 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Many cold-water sponges harbour microorganisms of which the role in the sponge host remains enigmatic. Here, we show a transfer of fixed inorganic carbon by sponge-associated microbes to its host, the cold-water coral encrusting sponge Hymedesmia (Stylopus) coriacea. Sponge were collected at approx. 100 m depth and incubated for 1.5–2.5 days with 13C labelled dissolved inorganic carbon (DIC) as tracer. Total DIC fixation rates ranged from 0.03–0.11 mmol C × mmol Csponge × d−1. 13C-tracer was recovered in bacterial-specific (i.e. short and branched) and sponge-specific (very long-chained) phospholipid-derived fatty acids (PLFA's), but was not incorporated into archaeal lipids. 13C-incorporation in biomarkers such as C16:1w7c and C18:1w7c indicated that nitrifying and/or sulphur-oxidizing bacteria (chemoautotrophs) were likely active in the sponge. Trophic transfer of microbially-fixed carbon to the sponge host was confirmed by recovery of label in very long chain fatty acids (VLCFA's) including C26:2 and C26:3. Tracer accumulation into several VLCFA's continued after removal of 13C-DIC, while tracer in most bacteria-specific PLFA's declined, indicating a transfer and elongation of bacterial-specific PLFA's to sponge-specific PLFA's. This implies that PLFA precursors released from chemo- as well as heterotrophic microbes in sponges contributed to the synthesis of VLCFA's, identifying sponge-associated bacteria as symbionts of the sponge.
Introduction
Many sponges live associated with a diverse and complex community of microorganisms (Taylor et al. Citation2007; Simister et al. Citation2012). Understanding the functional role of the associated microbes and the potential benefits for the host is a fundamental challenge in sponge biology. Evidence is accumulating from genomic studies and labelling studies that the sponge-microbe interaction is essential in the metabolism and nutrient cycles of sponges (Weisz et al. Citation2007; Hentschel et al. Citation2012; Webster and Thomas Citation2016; Rix et al. Citation2016a, Citation2016b). Photosynthetic products derived from sponge-associated microbial cells have been traced in sponge cells (Thacker and Freeman Citation2012). By using compounds enriched in heavy isotopes such as 13C-dissolved inorganic carbon, which can only be used by CO2 fixing microbes in the sponge, the transfer of organic carbon synthesized by the associated microbes to the host can be traced. A clear example hereof is the translocation of photosynthates from sponge-associated photoautotrophic microbes (e.g. cyanobacteria, zooxanthellae) to the sponge cells (Wilkinson and Fay Citation1979; Thacker Citation2005; Usher Citation2008; Weisz et al. Citation2010; Freeman and Thacker Citation2011; Fromont et al. Citation2015). Whether CO2 fixed by sponge-associated microbes in the dark is transferred to sponge host cells is as of yet unknown.
Chemoautotrophs such as nitrifying archaea, bacteria and mixotrophic Poribacteria have a cosmopolitan distribution in sponges and the genomic repertoire for chemotrophy (Preston et al. Citation1996; Hallam et al. Citation2006; Taylor et al. Citation2007; Lafi et al. Citation2009; Siegl et al. Citation2011; Simister et al. Citation2012; Cardoso et al. Citation2013; Li et al. Citation2014). They fix inorganic carbon by obtaining the energy from the oxidation of reduced inorganic compounds e.g. nitrification (e.g. Diaz and Ward Citation1997; Hoffmann et al. Citation2009; Radax et al. Citation2012). Many sponges live in the dark ocean in which CO2 fixation can only be performed by chemoautotrophic and mixotrophic microbes (bacteria and archaea). Direct CO2 fixation has been established for several cold-water coral reef sponges (Van Duyl et al. Citation2008) and although rates were low, transfer of specific compounds from the associated microbes to the host is expected to occur. In this study, we use CO2 fixation as a tool to assess general transfer between these microbes and the host sponge. To prove transfer, inorganic carbon exclusively fixed by microbes in the sponge needs to be recovered in sponge-specific compounds.
In particular, phospholipid fatty acids with very long chain fatty acids (i.e. >24 C atoms) are characteristic for demosponges, and have been used as biomarkers (Koopmans et al. Citation2015). Since sponges tend to lack the enzymes necessary to initiate fatty acid synthesis of short chain fatty acids or create branched fatty acids typically indicative of bacteria, they use enzyme systems for fatty acid elongation of bacterial-derived precursors to synthesize very long phospholipids characteristic for demosponges (Carballeira et al. Citation1986; Raederstorff et al. Citation1987; Hahn et al. Citation1988). Established precursors are the PLFA’s i-C15:0, aiC15, C16:0, C16:1ω7 (e.g. Hahn et al. Citation1988). Whether precursors are obtained from the diet of the sponge and/or the sponge-associated microbes is not known. Particularly under oligotrophic deep-sea conditions (low concentrations and poor quality of organic matter) it would be advantageous if sponge-associated microbes would provide the host with essential fatty acid building blocks. We hypothesize that sponge-associated microbes synthesize these building blocks, and that they are transferred and used by cold-water coral reef sponges for elongation of PLFA’s characteristic for demosponges. This would imply that there is a trophic transfer from sponge-associated microbes to the host.
For experiments we selected the deep-water demosponge Hymedesmia (Stylopus) coriacea (Fristedt, 1885, Subclass Ceractinomorpha, Order Poecilosclerida, Family Hymedesmidae). Hymedesmia (Stylopus) coriacea is a widely occurring encrusting sponge species in deep (>50 m) cold-water habitats in the NE Atlantic (Van Soest and De Voogd Citation2015) with a purported active associated microbial community (Rix et al. Citation2016a). It grows adjacent to living polyps of the cold-water coral Lophelia pertusa and is capable of overgrowing and killing it (Buhl-Mortensen and Buhl-Mortensen Citation2004), but CO2 fixations has not been documented for this species. We incubated H. coriacea with 13C-dissolved inorganic carbon in containers in the lab. To be sure that chemoautotrophic nitrifiers were not limited by ammonia, we added (NH4)2 SO4 to incubations. We determined the amount of 13C label incorporated in bacterial and archaeal lipids and in typical sponge-specific phospholipid-derived fatty acids. By adding high concentrations of 13C-DIC and applying long incubation times (1.5–2.5 days with 13C-DIC and chase periods of >15 days), we expected to obtain sufficient labelling of the microbial symbionts to detect incorporation and subsequent transfer. The aims of the present study are (1) to assess CO2 fixation by Hymedesmia (Stylopus) coriacea and (2) to detect transfer of fixed C from bacteria-specific phospholipid fatty acids (PLFA’s) to sponge-specific PLFA’s.
Material and methods
Tisler reef (58o59’ 50”N, 10o 57’ 39”E) is a 2 km long and maximally 200 m wide cold water coral reef in the NE Skagerrak on the Norwegian Shelf (Lavaleye et al. Citation2009). It is dominated by the cold-water coral Lophelia pertusa, which formed reef-like calcium carbonate structures protruding several metres above the sea floor. The skeleton of L. pertusa is locally overgrown by a sponge, which was originally identified by Van Soest as Hymedesmia (Stylopus) coriacea (). Sponge pieces of H. coriacea, were collected on the reef on 26 and 27 August 2010 between 90 and 115 m depth with a Sperre SUB-fighter 7500DC remotely operated vehicle (ROV) equipped with a manipulator arm and a sample collection tray. Lophelia pertusa pieces with overgrowing H. coriacea were transported to the lab in cooled, dark containers with seawater and transferred to aquaria in a climate room (air temperature and seawater temperature, 8°C). The climate room was supplied by a continuous flow of fresh seawater. The seawater was pumped to the lab via a sand-filter (1–2 mm particle size) from the entrance of the Koster Fjord adjacent to Tjärnö at 45 m depth (14.5°C). The seawater was cooled to 8°C before entering the climate room and was subsequently divided over the aquaria with the sponges. Sponge specimens were underwater-trimmed to appropriate sizes (2–5 cm wide and up to 8 cm high) for experiments and were kept in two 31 L aquaria under running seawater and continuous water movement by electric aquarium pumps. Sponges were left to recuperate from handling for minimally 2–3 days before incubation experiments started. The supply water, as well as the waste water of the experimental aquarium, was regularly sampled for inorganic nutrients, dissolved organic and inorganic carbon, oxygen and bacterial abundance. On 3 September 2010, water samples were collected on Tisler Reef (58o 59’ 49” N; 10o 57’ 32”E) at 123 m depth (2 m above the bottom) to compare its DOC, inorganic nutrients and DIC concentrations with that in the supply water.
Figure 1. In-situ benthic community with the coral Lophelia pertusa (white polyps) and the orange coral associated sponge Hymedesmia (Stylopus) coriacea overgrowing the coral (arrow is pointing to several coral polyps overgrown by the sponge).

