ABSTRACT
Phragmope discrepans (Lithothamnion discrepans) is recognized as a new monotypic genus endemic to South Africa on account of several active modes of growth, including apical meristem replacement, production of cell bars in canals of multiporate conceptacles, and development of a second compact, imperforate roof over multiporate conceptacles. Meristem replacement involves up to three layers. Development of cell bars starts with production of projecting cells from basal cells of lining filaments, and these cells subsequently coalesce to form a barrier. Subepithallial divisions produce a second compact roof that is composed of 1–2 perithallial cells and a layer of epithallial cells. It is assumed that these novelties reflect evolutionary advancements to overcome competition and the environmental pressure that Phragmope experiences in the intertidal zone. The new genus is taxonomically distant to the Northern Hemisphere genus Mesophyllum, and relates closer to taxa from the Southern Hemisphere, including Melyvonnea that occurs in South Africa and species of Mesophyllum sensu lato from Chatham-New Zealand-Australia, which collectively display a reduced fusion cell and lack of a pedestal in carposporangial conceptacles. Previous molecular studies based on SSU rDNA sequences and including an isolate of ‘M. engelhartii’ from South Africa support the same view. It is shown that the name ‘Fam.Mesophylleae Dum.’ Heeg [1891. Niederösterreiehische Lebermoose.- Verhandl. der k.k. zool.-botan. Gesellsch. in Wien 41:567–573] in Leverworts is invalid. Hence Mesophyllaceae Athanasiadis [2016. Phycologia Europaea Rhodophyta, Vols 1 & 2. Published by the author, Gothenburg. xlviii + 1504 pages] is available in the Corallinales (including Hapalidiales), while Mesophyllumaceae Schneider & Wynne (2019. Fourth addendum to the synoptic review of red algal genera. Botanica Marina. 62:355–367) becomes a later synonym.
Introduction
Mesophyllum engelhartii (Foslie) Adey was reported as a common intertidal alga in South Africa (Chamberlain and Keats Citation1995) and a DNA sequence attributed to it was included in several comparative studies (Bailey and Chapman Citation1998, table 1, figure 1; Broom et al. Citation2008, table 2, figure 5; Bittner et al. Citation2011, table 2, figure 1; Kato et al. Citation2011, figure 1; Peña et al. Citation2011, figure 3). The re-examination of the original material of Lithothamnion (Mesophyllum) engelhartii Foslie from Cape Jaffa (South Australia) showed, however, that records from other sites in the world were misidentifications (Athanasiadis Citation2017a). In fact, it was also found that the original material of L. engelhartii comprised two different species, actualizing the need to neo-lectotypify this taxon and emend its circumscription. In addition, Mesophyllum discrepans (Foslie) W.H. Adey and Mesophyllum speciosum (Foslie) W.H. Adey, two species indigenous to South Africa included in the synonymy of ‘Mesophyllum engelhartii’ (Chamberlain and Keats Citation1995), were considered to be candidates to replace the misapplied name. Access to several South African collections from the herbarium Chamberlain (presently at the BM), attributed to M. engelhartii or M. discrepans, actualized this study. The present investigation also provides information in the context of understanding the Southern Hemisphere members of Mesophyllaceae following the studies of Athanasiadis and Ballantine (Citation2014) and Athanasiadis (Citation2017a, Citation2017b, Citation2018b, Citation2019).
Material and methods
Collections made in South Africa by the late Dr Y.M. Chamberlain (YMC) and her co-workers during 1989 and 1991 were given to the author as a gift, during his visit to the Marine Laboratory of Portsmouth Polytechnic in October 2008. Specimens were examined under a Zeiss Stemi SV 6 microscope (Zeiss, Jena, Germany). For anatomical observations, transections (30–50 μm thick) were made using a Kryomat 1700 freezing microtome (Leitz, Stuttgart, Germany). The material was previously decalcified using acetic acid (20–45%, 5–24 h), stained and hardened in aniline blue (1–5% solution with alcohol 96% for at least 24 h), and sectioned in Hamilton’s freezing solution (1 g gum arabic: 30 g sucrose: one crystal of thymol: 100 ml distilled water). Microscopic preparations were mounted in 60% Karo® corn syrup (Best Food Division CPC International, Englewood Cliffs, NJ, USA) on microscope slides. Photomicrographs were taken using a camera (Nikon D7000, Nikon Europe B.V.) attached to a Zeiss Axiophot 2 microscope (Zeiss, Jena, Germany). Slides and specimens have been deposited in the herbarium of the author (herb.Athanas.). Herbarium abbreviations follow Holmgren et al. (Citation1990). Terminology follows Bold and Wynne (Citation1978) and Athanasiadis and Ballantine (Citation2014). The following abbreviations are used in the text: breadth (B), diameter (D), height (H), length (L), number of measurements (n), spermatangial mother cells (SMCs).
Comparative material examined
Mesophyllum (Lithothamnion) lichenoides (Ellis) Me Lemoine. Herbarium Suneson (GB – slides nos 131-a-3). The original specimens had been collected on Corallina L. at Roscoff (Atlantic France) in July–August 1932 and August 1936. For anatomical studies, specimens had been fixed for 12–24 h in a modified Fleming solution (including 1 g chromium acid: 1 cm3 acetic acid: 0,1 g osmium acid: 100 cm3 salt water), washed for 12–24 h, and hardened in alcohol, before embedded in paraffin and sectioned at 7 μm thickness (Suneson Citation1937, pp. 5–6).
Taxonomy
Family Mesophyllaceae Athanasiadis, Citation2016
Genus Phragmope gen. nov.
Type species Phragmope discrepans (Foslie) comb.nov.
Genus diagnosis
New monotypic genus of Mesophyllaceae (Corallinales, Rhodophyta) differing from other members of the family in undergoing regeneration of the terminal (hypothallial) meristem, in developing cell bars in canals of multiporate roofs and in producing a second compact, imperforate roof covering empty multiporate conceptacles.
