ABSTRACT
The non-native seagrass species Halophila stipulacea has spread throughout the Eastern Caribbean since 2002, and could potentially impact the functioning of local seagrass ecosystems. Important characteristics for invasiveness, such as dispersal, recruitment and expansion of H. stipulacea at a local scale, are unknown. We assessed H. stipulacea expansion rates within Lac Bay, Bonaire, Dutch Caribbean (7 km2), since its establishment in 2010 and tested the settlement potential of uprooted vegetative fragments of H. stipulacea. Using 49 fixed locations, we observed that between 2011 and 2015 the occurrence of H. stipulacea in the bay increased significantly from 6% to 20% while native Thalassia testudinum occurrence decreased significantly from 53% to 33%. Free-floating H. stipulacea fragments that were collected and tethered above the sediment rooted within 10 days with a settlement success rate of 100%. The growth of settled fragments was on average 0.91 shoots d−1. The ongoing shift from native T. testudinum to introduced H. stipulacea dominated meadows may have important consequences for multiple Caribbean seagrass ecosystem functions. Given the large difference in size between the two seagrass species, functions such as coastal protection, habitat structure, food availability, and the stability and resilience of these systems can be altered. The next steps towards modelling future expansion of H. stipulacea throughout the Caribbean and beyond should include the assessment of fragment viability and dispersal distance, and the impacts of natural and anthropogenic disturbance on vegetative fragment density, dispersion and settlement by this species.
RESPONSIBLE EDITOR:
Introduction
The rate at which non-native species are being introduced in aquatic ecosystems is increasing worldwide (Ruiz et al. Citation2000; Molnar et al. Citation2008). Some of these introduced species can rapidly spread and often become invasive when they negatively impact the stability and biological diversity of ecosystems (Williams Citation2007; Willette et al. Citation2014). For example, in Mediterranean and Australian seagrass ecosystems, the non-native green alga Caulerpa taxifolia (M.Vahl) C.Agardh has rapidly invaded and locally replaced native seagrass meadows (Williams Citation2007). Recent trans-oceanic introductions of seagrass species are rare, with only two documented species: Zostera japonica Acherson & Graebner and Halophila stipulacea (Forsskål) Ascherson. The introduction of Z. japonica from Japan to the North-Eastern Pacific led to increased seagrass habitat by colonization of bare mudflats (Posey Citation1988), although competition with Z. japonica in combination with disturbance significantly reduced native Zostera marina Linnaeus performance (Bando Citation2006). The other introduced seagrass species, H. stipulacea, originates from the Red Sea and the western Indian Ocean and settled in the Mediterranean Sea after the opening of the Suez Canal in 1869. From the early 2000s onwards, H. stipulacea spread to various islands in the Caribbean and was first reported on Bonaire in 2010 (Ruiz & Ballantine Citation2004; Debrot et al. Citation2011; Willette et al. Citation2014).
In the Mediterranean, the introduction of H. stipulacea did not negatively impact native seagrass meadows (Duarte Citation2002). However, this species can potentially have a large ecological impact at introduced locations due to its extensive range expansion and high tolerance to broad salinity, irradiance and temperature ranges and substrate types (Lipkin Citation1975; Georgiou et al. Citation2016). These qualities can favour the species in competition with native species and enable it to spread quickly. In the Caribbean, the potential invasiveness and ecological impact are currently unknown (Willette et al. Citation2014). However, recent observations in Dominica showed that H. stipulacea can outcompete the native pioneer species Syringodium filiforme Kützing, Halodule wrightii Ascherson and Halophila decipiens Ostenfeld (Willette & Ambrose Citation2012; Steiner & Willette Citation2015). Whether H. stipulacea can also replace Thalassia testudinum K.D.Koenig, the climax species that often dominates seagrass meadows in the Caribbean, remains to be studied. A potential shift from slow-growing, structurally complex T. testudinum meadows to fast-growing less complex meadows with H. stipulacea dominance could have a large impact on seagrass ecosystem services in this region and to the carrying capacity of seagrass meadows for recovering green turtle populations (Chelonia mydas (Linnaeus, 1758)). Besides invading existing seagrass beds, H. stipulacea may also locally increase the ecosystem services by colonizing areas which were previously bare. In Dominica, the total area covered by seagrass doubled from 2008 to 2013, exclusively due to H. stipulacea expansion (Steiner & Willette Citation2015).