Set-up of cylinder incubation (CY) experiments. Incubation experiments with single sponge specimens were conducted in 1 L cylindric incubation chambers (CY) consisting of an acrylic liner with a bottom and a top lid (). The top lid was equipped with a magnetic stirrer driven by an electric motor. The top contained a port for sampling and a port for the oxygen sensor. Three series of experiments with sponge pieces were conducted (CYa, CYb and CYc, ). 13C-labelled sodium bicarbonate (13C, 99%, Cambridge Isotope Laboratories, Inc.) was used as tracer. Label was dissolved in filtered seawater and added to the chambers, where it raised the concentrations of 13C with 600–900 μM. The series differed in incubation time and 13C-DIC concentration (CYa: 63 h, 600 μM; CYb: 44 h, 600 μM; CYc: 38 h, 900 μM 13C-DIC respectively, ). Each series was run with 6 incubation chambers with a single sponge piece. All 6 chambers received 13C-DIC of which 3–4 also received ammonium sulphate at a concentration of 100 μmol N L−1 ((NH4)2SO4, ACS reagent, >99%, Sigma) to obviate possible ammonium limitation of nitrification (). The oxygen concentration was measured with a Presens mini-optode (Fibox-3) to check whether the water remained well oxygenated in the separate incubation cylinders with sponge pieces. After the first experiment in which O2 concentrations dropped in a few chambers from 260 to <150 μmol L−1 during the incubation of 63 h, incubations were done with loose lids to allow O2 to enter the incubation cylinders. To stop the incubation, sponges were removed from the cylinders, flushed with fresh sea water, wrapped in aluminum foil and stored at −80°C. The increase in δ13C in sponge tissue relative to reference samples was determined in the bulk tissue, in archaeal lipids and phospholipid-derived fatty acids (PLFA).
Figure 2. Incubation cylinder with Hymedesmia (Stylopus) coriacea growing encrusting over branches of the cold-water coral Lophelia pertusa. Hymedesmia (Stylopus) coriacea has a light orange to light brown colour.
Table 1. Overview of sponge experiments conducted in cylinder incubation chambers (CY). Sponges in series CYa, CYb and CYc were incubated with 13C-DIC. To ten sponges (shaded in table) also 100 μM (NH4)2SO4 was added. Sponge with orange numbers (codes CY8-4, CY9-12, CY6-21) did not incorporate measureable amounts of 13C in bulk sponge tissue (see results CO2 fixation rates).
Set-up of pulse chase (PC) experiment. The PC experiment was conducted in a 31 L aquarium with 33 living sponge specimens. Sponge specimens were placed on a plastic grid ca 2 cm above the bottom of the aquarium. The water level over the sponges was lowered (15 L) and the water supply (not the water movement) was stopped during the initial 13C pulse phase. In total 3 g of 13C-labelled sodium bicarbonate was added after dissolution (2353 μmol L−1). The addition more than doubled the natural dissolved inorganic carbon (DIC) concentration (2122 μmol L−1). In addition, 200 mg ammonium sulphate was added increasing the ammonium concentration to 200 μmol N × L−1). The aquarium was largely covered by glass plates to reduce evaporation. During the pulse period, several O2 concentration measurements were done with a Presens mini-optode (Fibox-3) to check whether the water remained well oxygenated. After 49 h, the pulse period was stopped by quickly replacing the sponge-overlying water with supply water, and filling the aquarium up to the brim (31 L). Subsequently, the water supply of ca 2 L min−1 and throughflow were resumed which ran throughout the following 22-day ‘chase’ period of the experiment. Before the experiment started, two sponge samples were taken out of the aquarium for reference. Immediately after the pulse period of 49 h, two sponge specimens were removed from the aquarium, flushed with fresh seawater and stored at −80°C. In the following days, the rest of the sponges were removed and frozen, initially every day and gradually less frequently (t = 75, 98, 122, 148, 170, 197, 243, 289, 386, 459, 579 h). Last sponges were removed and stored 22 days (530 h) after the 13C-DIC pulse period. During the PC experiment, the inorganic nutrients, total and dissolved organic matter and the bacterial abundance in the water were monitored regularly in supply and outflow water. Samples for DIC concentrations (13C-DIC and total DIC) were taken before 13C-DIC addition, within 1 h after addition and just before removing the 13C-DIC enriched water.
Sponge tissue sampling. Two types of sponge tissue samples were prepared for analyses, pure sponge tissue samples and coral contaminated sponge samples. To obtain pure sponge tissue samples, the light-brown transparent sponge tissue was scraped from the coral branches, freeze-dried and grinded with mortar and pestle. The yield was however insufficient to perform all analyses with this material. To collect more sponge material from branches, pieces of coral skeleton with sponge tissue remains were used. After removal of coral polyps, the stripped corals with sponge remains were processed. These samples are referred to as coral contaminated sponge samples.
Samples used for bulk tissue δ13C and analysis method. Pure sponge tissue samples were used for bulk analysis of δ13C, C and N content of all sponges. Before measurements, samples were acidified with 2M HCl to remove calcium carbonate. Portions of up to 1100 μg DW were weighed in aluminum cups and measured for bulk δ13C and δ15N using a Thermo Electron FlashEA 1112 elemental analyzer (EA) coupled to a Delta V Advantage-isotope ratio mass spectrometer (IRMS, Thermo Scientific, Waltham, Bremen, Germany).
Samples used for archaeal lipids. The concentration of archaeal lipids was determined in one pure sponge tissue sample of the CYb series (CY8–11). To determine the 13C-DIC labelling of archaeal lipids, sponge tissue material of fifteen pure sponge tissue samples of the CY series was pooled to obtain sufficient material (> 120 mg DWsp) for label detection. For analysis of the natural 13C content of archaeal lipids, three sponge specimen (pure sponge tissue), of which the bulk tissue was not evidently labelled during the incubation with 13C-DIC (i.e. CY series sponges 4, 12 and 21, see ) were used as a reference.
Samples used for PLFA’s. The concentration and composition of PLFA’s in pure sponge tissue were measured in three sponge samples of the CY experiment: one labelled with 13C-DIC for 44 h in CYb (CY8–11) and two reference samples. The δ13C increase in PLFA’s in the labelled sample was only conducted for PLFA’s with equivalent chain lengths (ecl’s) of less than 24.9 C atoms. PLFA’s with longer ecl’s were not measured in pure tissue samples due to logistic constraints. All other PLFA analyses were conducted with coral contaminated sponge samples and covered the very long chain fatty acids (VLCFA) up to ecl’s of 29.3 carbon atoms. Of the CY series, three samples were analyzed for PLFA composition, concentration and 13C labelling (CYa:CY8–4 and CY9-5, incubated for 63 h; CYb series: CY8-11 incubated for 44 h). Of the pulse chase (PC) experiment, replicate coral contaminated sponge samples were pooled to collect sufficient sample of PLFA’s for analyses. Samples with insufficient material were not used. Consequently changes in labelling of PLFA’s in time in this experiment were available in four (0, 49, 148, 289 and 368/459 h) instead of eleven time intervals (as used for time series of δ13C of bulk tissue). For the last time step we combined samples which were incubated for 386 and 459 h (average 423 h). As reference samples (not incubated with 13C-DIC) for CY and PC experiments, we used the average δ13C of PLFA’s of two coral contaminated sponge samples.
Analyses of archaeal lipids and PLFA’s. Lipids were extracted using a modified Bligh and Dyer (Citation1959) method (Lengger et al. Citation2012). In short, archaeal lipids, GDGT’s (glycerol dibiphytanyl tetraether lipids) were separated into intact polar lipids, and simple (or core) lipids, and were quantified by HPLC-APCI-MS (Huguet et al. Citation2006; Schouten et al. Citation2007). To measure the 13C incorporation in archaeal membrane lipids, the total lipid extract was treated with HI/LiAlH4 to release the biphytanes, which were measured on a gas-chromatograph combustion-interface isotope-ratio mass spectrometer (GC-c-IRMS, Delta XP, Thermo Fisher Scientific Ltd., Waltham, MA) (Schouten et al. Citation1998; Lengger et al. Citation2014) and were analysed using ionOS stable isotope data processing software (Elementar UK Ltd., Cheadle, UK).
For phospholipid-derived fatty acid analysis, extracts (see above) were processed according to Boschker et al. (Citation1999) and Middelburg et al. (Citation2000). The phospholipid fatty acid fraction of the extract was obtained after elution over a silica column (Merck Kieselgel 60) with chloroform, acetone, and methanol and subsequently derivatized by mild alkaline methanolysis to obtain fatty acid methyl esters (FAME’s). Identification of the FAME’s was done by the equivalent chain length data comparison and by using C12:0 and C19:0 as standards for area corrections. In case FAME could not be identified, the equivalent chain length (ecl) was given. Particularly the very long fatty acids (VLCFA’s) present in H. coriacea with carbon chains of >24.9 were not identified with the exception of C26:2, C26:3 and C28:3. PLFA’s with slightly different ecl’s between separate samples. The carbon isotopic composition of individual FAME’s was determined with a gas-chromatograph combustion interface isotope mass spectrometer (GC-c-IRMS). Stable carbon isotope ratios (13C/12C) for individual FA’s were calculated from FAME’s by correcting for the one carbon atom in the methyl group that was added during derivatization (Middelburg et al. Citation2000).
Calculation of DIC incorporation rates and enrichments. Stable isotope ratios are given in the δ-notation for carbon:in which
To calculate the absolute amounts of 13C incorporated in sponge carbon (C) the following equation was used:
in which
and C presents the carbon concentration presented in μgC × gDW−1 or μmol × mmol Csponge−1.
For the latter, the TOC % of the sponge DW was used and the molecular weight of C.
For estimation of the total DIC fixation, we used:
Lipid concentrations were expressed in μg FA derived C × gDW−1 and μmolC × mmol Csponge−1. Contribution of separately-labelled PLFA's to the total pool of PLFA’s was expressed in %.
Bacterial abundance. The bacterial abundance in water samples was determined with flowcytometry. 1.5 ml unfiltered seawater (supply water) was fixed with 30 μl 25% glutaraldehyde in cryovials and placed in a 4–7°C refrigerator for 30 min and subsequently snap frozen in liquid nitrogen before storage in a −80°C freezer. Bacterial samples were stained with SYBR-I green at a final concentration of 10−4 of the commercial stock solution (Marie et al. Citation1997). The samples were incubated for 15 min in the dark, the discriminator was set on green fluorescence, and the bacteria in samples were counted for 1 min at a rate of 50 μl min−1 on a FACSort flow cytometer (Becton Dickinson, San Jose, Calif.) equipped with an air-cooled laser providing 15 mW at 488 nm and with the standard filter set-up.
