ABSTRACT
After being ecologically extinct for almost a century, the discovery of a shellfish reef with native European flat oysters (Ostrea edulis) in the Dutch coastal area of the North Sea by the authors of this study called for an extensive survey to better understand some of the key requirements for the return of the native oyster in coastal waters. We assessed habitat conditions, its potential for increasing biodiversity, and the role of substrate provision by other bivalves such as the invasive alien Pacific oyster (Crassostrea gigas). Using underwater visual census, O. edulis size-frequency distributions and attachment substrate was investigated, as well as the composition of the epibenthic community and substrata types inside quadrats that were distributed across the reef. This reef was found to be composed of native European flat oysters, invasive alien Pacific oysters and blue mussels (Mytilus edulis), alternated with sandy patches. The O. edulis population (6.8 ± 0.6 oysters m−2) consisted of individuals of different size classes. In quadrats with native and non-native oysters the number of epibenthic species was 60% higher compared to adjacent sand patches within the reef. Notably, our results showed that the native oyster predominantly used shell (fragments) of the invasive Pacific oyster as settlement substrate (81% of individuals). Our results optimistically show that conditions for native oyster restoration can be suitable at a local scale in the coastal North Sea area and suggest that the return of native oysters may be facilitated by novel substrate provided by invasive oysters at sites where their distribution overlap.
SUBJECT EDITOR:
Introduction
Until approximately one century ago, European flat oyster reefs were widely distributed over the North Sea, and covered ∼20% of the Dutch part of the North Sea floor (over 25,000 km2; Olsen Citation1883). However, overfishing exacerbated by habitat degradation and diseases have pushed native oyster reefs to become ecologically extinct in the North Sea and elsewhere across the globe (Lotze et al. Citation2006; Beck et al. Citation2011), leaving the area named ‘Central Oyster Grounds’ (Olsen Citation1883) empty of oysters. After the decline of the native European flat oyster (Ostrea edulis, Linnaeus, 1758, referred to as ‘native oyster’ below), the invasive alien Pacific oyster (Crassostrea gigas, Thunberg, 1793, also Magallana gigas, but see Bayne et al. Citation2017, referred to as ‘invasive oyster’ below) was introduced in the south-west of the Netherlands for farming in the 1970s (Reise et al. Citation2017a). This invasive oyster quickly expanded in the coastal waters of the North Sea and estuaries as this species was not susceptible to the Bonamia parasite, which caused mortality in native oysters, while it has a more opportunistic larval ecology and does not show brood care to the extent of the native oyster (Smaal et al. Citation2015). The historical locations of native oyster reefs offshore in the North Sea (Fariñas-Franco et al. Citation2018) and current invasive oyster reefs in the near-shore areas of the North Sea only show limited overlap (Smaal et al. Citation2015), suggesting that large unoccupied areas that were previously occupied are potentially available as a habitat for the native oyster. Restoration attempts, however, are hampered partly because detailed knowledge about the habitat requirements and suitable conditions for the restoration of the native oyster in the North Sea areas are scarce (Gercken and Schmidt Citation2014; Smaal et al. Citation2015).
Oyster reefs provide critical ecosystem functions, which have motivated multiple efforts to restore oyster ecosystems across the world. Native European flat oysters create biogenic reefs and thereby provide rare hard substrate in North Sea areas dominated by soft sediments. Aside from providing a fishery commodity since ancient times (Günther Citation1897), oysters can provide a number of critical ecosystem services as they can protect shorelines, filter the water and provide a nursery and habitat for associated commercial species, resulting in an estimated economic value of $5500–$99,000 per hectare per year (Grabowski et al. Citation2012). Additionally, oysters provide non-market ecosystem services including filtration benefits for submerged aquatic vegetation and by creating biogenic structures they provide habitat, settling substrate, nursery or foraging areas for other invertebrates and fish. This is also true for non-native oysters (Lemasson et al. Citation2017).
