ABSTRACT
Different studies on the position of the non-indigenous species Neogobius melanostomus within the coastal food web of the Pomeranian Bay (western Baltic) were performed, resulting in a quantitative and qualitative species list of prey organisms found in the stomachs of the invader and an estimation concerning the importance of round goby as prey for different resident predators. It seems that the colonization process is not fully completed yet, but the results reveal that the species is already established in the food web 16 years after the first observation within the study area. The results show that N. melanostomus feed upon a wide range of different resident organisms. While a direct predation effect on native fish species appears rather unlikely, indirect effects such as competition cannot yet be excluded. In addition, our results reveal an ontogenetic diet shift and that the round goby itself already serves as an important prey for piscivorous fish and seabirds. Finally, we formulate different hypotheses based on our results which will require further research.
RESPONSIBLE EDITOR:
Introduction
Non-indigenous species often pose a severe threat to the native brackish water fauna in the Baltic Sea (Leppäkoski & Olenin Citation2000, Citation2001). One of the most prominent invasive species is the euryhaline round goby Neogobius melanostomus (Pallas, 1814). Native to the Caspian Sea, Black Sea and the adjacent Sea of Azov (Svetovidov Citation1964; Miller Citation1986), it has been introduced into various locations (Kornis et al. Citation2012). In 1990, the round goby was first recorded in the St Clair River in North America and has spread throughout the Great Lakes since then (Jude et al. Citation1992, Citation1995). In Central Europe the species was observed first in the Baltic Sea (Skóra & Stolarski Citation1993) in 1990 and later in several river and canal systems (Wiesner Citation2005; van Beek Citation2006; Borcherding et al. Citation2011; Piria et al. Citation2011; Verreycken et al. Citation2011; Brunken et al. Citation2012; Hempel & Thiel Citation2013; Jacobs & Hoedemakers Citation2013; Schomaker & Wolter Citation2014). In the Baltic Sea, round gobies were first caught in the Gulf of Gdansk in 1990 (Skóra & Stolarski Citation1993, Citation1996) and had become one of the dominant species in the western part of the Gulf by 1999 (Sapota & Skóra Citation2005). The invasion expanded in a northeasterly direction towards the Curonian Lagoon (Rakauskas et al. Citation2013), the Gulf of Riga and the Gulf of Finland (Ojaveer Citation2006), as well as in a westerly direction (Winkler et al. Citation2000; Winkler Citation2006; Czugała & Woźniczka Citation2010; Schomaker & Wolter Citation2014). While several studies about the invasive process in the Great Lakes were published within recent decades (e.g. Barton et al. Citation2005; Lederer et al. Citation2006; Raby et al. Citation2010; Kipp et al. Citation2012; Kipp & Ricciardi Citation2012), the invasive process in the Baltic has been treated as a ‘Cinderella subject’ and in comparison fewer studies were published (e.g. Janssen & Jude Citation2001; Barton et al. Citation2005; Lederer et al. Citation2006; Copp et al. Citation2008; Raby et al. Citation2010; Kipp et al. Citation2012; Kipp & Ricciardi Citation2012; Sapota et al. Citation2014; Kotta et al. Citation2016). This has led to an opportunity to investigate the invasive and colonization process of a new species. However, in some areas the invasion process is still ongoing and should be investigated. One of those regions exhibiting an ongoing invasion process is the western part of the Baltic, where our studies were performed. The first occurrence of N. melanostomus in our study area was observed in 1999. In recent years, the species spread out rapidly and abundances increased (Winkler et al. Citation2015). Presuming that the species must already be established in the local food web to achieve the observed successful colonization of the area, we present a synergistic compilation of several case studies – each focusing on a particular aspect of the trophodynamic interactions between the round goby and the food web in the Pomeranian Bay and adjacent waters. Combining different adapted techniques, we focused on important native components of the investigated ecosystem and examined their specific interaction with N. melanostomus.
Materials and methods
Top-down effect
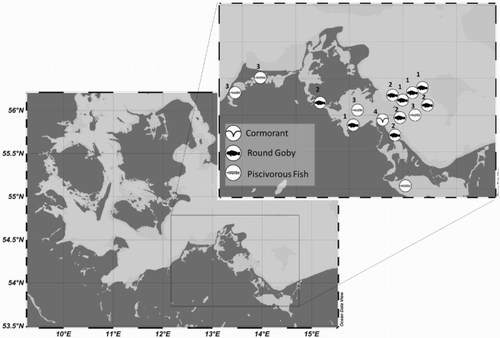
To investigate the prey of Neogobius melanostomus, two different study sites in the Pomeranian Bay and adjacent waters were investigated (, Case Study 1) including a semi-enclosed inshore lagoon (Greifswald Bay) and an area close to the Oderbank Plateau. At the first site (inshore), round goby samples were taken in three different habitat types using a beam trawl in August, October and November 2014. Habitat types included the ‘Potamogeton zone’ between 1 and 2 m water depth, the ‘Zostera zone’ at 3–4 m depth and the ‘Subphytal zone’ between 5 and 7 m water depth. Species from Oderbank Plateau were sampled with multi-mesh gillnets at around 5, 10 and 20 m depths in May and November 2014 (, Case Study 1).