Seagrasses can expand via sexual reproduction and vegetative growth. Long distance dispersal is facilitated by seeds and rhizomal fragments and local expansion by belowground rhizome elongation (Marbà & Duarte Citation1998; Kendrick et al. Citation2012). However, so far only male flowers and no seeds have been recorded for H. stipulacea in the Eastern Caribbean (Vera et al. Citation2014; Willette et al. Citation2014). Remarkable are the large amounts of fragments present in these bays, consisting of leaves, roots and rhizomes, of H. stipulacea (Ruiz & Ballantine Citation2004; Vera et al. Citation2014). These fragments apparently break off easily and are uprooted after disturbance such as grazing and waves (Steiner & Willette Citation2015). Fragments of colonizing seagrass species, i.e. H. wrightii and Halophila johnsonii N.J.Eiseman have been reported to settle and root within two weeks in mesocosms (Hall et al. Citation2006). So far, in situ studies on the potential of vegetative fragments as a dispersal mechanism for the non-native seagrass H. stipulacea are lacking.
In this study we aim (1) to quantify biannual changes (2011–2015) in seagrass occurrence in Lac Bay after establishment of the non-native seagrass species H. stipulacea and (2) to assess the colonization potential and rate of free floating H. stipulacea fragments in situ. Furthermore, we provide recommendations for future studies to model the expansion of this non-native seagrass and assess its potential invasiveness. We expect that H. stipulacea increased its number of colonized locations in Lac Bay from 2011 to 2015, given the rapid expansions reported elsewhere in the Caribbean. Secondly, we expect that colonized areas will show an increase in percentage occurrence of H. stipulacea and potentially a decrease in occurrence of T. testudinum. Additionally, H. stipulacea fragments are expected to be highly viable, reflected in the fast settlement (rooting) and subsequent growth from established fragments.
Material and methods
Lac Bay
Colonization of Halophila stipulacea and fragment settlement was determined in Lac Bay (7 km2, 12°10′N, 68°15′W), a shallow tropical clear-water bay with extensive seagrass meadows situated in the south-east of Bonaire, Dutch Caribbean (a). Seagrass meadows in the bay, which provide one of the most important foraging grounds for juvenile green turtles in the Caribbean, were dominated by Thalassia testudinum (Debrot et al. Citation2012; Stapleton et al. Citation2014). Mangrove vegetation surrounds the bay, with hypersaline backwaters at the north-western side (a). Near this mangrove border, dense and ungrazed T. testudinum meadows occurred in water as shallow as 0.3 m. In the deeper centre of the bay, sandy patches alternated with grazed mixed-species meadows at depths up to 6 m, consisting of T. testudinum, H. stipulacea, Syringodium filiforme and various macroalgae (a).
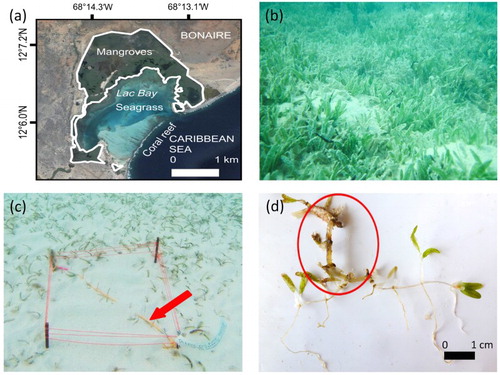
Figure 1. (a) Areal overview of Lac Bay, Bonaire, with an outline of the mangrove, seagrass and coral reef areas. (b) Seagrass meadow in Lac Bay dominated by Halophila stipulacea. (c) Set-up of the fragment settling experiment; the orange tether is indicated by the red arrow. (d) One of the experimental H. stipulacea fragments 6 days after settlement. The red circle indicates the original tethered fragment. The plant material outside the red circle was grown since settlement. Map (a) by Google Maps®, photos (b,c,d) by Fee Smulders.