Organic carbon, inorganic carbon, nitrogen and phosphorous. Total organic carbon (TOC), dissolved organic and inorganic carbon (DOC, DIC) and inorganic N and P concentrations were determined in water samples. DOC samples were filtered over a 0.2 μm polycarbonate filter (Millipore 47 mm) using a filter set-up with funnel, Erlenmeyer and vacuum hand pump. The filtration set-up was flushed three times with 10 ml filtered seawater before the sample water was collected. DOC samples (and unfiltered TOC samples) were transferred to (8 ml) combusted glass ampoules (4 h, 450°). After adding 1–2 drops of concentrated H3PO4, the ampoules were sealed and stored at 4–7°C. TOC and DOC were analysed by high-temperature combustion (Shimadzu, TOC Analyser, Model TOC-5000A). Each sample was measured in fivefold with an average analytical precision of 3%.
DIC samples were filtered over 0.2 μm Acrodisc syringe filters and transferred to small glass vials (5 ml) with screw caps, supplied in advance with 10 μl of saturated HgCL2. Vials were filled to the brim with filtered seawater without air inclusion. Samples for 13C-DIC were filtered over GF/F in a 20 ml headspace vial and fixed with 10 μl HgCl2, closed with an aluminum cap fitted with a rubber septum. All DIC samples were stored upside down until further analysis. DIC concentration in the small 5 ml vials was measured on a Technicon TRAACS 800 auto-analyzer (Technicon) following Stoll et al. (Citation2001). In 13C-DIC samples, a headspace of 3 ml was created by injecting N2 gas through the vial septum (Moodley et al. Citation2000). Samples were then acidified with 20 μl of concentrated H3PO4 to transform DIC into CO2 × 10 ml sample of the headspace gas was injected into an elemental analyzer isotope-ratio mass spectrometer (EA-IRMS).
Samples for inorganic N and P were filtered over 0.2 μm Acrodisc syringe filters and transferred to 6 ml plastic pony vials and stored at −20°C. Concentrations of PO43-, NH4+, NOx and NO2- (NOx-NO2-=NO3-) were measured on a Technicon TRAACS 800 auto-analyser.
Results
In-situ video observations. Underwater video images made during the collection showed that the sponge Hymedesmia (Stylopus) coriacea is, despite its patchy distribution, common on Tisler reef. It usually grows encrusting over the lower branches of the coral Lophelia pertusa extending towards the polyps (). Just below the coral polyp, the sponge extension tends to stop although complete overgrowth of the calices with live coral polyps was often observed on the underwater video as well as in the aquarium. In the aquarium, we also observed that the coral coenosarc is capable of overgrowing the sponge.
Supply water for incubation experiments. Water characteristics of Tisler reef water and the water used for the experiments (Bay of Tjärnö supply water) were roughly comparable taking into account that variations in the supply water covered variations between 25 August and 10 September 2010, while water at Tisler reef could only be sampled once (). The organic carbon (DOC and TOC) was on average higher in the supply water than at Tisler reef. Inorganic nutrients (PO43-, NOx) and bacterial abundance had lower average values in supply water than on Tisler reef.
Table 2. Concentrations of variables (with standard deviations) of In-situ water from Tisler reef (collected on 3 September 2010) and the Bay of Tjärnö (collected between 25 August and 10 September 2010). Bay of Tjärnö water was used for the incubations. DIC = dissolved inorganic carbon, TOC = total organic carbon, DOC = dissolved organic carbon.
CO2 fixation rates. Both incubation set-ups (CY and PC) showed 13C-DIC incorporation by the sponge () relative to reference samples of Hymedesmia (Stylopus) coriacea (δ13C −19.66‰ ± 0.37‰, n = 3).
Table 3. DIC fixation by Hymedesmia (Stylopus) coriacea with and without ammonium sulfate addition in cylinder (CY) and pulse-chace (PC) experiments with standard deviations.* Rate calculation made 3 days after pulse was removed, assuming that the 13C was only incorporated during pulse period.
For rate calculations per unit sponge carbon in tissue, we used the average organic carbon content of dry weight (DW) of H. coriacea, which was 41.27% ± 2.66 (n = 37). Rates in the CY experiment were slightly lower than in the PC experiment. The linear increase in 13C incorporation rates with 13C-DIC concentrations showed that the different treatments and variable conditions in experiments (e.g. lowered O2 concentrations in a few CY chambers of the CYa series) did not heavily affect the DIC fixation rates and that DIC fixation was not limited by DIC concentration (). Likewise, ammonia additions of 100–200 μM and its effect on other nutrients and organic matter over the incubation period (Table S1) did not influence 13C enrichment of the sponge (). Three of the 18 sponge pieces in cylinders did not incorporate measurable amounts of 13C in the bulk tissue and were not considered in rate calculations. These samples were used as reference samples for archaeal lipids (see M&M and ). The increase of δ13C in the remaining 15 sponge bulk samples (CY series) ranged from 1.2–5.7‰ (average 2.3‰, n = 15) after 38–63 h of labelling (reference samples −19.66‰ ± SD 0.37, n = 3). Sponge δ13C in the PC experiment increased with 7‰ during the pulse period (0–49 h), meaning that 34.71 μg13C × g DWsp−1 was incorporated representing a total CO2 fixation rate of 0.069 μmol C × mmolsp−1 × d−1 (32.54 μgC × gDWsp−1 × d−1, ). After removal of the pulse (δ13C= −12.53‰, 49 h), δ13C further increased with up to 33‰ (δ13C= +20.77‰, 98 h), with large variations between replicates (average 13C enrichment was 156 μg13C × g DWsp−1, ). The 13C-DIC concentration during the pulse period (2353 μM by addition) was 2.6–3.9 times higher than in the 1 L cylinder incubations and may have influenced the samples taken until two days (48 h) after the pulse period. Three days after the pulse (122 h after start of experiment), the 13C content returned to values found directly after the pulse (, ). Assuming that these values reflected the actual incorporation of 13C in constitutive cell compounds, the average C fixation in the PC experiment was 34.28 μgC × gDWsp−1 × d−1 (0.073 μmol C × mmol Csp−1 × d−1). Although slightly higher, this rate overlaps with the rates recorded in CY experiments (). In the chase period from 122 h onwards, values persisted until the end of the experiment with only a slight decrease of 1% × d−1 in 13C in the bulk tissue (based on data in , y = 34.99*e(−0.00041578x), R = 0.67) suggesting a turnover time of 100 days (2400 h) of the incorporated 13C.
Figure 3. Relation between sponge tissue fixation of 13C-DIC and 13C-DIC concentration with and without added ammonium sulfate. Linear regression lines are shown.
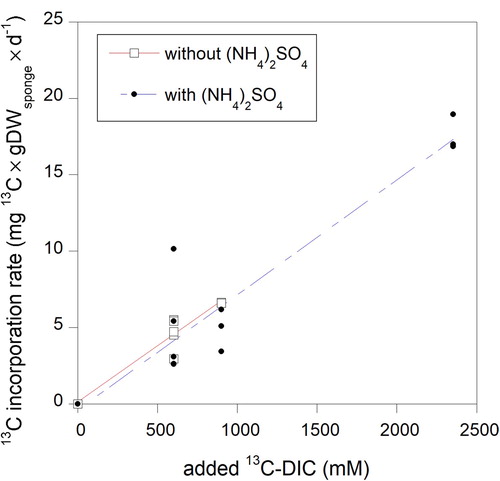
Figure 4. Changes in sponge bulk tissue 13C-enrichment after a pulse of 13C-labelled sodium bicarbonate (2353 μM 13C-DIC).
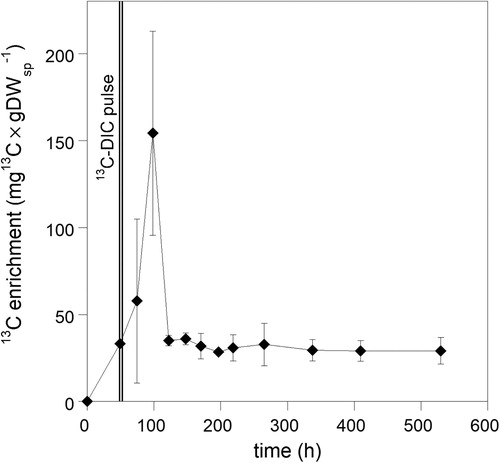
During the PC incubation, oxygen concentrations ranged between 250–256 μmol O2 × L−1 in the pulse period (23–33 h after starting the experiment). Ammonia concentrations increased with 100 μM (on top of the 200 μM added) and bacterial abundance increased more than 50 times exceeding increases of these variables in CY incubations (Table S2). Moreover, turbidity increased during the pulse period. These conditions apparently did not seriously affect the 13C incorporation rate during the pulse period, which spanned the rates obtained in the CY experiments. During the chase period, water quality conditions were comparable to the supply water ( and Table S2). The δ15N was not affected by added nutrients or labelling with 13C and varied between 14.77 and 17.01‰ for all samples analyzed (average 15.88 ± 0.51‰, n = 40). The average nitrogen content of the sponge dry weight (DW) was 7.75% ± SD 0.56 (n = 42). The average molar C:N ratio of H. coriacea was 6.2.