Figure 1. Sampling stations of cormorant pellets, round gobies and piscivorous fish in the Pomeranian Bay. Numbers indicate the case study number.
Another round goby sampling was performed from May 2011 until July 2012 at the inshore lagoon (‘Potamogeton zone’) and the area of the Oderbank Plateau at depths up to 14 m with a beach seine and trawl, respectively (, Case Study 2). In the laboratory, round gobies for both studies were measured for total length and stomach contents were examined. Gobies were dissected ventrally and the stomachs separated from the remaining digestive tract. Only prey organisms that had been in the stomach were identified to the lowest possible taxonomic level. For samples from 2014 the presence/absence of the single prey taxa was noted for each fish dissected and afterwards the percentage of specific prey taxa was calculated for each individual goby. For samples from 2011 and 2012, the frequency of occurrence of prey taxa was noted per length class.
Bottom-up effect
To examine the bottom-up effect of the invasive Neogobius melanostomus, stomach content analyses of piscivorous fish and analyses of pellets of the cormorant Phalacrocorax carbo (Linnaeus, 1758) from the Pomeranian Bay were investigated. The fish were sampled by different techniques (bottom trawl, gillnet) at several locations during the year where an occurrence of round gobies was noted (, Case Study 3). Total length of piscivorous fish was measured to the nearest 0.5 cm, individuals were dissected ventrally and the stomach was separated. Afterwards, stomach contents were identified to the lowest possible taxon. When prey individuals were intact, the wet weight and length were noted; otherwise, individual lengths and weights were estimated using empirically characterized relations between total length/biomass and otoliths or other skeleton fragments from the literature and/or the local reference collection (Härkönen Citation1986, Debus & Winkler Citation1996; Leopold et al. Citation2001; Myts Citation2012). The diet composition was calculated as N
i
(%) individual number, W
i
(%) as the reconstructed weight and F
i
(%) as the frequency of occurrence of prey taxon i. These three parameters were combined to calculate the Index of Relative Importance (IRI) as used by George & Hadley (Citation1979):
For the prey analysis of piscivorous birds, cormorant pellets were sampled between 2010 and 2015. The pellets were collected during the breeding season (between March and October) near Peenemünde in the Pomeranian Bay (, Case Study 4). When possible, 30 or more pellets were collected at least once a month with fresh and intact pellets being preferred to older and/or damaged pellets. After sampling, pellets were washed so that macerated pellet contents and prey items could be identified more efficiently. All prey items were identified to the lowest possible taxon. At least, length and biomass of prey individuals were recalculated via published and non-published regressions based on the identified otoliths and bony prey remains. Regressions for otoliths and lengths and weights for the round goby were calculated according to Menge (Citation2012).
Results
Top-down effect
Round gobies were found at each sample site except at a depth of 20 m on the Oderbank Plateau. The length and weight of each round goby was measured and individuals were assigned to different length classes (≤ 50 mm, 51–100 mm, 101–150 mm, 151–200 mm and 201–250 mm). When available, a maximum of 10 stomachs per length class and haul were analysed from the beam trawls and 20 stomachs per length class and depth contour were examined from the gillnet survey. A total of 1192 individuals were caught with 249 stomachs being analysed in the first case study, of which 47 were empty (). Individuals at both study sites from Case Study 1 consumed a variety of prey organisms. In general, polychaetes belonging to the family Nereididae Blainville, 1818 were identified in stomachs. Arthropod prey items included insects and crustaceans, which comprised several taxonomic groups as well. Neogobius melanostomus fed on cladocerans (Bosmina), ostracods and copepods, but also on amphipods such as Gammaridae, isopods as Idotea chelipes (Pallas, 1766), decapods including Crangon crangon (Linnaeus, 1758) and Palaemon sp., and Balanidae. The round goby diet also included bivalves such as Mya arenaria Linnaeus, 1758, Cerastoderma spp., Limecola balthica (Linnaeus, 1758) and Mytilus sp., whereas gastropods included hydrobiids Peringia ulvae (Pennant, 1777) and/or Ecrobia ventrosa (Montagu, 1803) and Littorina spp. On the Oderbank Plateau round gobies also consumed Halicryptus spinulosus (von Siebold, 1849), which belong to the family Priapulidae. In the inshore lagoon (Greifswald Bay), the diet of smaller individuals (< 50 mm TL) predominantly included ostracods, copepods and cladocerans. Larger round gobies (>100 mm TL) increasingly consumed polychaetes and molluscs. Neogobius melanostomus from the Pomeranian Bay (Oderbank Plateau), with a mean total length of 111.3 ± 13.5 mm for females and 150.5 ± 31.7 mm for males, fed on crustaceans such as C. crangon and Palaemon sp. However, molluscs were consumed more often than all other prey items such as arthropods, annelids and priapulids. Mytilus sp. and hydrobiid gastropods were identified as the most important prey items found in the stomachs of fish from the Oderbank Plateau. From the sampling in 2011 and 2012 (Case Study 2), a total of 115 round gobies were randomly selected depending on their length class so that around 20 stomachs per length class, if available, were analysed, resulting in 17 empty and 98 full or partly full stomachs. In addition to the above results, the analyses of prey occurrence of distinct length classes showed an ontogenetic diet shift. Crustaceans dominated the stomach contents of smaller individuals, while the occurrence of molluscs increased with body length ().