Seagrass occurrence in Lac Bay
To map the change in presence of the non-native seagrass species H. stipulacea and native seagrass species throughout Lac Bay, we assessed their occurrence in 2011, 2013 and 2015. Seagrass occurrence was assessed at 49 fixed sampling locations that were spaced evenly at 250 m intervals and marked with a handheld GPS (GPS 60Cx, Garmin). At each sampling location, we counted presence or absence of T. testudinum, H. stipulacea and S. filiforme in each of 100 equal squares within a one m2 quadrant. These measurements were taken in six adjacent plots per location by two observers wearing scuba or snorkelling equipment. The six outcomes were averaged per location, resulting in an average occurrence percentage per species per location. When seagrass was present, depth was measured twice per location, using a tape measure, and averaged. Maps were made in QGIS (Quantum GIS Development Team Citation2016) to visualize change in species occurrence over the years. The occurrence per species per year was averaged, as well as the percentage of locations colonized by either species. Additionally, the relative increase or decrease in occurrence of H. stipulacea between 2011 and 2015 was calculated per location. Because the data were not normally distributed, a non-parametric Friedman test for paired samples (P < 0.05) was used to test for differences over the years per species with separate post-hoc Wilcoxon signed-rank tests, using a Bonferroni adjustment (IBM SPSS Statistics 22.0 for Windows).
Halophila stipulacea fragment settlement
The colonization potential of detached H. stipulacea fragments was determined in situ in November 2015, in a period of intermediate wave action and wind speeds (average wind speed of 8.4 ± 0.17 m s−1, near the end of the hurricane season). A total of 25 rhizomal fragments was randomly collected from the water column (<1 m depth) in an area with an established H. stipulacea population (b). The fragments were characterized by on average 8.8 shoots (range 3–15), 15.5 leaves (5–28) and 2.0 roots (0–7). The fragments were attached to a rope (W:L; 6 mm:70 cm) that served as a tether to keep the fragments in place. Five fragments per tether were attached with cable ties and spaced evenly (at least 10 cm interval) along the rope. This was repeated for a total of five tethers. We assumed that the settlement of a single fragment was not influenced by the other fragments on the tether and therefore considered each fragment to be a unique individual measurement. The tethers were placed just above (<2 cm) the sediment surface so that the fragments were still hanging in the water and could move on the currents. Each tether was attached diagonally to two iron pins in a 0.25 m2 square patch of bare sand from which all seagrass biomass was removed (c). This patch was located within a shallow mixed seagrass meadow consisting of T. testudinum, H. stipulacea and S. filiforme (c). Every other day the fragments were checked for settlement. A small current was created by hand, and if the fragment remained in place and roots were visibly inserted in the sediment the fragment was considered to be settled. One fragment was lost from the tether, so calculations were based on 24 fragments. After 12 days the fragments were collected and the new shoots, leaves and roots were counted and photographed (d). The biomass produced since settlement could easily be distinguished from the material already present on the fragment at the start of the experiment. The old, large leaves lost their colouring compared with the young, green and small leaves. Old roots were lost and old rhizomes were thick and rigid, while new rhizomes and roots were flexible, thin and white. All produced biomass was subsequently dried at 70°C and weighed. The total biomass production per fragment and the production of new plant parts were calculated from the moment a fragment was settled. New shoot growth was proportional to new rhizome growth, as H. stipulacea is characterized by a mono-meristematic non-leaf-replacing growth form (Short & Coles Citation2001).
Results
Seagrass occurrence in Lac Bay
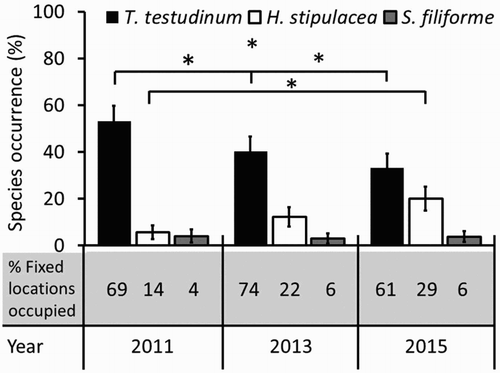