Concentration and labelling of archaeal lipids. Concentrations of archaeal lipids were measured in one sponge sample (CY 8–11). The GDGT-0 content was 160 μg × g DWsp−1 (0.38 μmol lipid derived C × mmol Csp−1) and the crenarchaeol content was 315 μg × g DWsp−1 (0.76 μmol lipid derived C × mmol Csp−1). A number of compounds eluted in the retention window, where archaeal lipids are normally found. Biphytanes, and other compounds, did not show 13C enrichment after background subtraction. This implies that no increase in δ13C in tetraether lipids of sponge-associated archaea could be detected after 38–63 h incubation with 600–900 μmol 13C-DIC.
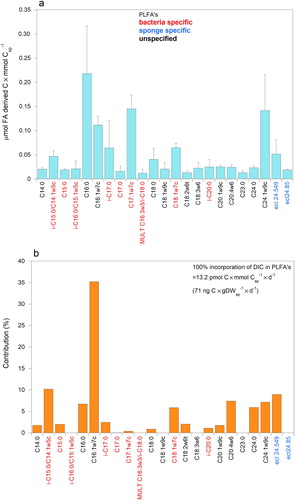
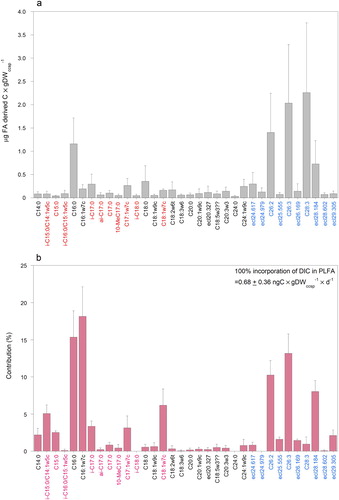
Composition and labelling of phospholipid-derived fatty acids (PLFA). In three pure sponge tissue samples 25–29 PLFA’s with equivalent chain lengths (ecl’s) of less than 24.9 carbon atoms (excluding PLFA’s with concentrations of less than 0.012 μmol FA derived C × mmol Csp−1) were detected. Samples had 23 PLFA’s in common (a), which weighed together 474 ± 157 μg FA derived C × gDWsp−1 (1.14 ± 0.38 μmol FA derived C × mmol Csp−1). Most of the PLFA’s were identified and the highest concentrations were recorded for C16:0, C16:1ω7c and C17:1ω7c and C24:1ω9 (a). Nine FA’s (35%± 1 of the weight of FA’s) were bacterial PLFA biomarkers in the sponge (a). The relatively high concentrations of unspecific C16:0, C16:1ω7c together with bacterial specific C18:1ω7c stood out. Seventeen of twenty-three PLFA’s increased in 13C content after labelling sponge CY 8–11 for 44 h with 13C-DIC (b). Recorded changes in δ13C (‰) in PLFA’s amounted up to + 20‰ (C16:1ω7c). Approximately 12% of the labelling of 23 PLFA’s was ascribed to bacteria-specific PLFA’s such as i-C15:0, C15:0, i-C17:0, C18:1ω7c and i-C20 (and 47% including C16:1ω7c). Strongest labelling was found in C16:1ω7c (b). Evident labelling of longer chain PLFA’s was found in C24:0, C24:1ω9 and ecl 24.55. Assimilation of 13C in PLFA’s (ecl < 25 C-atoms) was 0.015 ± 0.001 μg 13C × gDWsp−1 × d−1 (total DIC assimilation into the PLFA’s was 0.071 μg C × gDWsp−1 × d−1). Due to logistic constraints, the runs on the GC did not last long enough to measure longer chain fatty acids in these pure sponge tissue samples.
Figure 5. PLFA’s with equivalent chain length of less than 25 carbon atoms of pure tissue samples of the sponge Hymedesmia (Stylopus) coriacea in sample CY8-11. (a) Concentrations of PLFA’s. (b) Contribution (%) of 13C in PLFA’s after 44 h incubation with 13C-DIC.
Coral contaminated sponge (ccsp) samples showed a slightly different pattern in composition and distribution between PLFA’s (a) than the pure sponge tissue sample in a. Particularly PLFA’s with ecl’s between ecl 19.452 (i-C20:0) and 24.9 carbon atoms were found to differ between the two series, possibly related to remains of coral tissue. Despite the fact that in these coral contaminated sponge samples (ccsp) the concentrations of PLFA’s were lower per g DWccsp compared to the pure sponge samples, 34 different PLFA’s were distinguished that the four samples had in common. Because the dry weight was a mixture of coral skeleton and organic matter of sponge contaminated with coral (DWccsp), weights of PLFA’s per g DW sponge could not be determined. The average concentration of VLCFA’s (with ecl’s > 24.9 C-atoms) exceeded that of the shorter chain length FA’s (58% ± 24% versus 42 ± 24%). The concentration of bacteria-specific PLFA’s was approximately 12% (including C16:1ω7c) of all PLFA’s (a). Despite small differences, the 13C-labelling pattern of coral contaminated sponge samples in b confirms the pattern presented in b with clearly labelled bacteria-specific biomarkers (24 ± 5%) and C16:1ω7c (together 42 ± 3%) (b). With respect to longer chain PLFA’s, labelling of C24:0 was not found in coral contaminated sponge samples. Labelling of several very long chain PLFA’s (e.g. C26:2 and C26:3) was evident (b). On average 38 ± 6% of the incorporated 13C in PLFA was found in the VLCFA’s and 62% in the PLFA’s with ecl’s <24.9 after 38–64 h incubation. Applying this proportion to pure sponge tissue of which only PLFA’s <24.9C were analysed, we arrive at a total C uptake in PLFA’s of 0.115 μg C × g DWsp−1 × d−1.
Figure 6. PLFA results with equivalent chain lengths up to 29C-atoms of coral contaminated samples of the sponge Hymedesmia (Stylopus) coriacea. (a) Concentration of PLFA’s. (b) Contribution (%) of 13C in PLFA’s after 44-63 h incubation with 13C-DIC. For colour explanation of PLFA’s see a.
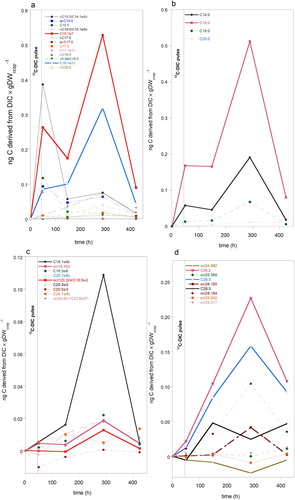
Pulse Chase experiment. Tracking 13C in PLFA’s in coral contaminated sponge sample in time. During the 13C-DIC pulse period (49 h) six of the thirteen bacterial- specific PLFA’s including C16:1ω7c were clearly enriched (a), indicating that bacteria fixed DIC and incorporated it in their PLFA’s. After the pulse was removed, labelling of most bacteria-specific PLFA’s dropped, with exception of C16:1ω7c and C18:1ω7c, which after a lag-period further increased (a). This latter pattern of accumulation of C derived from DIC was subsequently observed in C14:0 and C16:0 (b). DIC incorporation in unsaturated FAs with chain lengths of 10–24 carbon atoms (c) was relatively low compared to most other PLFA’s in a, b and d, with exception of oleic acid (C18:1ω9). This PLFA reached its highest incorporation approx. six days after removal of the pulse, comparable to other short chain fatty acids (a and b). Several polyunsaturated PLFA’s, probably not synthesized by bacteria, also peaked in this timeframe but not as pronounced as C18:1ω9. Most VLCFA’s (>24C) only started to increase after the pulse was removed and reached highest labelling approx. 250 h after removal of 13C-DIC. By the end of the experiment, labelling dropped but was still clearly present in C16:1ω7, C16:0 and several VLCFA’s in particular C26:2, C26:3, 28:3 and ecl 28.173 (a,b,d). It should be noted here that ecl values which slightly differed between separate runs on the GC-IRMS were lumped. In group ecl 29.305 atoms, ecl values ranged between 29.233 and 29.317 atoms. Group ecl 28.184 atoms contained PLFA’s with ecl’s between 28.111 and 28.184 carbon atoms. Variations in other groups (ecl 24.979, ecl 25.555, ecl 26.169 and ecl 28.602) were smaller than in ecl group 28.184. Groups may harbour different PLFA’s.
Figure 7. Temporal changes in 13C enrichment of PLFA’s of coral contaminated sponge samples of Hymedesmia (Stylopus) coriacea after a 13C-DIC pulse, which was removed 49 h after start of the pulse chase experiment. Concentration in ng C derived from DIC × g DWccsp−1 comprises 12C and 13C-DIC incorporated. (a) Bacteria-specific PLFA’s and C16:1ω7. (b) Saturated short chain PLFA’s (max 20 C-atoms chain lengths). (c) Mono- and poly-unsaturated PLFA with equivalent chain lengths of 18–25 carbon atoms. (d) Very long chain PLFA’s with equivalent chain lengths of 25 or more carbon atoms.
Discussion
We found dark CO2 fixation in Hymedesmia (Stylopus) coriacea by sponge-associated microbes and transfer of this fixed carbon to sponge-specific compounds. CO2 was mainly fixed by sponge-associated bacteria as evidenced by tracer incorporation into bacteria-specific phospholipid-derived fatty acids. Archaeal lipids were not labelled during incubations for up to 63 h. Transfer of short chain phospholipid-derived fatty acids characteristic for bacteria to very long chain sponge fatty acids (VLCFA’s) occurred, demonstrated by the appearance of label in desmsponge-characteristic PLFA’s such as C26:2 and C26:3. Labelled PLFA accumulation patterns in the pulse–chase experiment revealed increase of very long chain phospholipid fatty acids (≥24C) until 10 days after removal of the 13C pulse, indicating that the supply of precursors, such as C16:0 and C16:1ω7c synthesized by bacteria, also continued. Overall results suggest that sponges can acquire precursors for their PLFA synthesis from their associated microbes.