Etymology
The genus name is a new compound word of feminine gender, deriving from the neuter substantive phragma (hedge, barrier) and the feminine substantive οπη (opening, pore). The name denotes the closure of canals of multiporate roofs.
Phragmope discrepans (Foslie) comb. nov.
().
Figure 1. Phragmope discrepans gen. & comb.nov.; a, thalli overgrowing Scutellastra barbata (L.), annotated by Dr. Chamberlain (D16-YMC 89/300); b, thalli attached to rock fragments, annotated by Dr. Chamberlain (D14-YMC 89/211).
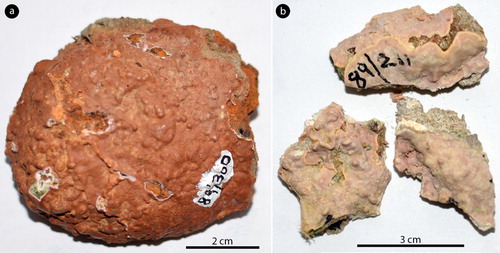
Figure 2. Phragmope discrepans gen. & comb.nov. Vegetative structures; a, section of thallus showing a predominantly coaxial hypothallium (short black arrow), a gradually ascending perithallium (long black arrow) supporting single epithalial cells (black arrowheads), and the active meristem (white arrowhead) covered by remains of two older (senescent) meristematic zones (white arrows); b, abutting lamellae (arrows); c, projecting lamellae back-to-back (arrows) forming a protuberance; d, synchronous anticlinal divisions (arrows) in terminal hypothallial cells; e-f, subdichotomous divisions (arrows) in terminal hypothallial cells; g, transition zone between terminal meristematic cells (white arrows) that progressively become wedge-shaped cells (black arrows) in ventrally displaced hypothallial filaments. Note the non-coaxial hypothallium; h, transition zone between terminal meristematic cells (long arrows) that progressively become epithallial cells (arrowheads) in dorsally displaced hypothallial filaments; i, synchronous anticlinal perithallial divisions (arrows), several cells below the epithallium (arrowheads), to produce a conceptacle. Materials: a,h, D8-YMC 91/2569; b, D16-YMC 89/300; c,d-g,i, D14-YMC 89/211.
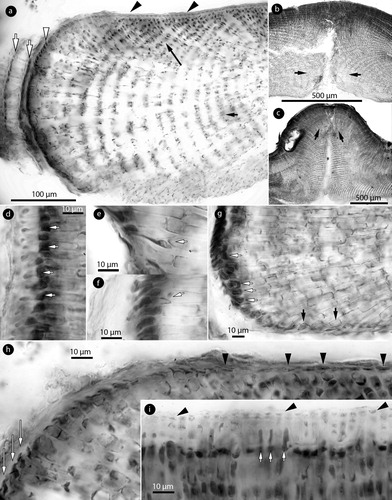
Figure 3. Phragmope discrepans gen. & comb.nov. Vegetative structures; a, section at the thallus margin showing three older (senescent) meristems (white arrows), covered by a cuticle (black arrow) and the active hypothallial meristem (arrowheads); b, thallus section showing a predominantly coaxial hypothallium (short arrow) supporting gradually ascending perithallial filaments (long arrow); c, magnification of the hypothallial core in Figure b, showing at least three layers of filaments (white arrows) running parallel to the substratum, and cells fusions (black arrows) between contiguous cells; d-e, sections at the thallus surface showing elongate subepithallial cells (arrows) supporting flattened to isodiametric epithallial cells (arrowheads); f, a terminal trichocyte amongst epithallial cells; g, internal trichocyte-like cells in the hypothallium. Materials: a,f, D8-YMC 91/2569; b-d, g, D14-YMC 89/211; e, D10-YMC 91/80.
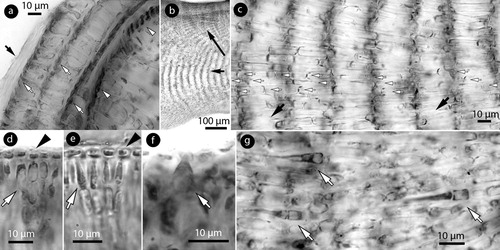
Figure 4. Phragmope discrepans gen. & comb.nov. Spermatangial structures; a-b, sections of conceptacles showing the development of predominantly unbranched (simple) spermatangial structures on the floor (arrows), the walls, and the roof; c-f, lunate SMCs (arrows) on the floor (Figures c-d), the roof (Figure e), and elongate SMCs on the walls (Figure f), developing spermatangia (white arrowheads) that release spermatia (black arrowheads); g-h, dendroid (branched) spermatangial structures with elongate SMCs (arrows) developing spermatangia (white arrowheads) that release spermatia (black arrowheads). Materials: a,e,f-h, D16-YMC 89/300; b-d, D8-YMC 91/2569.