Species occurrence in Lac Bay changed significantly through time for both Halophila stipulacea (χ2(2) = 7.475, P = 0.024, Friedman test) and Thalassia testudinum (χ2(2) = 18.721, P < 0.001, Friedman test). Following Bonferroni correction (P < 0.017) we found that in the 49 fixed locations, occurrence per m2 of the non-native seagrass H. stipulacea increased significantly from on average (±SE) 5.9% ± 3.1 in 2011 to 20.0% ± 5.2 in 2015 (Z = −2.947, P = 0.003, Wilcoxon signed-rank test) but not from 2011 to 2013 (12.8% ± 4.4) nor from 2013 to 2015 (Z = −2.080, P = 0.038; Z = 1.344, P = 0.179 respectively, Wilcoxon signed-rank tests, a,b,c). Native seagrass T. testudinum showed a significant decrease in occurrence per m2 from 53.2% ± 6.4 in 2011 to 33.2% ± 6.1 in 2015 (Z = −3.617, P < 0.001, Wilcoxon signed-rank test) and from 2011 to 2013 (40.4% ± 6.2) and 2013 to 2015 (Z = −3.878, P < 0.001; Z = −2.416, P = 0.016 respectively, Wilcoxon signed-rank tests). Syringodium filiforme remained at a constant low occurrence of 3.6% ± 1.4 per m2 (χ2(2) = 0.429, P = 0.807, Friedman test) over this period. The average increase in occurrence of H. stipulacea at already colonized locations was 42.0% per fixed location for 2011 to 2015 (range in changes from −15.8% to 99.0%). Halophila stipulacea settled first in the deeper, central area of the bay. Within four years it rapidly spread to 10 out of 49 additional locations that were situated in the shallower areas of the bay. Two of these locations recently colonized by H. stipulacea were previously devoid of seagrass presence. The number of fixed locations at which T. testudinum was present remained comparable over this period, while the average occurrence of T. testudinum at these colonized locations decreased by 17.5% for 2011 to 2015 (range in changes from –98.7% to 100.0%) (). The locations at which T. testudinum occurred had average depths of 2.1 m in 2011, 2.0 m in 2013 and 1.8 m in 2015, all ranging from 0.3 to 4.4 m. Halophila stipulacea was present on average at 3.4 m depth in 2011 (2.2–4.4 m), 3.1 m in 2013 (1.0–4.4 m) and 3.2 m in 2015 (1.5–4.4 m). The sampling locations directly adjacent to the mangroves showed the highest percentages of T. testudinum occurrence, with >90% at an average depth of 1.0 m in all years.
Figure 2. The occurrence and distribution of Thalassia testudinum, Halophila stipulacea and Syringodium filiforme in (a) 2011, (b) 2013 and (c) 2015 at the 49 fixed sampling locations in Lac Bay, Bonaire.
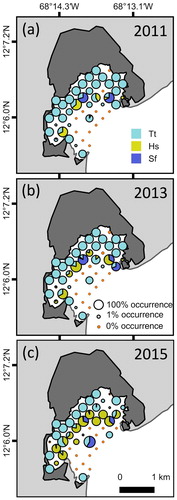
Figure 3. The average (±SE) percentage occurrence of each seagrass species per m2 per year; significant differences between years are indicated with *. The table below the occurrence graph displays the percentage of fixed sampling locations (n = 49) that is occupied per species in each year in Lac Bay.
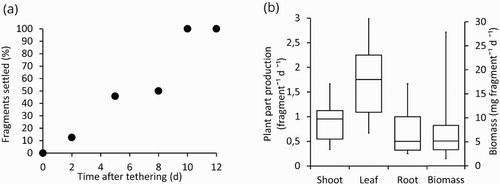
Halophila stipulacea fragment settlement
Within a week after tethering, 50% of the H. stipulacea fragments were rooted. After 10 days all 24 fragments were considered rooted and settled (d, 4a). Shoot growth, equal to rhizome growth, was on average 0.9 fragment−1 d−1. Leaf growth accounted for 1.8 fragment−1 d−1 and root growth for 0.7 fragment−1 d−1. Finally, total biomass production was 7.1 mg fragment−1 d−1. We observed a high variation in production of new shoots, leaves, roots and biomass per fragment (b).
Discussion
In this study we observed rapid expansion of the potentially invasive seagrass Halophila stipulacea between 2011 and 2015 throughout Lac Bay. Halophila stipulacea occurrence increased by about 350% within four years, and partially replaced or excluded native Thalassia testudinum within the fixed sampling locations. If these trends continue, this would result in an average occurrence of 26% for T. testudinum and 27% for H. stipulacea by 2017, hence potentially a shift in dominance of seagrass within seven years after settlement in this bay. Halophila stipulacea colonization and expansion was more extensive in the deeper parts of the bay. Here, T. testudinum and S. filiforme are intensively cropped by grazing green turtles, resulting in low-density T. testudinum meadows that may be more easily colonized by the fast-growing H. stipulacea than ungrazed, more structurally complex seagrass canopies. However, H. stipulacea expansion is not limited to these particular conditions, as more shallow sites have also recently been colonized and it has been observed to grow in between dense, ungrazed T. testudinum meadows at depths up to 0.2 m (Fee Smulders, personal observation in 2015).