CO2 fixation. Although the microbiome of deep-water sponges harbours various CO2 fixing microbes (e.g. Meyer and Kuever Citation2008; Radax et al. Citation2012; Cardoso et al. Citation2013; Li et al. Citation2014), dark CO2 fixation by deep-water sponges was as far as we know only directly measured in cold-water coral reef sponges by Van Duyl et al. (Citation2008). They assessed CO2 fixation rates of sponges collected between 570 and 785 m depth on Rockall Bank (NE Atlantic) and reported rates of 0.7 and 4.5 nmol cm−3 × d−1 (0.005 and 0.036 μgC × gDW−1 × d−1) for the cold water coral reef sponges Higginsia thielei and Nodastrella nodastrella (formerly Rossella nodastrella). Rates for Hymedesmia (Stylopus) coriacea in this study were higher (range 20–34 μgC × gDWsp−1 × d−1, ) and comparable to estimated CO2 fixation rates (based on nitrification rate) for Geodia barretti (Hoffmann et al. Citation2009). Higher rates were not unexpected considering the fact that the depth at which the latter sponges were collected was less (100–200 m) and closer to the mainland. The higher metabolic activity of these shallower sponges probably results in an increased supply of reduced inorganic compounds required for CO2 fixation. The addition of NH4+ in the experiments did not stimulate the fixation rates, suggesting that there was sufficient ammonia available for nitrification by chemoautotrophic nitrifying bacteria and archaea. It is also possible that the added ammonia which increased the concentration with 100–200 μM in incubations, inhibited the increase in nitrification and thus also CO2 fixation due to toxicological effects (Camargo and Alonso Citation2006). Variations in CO2 fixation rates of sponges measured in cylinders were slightly lower than CO2 fixation rates in the pulse-chased experiment, which fluctuated heavily in the three days after the 13C-DIC pulse was flushed away. The high concentration of 13C-DIC applied during the pulse period of the pulse-chase experiment (2353 μM, which was 2.6–3.9 times higher than in the 1 L cylinder incubations) may have influenced the samples taken until two days (48 h) after the pulse period. Unincorporated 13C might have been still present in the sponge at the time of sampling. Comparison of rates with CO2 fixation rates of Lophelia pertusa (Middelburg et al. Citation2015) showed that the CO2 fixation rate by sponge tissue was approximately 20 times higher than that of coral tissue of Lophelia pertusa on Tisler reef. The sponge apparently provides a more suitable environment to support CO2 fixing microbes than the coral.
CO2 fixation rates represented a minor fraction (approximately 0.2–2.1%) of total estimated carbon requirements considering DOM and particulate feeding experiments with H. coriacea from the same location (Rix et al. Citation2016a; Van Oevelen et al. Citation2018) and oxygen consumption rates as reported for other cold-water sponges (e.g. Tjensvoll et al. Citation2013; Kutti et al. Citation2015; Kazanidis et al. Citation2018). This range of 0.2–2.1% also covers the estimates for contribution of CO2 fixation to total carbon uptake of Geodia barretti, Higginsia thielei, and Nodastrella nodastrella and the tissue of the cold-water coral L. pertusa (Van Duyl et al. Citation2008; Hoffmann et al. Citation2009; Middelburg et al. Citation2015). It is evident that cold-water sponges rely only for a small fraction of their carbon needs on CO2 fixation. The observation that the incorporated tracer carbon remained in their tissue long after the 13C-DIC was removed implies that it was incorporated in constructive compounds such as proteins, nucleic acids and phospholipids. The contribution of label in PLFA’s to bulk tissue labelling of the sponge with 13C-DIC was low, however, approximately 0.7%. This suggested that the majority of label was incorporated in other compounds such as amino acids, proteins, nucleic acids. It should be noted here that total 13C labelling of the sponge may have been slightly affected by anaplerotic reactions (Dijkhuizen and Harder Citation2000; Roslev et al. Citation2004; Alonso-Saez et al. Citation2010). Anaplerotic reactions replenish the tricarboxylic acid (TCA) cycle intermediates which are used for synthesis of amino acids, nucleic acids precursors, and biosynthesis of fatty acids. This could have led to labelling of the TCA pathway (pyruvate + CO2 + H2O + ATP > pyruvate carboxylase > oxaloacetate+ ADP + Pi + 2H+) in the microbial community as well as in the sponge cells. However, ammonia oxidizers and other obligate chemoautotrophs do not express all enzyme activities needed for a complete TCA cycle (Boschker et al. Citation2014). Moreover, carbon fixation through such reactions is not directly utilized in the synthesis of PLFA’s and archaeal lipids (Wuchter et al. Citation2003; Feisthauer et al. Citation2008). Therefore, the lipid labelling is considered to originate from the active CO2 fixation and transfer of this C to lipids.
Lipid composition. Concentrations of archaeal lipids (GDGT-0 and crenarchaeol, together 475 μg × gDWsp−1) in H. coriacea were high in comparison with GDGTs/biphytanes in other Demosponges (Pape et al. Citation2004). They found amounts of 0.8 μg × gDWsp−1 or less in sponges collected in a comparable depth range and habitat. However, as our sample size was low, and only a single analysis was conducted, these values are associated with a larger amount of uncertainty. Assuming that one archaeal cell contains 1*10−15 g GDGTs (Sinninghe Damsté et al. Citation2002), H. coriacea might harbour 4.8 *1011 cells × gDWsp−1 (23 μmolC × mmol Csp−1 based on 20 fg C × cell−1). High abundances of archaea have been reported for various sponge species (Preston et al. Citation1996; Jackson et al. Citation2013). The majority of these archaea are likely nitrifiers because crenarchaeol is a biomarker for ammonia oxidizing archaea (AOA), that also synthesize GDGT-0, a common, non-source–specific compound (Rush and Sinninghe Damsté Citation2017). These archaea belong to the Phylum Thaumarchaeota (Spang et al. Citation2010), convert ammonia to nitrite and fix CO2 (Preston et al. Citation1996). Even though no label was detected in archaeal lipids, it is likely that ammonia-oxidizing archaea contributed to the CO2 fixation of H. coriacea. This is because ammonia-oxidizing Thaumarchaeota have slow growth, and their lipids are known to possess slow turnover times (Xie et al. Citation2013; Lengger et al. Citation2014).
The PLFA profile of H. coriacea is characterized by bacteria-specific PLFA’s and VLCFA’s typical for sponges. The bacterial fingerprint comprised odd-numbered and branched, short-chain saturated and mono-unsaturated fatty acids of 15C, 17C and C18:1ω7c, which are considered biomarkers for bacteria (e.g. Volkman et al. Citation1980; Gillan et al. Citation1988). Also C16:0, C16:1ω7c and C18:1ω9 have been reported as common PLFA’s, albeit not specific for bacteria (Kaneda Citation1991; Boschker and Middelburg Citation2002). Striking was the dominance of C16:1ω7c in combination with C16:0 and C18:1ω7c, which may point to presence of chemoautotrophic nitrifying and sulphur-oxidizing bacteria (e.g. Blumer et al. Citation1969; Lipski et al. Citation2001; Van Gaever et al. Citation2009; Veuger et al. Citation2013; Boschker et al. Citation2014). In various sponge species, enhanced concentrations of these PLFA’s have been found (e.g. Blumenberg Citation2003; Van Duyl et al. Citation2011; Koopmans et al. Citation2015), suggesting that presence of chemoautotrophs is not unusual in sponges. The longest sponge-specifics PLFA in the pure sponge tissue sample which contributed substantially to the signal was C24:1ω9c, which has been reported for H. coriacea (Rix et al. Citation2016a) and has been observed in other sponges (e.g. Koopmans et al. Citation2011, Citation2015). In coral contaminated sponge samples, the bacterial signal was largely comparable to that in pure sponge tissue, albeit less pronounced. Contribution of typical PLFA’s of L. pertusa with 20–22 C atoms (Mueller et al. Citation2014; Middelburg et al. Citation2015) in coral contaminated sponge samples was small and therefore comparable to the pure sponge tissue sample. This implies that the recorded signals were mainly derived from the sponge. As expected, H. coriacea harboured very long chain PLFA’s of ≥24 C-atoms, which are characteristic of sponges (e.g. Litchfield et al. Citation1976; Djerassi and Lam Citation1991; Thiel et al. Citation1999; Blumenberg Citation2003; Mishra et al. Citation2015). The contribution of VLCFA’s to total PLFA’s in H. coriacea was on average more than 50%, in agreement with PLFA profiles of other sponges (Litchfield et al. Citation1976; Djerassi and Lam Citation1991). Sponge-specific VLCFA’s identified in this study (C24:1ω9, C26:2, C26:3 and C28:3) have been found in various other sponge species as well (e.g. Carballeira et al. Citation1986; Raederstorff et al. Citation1987; Hahn et al. Citation1988; Blumenberg Citation2003; De Goeij et al. Citation2008; Koopmans et al. Citation2011).