Figure 2. Frequency of occurrence of different prey taxa for different length classes, Case Study 2. Numbers in brackets indicate the number of non-empty analysed round goby stomachs.
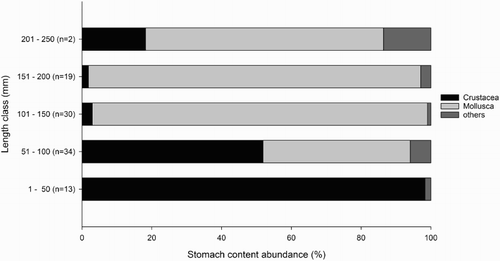
Table I. Detailed information about investigated round gobies (RG) and respective stomach contents from Case Study 1.
Bottom-up effect
In addition to the stomach analysis of round gobies, a total of 321 individuals of piscivorous fish were analysed (, Case Study 3). Stomachs from Scophthalmus maximus (Linnaeus, 1758) and Gymnocephalus cernuus (Linnaeus, 1758) contained prey items, but no Neogobius melanostomus were detected. However, stomachs from Perca fluviatilis Linnaeus, 1758 and Sander lucioperca (Linnaeus, 1758) included N. melanostomus besides other prey species. The IRI shows that N. melanostomus became an important prey item for S. lucioperca and P. fluviatilis during the last years of the invasion process (). These results were verified by determining the number, the frequency of occurrence and the biomass of the food items. Furthermore, N. melanostomus was found in the stomachs of S. lucioperca and P. fluviatilis at all sampling sites. Whereas S. lucioperca caught in the Odra estuary had the highest number of N. melanostomus in their stomachs, the IRI of N. melanostomus calculated for P. fluviatilis was highest from this location. In this study, S. lucioperca with a total length of 30–38 cm and P. fluviatilis with a total length of 20–30 cm had ingested the highest number of N. melanostomus.
Figure 3. Diet composition of Perca fluviatilis in black (n = 104) and Sander lucioperca in grey (n = 57) expressed as Index of Relative Importance.
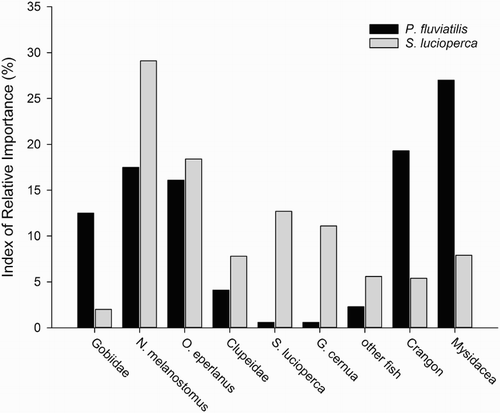
Table II. Information about analysed piscivorous fish species (Case Study 3).
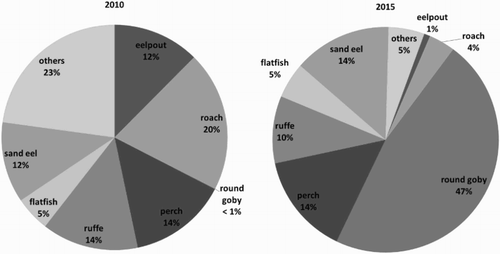
Moreover, the analysis of 1048 (103–263 per year) cormorant pellets (Case Study 4) shows that the occurrence of N. melanostomus in the pellets has increased significantly over recent years (Pearson’s r, p = 0.009). In 2010, the first round gobies were observed in the pellets; however, the percentage of biomass was very small. Over the following three years, the occurrence of N. melanostomus within the pellets increased considerably, especially between 2012 and 2013 (). In recent years, the biomass of N. melanostomus represented about 35% of the estimated pellet biomass. This shows that N. melanostomus became an important, perhaps the most important, prey of the cormorants at that location during the breeding season. In contrast, the biomass of other prey organisms decreased over the last six years. With an occurrence of 20%, for example, roach Rutilus rutilus (Linnaeus, 1758) was the most important prey in 2010, while the percentage was only around 4% in 2015 ().