The groundwork for successful oyster reef restoration has been laid in large-scale projects that showed initial success including those for Crassostrea virginica in Chesapeake Bay, Pamlico Sound (North Carolina), and O. edulis in Strangford Lough (Northern Ireland) and Limfjord (Denmark) (Brumbaugh et al. Citation2000; Dolmer and Hoffmann Citation2004; Kennedy and Roberts Citation2006; Brett et al. Citation2011). Together, these restoration projects show that the return and restoration of native oyster beds is feasible but only when conducted on a large scale, with high investments, when following a critical assessment of location suitability (e.g. temperature, low bottom shear stress), under presence of a substantial source of larvae in close proximity, with high availability of suitable substrate, and when located in areas without bottom disturbance (Beck et al. Citation2011). Restoration requirements for the native oyster O. edulis are expected to be stricter compared with other oyster species as their dispersal distance is more limited due to their mechanism of brood care (up to 10 km (Berghahn and Ruth Citation2005), and the settlement of spat requires relatively smooth and clean settlement substrates (e.g. clean surface of empty shells), also referred to as ‘cultch’, which can potentially limit recruitment due to limited substrate availability (Brumbaugh et al. Citation2000; Kennedy and Roberts Citation2006; Brett et al. Citation2011). The provisioning of bivalve settling substrate (such as live shells and shell fragments of native mussels Mytilus edulis, Linnaeus, 1758, and invasive oyster C. gigas) in the North Sea may potentially facilitate native O. edulis settlement in areas where the distribution of both oysters overlaps.
After being ecologically extinct for almost a century, the discovery of a shellfish reef with native oysters in the coastal ‘Voordelta’ area of the Dutch North Sea by the authors suggested that habitat conditions are locally still favourable. In this study, the conditions at this reef were investigated to better understand the requirements for large-scale restoration and the return of the native oyster. The restoration of the threatened and protected native oyster (reefs) is supported in (inter-) national government policy (EU Red List of habitats, Gubbay et al. Citation2016) and plans are being developed to restore and protect these reefs (OSPAR, Ministry of Infrastructure and Environment Citation2015; Sundseth and Creed Citation2008). Using underwater visual census O. edulis size-frequency distributions and attachment preference was measured, as well as the composition of the bivalve community, epibenthic community and substrata types inside quadrats that were distributed across the reef. Specifically, the importance of invasive oyster shell fragments as a settlement substrate for native oyster was investigated.
Materials and methods
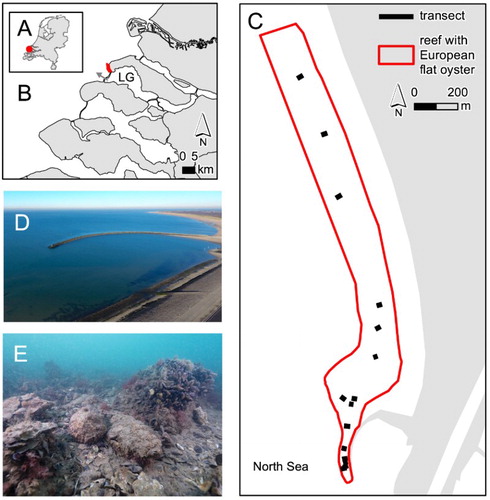
Fieldwork and measurements were performed at a recently discovered shellfish reef with native European flat oysters, located in the coastal zone near Zeeland, the Netherlands (the so-called Voordelta) (range latitude, longitude; 51.77N, 3.85E – 51.79N, 3.85E, WGS84) in the Dutch part of the North Sea (). Scuba divers discovered the European flat oysters in a Pacific oyster reef, which was already known, after pre-selecting a search area based on favourable conditions for oyster presence. These conditions included the availability of suitable substrate for settlement of oyster larvae such as empty shells of mussels and oysters, a nearby source of oyster larvae and the absence of bottom disturbing fishing activities caused by the presence of big stones. The oyster reef is located at close proximity to the ‘Brouwerssluis’, a water outlet of Lake Grevelingen, a saline lake with limited tidal range. This sluice has been open year-round since 1998 enabling oyster larvae to travel from Lake Grevelingen, where native O. edulis occur naturally and in aquaculture plots, to enter the coastal area of the North Sea (Sas et al. Citation2016). Additionally, the reef is located close to the shore and is bordered by breakwaters on the south side. Various, seemingly random artificial heaps of large boulders are locally scattered over the sea floor, possibly as a result of spilling or dumping at the time of constructing the Brouwersdam (finished in 1971), which likely prevents bottom disturbance by fishing activities. These breakwaters are colonized by blue mussels (M. edulis) and Pacific oysters (C. gigas), and the empty shells of these bivalves are deposited as substrate in the surrounding soft sediments.