PLFA labelling. Besides labelling of bacteria-specific PLFA’s, strong increase of label in other short-chain saturated and monounsaturated lipids like C14, C16, C16:1ω7c was remarkable, which likely confirms their microbial source. Strong labelling of C16:1ω7c, in combination with labelling of C16:0 and C18:1ω7c suggested CO2 fixation activity by chemoautotrophic nitrifying and sulphur–oxidizing bacteria in the sponge (e.g. Lipski et al. Citation2001; Van Gaever et al. Citation2009; Middelburg et al. Citation2015). Abundances and activity of CO2 fixing microbes in the sand-filtered overlying water during the incubations, and their uptake by the sponge, were considered negligible. Therefore the 13CO2 fixation was ascribed to the sponge-associated microbes. The appearance of 13C label in PLFA’s with chain lengths of 24 carbon atoms or more showed that fixed inorganic carbon by bacteria was transferred to the sponge host. Since sponges tend to lack critical genes necessary to synthesize short chain PLFA’s, they use precursors from their diet for synthesis of the characteristic VLCFA’s such as C26:2, C26:3 and C28:3 (Carballeira et al. Citation1986; Hahn et al. Citation1988; Gold et al. Citation2017). Typical precursors for sponge fatty acids are C14, C16, C16:ω7, C18:1ω7 (Morales and Litchfield Citation1977; Walkup et al. Citation1981; Hahn et al. Citation1988; Djerassi and Lam Citation1991; Kornprobst and Barnathan Citation2010). Label was recovered in these PLFA’s, implying that CO2 fixing bacteria (and also other sponge-associated bacteria) synthesize them and might provide the sponge with these compounds. The pulse-chase experiment provided insight in the fate of short chain PLFA in time. Interestingly, after a clear initial increase in label of short chain FA’s during and directly after the pulse, labelling clearly dropped for most short chain PLFA’s, but dwindled for certain precursors e.g. C14, C16:0 and C16:1ω7. Precursors picked up labelling again 4–5 days after the pulse. The observed relay in precursor labelling suggests that besides labelled PLFA’s, other labelled compounds such as carbohydrates and amino acids were derived from CO2 fixing microbes. These compounds were likely used by heterotrophic bacteria in the sponge to produce building blocks for fatty acids (e.g.13C-acetate) and synthesize additional precursors for synthesis of VLCFA’s. This might explain the bi-phasic labelling pattern of various common short chain PLFA’s in this study. A comparable phenomenon was observed in pulse-chase experiments with 13C-labelled DIC and 13C-acetate in microbial mats showing transfer of metabolites from chemo- to heterotrophic microbes (Van der Meer et al. Citation2005, Citation2007). This implies that many more sponge-associated microbes than only CO2 fixing microbes can contribute to the supply of PLFA precursors for sponge-mebrane lipid synthesis. Results indicate that, although DIC fixation rates by sponges are low, the approach provides a useable tool to demonstrate transfer of compounds from the sponge microbiome to the sponge host. Essential products, such as short chain phospholipid fatty acids synthesized by CO2 fixing microbes, are also synthesized by heterotrophs. Results may therefore, with some reservation, be extrapolated to the whole bacterial community in sponges. Growth rates of chemoautotrophs are quite slow compared to heterotrophic bacteria, implying that actual transfer may be underestimated.
The build-up of label in VLCFA’s, C26:2, C26:3, C28:3 and the ecl 28.184 group, steadily increased and most VLCFA’s reached their highest labelling approximately 10 days after removal of the 13C-pulse together with the precursors. The turnover of VLCFA’s appeared, however, to be slower than that of the precursors with a decrease of 0–60% towards the end of the experiment depending on PLFA type, while labelling of precursors dropped with approximately 80% in this period. Hymedesmia (Stylopus) coriacea apparently continuously processed compounds derived from its associated microbes.
Whether the transfer of labelled compounds to the sponge host was via consumption of associated microbes or uptake of metabolites released by the microbes, which were assimilated by the sponge is still unknown. Rapid disappearance of bacteria-specific biomarkers suggests that available labelled metabolites were more directed to synthesis of PLFA precursors for the sponge than for synthesis of bacteria biomarkers. This suggests that the sponge host is able to identify and stimulate its associated microbes to produce compounds required by the sponge host (e.g. Webster and Taylor Citation2012).
We conclude that there is transfer of compounds synthesized by sponge-associated bacteria to the sponge host and that these microbes provide precoursors/building blocks for the synthesis of VLCFA’s characteristic for the sponge. It is evident that sponge-associated bacteria can be considered as true symbionts of the host. The extent to which archaea contribute to compound transfer could not be determined due to the short labelling times and remains to be investigated.
Supplemental Material
Download MS Word (19.3 KB)Acknowledgements
Support was given by the European Community for the access provided at the Sven Lovén Centre for Marine Sciences, Tjärnö, Sweden (ASSEMBLE grant agreement no. 227799). We thank the local technical and administrative staff for assistance and logistic support during the sponge experiments in Sweden. Karel Bakker, Santiago Gonzalez, Richard Doggen and Kirsten Kooijman and Jort Ossebaar of NIOZ-Texel are thanked for their analyses of inorganic nutrients, dissolved organic matter analyses, bacterial abundance and bulk stable isotope analyses. The analytical lab of NIOZ-Yerseke is thanked for PLFA analyses.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alonso-Saez L, Galand PE, Casamayor EO, Pedros-Alio C, Bertilsson S. 2010. High bicarbonate assimilation in the dark by Arctic bacteria. The ISME Journal. 4:1581–1590. doi: 10.1038/ismej.2010.69
- Bligh EG, Dyer WJ. 1959. A rapid method for total lipid extraction and purification. Canadian Journal Biochemistry and Physiology. 37:911–917. doi:10.1139/y59-099 doi: 10.1139/o59-099
- Boschker HTS, de Brouwer JFC, Cappenberg TE. 1999. The contribution of macrophyte-derived organic matter to microbial biomass in salt-marsh sediments: stable carbon isotope analysis of microbial biomarkers. Limnology and Oceanography. 44:309–319. doi: 10.4319/lo.1999.44.2.0309
- Boschker HTS, Middelburg JJ. 2002. Stable isotopes and biomarkers in microbial ecology. FEMS Microbiology Ecology. 40:85–95. doi: 10.1111/j.1574-6941.2002.tb00940.x
- Boschker HTS, Vasquez-Cardenas D, Bolhuis H, Moerdijk-Poortvliet TWC, Moodley L. 2014. Chemoautotrophic carbon fixation rates and active bacterial communities in intertidal marine sediments. PlosOne. 9:e101443. doi: 10.1371/journal.pone.0101443
- Blumenberg M. 2003. Biomarker aus Kaltwasser- und Tiefseekieselschwammen.phylogenie, Chemotaxonomie und chemische Ökologie der Demospongiae und der Hexactinellida [PhD thesis]. Hamburg, Universität Hamburg.
- Blumer M, Chase T, Watson SW. 1969. Fatty acids in the lipids of marine and terrestrial nitrifying bacteria. Journal Bacteriology. 99:366–370. doi: 10.1128/JB.99.2.366-370.1969
- Buhl-Mortensen L, Buhl-Mortensen P. 2004. Symbiosis in deep water corals. Symbiosis. 37:33–61.
- Camargo JA, Alonso A. 2006. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: a global assessment. Environment International. 32:831–849. doi: 10.1016/j.envint.2006.05.002
- Carballeira N, Thompson JE, Ayanoglu E, Djerassi C. 1986. Biosynthetic studies of marine lipids. 5.1 The biosynthesis of long-chain branched fatty acids in marine sponges. Journal Organic Chemistry. 51:2751–2756. doi: 10.1021/jo00364a024
- Cardoso JFMF, Van Bleijswijk JDL, Witte H, Van Duyl FC. 2013. Diversity and abundance of ammonia oxidizing archaea and bacteria in tropical and cold-water coral reef sponges. Aquatic Microbial Ecology. 68:215–230. doi: 10.3354/ame01610
- De Goeij JM, Moodley L, Houtekamer M, Carballeira NM, Van Duyl FC. 2008. Tracing 13C-enriched dissolved and particulate organic carbon in the bacteria containing coral reef sponge Halisarca caerulea: Evidence for DOM-feeding. Limnology and Oceanography. 53:1376–1386. doi: 10.4319/lo.2008.53.4.1376
- Diaz MC, Ward BB. 1997. Sponge-mediated nitrification in tropical benthic communities. Marine Ecology Progress Series. 156:97–107. doi: 10.3354/meps156097
- Dijkhuizen L, Harder W. 2000. Microbial metabolism of carbon dioxide. In: Dalton H, editor. Comprehensive bio/technology. Oxford: Pergamon Press Ltd; p. 409–423.
- Djerassi C, Lam W-K. 1991. Sponge phospholipids. Accounts of Chemical Research. 24:69–75. doi: 10.1021/ar00003a002
- Feisthauer S, Wick LY, Kastner M, Kaschabek SR, Schloman M, Richnow HH. 2008. Differences of heterotrophic 13CO2 assimilation by Pseudomonas knackmussii strain B13 and Rhodococcus opacus 1CP and potential impact on biomarker stable isotope probing. Environmental Microbiology. 10:1641–1651. doi: 10.1111/j.1462-2920.2008.01573.x
- Freeman CJ, Thacker RW. 2011. Complex interactions between marine sponges and their symbiotic microbial communities. Limnology and Oceanography. 56:1577–1586. doi: 10.4319/lo.2011.56.5.1577
- Fromont J, Huggett MJ, Lengger SK, Grice K, Schönberg CHL. 2015. Characterization of Leucetta prolifera, a calcarean cyanosponge from south-western Australia, and its symbionts. Journal of the Marine Biological Association of the United Kingdom. 96:541–552. doi:10.1017/S0025315415000491.