Figure 4. Number of individuals and estimated biomass of round goby as percentage of the total number and biomass of the cormorant pellets for 2010–2015 in Peenemünde (Case Study 4) (Stark Citation2011; Myts Citation2012; Winkler et al. Citation2014). Number of analysed cormorant pellets: 2010 = 263, 2011 = 221, 2012 = 184, 2013 = 164, 2014 = 103, 2015 = 113.
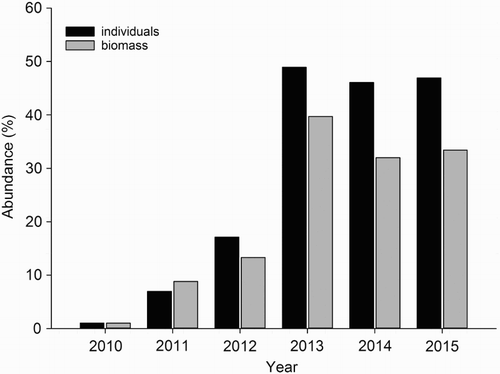
Figure 5. Abundance of fish species in cormorant pellets from 2010 (first appearance of round goby in prey) compared to 2015 (Case Study 4). Data for 2010 modified from Stark (Citation2011). Number of analysed cormorant pellets: 2010 = 263, 2015 = 113.
Discussion
Despite the emergence of modern techniques such as measuring stable isotopes and fatty acid analyses, classical stomach content analysis remains an irreplaceable method to investigate fish feeding ecology, mainly due to the advantage that prey items can be determined to lower taxonomic levels (Cresson et al. Citation2014). However, it has to be considered that prey items are sometimes very difficult to identify, depending on their degree of digestion (Hyslop Citation1980), and that some species could be overestimated due to indigestible fractions (Hyslop Citation1980; Baker et al. Citation2014). In our study, the percentage of bivalves might be overvalued in the diet of round goby because their shells are indigestible and have a longer retention time in stomachs compared to soft-bodied prey. Moreover, they are easier to identify (Coulter et al. Citation2011; Brush et al. Citation2012). Another critical aspect is that the digestive process continues during the catch period (Baker et al. Citation2014); therefore, fish were frozen immediately after capture and the fishing time of the gillnets was minimized to less than 24 hours. However, even if the stomach content analyses present only a snapshot of the species’ diet and describe what the individual has consumed over a certain time span, the results of this study regarding dietary composition of the round goby and round goby predators are assumed to represent a reasonably accurate picture within the different case studies.
Our results show that the round goby preys upon a variety of resident species and shifts the choice of its prey depending on its size. The study reveals that mainly crustaceans and molluscs were impacted directly by the introduction of round goby while fish were rarely found in the stomachs. Therefore, a significant and direct impact on fish species appears rather unlikely while an indirect impact on the resident fish community due to competition could not be excluded at this stage and will be discussed later. On the other hand, some piscivorous fish and bird species adapted to the new food resource so that Neogobius melanostomus became an important food item.
The percentage of successful invaders is difficult to determine. Statistics are rare and unsuccessful Non-Indigenous Species (NIS) are underrepresented, as well as the more easily observable species being overrepresented (Lodge Citation1993). A review conducted by Lodge (Citation1993) presents a minimum rate of NIS establishment of 35%, but others assume a rate of 10% (Williamson & Brown Citation1986; Williamson Citation1989). The colonization success of NIS depends on different factors, but it is assumed that climate and predation are the most important factors influencing the invasion process; however, competition, diseases and other factors are difficult to analyse and could therefore be undervalued (Crawley et al. Citation1986; Lodge Citation1993). On the other hand, the most important impact of introduced mammals on native species, for example, is caused by predation and habitat changes. Ecological changes due to competition are less frequently documented, probably due to the challenging task of proving their actual significance (Ebenhard Citation1988). Based on different types of fishing gear (e.g. bottom trawls, beach seine) and interviews with fishermen, a chronological analysis of the round goby invasion in the eastern German part of the Baltic shows that N. melanostomus has occurred in the German part of the Pomeranian Bay since 1999 and spread out rapidly over recent years with an increasing abundance at some sites (Winkler Citation2006; Winkler et al. Citation2015). Such a fast and successful colonization can only be feasible with a successful niche partitioning together with the factors described above. In addition, our case studies reveal that N. melanostomus is already successfully established in the food web of our study area and has already or is well on the way to establishing itself within the ecosystem in the investigated area. Therefore, future work should focus on the description of the niche, and the direct and indirect influence on native species and habitats. As an example, apart from N. melanostomus, we identified 12 other fish species within the inshore and 19 other fish species within the offshore study area. While our results support the findings of Thiel et al. (Citation2014) that it can be excluded that round gobies exert a high predation pressure on native fish species, it can be assumed that