In Lac Bay, H. stipulacea is increasing in abundance due to rapid clonal growth. In addition, this species is probably able to spread both inside and outside the bay, as fragments easily detach, float and are able to quickly root when close to the sediment. The H. stipulacea fragments were abundant, drifting both on the water surface, in the water column and near the sediment. Randomly collected fragments of different sizes and lifespans all appeared viable and after tethering the fragments started to grow new roots and leaves within 10 days. Apart from our study, clonal growth rates of H. stipulacea have been reported only once from a mesocosm study in the Mediterranean (Georgiou et al. Citation2016). Reported maximum growth rates are one shoot every two days at the maximum studied temperature of 30°C (Georgiou et al. Citation2016). Given our average production of 0.9 shoots per day, Caribbean H. stipulacea appears to grow remarkably faster, possibly due to the high temperatures and light availability in shallow Caribbean bays (Debrot et al. Citation2012). This short-term rooting of vegetative H. stipulacea fragments from the first field experiments are in line with ex situ experiments where H. wrightii and H. johnsonii fragments settled on the sediment and started rooting within two weeks (Hall et al. Citation2006). In another experiment from the same study where settlement was prevented, individual H. wrightii fragments remained viable for four weeks after being fragmented, and H. johnsonii for eight days at most (Hall et al. Citation2006). During this time the fragments can travel and reach new grounds to colonize.
The dispersal method via fragments might explain the past and predicted future expansion of H. stipulacea. Through this efficient method, H. stipulacea can thrive in meadows prone to natural and human-induced disturbances such as turtle grazing, storms, damage by propellers or anchors, eutrophication, and bioturbation from shrimps or rays (Steiner & Willette Citation2015; van Tussenbroek et al. Citation2016). These factors can create open patches in native beds and stimulate the release of vegetative fragments of the non-native species with the potential to settle at another location, as observed for the invasive macroalgal species Caulerpa taxifolia in Mediterranean meadows (West et al. Citation2007, Citation2009). Comparably, the invasive seagrass species Z. japonica was also reported to positively respond to disturbance by increasing invasion rates and therefore become competitively superior over Z. marina in the Pacific Ocean (Bando Citation2006).
Expansion of H. stipulacea into native seagrass meadows and the potential replacement of T. testudinum may compromise seagrass ecosystem functioning. Thalassia testudinum provides a dense canopy structure to support faunal assemblages, including green turtles, and forms a thick rhizome mat to stabilize the sediment (Patriquin Citation1973; van Tussenbroek et al. Citation2006). Halophila stipulacea has smaller leaves than T. testudinum (Den Hartog Citation1970) and the leaf canopy of this potentially invasive species thus likely provides less shelter for fauna (Debrot et al. Citation2012). Even more noteworthy are differences in belowground structure; H. stipulacea is not as firmly rooted in the sediment and lower belowground biomass may result in decreased coastal protection (Christianen et al. Citation2013) and eventually to changed stability and resilience of the meadows (Vonk et al. Citation2015). Shifts in species composition as a result of seagrass invasion are therefore expected to have a major impact on productivity, habitat structure, food availability and carbon sequestration (Marbà et al. Citation2015; Unsworth et al. Citation2015). However, H. stipulacea has also colonized two locations that previously consisted solely of bare sediment in Lac Bay. Therefore, H. stipulacea can locally increase the total cover of seagrass area and enhance ecosystem functioning, especially if this species continues to colonize unvegetated areas throughout the Caribbean (Steiner & Willette Citation2015).
Although H. stipulacea has been reported to be invasive, there are no unequivocal results supporting this claim. Our first quantification of properties of introduced seagrass fragments calls for further investigation of fragment characteristics and interspecies competition in order to determine the invasiveness of H. stipulacea and be able to model non-native seagrass expansion. To fill in the most important gaps of knowledge around H. stipulacea invasiveness potential and expansion, more knowledge is needed on fragment properties (viability, floating duration, dispersal distance, uprooting factors, density, lifespan; Ceccherelli & Cinelli Citation1999; Smith & Walters Citation1999; Hall et al. Citation2006; Grech et al. Citation2016) and the impact of natural and human-induced stressors (e.g. grazing, storms, anchor or propeller damage; Bando Citation2006; West et al. Citation2007, Citation2009). From the parameters obtained, we can model the rate of expansion, predict changes in meadow composition, and provide management recommendations to maintain key ecosystem services of Caribbean seagrass meadows.