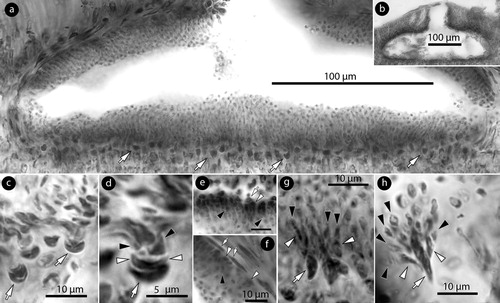
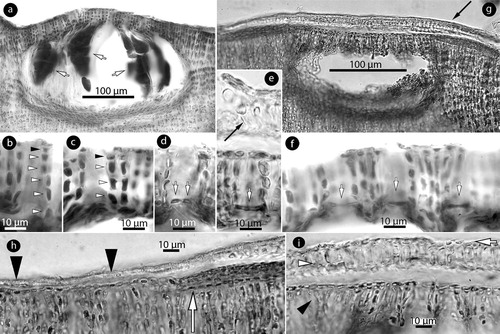
Figure 5. Phragmope discrepans gen. & comb.nov. Carpogonial and carposporangial structures; a, carpogonial conceptacle with carpogonial branches (arrows) extending along the flattened floor; b-c, three-celled carpogonial branches composed of a carpogonium (ca), a hypogynous (h), and a supporting cell (s) attached to a basal (b) cell. Note the sterile cell (broken lines) attached to the hypogynous cell; d, carposporangial conceptacle with peripheral development of carposporangia (c) (the fertile zone magnified in Figure h); e-h, post fertilization stages showing a fusion cell (f), gonimoblast filaments (g.f.), and neighbouring hypogynous (short arrows) and basal (b) cells (long arrows in Figure h) connected to gonimoblast filaments; i, carposporangial conceptacle becoming embedded; j, embedded carposporangial chamber (arrow); k, removed chamber. Materials: a-k, D14-YMC 89/211. Abbreviations: b, basal cell; c, carpospore; ca, carpogonium; g.f.: gonimoblast filament; f, fusion cell; h, hypogynous cell; s, supporting cell.
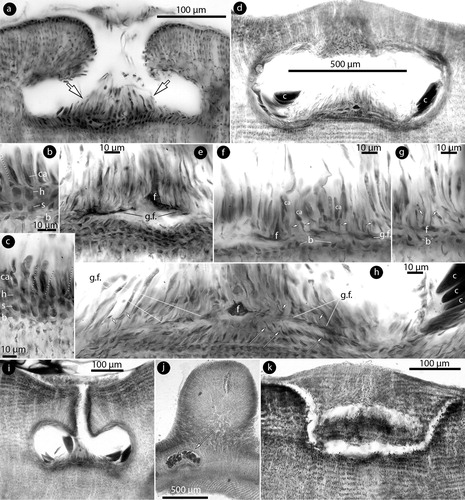
Figure 6. Phragmope discrepans gen. & comb.nov. Tetrasporangial structures; a, multiporate conceptacle with tetrasporangia (arrows); b-f, sections of canals of multiporate roofs. Canals are straight and lined by 4–6 non-differentiated cells (arrowheads), inclusive epithallial cells (black arrowheads). Canal closure initiates with cells projecting from the opposite basal cells (white arrows in Figure d), and the projecting cells subsequently coalesce to form a barrier (white arrows in Figures e-f). A new compact ‘roof’ (black arrow in Figure e) is formed; g, development of a compact ‘roof’ (arrow) above an old multiporate conceptacle; h, margin of a compact ‘roof’ (arrowheads), and a putative initial of a similar formation below (arrow); i, compact ‘roof’ composed of 1–2 perithallial (white arowhead) and a layer of epithallial cells (arrow) above the old roof (black arrowhead). Materials: a-c, e-f, D16-YMC 89/300; d, g-i, D10-YMC 91/80.
Basionym
Lithothamnion discrepans Foslie (Citation1907b, p. 8).
Homotypic synonym
Mesophyllum discrepans (Foslie) W.H. Adey (Citation1970, p. 23).
Type locality
Grahamstown [‘presumably referring to the shores of Algoa Bay’ (Chamberlain and Keats Citation1995, p. 141)], South Africa, coll. H.Becker, May 1899.
Lectotype
In TRH (C18-3332), examined by Chamberlain and Keats (Citation1995, pp. 141–143, figures 5, 41–45, table 1).
Isolectotype
In TRH (C18-3333), examined by Chamberlain and Keats (Citation1995, pp. 141–143, figures 6, 36–40, table 1).
Heterotypic synonyms
Lithothamnion synanablastum f. speciosum Foslie (Citation1900, p.11), ‘speciosa’; type locality: Grahamstown, South Africa, coll. H.Becker, May 1899, growing on an algal ball; holotype: in TRH, B17-2591, examined by Chamberlain and Keats (Citation1995, figures 4, 46–51, table 1).
Lithothamnion speciosum (Foslie) Foslie (Citation1907b, p. 16)
Mesophyllum speciosum (Foslie) W.H. Adey (Citation1970, p. 26)
Material examined
D16-YMC 89/300 (herb.Athanas.). South Africa, Doring Baai, Cape west coast, collected by Y.M.C. 18 November 1989, annotated ‘Mesophyllum engelhartii … on Patella barbata’, gametophytes & tetrasporophytes – collection not cited in Chamberlain and Keats (Citation1995) (a, 2b, 4a,e,f–h, 6a–c,e–f); D14-YMC 89/211 (herb.Athanas.). South Africa, Brandfontein, West of Cape Aguilhas, collected by Y.M.C. 11 Nov.1989, annotated ‘Mesophyllum engelhartii … on Patella miniata’, gametophytes – collection cited in Chamberlain and Keats (Citation1995, p. 136) (b, 2c,d–g,i, 3b–d,g, 55a–k); D10-YMC 91/80 (herb.Athanas.). South Africa, Holbaipunt, SE of False Bay, West Cape, on rocks, shells etc., collected by Y.M.C. & D.W.K. 25 October 1991, annotated ‘Mesophyllum discrepans’, tetrasporophytes – collection cited in Chamberlain and Keats (Citation1995, p.136) (e, 6d,g–i); D8-YMC 91/2569 (herb.Athanas.). South Africa, Holbaipunt, SE of False Bay, West Cape, collected by Y.M.C. 26 nov.1991, annotated ‘Mesophyllum discrepans … on Patella S.’, gametophytes – collection not cited in Chamberlain and Keats (Citation1995) (a,h, 3a,f, 4b–d).