there is already a potential competition concerning space and food resources at least with some of the native species. As our results show, smaller N. melanostomus feed upon crustaceans while larger individuals prefer molluscs. A similar feeding behaviour and therefore a potential competition is assumed for Platichthys flesus (Linnaeus, 1758) and Vimba vimba (Linnaeus, 1758) in the Baltic Sea. Young of the year (YOY) flounders start feeding on small crustaceans, larvae of chironomids and oligochaetes, whereas they switch to polychaetes and molluscs at a body length of around 10 cm (Ojaveer & Drevs Citation2003). YOY vimba bream feed on small crustaceans, molluscs (Hydrobia) and mainly on the polychaete Hediste diversicolor (O.F. Müller, 1776) in the Pärnu Bay of the Baltic Sea. Limecola balthica is dominant in the diet of larger vimba bream (Erm et al. Citation2003). In addition, molluscs are also an important food for Rutilus rutilus (Vetemaa et al. Citation2003), whereby a competition with N. melanostomus may exist. Karlson et al. (Citation2007) describe a dietary overlap between small flounders and round gobies based on stomach contents, stable isotope analyses and lab experiments and reveal a decrease of the flounder population following the establishment of round goby in the area. A comparison between stomach contents from 0 age flounders (Andersen et al. Citation2005) and our results, in addition to the known temporal and spatial overlap at our study sites, supports the potential diet competition. However, besides the indirect diet competition with other fish species, direct feeding consequences exerted by N. melanostomus within the ecosystem are still unknown. As an example, our data show that isopods (Idotea chelipes) and hydrobiid gastropods were regularly found in stomach contents of round goby within the inshore area. Both prey species are grazers within the macrophyte area (Schaffelke et al. Citation1995; Schanz et al. Citation2000) and an induced decrease of these grazers due to N. melanostomus predation could have extensive consequences for the filamentous algae fouling on seagrass and other macrophytes and thus for the whole ecosystem. Besides the negative effects on the native biodiversity, our analyses of the cormorant pellets and stomach contents of piscivorous fishes show that the biomass of native species within the prey decreased, which may have positive effects on the population of those resident species. For example, round goby is one of the most important food items for pikeperch in the Sea of Azov (Maiskij Citation1955). Therefore, it is not surprising that round goby is now the most important food item for pikeperch in the newly colonized Kiel Canal (Thiel et al. Citation2014). Gobies also serve as an important food especially for young sea mammals. According to Behnke et al. (Citation1998), 50% of the food biomass of the harbour porpoises Phocoena phocoena (Linnaeus, 1758) in the Baltic Sea consists of gobies. Hepner et al. (Citation1976) reviewed literature concerning the diet of harbour porpoises in the Black Sea and found that a total of 36% of the porpoises’ food biomass consisted of gobies, including N. melanostomus. However, an assumption about future consequences would be purely speculative. Therefore, more fieldwork and experiments are necessary to rate the consequences of the invasion of round goby for native biodiversity in the coastal area in order to learn more about the impact of general invasion processes.
Acknowledgements
We thank the BONUS Inspire project for the chance to sample the round gobies from gillnets as well as Christoph Stark and Dorothee Moll for their work and support. We furthermore thank Dr Michael L. Zettler for his taxonomic support and advice as well as two unknown reviewers for their constructive criticism.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Andersen BS, Carl JD, Grønkjær P, Støttrup JG. 2005. Feeding ecology and growth of age 0 year Platichthys flesus (L.) in a vegetated and a bare sand habitat in a nutrient rich fjord. Journal of Fish Biology 66:531–52. doi:10.1111/j.0022-1112.2005.00620.x
- Baker R, Buckland A, Sheaves M. 2014. Fish gut content analysis: robust measures of diet composition. Fish and Fisheries 15:170–77. doi:10.1111/faf.12026
- Barton DR, Johnson RA, Campbell L, Petruniak J, Patterson M. 2005. Effects of round gobies (Neogobius melanostomus) on dreissenid mussels and other invertebrates in eastern Lake Erie, 2002–2004. Journal of Great Lakes Research 31(Suppl 2):252–61. doi:10.1016/S0380-1330(05)70318-X
- Behnke H, Siebert U, Lick R, Bandomir B, Weiss R. 1998. The current status of harbour porpoises (Phocoena phocoena) in German waters. Archive of Fishery and Marine Research 46(2):97–123.
- Borcherding J, Staas S, Krüger S, Ondračková M, Šlapanský L, Jurajda P. 2011. Non-native gobiid species in the lower River Rhine (Germany): recent range extensions and densities. Journal of Applied Ichthyology 27:153–55. doi:10.1111/j.1439-0426.2010.01662.x
- Brunken H, Castro J, Hein M, Verwold A, Winkler HM. 2012. First records of round goby Neogobius melanostomus (Pallas, 1814) in the river Weser. Lauterbornia 75:31–37.