Figure 1. (A) The Netherlands with (B) the location of the shellfish reef with native European flat oysters (O. edulis) that was recently discovered in the Haringvliet coastal zone (Voordelta) in the Dutch part of the North Sea. Note the tidal water outlet (marked with a grey arrow) of Lake Grevelingen (‘LG’). (C) A detailed map of the extent of the shellfish reef with native oysters, with transect locations, note that the bank's northern border has not yet been confirmed (indicated by a dashed line). (D) an aerial photo of the breakwater ‘Blokkendam’. (E) An impression of the shellfish reef with native O. edulis that is also inhabited by Pacific oysters (C. gigas), blue mussels (M. edulis) and epibionts (photos D and E by Peter van Rodijnen).
The extent of the oyster reef (c) was surveyed by a scuba diver following the outer edges of the reef while deploying small buoys at regular intervals. The outer reef edge was drawn around shellfish patches with five living oysters per m2 or more, following the native O. edulis oyster bed habitat definition (Haelters and Kerckhof Citation2009; OSPAR Citation2009). These buoys were then plotted using a handheld GPS by the boat crew. Subsequently, the presence of the reef within the extent was validated by a haphazardly placed series of short dives at point locations and later also by the visual census transect measurements.
Measurements were taken of O. edulis size-frequency distributions and attachment preference, as well as the composition of the bivalve community, epibenthic community and substrata types. These were conducted by scuba divers in 75 quadrats that were spread across the oyster bed along 15 transect lines (). Along a transect line of 25 m, five quadrats (50 cm × 50 cm) were placed 5 m apart. For two transects the distance between quadrats was adjusted to 2.5 m to fit five quadrats within the extent of the reef. All transect lines were positioned perpendicular to the coast and were randomly set up across the oyster reef in between the reef’s extent. The positions of the start and endpoint of the transect were marked by handheld GPS. All measurements were conducted simultaneously in each quadrat by two observers using underwater visual census and scuba. One observer was measuring species richness, while the other performed the other measurements. To assess the population composition, the density and cover of live O. edulis, C. gigas and M. edulis was estimated. All other bivalves were grouped into other bivalves. Additionally, the size-frequency distribution was determined from the 122 O. edulis individuals found in the quadrats. Oyster shell width was measured as the longest axis of the right valve (distance between the anterior and posterior tip) with a calliper to the nearest millimetre. To assess the species richness, epibenthic invertebrates (>5 mm) were identified within the quadrat in the field. For colony forming organisms only the percentage cover was estimated and only abundance was collected for solitary invertebrates. The observed species were identified to species level or the finest taxonomic level possible. Species richness was compared between patches with native oysters and bare sand patches within the reef. The substrate preference for O. edulis settlement was investigated by careful visual inspection of all individual native O. edulis found in the quadrats. The (shell) material attached to the umbonal area (or dorsal tip) of the left concave valve of each oyster was identified to species level. After this underwater inspection, the oysters were carefully placed back into its original position. For each quadrat the dominant sediment type was noted; hard substrate (rock, stones), coarse sandy substrate and fine mud and clay substrate. Quadrats were sampled between August and October 2017. Additional observations on fish and epibenthic species presence were collected between 2015 and 2017 using (video-) monitoring.
Prior to statistical analysis, data were checked for normality using Shapiro–Wilk tests (P = 0.05) and further confirmation by graphical validation. We have averaged the quadrat data within transects to include the spatial structure of the transect data. No transformations were needed. The differences in O. edulis number of species per habitat were analysed with one-way ANOVA. The data on substrate settlement were not normally distributed. A non-parametric Kruskal–Wallis test for paired samples (P < 0.05) was used to test for differences in O. edulis settling substrates with post-hoc pairwise comparisons using Wilcoxon rank tests with Bonferroni adjustment for multiple testing. Average values are presented with standard errors (SE).