Description
Thalli encrusting, lacking unattached superimposed growth and expanding horizontally, to at least 7 cm in diameter (a–b). Individual lamellae, 200–900 µm thick (excluding protuberances; a – to 1.5 mm according to Chamberlain and Keats Citation1995, table 1). Meeting lamellae grow back-to-back and form erect protuberances up to 0.8 mm high and 1.5 mm in diameter (b–c). Thallus organization dorsiventral with a predominantly arching, coaxial hypothallium (with non-coaxial patches; g), 100–550 µm in thickness (a), dominating at the margin and progressively forming about 1/4 of the thallus thickness (b,c, 5j) – forming up to 80% of the thallus, and hypothallium ‘mainly to non-coaxial’ or ‘partly’ coaxial, according to Chamberlain and Keats (Citation1995, table 1). Hypothallium growing by terminal meristematic cells that undergo synchronous divisions and elongations (a,d). Anticlinal divisions (d) contribute to thallus elongation. Subdichotomous divisions (e,f) add to the thallus thickness, and gradually displace peripheral filaments dorsally (to form the perithallium) or ventrally (to form descending hypothallial filaments). Terminal meristematic cells displaced ventrally become wedge-shaped cells (g). Terminal meristematic cells displaced dorsally become epithallial cells (h). Conceptacles initiate about 3–5 cells below the epithallium via anticlinal perithallial divisions (i). Remains of older meristematic zones, attached to the active meristem, exist below a cuticle (a, 3a). The hypothallium is composed of elongate cells, 18–30 × 5–8 µm (L x B) (11–50 × 4–13 µm, according to Chamberlain and Keats Citation1995, table 1). A polystromatic core of at least three hypohallial filaments runs parallel to the substratum (b,c). The perithallium is 160 to at least 400 µm thick, stratified (b, 5d,i) and composed of elongate cells 15–35 × 5–9 µm (L x B) (5–20 × 2–11 µm, according to Chamberlain and Keats Citation1995, table 1). Subepithallial cells are elongate, to 35 µm long and slightly longer than those below during division (d,e). Epithallial cells are flattened to isodiametric, 5–10 × 2–3 (D x H), and singly borne (d,e) (2.5–5 × 2.5–4 µm, and up to 3 cells according to Chamberlain and Keats Citation1995, table 1).
Trichocytes, c. 25 × 15 µm (L x B), occur rarely and terminally amongst epithallial cells (f). Trichocyte-like cells occur in the hypothallium (g). Cell fusions occur between contiguous vegetative cells (c). Secondary pit connections are absent.
Gametophytes dioecious. Male conceptacles, 230–560 × 30–90 µm (D x H; n: 6), exhibit oblong chambers 210–500 × 50–80 µm (L x H; n: 6) (218–442 × 52–104 µm, according to Chamberlain and Keats Citation1995, table 1), with a central ostiole c. 60 × 80 µm (D x H). Roof 60–80 µm thick (a,b) (52–105 µm, according to Chamberlain and Keats Citation1995, table 1). Mature spermatangial structures predominantly unbranched (simple), composed of lunate SMCs cutting off spermatangia which liberate roundish spermatia (b-f). Simple spermatangial structures occur on the floor, the walls and the roof, and exhibit lunate SMCs on the floor (b-d) and the roof (e), and elongate SMCs on the walls (f). Dendroid (branched) spermatangia may occur rarely in the centre of the fertile floor (g,h).
Carpogonial conceptacles, c. 430 × 45 µm (D x H), exhibit oblong chambers c. 260 × 65 µm (D x H) with a central ostiole c. 70 × 90 µm (D x H). Roof 70–90 µm thick (a). The fertile zone develops carpogonial branches and extends 100–120 µm across the flattened floor (a). Carpogonial branches are 3-celled, composed of a carpogonium, the hypogynous and the supporting cell (b,c) which stain similarly. A sterile cell is usually attached to the hypogynous cell, next to the carpogonium (b,c). The basal cell which supports the carpogonial branch may be similarly or less stained (b,c) and makes a part of the vegetative floor (below).
Carposporangial conceptacles, 430–630 × 40–240 µm (D x H; n: 6), exhibit oblong chambers 340–590 × 130–160 µm (D x H; n: 7) (286–520 × 78–195 µm, according to Chamberlain and Keats Citation1995, table 1). The fertile zone can be slightly elevated centrally but lacks a distinctive pedestal (d,h,i). The roof is 70–130 µm thick (65–130 µm, according to Chamberlain and Keats Citation1995, table 1), provided with a central ostiole 50–70 × 85–130 µm (D x H; n: 3). The mode of zygote transfer was not observed. Following fertilization a fusion cell composed of at least two-three supporting cells and occasionally a carpogonium and a hypogynous cell was seen (e,f). Yet, in most case the fusion is limited to a few supporting cells (g,h). Basal cells remain in contact to the vegetative floor, revealing that they are not part of the carpogonial branches (e–h). Gonimoblast filaments develop at the level of the fusion cell (i.e. the supporting cells) and radiate to produce carposporangia from the periphery (d,h). At this stage, basal and neighbouring hypogynous cells (on either side of the gonimoblast filaments) may be connected to the gonimoblast filaments, apparently transferring part of their cytoplasm and leaving cell wall remains attached (h, arrows). Older conceptacles become gradually embedded in the perithallium (i,j), or removed with part of the surrounding perithallial tissue (k).
Tetrasporangial conceptacles, 300–400 × 40–100 µm (D x H; n: 4), exhibit oblong chambers 180–360 × 80–160 µm (D x H; n: 4) (182–338 × 104–208 µm, according to Chamberlain and Keats Citation1995, table 1), with convex to flattened multiporate roof, 30–40 µm thick, composed of 5–7-celled filaments. Tetrasporangia 60–100 × 20–35 µm (H x B; n: 7) (a) (65–120 × 23–104 µm, according to Chamberlain and Keats Citation1995, table 1). Canals are straight, 10–12 µm wide, with 4–6-celled non-differentiated lining cells (b,c). Following spore release, canals develop a barrier blocking the canal function. The barrier originates as elongate projecting cells produced from opposite basal cells lining the canals (d). The projecting cells subsequently merge to form a bar (e,f). A second compact, imperforate roof, 15–22 µm thick, is formed above empty multiporate conceptacles (e,g). This second roof is composed of 1–2 perithallial cells and a single layer of epithallial cells (g–i). Hypothallial filaments are not involved in this formation. This second roof presumably develops from a subepithallial meristem (h,i), in a similar manner to conceptacle initiation (i), and forms a vault (seen as an arch in section; g).