- Gillan FT, Stoilov IL, Thompson JE, Hogg RW, Wilkinson CR. 1988. Fatty acids as biological markers for bacterial symbionts in sponges. Lipids. 23:1139–1145. doi: 10.1007/BF02535280
- Gold DA, O’Reilly SS, Watson J, Degnan BM, Degnan SM, Krömer JO, Summons RE. 2017. Lipidomics of the sea sponge Amphimedon queenslandica and implication for biomarker geochemistry. Geobiology. 15:835–843. doi: 10.1111/gbi.12253
- Hahn S, Stoilov IL, Tam Ha TB, Raederstorff D, Doss GA, Li H-T, Djerassi C. 1988. Biosynthetic studies of marine lipids. 17.1 The course of chain elongation and desaturation in long-chain fatty acids of marine sponges. Journal American Chemical Society. 110:8117–8124. doi: 10.1021/ja00232a025
- Hallam SJ, Mincer TJ, Schleper C, Preston CM, Roberts K, Richardson PM, DeLong EF. 2006. Pathways of carbon assimilation and ammonia oxidation suggested by environmental genomic analyses of marine Crenarchaeota. PLOS Biology. 4:520–536. doi: 10.1371/journal.pbio.0040095
- Hentschel U, Piel J, Degnan SM, Taylor MW. 2012. Genomic insights into the marine sponge biome. Nature Reviews Microbiology. 10:641–654. doi: 10.1038/nrmicro2839
- Hoffmann F, Radax R, Woebken D, Holtappels M, Lavik G, Rapp HT, Schläppy M-L, Schleper C, Kuypers MM. 2009. Complex nitrogen cycling in the sponge Geodia barretti. Environmental Microbiology. 11:2228–2243. doi: 10.1111/j.1462-2920.2009.01944.x
- Huguet C, Hopmans EC, Febo-Ayala W, Thompson DH, Sinninghe Damsté JS, Schouten S. 2006. An improved method to determine the absolute abundance of glycerol dibiphytanyl glycerol tetraether lipids. Organic Geochemistry. 37:1036–1041. doi:10.1016/j.orggeochem.2006.05.008.
- Jackson SA, Flemer B, McCann A, Kennedy J, Morrissey JP, O’Gara F, Dobson ADW. 2013. Archaea appear to dominate the microbiome of inflatella pellicula deep sea sponges. PlosOne. 8:e84438. doi: 10.1371/journal.pone.0084438
- Kazanidis G, van Oevelen D, Veuger B, Witte UFM. 2018. Unravelling the versatile feeding and metabolic strategies of the cold-water ecosystem engineer Spongosorites coralliophaga (Stephens, 1915). Deep-Sea Research Part I: Oceanographic Research Papers 141:71-82.
- Kaneda T. 1991. Iso- and Anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiological Reviews. 55:288–302. doi: 10.1128/MMBR.55.2.288-302.1991
- Kornprobst J-M, Barnathan G. 2010. Demospongic acids revisited. Marine Drugs. 8:2569–2577. doi: 10.3390/md8102569
- Koopmans M, Van Rijswijk P, Martens D, Egorova-Zachernyuk TA, Middelburg JJ, Wijffels RH. 2011. Carbon conversion and metabolic rate in two marine sponges. Marine Biology. 158:9–20. doi: 10.1007/s00227-010-1538-x
- Koopmans M, Van Rijswijk P, Boschker HTS, Houtekamer M, Martens D, Wijffels RH. 2015. Seasonal variation of fatty acids and stable carbon isotopes in sponges as indicators for nutrition: biomarkers in sponges identified. Marine Biotechnology. 17:43–54. doi: 10.1007/s10126-014-9594-8
- Kutti T, Bannister RJ, Fosså JH, Krogness CM, Tjensvoll I, Søvik G. 2015. Metabolic responses of the deep-water sponge Geodia barretti to suspended bottom sediment, simulated mine tailings and drill cuttings. Journal of Experimental Marine Biology and Ecology. 473:64–72. doi: 10.1016/j.jembe.2015.07.017
- Lafi FF, Fuerst JA, Fieseler L, Engels C, Wei Ling Goh W, Hentschel U. 2009. Widespread distribution of Poribacteria in Demospongiae. Applied Environmental Microbiology. 75:5695–5699. doi: 10.1128/AEM.00035-09
- Lavaleye MSS, Duineveld GCA, Lundälv T, White M, Guihen D, Kiriakoulakis K, Wolff GA. 2009. Cold water corals on Tisler reef: Preliminary observations on the dynamic reef environment. Oceanography. 22:76–84. doi: 10.5670/oceanog.2009.08
- Lengger SK, Hopmans EC, Sinninghe Damsté JS, Schouten S. 2012. Comparison of extraction and work up techniques for analysis of core and intact polar tetraether lipids from sedimentary environments. Organic Geochemistry. 47:34–40. doi: 10.1016/j.orggeochem.2012.02.009
- Lengger SK, Lipsewers YA, de Haas H, Sinninghe Damsté JS, Schouten S. 2014. Lack of 13C-label incorporation suggests low turnover rates of thaumarchaeal intact polar tetraether lipids in sediments from the Iceland shelf. Biogeosciences. 11:201–216. doi:10.5194/bg-11-201-2014.
- Li Z-Y, Wang Y-Z, He L-M, Zheng H-J. 2014. Metabolic profiles of prokaryotic and eukaryotic communities in deep-sea sponge Neamphius huxleyi indicated by metagenomics. Scientific Reports. 4:3895. doi: 10.1038/srep03895
- Lipski A, Spieck E, Makolla A, Altendorf K. 2001. Fatty acid profiles of nitrite-oxidizing bacteria reflect their phylogenetic heterogeneity. System Applied Microbiology. 24:377–384. doi: 10.1078/0723-2020-00049
- Litchfield C, Greenberg AJ, Noto G, Morales RW. 1976. Unusually high levels of C24−C30 fatty acids in sponges of the class Desmospongiae. Lipids. 11:567–570. doi: 10.1007/BF02532903
- Marie D, Partensky F, Jacquet S, Vaulot D. 1997. Enumeration and cell cycle analysis of natural populations of marine picoplankton by flow cytometry using the nucleic acid stain SYBR green I. Applied Environmental Microbiology. 63:186–193. doi: 10.1128/AEM.63.1.186-193.1997
- Meyer B, Kuever J. 2008. Phylogenetic diversity and spatial distribution of the microbial community associated with the Caribbean deep-water sponge Polymastia cf. corticata by 16S rRNA, aprA, and amoA gene analysis. Microbial Ecology. 56:306–321. doi: 10.1007/s00248-007-9348-5
- Middelburg JJ, Barranguet C, Boschker HTS, Herman PMJ, Moens T, Heip C. 2000. The fate of intertidal microphytobenthos carbon: An in situ 13C-labeling study. Limnology and Oceanography. 45:1224–1234. doi: 10.4319/lo.2000.45.6.1224
- Middelburg JJ, Mueller CE, Veuger B, Larsson AI, Form A, Van Oevelen D. 2015. Discovery of symbiotic nitrogen fixation and chemoautotrophy in cold-water corals. Scientific Reports. 5:17962. doi: 10.1038/srep17962
- Mishra PM, Sree A, Panda PK. 2015. Fatty acids of marine sponges. In: Kim S-K, editor. Handbook of marine biotechnology. Berlin, Heidelberg: Springer; p. 851–868.
- Moodley L, Boschker HTS, Middelburg JJ, Pel R, Herman PMJ, Heip CHR. 2000. Ecological significance of benthic foraminifera: 13C labelling experiments. Marine Ecology Progress Series. 202:289–295. doi: 10.3354/meps202289
- Morales RW, Litchfield C. 1977. Incorporation of 1-14C-acetate into C26 fatty acids of the marine sponge Microciona prolifera. Lipids. 12:570–576. doi: 10.1007/BF02533383
- Mueller CE, Larsson AI, Veuger B, Middelburg JJ, Van Oevelen D. 2014. Opportunistic feeding on various organic food resources by the cold-water coral Lophelia pertusa. Biogeosciences. 11:123–133. doi: 10.5194/bg-11-123-2014
- Pape T, Blumenberg M, Thiel V, Michaelis W. 2004. Biphytanes as biomarkers for sponge associated archaea. In: Pansini M, Pronzato R, Bavestrello G, Manconi R, Sarà M, editor. Sponge Science in the New Millenium. Genua: Bollettino dei Musei e degli Istituti Biologici dell'Universitá di Genova 68. p. 509–515.
- Preston CM, Wu KY, Miolinski TF, DeLong EF. 1996. A psychrophilic crenarchaeon inhabits a marine sponge: Cenarchaeum symbiosum gen. nov., sp. nov. Proceedings of the National Academy of Sciences. 93:6241–6246. doi: 10.1073/pnas.93.13.6241
- Radax R, Hoffmann F, Rapp HT, Leininger S, Schleper C. 2012. Ammonia-oxidizing archaea as main drivers of nitrification in cold-water. Environmental Microbiology. 4:909–923. doi:10.1111/j.1462-2920.2011.02661.x.