- Brush JM, Fisk AT, Hussey NE, Johnson TB. 2012. Spatial and seasonal variability in the diet of round goby (Neogobius melanostomus): stable isotopes indicate that stomach contents overestimate the importance of dreissenids. Canadian Journal of Fisheries and Aquatic Sciences 69:573–86. doi:10.1139/f2012-001
- Copp G, Kováč V, Zweimüller I, Dias A, Nascimento M, Balážová M. 2008. Preliminary study of dietary interactions between invading Ponto-Caspian gobies and some native fish species in the River Danube near Bratislava (Slovakia). Aquatic Invasions 3:193–200. doi:10.3391/ai.2008.3.2.10
- Coulter DP, Murry BA, Webster WC, Uzarski DG. 2011. Effects of dreissenid mussels, chironomids, fishes, and zooplankton on growth of round goby in experimental aquaria. Journal of Freshwater Ecology 26:155–62. doi:10.1080/02705060.2011.553987
- Crawley MJ, Kornberg H, Lawton TH, Usher MB, Southwood R, O'Connor RJ, Gibbs A. 1986. The population biology of invaders: Discussion. Philosophical Transactions of the Royal Society B 314:711–31. doi:10.1098/rstb.1986.0082
- Cresson P, Ruitton S, Ourgaud M, Harmelin-Vivien M. 2014. Contrasting perception of fish trophic level from stomach content and stable isotope analyses: a Mediterranean artificial reef experience. Journal of Experimental Marine Biology and Ecology 452:54–62. doi:10.1016/j.jembe.2013.11.014
- Czugała A, Woźniczka A. 2010. The river Odra estuary – another Baltic Sea area colonized by the round goby Neogobius melanostomus Pallas, 1811. Aquatic Invasions 5(1):561–65. doi:10.3391/ai.2010.5.S1.014
- Debus L, Winkler HM. 1996. Hinweise zur computergestützten Auswertung von Nahrungsanalysen. Rostocker Meeresbiologogische Beiträge 4:97–110.
- Ebenhard T. 1988. Introduced birds and mammals and their ecological effects. Swedish Wildlife Research 13(4):1–107.
- Erm V, Turovski A, Paaver T. 2003. Vimba bream, Vimba vimba (L.). In: Ojaveer E, Pihu E, Saat T, editors. Fishes of Estonia. Tallin: Estonian Academy Publishers, p 223–25.
- George EL, Hadley WF. 1979. Food and habitat partitioning between rock bass (Ambloplites rupestris) and smallmouth bass (Micropterus dolomieui) young of the year. Transactions of the American Fisheries Society 108:253–61. doi:10.1577/1548-8659(1979)108<253:FAHPBR>2.0.CO;2
- Härkönen T. 1986. Guide to the Otoliths of the Bony Fishes of the Northeast Atlantic. Hellerup, Denmark: Danbiu Aps. 256 pages.
- Hempel M, Thiel R. 2013. First records of the round goby Neogobius melanostomus (Pallas, 1814) in the Elbe River, Germany. BioInvasions Records 2:291–95. doi:10.3391/bir.2013.2.4.05
- Heptner WG, Tshapskii KK, Arsenev VA, Sokolov VE. 1976. Mlekopitajushtshie Sovietskogo Sojusa. Volume 2, Part 3. Lastonogie i Zubatye Kity [Mammals of the Soviet Union, Pinnipeds and Toothed Whales]. Moscow: Vysshaja Skola, 718 pages. (in Russian).
- Hyslop EJ. 1980. Stomach contents analysis – a review of methods and their application. Journal of Fish Biology 17:411–29. doi:10.1111/j.1095-8649.1980.tb02775.x
- Jacobs P, Hoedemakers K. 2013. The round goby Neogobius melanostomus (Pallas, 1814) (Perciformes: Gobiidae), an invasive species in the Albert Canal (Belgium). Belgian Journal of Zoology 143:148–53.
- Janssen J, Jude DJ. 2001. Recruitment failure of mottled sculpin Cottus bairdi in Calumet Harbor, Southern Lake Michigan, induced by the newly introduced round goby Neogobius melanostomus. Journal of Great Lakes Research 27:319–28. doi:10.1016/S0380-1330(01)70647-8
- Jude DJ, Reider RH, Smith W. 1992. Establishment of Gobiidae in the Great Lakes Basin. Canadian Journal of Fisheries and Aquatic Sciences 49:416–21. doi:10.1139/f92-047
- Jude DJ, Janssen J, Crawford G. 1995. Ecology, distribution, and impact of the newly introduced round & tubenose gobies on the biota of the St. Clair & Detroit rivers. In: Munawar M, Edsall T, Leach J, editors. The Lake Huron Ecosystem: Ecology, Fisheries and Management. Amsterdam: SPB Academic Publishing, p 447–60.