The above citations from Chamberlain and Keats (Citation1995, table 1) include data from the types of Lithothamnion discrepans and L. synanablastum f. speciosa.
Distribution and habitat
Chamberlain and Keats (Citation1995, p. 136) recognized the species as ‘one of the commonest crustose coralline species on rocks and Patella shells..at low intertidal level where it forms mainly brownish thalli.’, ‘ … also common in the subtidal to at least 8 m depth, growing on stones, shells, Ecklonia holdfasts and rock faces..’. The species ‘..is perennial and [abundantly fertile]..in March, April, May, July, October and November. Data..not available for other months.’ Listed specimens were collected at Eastern Cape (Cintsa, Three sisters, Skoenmakerskop) and Western Cape (Cape Agulhas, Brandfontein, Cape of Good Hope, Oudekraal, Bakoven, Holbaiipunt, Abdolsbaai, Doringbaai, Groenriviermond). The hitherto data suggest that Phragmope discrepans is endemic to South Africa.
Mesophyllum lichenoides (Ellis) Me Lemoine (Citation1928)
()
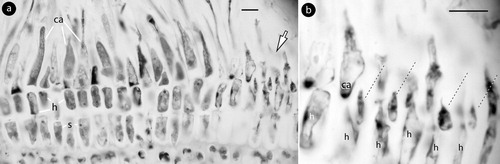
Figure 7. Mesophyllum lichenoides. Carpogonial structures; a, two-celled carpogonial branches, each composed of a carpogonium (ca) and a hypogynous (h) cell connected to a supporting (s) cell. Arrow points to the area magnified in Figure b, showing single sterile cells (broken lines) attached to hypogynous (h) cells. Material: Suneson slide 131-a-3 (GB). Abbreviations: ca, carpogonium; h, hypogynous cell; s, supporting cell.
Remarks
Zygote transfer and post-fertilization stages have been recently described in material from Roscoff (Atlantic France), and compared to Mesophyllum philippii (Foslie) W.H. Adey from Catalonia (Mediterranean Spain) (Athanasiadis Citation2018a). The present observations of carpogonial conceptacles revealed that the two-celled carpogonial branches occasionally develop a sterile cell attached to the hypogynous cell, next to the carpogonium (a,b).
Discussion
Phragmope displays characters not previously observed in members of the family Mesophyllaceae. Meristem regeneration of hypothallial filaments (a, 3a) is apparently a mode to repair injuries of the growing margin, and has not been previously described in any member from the Northern Hemisphere (Athanasiadis et al. Citation2004; Athanasiadis and Adey Citation2006; Athanasiadis and Ballantine Citation2014, and references therein). Moreover, Phragmope develops cell bars in canals of multiporate conceptacles after spore release. This process is initiated from the basal cells (that line the canals) which produce a cell projecting in the canal (d). Subsequently, the opposite cells merge to form a solid tissue (e,f), here named cell bar. This formation presumably keeps out organisms, that may penetrate and injure the soft tissue of the chamber that lacks calcification, and is essential for the species survival since older conceptacles may become embedded in the thallus. Thallus protection is reinforced by the development of a second compact roof, lacking canals and covering the old multiporate roof (e, g-l). This second roof does not involve hypothallial filaments and should not be confused with the standard mode of conceptacle embedment via thallus regeneration (e.g. see Athanasiadis et al. Citation2004, figure 72). In the case of carposporangial conceptacles, the entire chamber can be removed (k), possibly as a protection from injuries already caused by penetrating organisms. Being a common species in the intertidal zone (Chamberlain and Keats Citation1995, p.136), these mechanisms have presumably evolved facing the dynamic changes of the environment and the grazing pressure of animals. Meristem replacement and development of a second compact roof were also documented by Chamberlain and Keats (Citation1995, figures 15, and 33, 43 respectively) and commented as ‘senescent cells attached externally’ or ‘conceptacle shedding’, ‘thallus shedding’.
Most other vegetative and reproductive characters of Phragmope have been previously documented, including the development of trichocytes (Chamberlain and Keats Citation1995, figure 17), a predominantly coaxial hypothallium (with non-coaxial patches) that originally dominates the thallus (but ‘mainly to non-coaxial’ or ‘partly’ coaxial, and up to 80% of the thallus according to Chamberlain and Keats Citation1995, figures 14, 16, 36, 46, table 1), embedding of older conceptacles in the perithallium (Chamberlain and Keats Citation1995, figures 18, 31, table 1 ‘sometimes’), conceptacle initiation below the epithallium (Chamberlain and Keats Citation1995, figure 31), straight canals of multiporate roofs lined by non-differentiated pore cells (Chamberlain and Keats Citation1995, figures 45, 50), elongate subepithallial initials which support single isodiametric to flattened epithallial cells (Chamberlain and Keats Citation1995, figures 16, 17, 41, 47), flattened fertile zone in carposporangial conceptacles with peripheral development of carposporangia (Chamberlain and Keats Citation1995, figures 24, 25), wedge-shaped cells ending descending hypothallial filaments (Chamberlain and Keats Citation1995, figure 16), dioecious (‘usually’) gametophytes (Chamberlain and Keats Citation1995, p. 140, figures 18, 36), convex tetrasporangial conceptacle roofs lacking a rim (Chamberlain and Keats Citation1995, figures 33, 34, 42, 43, 48, 49), predominantly simple (unbranched) spermatangial structures (Chamberlain and Keats Citation1995, figure 21), and most significantly absence of a prominent fusion cell (Chamberlain and Keats Citation1995, p.141, figure 24, ‘possible discontinuous cell’). In addition, the two authors also described and/or illustrated seven to eight rosette cells, up to 60 canals in multiporate roofs (Chamberlain and Keats Citation1995, figures 35, 51 and table 1), and two-celled carpogonial branches (Chamberlain and Keats Citation1995, pp. 140–1, figure 23).