- Raederstorff D, Shu AYL, Thompson JE, Djerassi C. 1987. Biosynthetic studies of marine lipids. 11.1 synthesis, biosynthesis, and absolute configuration of the internally branched demospongic acid, 22-methyl-5,9-octacosadienoic acid. Journal Organic Chemistry. 52:2337–2346. doi: 10.1021/jo00388a001
- Rix L, De Goeij JM, Mueller CE, Struck U, Middelburg JJ, van Duyl FC, Al-Horani FA, Wild C, Naumann MS, van Oevelen D. 2016a. Coral mucus fuels the sponge loop in warm-and cold-water coral reef ecosystems. Scientific Reports. 6:18715. doi: 10.1038/srep18715
- Rix L, De Goeij JM, Van Oevelen D, Struck U, Al-Horani FA, Wild C, Naumann MS. 2016b. Differential recycling of coral and algal dissolved organic matter via the sponge loop. Functional Ecology. 31:778–789. doi: 10.1111/1365-2435.12758
- Roslev P, Brøndum M, Jørgensen D, Hesselhoe M. 2004. Use of heterotrophic CO2 assimilation as a measure of metabolic activity in planktonic and sessile bacteria. Journal Microbiological Methods. 59:381–393. doi: 10.1016/j.mimet.2004.08.002
- Rush D, Sinninghe Damsté JS. 2017. Lipids as paleomarkers to constrain the marine nitrogen cycle. Environmental Microbiology. 19:2119–2132. doi: 10.1111/1462-2920.13682
- Sinninghe Damsté JS, Rijpstra WIC, Hopmans EC, Prahl FG, Wakeham SG, Schouten S. 2002. Distribution of membrane lipids of planktonic Crenarchaeota in the Arabian Sea. Applied Environmental Microbiology. 68:2997–3002. doi: 10.1128/AEM.68.6.2997-3002.2002
- Schouten S, Hoefs MJL, Koopmans MP, Bosch H-J, Sinnighe Damsté JS. 1998. Structural characterization, occurrence and fate of archaeal ether-bound acyclic and cyclic biphytanes and corresponding diols in sediments. Organic Geochemistry. 29:1305–1319. doi: 10.1016/S0146-6380(98)00131-4
- Schouten S, Huguet C, Hopmans EC, Kienhuis MVM, Sinninghe Damsté JS. 2007. Analytical methodology for TEX 86 paleothermometry by High-Performance Liquid Chromatography/atmospheric Pressure Chemical Ionization-mass Spectrometry. Analytical Chemistry. 79:2940–2944. Doi:10.1021/ac062339v.
- Siegl A, Kamke J, Hochmuth T, Piel J, Richter M, Liang C, Dandekar T, Hentschel U. 2011. Single-cell genomics reveals the lifestyle of Poribacteria, a candidate phylum symbiotically associated with marine sponges. ISME Journal. 5:61–70. doi: 10.1038/ismej.2010.95
- Simister RL, Deines P, Botte ES, Webster NS, Taylor MW. 2012. Sponge-specific clusters revisited: a comprehensive phylogeny of sponge-associated microorganisms. Environmental Microbiology. 14:517–524. doi: 10.1111/j.1462-2920.2011.02664.x
- Sinninghe Damsté JS, Rijpstra WIC, Hopmans EC, Wakeham SG, Prahl FG, Schouten S. 2002. Distribution of intact core ether lipids of planktonic Crenarchaeota in the Arabian Sea. Applied Environmental Microbiology. 68:2997–3002. doi: 10.1128/AEM.68.6.2997-3002.2002
- Stoll MHC, Bakker K, Nobbe GH, Haese RR. 2001. Continuous-flow analysis of dissolved inorganic carbon content in seawater. Analytical Chemistry. 73:4111–4116. doi: 10.1021/ac010303r
- Spang A, Hatzenpichler R, Brochier-Armanet C, Rattei T, Tischler P, Spieck E, Streit W, Stahl DA, Wagner M, Schleper C. 2010. Distinct gene set in two different lineages of ammonia-oxidizing archaea supports the phylum Thaumarchaeota. Trends in Microbiology. 18:331–340. doi: 10.1016/j.tim.2010.06.003
- Taylor MW, Radax R, Steger D, Wagner M. 2007. Sponge-associated microorganisms: Evolution, ecology, and biotechnological potential. Microbiology and Molecular Biology Reviews. 71:295–347. doi: 10.1128/MMBR.00040-06
- Thacker RW. 2005. Impacts of shading on sponge-cyanobacteria symbioses: A comparison between host-specific and generalist associations. Integrative and Comparative Biology. 45:369–376. doi: 10.1093/icb/45.2.369
- Thacker RW, Freeman CJ. 2012. Sponge-microbe symbioses: recent advances and new directions. Advances in Marine Biology. 62:57–111. doi: 10.1016/B978-0-12-394283-8.00002-3
- Thiel V, Jenisch A, Wörheide G, Lowenberg A, Reitner J, Michaelis W. 1999. Mid-chain branched alkanoic acids from “living fossil” demosponges: a link to ancient sedimentary lipids? Organic Chemistry. 30:1–14.
- Tjensvoll I, Kutti T, Fosså JH, Bannister RJ. 2013. Rapid respiratory responses of the deep-water sponge Geodia barretti exposed to suspended sediments. Aquatic Biology. 19:65–73. doi: 10.3354/ab00522
- Usher KM. 2008. The ecology and phylogeny of cyanobacterial symbionts in sponges. Marine Ecology Progress Series. 29:178–192. doi: 10.1111/j.1439-0485.2008.00245.x
- Van der Meer MTJ, Schouten S, Bateson MM, Nübel U, Wieland A, Kühl M, De Leeuw JW, Sinninghe Damsté JS, Ward DM. 2005. Diel variations in carbon metabolism by green nonsulfur-like bacteria in alkaline siliceous hot spring microbial mats from Yellowstone National Park. Applied and Environmental Microbiology. 71:3978–3986. doi: 10.1128/AEM.71.7.3978-3986.2005
- Van der Meer MTJ, Schouten S, Sinninghe Damsté JS, Ward DM. 2007. Impact of carbon metabolism on 13C signatures of cyanobacteria and green non-sulfur-like bacteria inhabiting a microbial mat from an alkaline siliceous hot spring in Yellowstone National Park (USA). Environmental Microbiology. 9:482–491. doi: 10.1111/j.1462-2920.2006.01165.x
- Van Duyl FC, Hegeman J, Hoogstraten A, Maier C. 2008. Dissolved carbon fixation by sponge-microbe consortia of deep water coral mounds in the northeastern Atlantic Ocean. Marine Ecology Progress Series. 358:137–150. doi: 10.3354/meps07370
- Van Duyl FC, Moodley L, Nieuwland G, Van Ijzerloo L, Van Soest RWM, Houtekamer M, Meesters EH, Middelburg JJ. 2011. Coral cavity sponges depend on reef-derived food resources: stable isotope and fatty acid constraints. Marine Biology. 158:1653–1666. doi: 10.1007/s00227-011-1681-z
- Van Gaever S, Moodley L, Pasotti F, Houtekamer M, Middelburg JJ, Danovaro R, Vanreusel A. 2009. Trophic specialisation of metazoan meiofauna at the Håkon Mosby Mud Volcano: fatty acid biomarker isotope evidence. Marine Biology. 156:1289–1296. doi: 10.1007/s00227-009-1170-9
- Van Oevelen D, Mueller CE, Lundälv T, Van Duyl FC, De Goeij JM, Middelburg JJ. 2018. Niche overlap between a cold-water coral and an associated sponge for isotopically-enriched particulate food sources. PLoS One. 13:e0194659. doi: 10.1371/journal.pone.0194659
- Van Soest RWM, De Voogd NJ. 2015. Sponge species composition of north-east Atlantic cold-water coral reefs compared in a bathyal to inshore gradient. Journal of the Marine Biological Association of the United Kingdom. 95:1461–1474. doi: 10.1017/S0025315413001410
- Veuger B, Pitcher A, Schouten S, Sinninghe Damsté JS, Middelburg JJ. 2013. Nitrification and growth of autotrophic nitrifying bacteria and Thaumarchaeota in the coastal North Sea. Biogeosciences. 10:1775–1785. doi: 10.5194/bg-10-1775-2013
- Volkman JK, Johns B, Gillan FT, Perry JF, Bavor JrHJ. 1980. Microbial lipids of an intertidal sediment-I. fatty acids and hydrocarbons. Geochimica Cosmochimica Acta. 44:1133–1143. doi: 10.1016/0016-7037(80)90067-8
- Walkup RD, Jamieson GC, Ratcliff MR, Djerassi C. 1981. Phospholipid studies of marine organisms: 2.1. phospholipids, phospholipid-bound fatty acids and free sterols of the sponge Aplysina fistularis (Pallas) forma fulva (Pallas) (=verongia thiona) 2. Isolation and structure elucidation of unprecedented branched fatty acids. Lipids. 16:631–646. doi: 10.1007/BF02535058
- Webster NS, Taylor MW. 2012. Marine sponges and their microbial symbionts: love and other relationships. Environmental Microbiology. 14:335–346. doi: 10.1111/j.1462-2920.2011.02460.x
- Webster NS, Thomas T. 2016. The sponge Hologenome. mBio. 7:e00135–00116. doi: 10.1128/mBio.00135-16
- Weisz JB, Hentschel U, Lindquist N, Martens CS. 2007. Linking abundance and diversity of sponge-associated microbial communities to metabolic differences in host sponges. Marine Biology. 152:475–483. doi: 10.1007/s00227-007-0708-y
- Weisz JB, Massaro AJ, Ramsby BD, Hill MS. 2010. Zooxanthellate symbionts shape host sponge trophic status through translocation of carbon. Biological Bulletin. 219:189–197. doi: 10.1086/BBLv219n3p189
- Wilkinson C, Fay P. 1979. Nitrogen fixation in coral reef sponges with symbiotic cyanobacteria. Nature. 279:527–529. doi: 10.1038/279527a0
- Wuchter C, Schouten S, Boschker HTS, Sinninghe Damste J. 2003. Bicarbonate uptake by marine Crenarchaeota. FEMS Microbiology Letters. 219:203–207. doi: 10.1016/S0378-1097(03)00060-0
- Xie S, Lipp JS, Wegener G, Ferdelman TG, Hinrichs K-U. 2013. Turnover of microbial lipids in the deep biosphere and growth of benthic archaeal populations. Proceedings of the National Academy of Sciences. 110:6010–6014. doi:10.1073/pnas.1218569110.