- Karlson AML, Almqvist G, Skóra KE, Appelberg M. 2007. Indications of competition between non-indigenous round goby and native flounder in the Baltic Sea. ICES Journal of Marine Science 64:479–86. doi:10.1093/icesjms/fsl049
- Kipp R, Ricciardi A. 2012. Impacts of the Eurasian round goby (Neogobius melanostomus) on benthic communities in the upper St. Lawrence River. Canadian Journal of Fisheries and Aquatic Sciences 69:469–86. doi:10.1139/f2011-139
- Kipp R, Hébert I, Lacharité M, Ricciardi A. 2012. Impacts of predation by the Eurasian round goby (Neogobius melanostomus) on molluscs in the upper St. Lawrence River. Journal of Great Lakes Research 38:78–89. doi:10.1016/j.jglr.2011.11.012
- Kornis MS, Mercado-Silva N, vander Zanden MJ. 2012. Twenty years of invasion: a review of round goby Neogobius melanostomus biology, spread and ecological implications. Journal of Fish Biology 80:235–85. doi:10.1111/j.1095-8649.2011.03157.x
- Kotta J, Nurkse K, Puntila R, Ojaveer H. 2016. Shipping and natural environmental conditions determine the distribution of the invasive non-indigenous round goby Neogobius melanostomus in a regional sea. Estuarine, Coastal and Shelf Science 169:15–24. doi:10.1016/j.ecss.2015.11.029
- Lederer A, Massart J, Janssen J. 2006. Impact of round gobies (Neogobius melanostomus) on dreissenids (Dreissena polymorpha and Dreissena bugensis) and the associated macroinvertebrate community across an invasion front. Journal of Great Lakes Research 32:1–10. doi:10.3394/0380-1330(2006)32[1:IORGNM]2.0.CO;2
- Leopold MF, van Damme CJ, Philippart CJM, Winter CJN. 2001. Otoliths of North Sea Fish – Fish Identification Key by Means of Otoliths and Other Hard Parts. World Biodiversity Database CD-ROM series. Amsterdam: ETI BioInformatics. CD-ROM.
- Leppäkoski E, Olenin S. 2000. Non-native species and rates of spread: lessons from the brackish Baltic Sea. Biological Invasions 2:151–63. doi:10.1023/A:1010052809567
- Leppäkoski E, Olenin S. 2001. The meltdown of biogeographical peculiarities of the Baltic Sea: the interaction of natural and man-made processes. AMBIO: A Journal of the Human Environment 30:202–09. doi:10.1579/0044-7447-30.4.202
- Lodge DM. 1993. Biological invasions: lessons for ecology. Trends in Ecology & Evolution 8(4):133–37. doi:10.1016/0169-5347(93)90025-K
- Maiskij VN. 1955. Pitanie i kormovaja baza sudaka v Azovskom More [Feeding and food base of pikeperch in the Azov Sea]. Trudy Vsesojuznyj Naucno-Issledovatel’skij Institut Morskogo Rybnogo Chozjajstva i Okeanografii (VNIRO) 31:337–55. (in Russian)
- Menge M. 2012. Untersuchungen zur Ökologie der Schwarzmundgrundel (Neogobius melanostomus) in Mecklenburg-Vorpommern. Diploma Thesis. University of Rostock. 101 pages.
- Miller P. 1986. Gobiidae. In: Whitehead PJP, Bauchot M-L, Hureau J-C, Nielsen J, Tortonese E, editors. Fishes of the North-Eastern Atlantic and the Mediterranean, Volume 3. Paris: Unesco, p 1019–85.
- Myts D. 2012. Nahrungsökologie des Kormorans (Phalacrocorax carbo sinensis). Diploma Thesis. University of Rostock. 78 pages.
- Ojaveer H. 2006. The round goby Neogobius melanostomus is colonising the NE Baltic Sea. Aquatic Invasions 1:44–45. doi:10.3391/ai.2006.1.1.11
- Ojaveer E, Drevs T. 2003. Flounder, Platichthys flesus trachurus (Duncker). In: Ojaveer E, Pihu E, Saat T, editors. Fishes of Estonia. Tallin: Estonian Academy Publishers, p 367–68.
- Piria M, Šprem N, Jakovlić I, Tomljanović T, Matulić D, Treer T, et al. 2011. First record of round goby, Neogobius melanostomus (Pallas, 1814) in the Sava River, Croatia. Aquatic Invasions 6(Suppl 1):153–57. doi:10.3391/ai.2011.6.S1.034
- Raby GD, Gutowsky LFG, Fox MG. 2010. Diet composition and consumption rate in round goby (Neogobius melanostomus) in its expansion phase in the Trent River, Ontario. Environmental Biology of Fishes 89:143–50. doi:10.1007/s10641-010-9705-y
- Rakauskas V, Pūtys Ž, Dainys J, Lesutiene J, Ložys L & Arbačiauskas K. 2013. Increasing population of the invader round goby, Neogobius melanostomus (Actinopterygii: Perciformes: Gobiidae), and its trophic role in the Curonian Lagoon, SE Baltic Sea. Acta Ichthyologica et Piscatoria 43:95–108. doi:10.3750/AIP2013.43.2.02
- Sapota M, Skóra K. 2005. Spread of alien (non-indigenous) fish species Neogobius melanostomus in the Gulf of Gdańsk (south Baltic). Biological Invasions 7:157–64. doi:10.1007/s10530-004-9035-0
- Sapota MR, Balazy P, Mirny Z. 2014. Modification in the nest guarding strategy – one of the reasons of the round goby (Neogobius melanostomus) invasion success in the Gulf of Gdansk. International Journal of Oceanography and Hydrobiology 43:21–28.