Results
In October 2015, during a field survey of sites suitable for oyster reintroduction, we discovered a mixed shellfish reef consisting of a substantial population of native oysters (O. edulis), mixed with Pacific oysters (invasive oyster, C. gigas), blue mussels (M. edulis), but also bare substrate patches. The total area of the O. edulis reef measured at least 39.6 hectare, with the northern boundary still not determined (). The reef was found at water depths between 2 and 5 metres below Amsterdam Ordinance Datum (NAP). Three bivalve species dominated the reef. Ostrea edulis density was 6.8 ± 0.6 oysters m−2 in shellfish patches within this reef, C. gigas density was 19.4 ± 1.8 oysters m−2 and M. edulis density was 8.8 ± 1.9% cover. Although a relatively low density of living native oysters was observed on this reef, large quantities of dead shell of mixed species provided a substantial reef structure. Ostrea edulis were located in areas with (but not attached to) hard substrate (rock, stones in 65.7 ± 3.4% of quadrats), but also on coarse sandy sediment (14.5 ± 2.4%) and on fine mud and clay sediment (27.8 ± 3.9%). The size-frequency distribution of native O. edulis showed that the native oysters on this reef consisted of individuals from different size classes (a). Ostrea edulis shell width ranged from 1.0 cm to 11.2 cm and averaged 6.6 ± 0.2 cm. Oysters were often found to grow on hard substrate together with invasive Pacific oysters and blue mussels. Native oysters were predominantly attached to the shell (fragments) of invasive oyster (81.3 ± 5.7%) and were significantly less often attached to shell fragments of other bivalves (12.7 ± 4.7%) such as the blue mussels, common cockle and native oyster (b, P < 0.001; ). For a small number of native oysters (5.8 ± 3.0%) no (shell) fragment could be found attached. The epibenthic community field assessment of the shellfish reef with O. edulis yielded 74 species (Appendix 1), endobenthos was not sampled. As invasive Pacific oysters were mixed with native oysters in most quadrats their individual impact on species richness could not be determined. The species list included some species of conservation interest that all used the native oyster as a habitat (). The number of epibenthic species was significantly increased by 60% in quadrats with native and invasive oysters (14.9 ± 1.8 species m−2) when compared with adjacent sand patches (9.3 ± 0.7 species m−2) (c, P < 0.001).
Figure 2. (A) Size-frequency diagram of O. edulis (n = 122), (B) settlement substrate preferences (n = 75) of O. edulis, P < 0.001. Inset: native oyster (‘NO’) settled on invasive Pacific oyster (‘PO’). (C) Comparison of the average species richness of epibenthic species on the shellfish reef (>5 oysters m−2) per quadrat compared with adjacent bare soft sediment areas in the North Sea Voordelta area, P < 0.001.
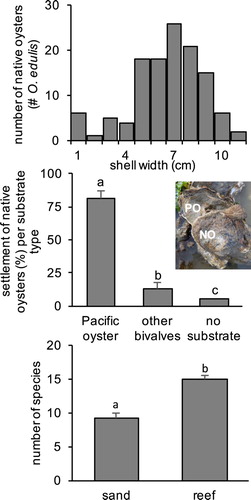
Figure 3. Native oysters (O. edulis) were found attached to multiple bivalve species that provided settling substrate for the native oyster. (A) Detail of a large O. edulis with several smaller native oysters of different sizes attached on top, showing that the reef includes native oysters of different size classes. All oysters have the flat right valve facing upwards (photo by Wouter Lengkeek). (B) O. edulis in between and attached to blue mussels. The oyster is positioned both standing straight up as well as C) positioned flat on the ground. Smaller native oysters (recruits) were found attached to larger native European flat oysters (C, E, G), to (D) Pacific oysters (C. gigas) and to (F) American razor clams (Ensis leei).
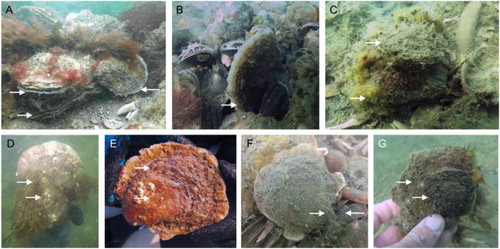
Table I. Species with special conservation status, which were found on the shellfish reef with native oysters (O. edulis) between 2015–2017, in the Dutch Voordelta area of the North Sea. Conservation status ‘H1110A’; typical species of Sundseth and Creed (Citation2008) habitat type subtidal sandbanks. German Red List scores; ‘2’ highly endangered; ‘3’ endangered; ‘R’ extremely rare; ‘G’ unknown. EU Red List of habitats ‘A3’; critically endangered habitat for both the EU 28 and EU 28+.