The present study documented three-celled carpogonial branches and also showed the presence of a small fusion cell composed of 2–3 supporting cells and occasionally involving the carpogonium and the hypogynous cells. In fact, the fusion cell of Phragmope is similar to that described as a ‘discontinuous fusion cell’ in other Mesophyllaceae of the Southern Hemisphere, including members of Synarthrophyton R.A. Townsend (Townsend Citation1979; May and Woelkerling Citation1988; Woelkerling and Foster Citation1989; Maneveldt et al. Citation2007; Athanasiadis Citation2018b), Amphithallia Athanasiadis (Citation2019), Melyvonnea Athanasiadis & D.L Ballantine (Keats and Chamberlain Citation1994, figure 25, as Mesophyllum), and Capensia Athanasiadis (Citation2017b). On the other hand, postfertilization studies in Mesophyllum lichenoides and M. philippii (Athanasiadis Citation2018a) confirmed Suneson’s (Citation1937) and Lebednik’s (Citation1977) findings of a prominent fusion cell in the genus Mesophyllum comprising some 10 hypogynous and 10 supporting cells. It was further concluded that the term ‘discontinuous fusion cell’ should be abandoned, being based on observations from sections that cross the radiating gonimoblast filaments and not the fusion cell, and attention should rather concentrate to which cells are involved in the fusion cell and whether multiple fusions (independent fertilizations) take place (Athanasiadis Citation2018a, p. 528, figures 12, 13). An intermediate condition, involving the participation of a hypogynous and at least four or five supporting cells was documented in the Southern Hemisphere genus Carlskottsbergia Athanasiadis (Citation2018b).
The presence of a sterile cell, borne next to the carpogonium, is here documented both in Phragmope (b,c) and in Mesophyllum lichenoides (Ellis) Me Lemoine () – the latter observation correcting Suneson's (Citation1937, p. 77, figure 42g) report. Thereby, within the Mesophyllaceae, sterile cells appear to have been documented on hypogynous cells, except in Amphithallia crassiuscula (Foslie) Athanasiadis (Citation2019, figure 4b-d) and Leptophytum laeve (Foslie) W.H. Adey (Citation1966, figures 75, 79) where they occur on the supporting cell that may occasionally develop even a second carpogonial branch.
Taxonomic affinities
The re-examination of the original material of Lithothamnion (Mesophyllum) engelhartii Foslie (Citation1900, p. 18; type locality: Cape Jaffa, South Australia), showed that it was based on heterogenous collections (Athanasiadis Citation2017a). The generic position of the selected lectotype and a second species included in the original material remained, however, uncertain (within the family Mesophyllaceae). Lithothamnion (Mesophyllum) engelhartii is here considered to be remotely related to Phragmope differing in several vegetative and reproductive features, including the presence of an unattached superimposed growth, the lack of a predominantly coaxial hypothallium, limited perithallial development (and as a result lack of embedded conceptacles), development of conical to pyriform canals of multiporate roofs, and finally in having differentiated lining (basal and subbasal) cells in the canals of multiporate roofs. The second species included in the original material of L. engelhartii comes significantly closer sharing with Phragmope the presence of straight canals of multiporate roofs, the production of cell bars in the canals, and in having embedded conceptacles in a well developed perithallium (Athanasiadis Citation2017a, figures 29, 24, 25). It differs though from Phragmope in lacking entirely a coaxial hypothallial growth. The identity of this second species of Mesophyllaceae remained, however, unknown pending the re-examination of three other species from South Australia and the Auckland Islands, included in the synonymy of ‘M. engelhartii’ by Woelkerling and Harvey (Citation1993, pp. 581–582, 597, 600).
Phragmope is clearly separated from the Northern Hemisphere genus Mesophyllum that develops two-celled carpogonial branches and a prominent fusion cell that incorporates at least 10 hypogynous and 10 supporting cells (Suneson Citation1937; Lebednik Citation1977; Athanasiadis Citation2018a). Mesophyllum also differs in lacking trichocytes and dendroid spermatangial structures and in possessing differentiated (thinner-wider) pore cells in canals of multiporate roofs and a distinctive pedestal in carposporangial conceptacles (). These differences most likely reflect a long time isolation of Phragmope – the two genera currently displaying a disjunct distribution with Mesophyllum apparently lacking members in the Southern Hemisphere (Athanasiadis and Ballantine Citation2014).
Table I. Comparative data between Phragmope discrepans and related species and genera of Mesophyllaceae.
Amongst the other South African Mesophyllaceae, Melyvonnea is a putative sister-taxon, sharing with Phragmope the absence of both a prominent fusion cell and a pedestal in carposporangial chambers (Keats and Chamberlain Citation1994, figure 25), and the presence of trichocytes and a predominantly coaxial hypothallium (Athanasiadis and Ballantine Citation2014). Still, Melyvonnea differs in possessing differentiated (elongate) basal cells in canals of multiporate roofs and in lacking dendroid spermatangia ().
Two other Mesophyllaceae recorded from South Africa are Mesophyllum funafutiense (Foslie) E. Verheij, originally described from Funafuti Atoll (Polynesia) and Mesophyllum incisum (Foslie) W.H. Adey originally described from North Island (New Zealand) and also recorded from southern Australia. In M. funafutiense gametophytes remain unknown and this species also lacks trichocytes or a predominantly coaxial hypothallium, and possesses 9–12-celled pore filaments with differentiated basal cells (Keats and Chamberlain Citation1994; Athanasiadis and Ballantine Citation2014, Appendix II). Mesophyllum incisum comes closer in possessing trichocytes and lacking a prominent fusion cell (Woelkerling and Harvey Citation1992, figs 29, 30). It still differs in having 2-celled carpogonial branches (Woelkerling and Harvey Citation1992, figure 27), highly specialized (4-celled) pore filaments (Woelkerling and Harvey Citation1993, figure 14D; Keats and Maneveldt Citation1997a, figure 17), and in lacking dendroid spermatangia. Moreover, the South African specimens seem to lack a predominantly coaxial hypothallium (Keats and Maneveldt Citation1997a, figures 5, 9–10).