Acknowledgements
We would like to thank Caren Eckrich (STINAPA) for assisting in the field with the seagrass mapping, and Lisa Becking for suggestions on the research setup and logistic support.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Fee O. H. Smulders http://orcid.org/0000-0003-4124-8355
Additional information
Funding
References
- Bando KJ. 2006. The roles of competition and disturbance in a marine invasion. Biological Invasions 8:755–763. doi: 10.1007/s10530-005-3543-4
- Ceccherelli G, Cinelli F. 1999. The role of vegetative fragmentation in dispersal of the invasive algae Caulerpa taxifolia in the Mediterranean. Marine Ecology Progress Series 182:299–303. doi: 10.3354/meps182299
- Christianen MJA, van Belzen J, Herman PMJ, van Katwijk MM, Lamers LPM, van Leent PJM, Bouma TJ. 2013. Low-canopy seagrass beds still provide important coastal protection services. PLoS One 8:e62413. 8 pages. doi: 10.1371/journal.pone.0062413
- Debrot AO, Hylkema A, Vogelaar W, Meesters HWG, Engel MS, de León R, et al. 2012. Baseline surveys of Lac Bay benthic and fish communities, Bonaire. Journal of Experimental Marine Biology and Ecology 376:17–25.
- Debrot AO, van Buurt G, Vermeij MJA. 2011. Preliminary overview of the exotic and invasive marine species in the Dutch Caribbean. Report C188/11. Wageningen, the Netherlands: IMARES. 29 pages.
- Den Hartog C. 1970. The Sea-Grasses of the World. Amsterdam: North-Holland Publishing Company. 275 pages.
- Duarte CM. 2002. The future of seagrass meadows. Environmental Conservation 29:192–206. doi: 10.1017/S0376892902000127
- Georgiou D, Alexandre A, Luis J, Santos R. 2016. Temperature is not a limiting factor for the expansion of Halophila stipulacea throughout the Mediterranean Sea. Marine Ecology Progress Series 544:159–167. doi: 10.3354/meps11582
- Grech A, Wolter J, Coles R, McKenzie L, Rasheed M, Thomas C, et al. 2016. Spatial patterns of seagrass dispersal and settlement. Diversity and Distributions 22:1150–1162. doi: 10.1111/ddi.12479
- Hall LM, Hanisak MD, Virnstein RW. 2006. Fragments of the seagrasses Halodule wrightii and Halophila johnsonii as potential recruits in Indian River Lagoon, Florida. Marine Ecology Progress Series 310:109–117. doi: 10.3354/meps310109
- Kendrick GA, Waycott M, Carruthers TJB, Cambridge ML, Hovey R, Krauss SL, et al. 2012. The central role of dispersal in the maintenance and persistence of seagrass populations. BioScience 62:56–65. doi: 10.1525/bio.2012.62.1.10
- Lipkin Y. 1975. Halophila stipulacea, a review of a successful immigration. Aquatic Botany 1:203–215. doi: 10.1016/0304-3770(75)90023-6
- Marbà N, Arias-Ortiz A, Masqué P, Kendrick GA, Mazarrasa I, Bastyan GR, et al. 2015. Impact of seagrass loss and subsequent revegetation on carbon sequestration and stocks. Journal of Ecology 103:296–302. doi: 10.1111/1365-2745.12370
- Marbà N, Duarte CM. 1998. Rhizome elongation and seagrass clonal growth. Marine Ecology Progress Series 174:269–280. doi: 10.3354/meps174269
- Molnar JL, Gamboa RL, Revenga C, Spalding MD. 2008. Assessing the global threat of invasive species to marine biodiversity. Frontiers in Ecology and the Environment 6:485–492. doi: 10.1890/070064
- Patriquin DG. 1973. Estimation of growth rate, production and age of the marine angiosperm Thalassia testudinum König. Caribbean Journal of Science 13:111–123.
- Posey MH. 1988. Community changes associated with the spread of an introduced seagrass, Zostera japonica. Ecology 69:974–983. doi: 10.2307/1941252
- Quantum GIS Development Team. 2016. Quantum GIS Geographic information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org.
