveness of marine reserves on unsustainably harvested long-lived sessile invertebrates. Conservation Biology 26:88–96. doi:10.1111/j.1523-1739.2011.01795.x
- Linnaeus C. 1758. Systema Naturae, 10th edition. Stockholm: Salvii Press. 824 pages. (in Latin)
- Nonaka M, Muzik K, Iwasaki N. 2012. Descriptions of two new species and designation of three neotypes of Japanese Coralliidae from recently discovered specimens that were collected by Kishinouye, and the introduction of a statistical approach to sclerite abundance and size. Zootaxa 3428:1–67.
- Nonaka M, Nakamura M, Muzik K. 2015. Sexual reproduction in precious corals (Coralliidae) collected in the Ryukyu Archipelago. Pacific Science 69:15–46. doi:10.2984/69.1.2
- Parrish FA, Grigg RW, DeMello JK, Montgomery AD. 2009. The status of Corallium spp. in Hawaii and the US Pacific: population status, trends and threats, fisheries, trade, management, enforcement and conservation. In: Bruckner AW, Roberts GG, editors. International Workshop on Corallium Science, Management, and Trade. Silver Spring, MD: NOAA Technical Memorandum NMFS-OPR-43 and CRCP-8, p 87–107.
- Pasternak AP. 1981. Benthos of the submarine mountains Marcus-Necker and adjacent Pacific regions. In: Kuznetsov AP, Mironov AN, editors. Alcyonacea and Gorgonacea. Moscow: Akademiya Nauk, p 40–55. (in Russian)
- Rasband WS. 2012. ImageJ: Image processing and analysis in Java. Astrophysics Source Code Library 1206.013.
- Ridley SO. 1882. On the arrangement of the Coralliidae, with descriptions of new or rare species. Proceedings of the Zoological Society of London 50:221–34. doi:10.1111/j.1096-3642.1882.tb02738.x
- Sampaio I, Ocana O, Tempera F, Baraga Henriques A, Matos V. 2009. New occurrences of Corallium spp. (Octocorallia, Coralliidae) in the Central Northeast Atlantic. Arquipélago Life and Marine Sciences 26:73–78.
- Simpson A, Watling L. 2011. Precious corals (Coralliidae) from north-western Atlantic seamounts. Journal of the Marine Biological Association of the United Kingdom 91:369–82. doi:10.1017/S002531541000086X
- Thomson JA, Henderson WD. 1906. An Account of the Alcyonarians Collected by the Royal Indian Marine Survey Ship Investigator in the Indian Ocean. Calcutta: Trustees of the Indian Museum. 384 pages.
- Thomson JA, Mackinnon DL. 1910. Alcyonarians collected on the Percy Sladen Trust Expedition by Mr. J. Stanley Gardiner. Part 2, the Stolonifera, Alcyonacea, Pseudaxonia, and Stelechotokea. Transactions of the Linnaen Society of London 2:165–211.
- Tu TH, Dai CF, Jeng MS. 2012. Precious corals (Octocorallia: Coralliidae) from the northern West Pacific region with descriptions of two new species. Zootaxa 3395:1–17.
- Tu TH, Altuna A, Jeng MS. 2015a. Coralliidae (Anthozoa: Octocorallia) from the INDEMARES 2010 expedition to north and northwest Spain (northeast Atlantic), with delimitation of a new species using both morphological and molecular approaches. Zootaxa 3926:301–28. doi:10.11646/zootaxa.3926.3.1
- Tu TH, Dai CF, Jeng MS. 2015b. Phylogeny and systematics of deep-sea precious corals (Anthozoa: Octocorallia: Coralliidae). Molecular Phylogenetics and Evolution 84:173–84. doi:10.1016/j.ympev.2014.09.031
- Verrill AE. 1865. Classification of polyps (extract condensed from Synopsis of the Polyps and Corals of the North Pacific Exploring Expedition under Commodore C. Ringgold and Captain John Rodgers, U.S.N.). Communications of the Essex Institute 4:145–52.