Discussion
The reef that was found in the coastal zone of the North Sea near Zeeland is the first shellfish reef with native European flat oysters that has returned in the Dutch part of the North Sea to our knowledge. At this reef, O. edulis oysters of multiple size classes were found. Together with observed gonad development in caged oysters (Sas et al. Citation2016) this shows that environmental conditions for these native oysters were suitable to allow their survival, growth, reproduction and recruitment (‘SGRR’). This discovery shows that conditions for native oyster restoration can be suitable at a local scale and suggests that the return of native oysters is possible in the coastal North Sea area. Recent findings in a national shellfish survey suggests that success is not limited to this single oyster bed as a small number of native oysters were also found in an area more than 6 km north-west of the first bank (Sas et al. Citation2018).
Our results may help in deriving guiding principles for restoration and conservation of native European flat oyster reefs elsewhere in the Voordelta and in the North Sea. Native oyster used shell fragments of bivalves, predominantly invasive oyster, but also blue mussels, American razor clams (Ensis leei), and common cockle (Cardium edule) as a settlement substrate (). These observations not only indicate the importance of substrate availability to restore oyster stocks, but also indicate the biocoenosis of multiple bivalves that were living in the same habitat. Visual observations of subfossils of O. edulis brought up by trawlers supported this observation for the North Sea. These fossil oysters were attached to shells of other molluscs including Buccinum undatum, Neptunea antiqua and O. edulis (personal communication G. Beuker). Hence, to better pinpoint areas for oyster restoration, the ‘shelliness’, the availability of shell material in an area, should be taken into account. Areas with high availability of shell material are most suitable when selecting sites for oyster (larvae) introduction. Furthermore, shells (e.g. of invasive oysters) can be introduced as settlement substrate. Additionally, as a copious novel source of substrate Pacific oysters can facilitate native oysters wherever their species distributions overlap or shells are introduced; these Pacific oyster reefs can form important focal areas for restoration projects.
Invasive alien Pacific oysters were found to co-occur with native bivalves in other locations. For example, co-existence between native and invasive oysters is reported in Ireland (Zwerschke et al. Citation2017) in the North Sea (this study) and the Wadden Sea (personal observation authors). Also, in Lake Grevelingen co-existence of both species is well documented on the basis of landing data of both species that are cultivated on bottom plots in the area. In both the Dutch as well as the German part of the Wadden Sea, mussel beds were invaded by alien Pacific oysters, changing the reef from a mono-species (M. edulis) bed into a two-species multi-layered mixed reef of oysters and mussels (around 1990; Reise et al. Citation2017b; Nieuwhof Citation2018). This co-existence of native and invasive bivalves provided refuge against predation for a higher abundance of the native mussel, and possibly also for the native oyster. Furthermore, these reefs with invasive oysters may improve structural stability and a natural no-fishing zone that protect native oysters (Reise et al. Citation2017a). Therefore, it is hypothesized that the facilitation of native oysters by invasive oysters may also increase survival of native oyster not only during the settlement phase but also during their adult lives.
The potential positive impact of invasive oysters is in line with recent discussions that highlight the role of non-native species in marine ecosystems and how their effect is not always negative (threatening biodiversity, ecosystem function, human well-being) but can sometimes be positive, especially when non-native foundation species establish in areas where the native ecosystem is very degraded (Thomsen et al. Citation2011; Byers et al. Citation2012; Ramus et al. Citation2017). The bottom of the North Sea is an example of such a degraded system. Although we did not experimentally identify preferences for substrate settlement for native European flat oysters, our results suggest that, by providing a critical novel source of substrate, Pacific oysters may facilitate the return of native oysters wherever their shells are available or provided.
Our results also underpin the importance of the oyster reefs with native oysters as key habitat species to increase biodiversity in the Voordelta and elsewhere in the North Sea. This study demonstrates that these reefs not only enhance the available hard substrate in soft sediment ecosystems but also increase the species richness of associated assemblages, including several species of conservation interest (). These results support the value of restoring O. edulis reefs for biodiversity in general and specific conservation goals in the North Sea. Although benthic assemblages associated with native and non-native oysters can be similar (Zwerschke et al. Citation2016), their net biomass and biodiversity is expected to increase when restoring native oysters due to the different spatial distribution of both oysters. In contrast to native oysters, invasive oysters are restricted to a coastal (shallow) habitat (personal observation authors), and therefore only native oysters are available for restoration of oyster reefs in deeper offshore habitats. When native oyster reefs return to offshore habitats in the North Sea, the biodiversity and biomass of associated assemblages is expected to increase at a seascape scale, as epibenthic biogenic reefs are currently rare.