- Schaffelke B, Evers D, Walhorn A. 1995. Selective grazing of the isopod Idothea baltica between Fucus evanescens and F. vesiculosus from Kiel Fjord (Western Baltic). Marine Biology 124:215–18. doi:10.1007/BF00347125
- Schanz A, Polte P, Asmus H, Asmus R. 2000. Currents and turbulence as a top-down regulator in intertidal seagrass communities. Biologia Marina Mediterranea 7:278–81.
- Schomaker C, Wolter C. 2014. First record of the round goby Neogobius melanostomus (Pallas, 1814) in the lower River Oder, Germany. BioInvasions Records 3:185–88. doi:10.3391/bir.2014.3.3.08
- Skóra KE, Stolarski J. 1993. New fish species in the Gulf of Gdańsk, Neogobius sp. [cf. Neogobius melanostomus (Pallas 1811)]. Bulletin of the Sea Fisheries Institute, Gdynia 1:83.
- Skóra KE, Stolarski J. 1996. Neogobius melanostomus (Pallas 1811), a new immigrant species in the Baltic Sea. Proceedings of the Second International Symposium held in Gdansk, 18–22 October 1993. Crangon No. 1, Issues of the Marine Biology Centre in Gdynia, p 101–08.
- Stark K. 2011. Nahrungsanalyse beim Kormoran (Phalacrocorax carbo). Diploma Thesis. University of Rostock. 106 pages.
- Svetovidov AN. 1964. Ryby Tshernogo Morja [Fishes of the Black Sea]. Moscow, Leningrad: ANSSSR izd. Nauka, p 435–40. (in Russian)
- Thiel R, Horn L, Knörr C, Tonn M. 2014. Analyse der ökologischen Einnischung der invasiven Schwarzmundgrundel in relevanten Brack und Süßgewässerhabitaten Schleswig Holsteins. Final Report. Landesamt für Landwirtschaft, Umwelt und ländliche Räume Schleswig Holstein. 199 pages.
- van Beek G. 2006. The round goby Neogobius melanostomus first recorded in the Netherlands. Aquatic Invasions 1:42–43. doi:10.3391/ai.2006.1.1.10
- Verreycken H, Breine JJ, Snoeks J, Belpaire C. 2011. First record of the round goby, Neogobius melanostomus (Actinopterygii: Perciformes: Gobiidae) in Belgium. Acta Ichthyologica et Piscatoria 41:137–40. doi:10.3750/AIP2011.41.2.11
- Vetemaa M, Saat T, Paaver T, Turovski A. 2003. Roach, Rutilus rutilus (L.). In: Ojaveer E, Pihu E, Saat T, editors. Fishes of Estonia. Tallin: Estonian Academy Publishers, p 167–68.
- Wiesner C. 2005. New records of non-indigenous gobies (Neogobius spp.) in the Austrian Danube. Journal of Applied Ichthyology 21:324–27. doi:10.1111/j.1439-0426.2005.00681.x
- Williamson M. 1989. Mathematical models of invasion. In: Drake JA, Mooney HA, di Castri F, Groves RH, Kruger FJ, Rejmanek M, Williamson M, editors. Biological Invasions: A Global Perspective. London: John Wiley and Sons, p 329–50.
- Williamson MH, Brown KC, Holdgate MW, Kornberg H, Southwood R, Mollison D. 1986. The analysis and modelling of British invasions (and discussion). Philosophical Transactions of the Royal Society B 314:505–22. doi:10.1098/rstb.1986.0070
- Winkler HM. 2006. Die Fischfauna der südlichen Ostsee, Bemerkungen zum gegenwärtigen Kenntnisstand. Meeresangler-Magazin 16:17–18.
- Winkler HM, Skora K, Repecka R, Pliks M, Neelov A, Urho L, et al. 2000. Checklist and status of fish species in the Baltic Sea. CM 2000/Mini: 11, ICES Paper. 6 + 9 pages.
- Winkler HM, Myts D, Lüttkemöller E, Gröger J. 2014. Ernährung des Kormorans und sein Einfluss auf die Fischbestände der Küstengewässer Vorpommerns. In: Populationsanalyse und Erprobung von Maßnahmen zur Reduzierung des Bruterfolges beim Kormoran (Phalacrocorax carbo sinensis) in M-V sowie Untersuchungen über seinen Einfluss auf freilebende Fischbestände. Landesförderinstitut Mecklenburg-Vorpommern, Abteilung Agrar-, Forst- u. Fischereiförderung.
- Winkler H, Kotterba P, Oesterwind D. 2015. Round goby: a story of invasion success in the Baltic. Poster in: ICES Annual Science Conference 2015, 21–25 September, Copenhagen, Denmark.