The remaining Mesophyllaceae from South Africa include a highly specialized epiphyte [Amphithallia crassiuscula (Athanasiadis Citation2019)], a parasite [Capensia fucorum (Esper) Athanasiadis (Citation2017b)], five species included in the mainly Southern Hemisphere genus Synarthrophyton (Maneveldt et al. Citation2008, table 1), and four species placed in the Arctic genus Leptophytum W.H. Adey [Chamberlain and Keats Citation1994; Adey Citation1970, p. 30, as regards L. repandum (Foslie) W.H. Adey]. The South African members of all these genera are here considered to be remotely related to Phragmope, differing by significant characters such as thallus organization (bilateral in Synarthrophyton and Amphithallia, sympodial growth in Capensia) and short subepithallial initials (species of Leptophytum). Moreover, dendroid spermatangia occur predominantly in most of these taxa (but Capensia). Yet, like Phragmope, they all display poorly developed (or lack entirely?) fusion cells (as discussed above). It could be added that, on account of short subepithallial initials and dendroid spermatangia, the four species of Leptophytum have been transferred to Phymatolithon Foslie (Wilks and Woelkerling Citation1994; van der Merwe and Maneveldt Citation2014), a genus sharing most of its reproductive characters with Lithothamnion Heydrich, and whose remote systematic position has been confirmed by several molecular investigations (e.g. Bailey and Chapman Citation1998; Adey et al. Citation2015). The generic position of the latter four species remains however enigmatic, requiring further efforts to clarify their geographically and systematically disjunct relationship to the Northern Hemisphere genus Leptophytum, whose members display predominantly simple (unbranched) spermatangial structures (Athanasiadis and Adey Citation2006).
Rather surprisingly, three molecular studies clustered Phragmope with an unidentified species of ‘Mesophyllum’ from the Chatham Islands (Broom et al. Citation2008; Bittner et al. Citation2011; Peña et al. Citation2011; c–e). Dr Jennifer Dalen kindly provided on loan a part of the original Chatham collection (WELT A027265/A), and three other samples from North Island [New Zealand; WELT A029290 (NZC 2057); WELT A029292 (NZC 2600); WELT A029295 (NZC 2134)], all labelled ‘Mesophyllum macroblastum’, but the identification with the Mediterranean Mesophyllum macroblastum (Foslie) W.H. Adey could not be confirmed. In particular, the Chatham-New Zealand material was found to be in agreement with accounts of ‘Mesophyllum macroblastum’ from Australia (Woelkerling and Harvey Citation1993; Harvey et al. Citation2003) and New Zealand (Harvey et al. Citation2005), differing from the Mediterranean species in lacking a predominantly coaxial hypothallium and differentiated pore cells of multiporate roofs, and in possessing smaller tetra/bisporangial chambers (145–330 µm according to Woelkerling and Harvey Citation1993, p. 590, and Harvey et al. Citation2003, p. 670, vs 230–400 µm in the Mediterranean species; Kaleb et al. Citation2011, table 2). The two species also differ in ecological preferences, as the Chatham – New Zealand – Australian species grows in the exposed intertidal and sublittoral down to 27 m depth, while the Mediterranean species is restricted to the sublittoral (to 90 m depth) and shallower habitats with similar conditions (i.e. in caves and crevices protected from direct light and wave motion; see Athanasiadis Citation2016, p. 266, and references therein). Moreover, based on a comparison of psbA sequences, Peña et al. (Citation2014, figure 11) showed that a Mediterranean isolate was distantly related to the New Zealand counterpart. Phragmope shares with the Australian – New Zealand – Chatham taxon most of its reproductive characters, including the lack of a prominent fusion cell and a pedestal in carposporangial conceptacles, and even the rare presence of dendroid spermatangia (). Yet, the two species do not share any of the apomorphies of Phragmope, and still differ in the development of trichocytes (in Phragmope), and also in the structure of the multiporate conceptacles (convex roof in Phragmope vs rimmed roof in the Australian – New Zealand – Chatham species). In addition, ‘Mesophyllum macroblastum’ has not been recorded from South Africa, which suggests that a putative relationship would be nested in a remote ancestor in the very distant past.