Underlying research materials
The underlying research materials for this article can be accessed at <https://doi.org/10.4121/uuid:62dcccd6-6dc4-47c4-9ef8-09afa163cf76>/ 4TU Centre for Research Data.
Supplemental Material
Download MS Word (27.9 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
M. J. A. Christianen http://orcid.org/0000-0001-5839-2981
Additional information
Funding
References
- Bayne BL, Ahrens M, Allen SK., D'auriac MA, Backeljau T, Beninger P, Bohn R, Boudry P, Davis J, Green T, et al. 2017. The proposed dropping of the genus Crassostrea for all Pacific cupped oysters and its replacement by a new genus Magallana: a dissenting view. Journal of Shellfish Research. 36:545–547. doi: 10.2983/035.036.0301
- Beck MW, Brumbaugh RD, Airoldi L, Carranza A, Coen LD, Crawford C, Defeo O, Edgar GJ., Hancock B, Kay MC, et al. 2011. Oyster reefs at risk and recommendations for conservation, restoration, and management. Bioscience. 61:107–116. doi: 10.1525/bio.2011.61.2.5
- Berghahn R, Ruth M. 2005. The disappearance of oysters from the Wadden Sea: a cautionary tale for no-take zones. Aquatic Conservation: Marine and Freshwater Ecosystems. 15:91–104. doi: 10.1002/aqc.635
- Brett CE, Parsons-Hubbard KM, Walker SE, Ferguson C, Powell EN, Staff G, Ashton-Alcox KA, Raymond A. 2011. Gradients and patterns of sclerobionts on experimentally deployed bivalve shells: synopsis of bathymetric and temporal trends on a decadal time scale. Palaeography, Palaeoclimatology, Palaeoecology. 312:278–304. doi: 10.1016/j.palaeo.2011.05.019
- Brumbaugh RD, Sorabella LA, Garcia CO, Goldsborough WJ, Wesson JA. 2000. Making a case for community-based oyster restoration: an example from Hampton Roads, Virginia, USA. Journal of Shellfish Research. 19:467–472.
- Byers JE, Gribben PE, Yeager C, Sotka EE. 2012. Impacts of an abundant introduced ecosystem engineer within mudflats of the southeastern US coast. Biological Invasions. 14: 2587–2600. doi: 10.1007/s10530-012-0254-5
- Dolmer P, Hoffmann E. 2004. Østersfiskeri i Limfjorden-sammenligning af redskaber. Danmarks Fiskeriundersøgelser. 43 pages.
- Fariñas-Franco JM, Pearce B, Mair JM, Harries DB, MacPherson RC, Porter JS, Reimer PJ, Sanderson WG. 2018. Missing native oyster (Ostrea edulis L.) beds in a European marine protected area: should there be widespread restorative management? Biological Conservation. 221:293–311. doi: 10.1016/j.biocon.2018.03.010
- Gercken JS, Schmidt A. 2014. Current status of the European Oyster (Ostrea edulis) and Possibilities for Restoration in the German North Sea. 96 pages.
- Grabowski JH, Brumbaugh RD, Conrad RF, Keeler AG, Opaluch JJ, Peterson CH, Piehler MF, Powers SP, Smyth AR. 2012. Economic valuation of ecosystem services provided by oyster reefs. Bioscience. 62:900–909. doi: 10.1525/bio.2012.62.10.10
- Gubbay S, Sanders N, Haynes T, Janssen JAM, Rodwell JR, Nieto A, García Criado M, Beal S, Borg J, Kennedy M, et al. 2016. European red list of habitats. Part 1. Marine habitats. Brussels: European Commission.
- Günther RT. 1897. The oyster culture of the ancient Romans. Journal of the Marine Biological Association of the United Kingdom. 4:360–365. doi: 10.1017/S0025315400005488
- Haelters J, Kerckhof F. 2009. Background document for Ostrea edulis and Ostrea edulis beds. 22 pages.