- Ruiz GM, Fofonoff PW, Carlton JT, Wonham MJ, Hines AH. 2000. Invasion of coastal marine communities in North America: apparent patterns, processes, and biases. Annual Review of Ecology and Systematics 31:481–531. doi: 10.1146/annurev.ecolsys.31.1.481
- Ruiz H, Ballantine DL. 2004. Occurrence of the seagrass Halophila stipulacea in the tropical West Atlantic. Bulletin of Marine Science 75:131–135.
- Short FT, Coles RG. 2001. Methods for the measurement of seagrass growth and production. In: Short FT, Coles RG, editors. Global Seagrass Research Methods. Amsterdam: Elsevier Science, p 155–182.
- Smith CM, Walters LJ. 1999. Fragmentation as a strategy for Caulerpa species: fates of fragments and implications for management of an invasive weed. Marine Ecology 20:307–319. doi: 10.1046/j.1439-0485.1999.2034079.x
- Stapleton S, Nava M, Willes S, Brabec B. 2014. Research and Monitoring of Bonaire’s Sea Turtles: 2014 Technical Report. Kralendijk, Bonaire: Sea Turtle Conservation Bonaire. 22 pages.
- Steiner SCC, Willette DA. 2015. The expansion of Halophila stipulacea (Hydrocharitaceae, Angiospermae) is changing the seagrass landscape in the commonwealth of Dominica, Lesser Antilles. Caribbean Naturalist 22:1–19.
- van Tussenbroek BI, van Katwijk MM, Bouma TJ, van der Heide T, Govers LL, Leuven RSEW. 2016. Non-native seagrass Halophila stipulacea forms dense mats under eutrophic conditions in the Caribbean. Journal of Sea Research 115:1–5. doi: 10.1016/j.seares.2016.05.005
- van Tussenbroek BI, Vonk JA, Stapel J, Erftemeijer PLA, Middelburg JJ, Zieman JC. 2006. The biology of Thalassia: paradigms and recent advances in research. In: Larkum AWD, Orth RJ, Duarte DM, editors. Seagrasses: Biology, Ecology and Conservation. Dordrecht, the Netherlands: Springer, p 409–439.
- Unsworth RKF, Collier CJ, Waycott M, Mckenzie LJ, Cullen-Unsworth LC. 2015. A framework for the resilience of seagrass ecosystems. Marine Pollution Bulletin 100:34–46. doi: 10.1016/j.marpolbul.2015.08.016
- Vera B, Collado-Vides L, Moreno C, van Tussenbroek BI. 2014. Halophila stipulacea (Hydrocharitaceae): a recent introduction to the continental waters of Venezuela. Caribbean Journal of Science 48:66–70. doi: 10.18475/cjos.v48i1.a11
- Vonk JA, Christianen MJA, Stapel J, O’Brien KR. 2015. What lies beneath: why knowledge of belowground biomass dynamics is crucial to effective seagrass management. Ecological Indicators 57:259–267. doi: 10.1016/j.ecolind.2015.05.008
- West EJ, Barnes PB, Wright JT, Davis AR. 2007. Anchors aweigh: fragment generation of invasive Caulerpa taxifolia by boat anchors and its resistance to desiccation. Aquatic Botany 87:196–202. doi: 10.1016/j.aquabot.2007.06.005
- West EJ, Davis AR, Barnes PB, Wright JT. 2009. The role of recreational activities in creating fragments of invasive Caulerpa taxifolia. Journal of Experimental Marine Biology and Ecology 376:17–25. doi: 10.1016/j.jembe.2009.05.015
- Willette DA, Ambrose RF. 2012. Effects of the invasive seagrass Halophila stipulacea on the native seagrass, Syringodium filiforme, and associated fish and epibiota communities in the Eastern Caribbean. Aquatic Botany 103:74–82. doi: 10.1016/j.aquabot.2012.06.007
- Willette DA, Chalifour J, Debrot AOD, Engel MS, Miller J, Oxenford HA, et al. 2014. Continued expansion of the trans-Atlantic invasive marine angiosperm Halophila stipulacea in the Eastern Caribbean. Aquatic Botany 112:98–102. doi: 10.1016/j.aquabot.2013.10.001
- Williams SL. 2007. Introduced species in seagrass ecosystems: status and concerns. Journal of Experimental Marine Biology and Ecology 350:89–110. doi: 10.1016/j.jembe.2007.05.032