- Kennedy RJ, Roberts D. 2006. Commercial oyster stocks as a potential source of larvae in the regeneration of Ostrea edulis in Strangford Lough, Northern Ireland. Journal of the Marine Biological Association of the United Kingdom. 86:153–159. doi: 10.1017/s0025315406012963
- Lemasson, A.J, Fletcher, S, Hall-Spencer, JM, Knights, AM. 2017. Linking the biological impacts of ocean acidification on oysters to changes in ecosystem services: a review. Journal of Experimental Marine Biology and Ecology. 492:49–62. doi: 10.1016/j.jembe.2017.01.019
- Lotze HK, Lenihan HS, Bourque BJ, Bradbury RH, Cooke RG, Kay MC, Kidwell SM, Kirby MX, Peterson CH, Jackson JBC. 2006. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science. 312:1806–1809. doi: 10.1126/science.1128035
- Ministry of Infrastructure and Environment. 2015. Mariene Strategie voor het Nederlandse deel van de Noordzee 2012–2020. Deel 3 KRM-programma van maatregelen, Bijlage 5 bij het Nationaal Waterplan 2016–2021. Den Haag: Ministerie van Infrastructuur en Milieu. 152 pages.
- Nieuwhof S. 2018. The use of remote sensing to reveal landscape-scale ecosystem engineering by shellfish reefs [PhD thesis]. University of Twente. 150 pages.
- Olsen OT. 1883. The Piscatorial Atlas of the North Sea, English and St. George's Channels: Illustrating the Fishing Ports, Boats, Gear, Species of Fish (How, Where, and when Caught), and Other Information Concerning Fish and Fisheries. Olsen. 50 pages.
- OSPAR Commission. 2009. Background document for Ostrea edulis and Ostrea edulis beds. 22 pages.
- Ramus AP, Silliman BR, Thomsen MS, Long ZT. 2017. An invasive foundation species enhances multifunctionality in a coastal ecosystem. Proceedings of the National Academy of Sciences. 114:8580–8585. doi: 10.1073/pnas.1700353114
- Reise K, Buschbaum C, Buttger H, Rick J, Wegner KM. 2017a. Invasion trajectory of Pacific oysters in the northern Wadden Sea. Marine Biology. 164:68. doi: 10.1007/s00227-017-3104-2
- Reise K, Buschbaum C, Büttger H, Wegner MK. 2017b. Invading oysters and native mussels: from hostile takeover to compatible bedfellows. Ecosphere. 8:e01949. doi:10.1002/ecs2.1949
- Sas H, Kamermans P, van der Have T, Lengkeek W, Smaal A. 2016. Shellfish reef restoration pilots: Voordelta, The Netherlands. Annual report. Pacific Oyster Consortion. Commissioned by ARK Natuurontwikkeling and WWF Netherlands. 45 pages.
- Sas H, van der Have T, Kamermans P. 2018. Shellfish reef restoration pilots 2017, Voordelta, The Netherlands. Annual report. Pacific Oyster Consortion. Commissioned by ARK Natuurontwikkeling and WWF Netherlands. 45 pages.
- Smaal AC, Kamermans P, van der Have T, Engelsma MY, Sas H. 2015. Feasibility of Flat Oyster (Ostrea edulis L.) restoration in the Dutch part of the North Sea. IMARES. c028–15. 48 pages.
- Sundseth K, Creed P. 2008. Natura 2000: protecting Europe's biodiversity. European Commission. Directorate-General for the Environment. 292 pages. doi:10.2779/45963
- Thomsen MS, Wernberg T, Olden JD, Griffin JN, Silliman BR. 2011. A framework to study the context-dependent impacts of marine invasions. Journal of Experimental Marine Biology and Ecology. 400:322–327. doi:10.3354/meps10566
- Zwerschke N, Emmerson MC, Roberts D, O'Connor NE. 2016. Benthic assemblages associated with native and non-native oysters are similar. Marine Pollution Bulletin. 111:305–310. doi: 10.1016/j.marpolbul.2016.06.094
- Zwerschke N, Kochmann J, Ashton EC, Crowe TP, Roberts D, O'Connor NE. 2017. Co-occurrence of native Ostrea edulis and non-native Crassostrea gigas revealed by monitoring of intertidal oyster populations. Journal of the Marine Biological Association of the United Kingdom. 1–10. doi: 10.1017/S0025315417001448