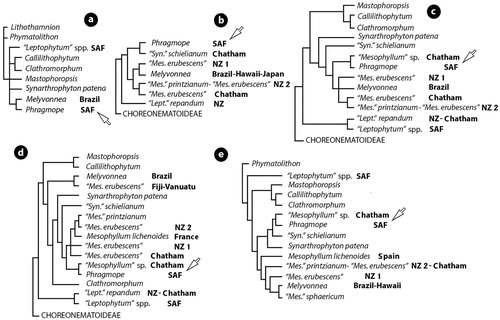
Figure 8. Relationships between species and genera of Mesophyllaceae as indicated by parsimony analysis (a), maximum likelihood (b-d), and Bayesian inference (e) of SSU rDNA sequences. a, after Bailey & Chapman (Citation1998, table 1, figure 1); b, after Kato et al. (Citation2011, figure 1); c, after Broom et al. (Citation2008, table 2, figure 5); d, after Bittner et al. (Citation2011, table 2, figure 1); e, after Peña et al. (Citation2011, figure 3). Unidentified species have been excluded in the here shown reproductions. Ingroup members polarized by the closest outgroup taxon: Lithothamnion or Phymatolithon (a,e), or Choreonematoideae (b-d). Phragmope replaces the sequence of ‘Mesophyllum engelhartii’ from South Africa and is indicated by arrow. Melyvonnea replaces the sequence of ‘Mesophyllum’ erubescens from Brazil (Athanasiadis and Ballantine Citation2014, figure 2). Accession numbers for outgroup taxa: Lithothamnion (U60738); Phymatolithon (U60740 & U60741); Choreonematoideae (AY221254). Accession numbers for ingroup taxa (in alphabetic order): Callilithophytum (U61252); Clathromorphum (U60742);‘Leptophytum’ spp. SAF (U62119 & U62120); ‘Leptophytum’ repandum NZ (EF628216);‘Leptophytum’ repandum Chatham (EF628215); Mastophoropsis (U62118); Melyvonnea Brazil (U61257); Melyvonnea Hawaii (DQ629011, DQ629012); Melyvonnea Japan (AB576027, AB576028);‘Mesopyllum’ sphaericum (CH1814, CH1891); ‘Mesophyllum erubescens’ NZ 1 (EF628219 – Wharariki Beach; EF628220, EF628221- Golden Bay); ‘Mesophyllum erubescens’ NZ 2 (EF628223 - Wellington); ‘Mesophyllum erubescens’ Chatham (EF628222); ‘Mesophyllum erubescens’ Fiji-Vanuatu (GQ917405, GQ917427); Mesophyllum lichenoides France (GQ917384); Mesophyllum lichenoides Spain (CH1992); ‘Mesophyllum’ printzianum (EF628224-Chatham); ‘Mesophyllum sp.’ Chatham (EF628218); Phragmope (U61256); Synarthrophyton patena (U61255); ‘Synarthrophyton’ schielianum (EF628217).
Molecular systematics
()
In five studies, the single SSU rDNA sequence from South Africa (Acc.no U61256), attributed to ‘Mesophyllum engelhartii’ and most likely representing Phragmope, clustered with three different sister-taxa: Melyvonnea (Bailey and Chapman Citation1998, figure 1), ‘Synarthrophyton’ schielianum Woelkerling & Foster (Kato et al. Citation2011, figure 1), and a species of ‘Mesophyllum’ – possibly the Australian – New Zealand – Chatham ‘Mesophyllum macroblastum’ (Broom et al. Citation2008, figure 5; Bittner et al. Citation2011, figure 1; Peña et al. Citation2011, figure 3). The last two studies included the largest number of (the same) ingroup taxa, but also several unidentified species – the latter excluded in the here shown reproductions (). Melyvonnea was significantly distanced from Phragmope in the last two studies, with Synarthrophyton patena (Hooker fil. & Harv.) R.A. Towns. (bilateral thallus organization) and ‘Synarthrophyton’ schielianum (dorsiventral thallus organization) being placed between (d,e). The type of Mesophyllum, M. lichenoides (Ellis) Me Lemoine, resolved closer to the Australian-New Zealand ‘Mesophyllum’ printzianum Woelkerling & A.S. Harvey (d,e), or to Melyvonnea (e), than to Phragmope. In a later study (Peña et al. Citation2014, S3), a new sequence was included, attributed to ‘Mesophyllum engelhartii’ from New Zealand (Moturoa, FJ361517) and previously listed by Farr et al. (Citation2009, p. 123, WELT A029456). Nevertheless, the morphological and anatomical characters attributed to this taxon by Farr et al. (Citation2009, p. 94) differ from the lectotype as regards thallus habit and even shape and structure of canals of multiporate roofs ().
The significance of the above five molecular studies, in the context of the present investigation, is that they provide independent data sets for comparison, which do not contradict recognition of Phragmope as a new genus genetically remotely related to its closest generic allies (Melyvonnea, Mesophyllum, Synarthrophyton).
Selecting the nomenclatural type of Phragmope
The here studied specimens originally attributed to Mesophyllum discrepans by Chamberlain (in sched.) or ‘Mesophyllum engelhartii’ by Chamberlain and Keats (Citation1995, p. 136) are in agreement with the types of Lithothamnion discrepans Foslie (Citation1907b) and Lithothamnion synanablastum f. speciosa Foslie (Citation1900) [=L. speciosum (Foslie) Foslie (Citation1907b)]. The latter two taxa were originally described from collections made at Grahamstown, and their types were re-examined by Chamberlain and Keats (Citation1995, pp. 141–143, figures 5, 36–45, respectively pp.143–4, figures 4, 46–51). At species level, these two taxa have the same nomenclatural priority and since the type of L. discrepans was best documented (Chamberlain Keats Citation1995), this binomial was chosen (Turland et al. Citation2018, Art.11.5) as the basionym of Phragmope discrepans. Apart from habitat and locality attributes, the new collections of Phragmope, share with the type of L. discrepans at least the presence of: (1) elongate subepithallial initials supporting single isodiametric to flattened epithallial cells, (2) multiporate conceptacles with convex roof (lacking a rim), (3) straight pore canals of multiporate roofs with non-differentiated pore cells, (4) flattened floor in carposporangial conceptacles (with peripheral production of carposporangia), and (5) most importantly, the development of a second compact roof above older multiporate conceptacles (Chamberlain and Keats Citation1995, figures 40–43, 45). In addition, the type specimen was said to posses a partly coaxial hypothallium (Chamberlain and Keats Citation1995, table 1), while the here studied collections showed the presence of non-coaxial patches (g) in an otherwise predominantly coaxial tissue (a-c, 3a-c), and Chamberlain and Keats (Citation1995, table 1) concluded that the species possesses a mainly coaxial to non-coaxial hypothallium. The frequency of non-coaxial patches vs non-coaxial regions (or specimens?) remains to be settled studying further collections.
A third earlier described species from South Africa, included in the synonymy of ‘Mesophyllum engelhartii’ by Chamberlain and Keats (Citation1995, p. 134), is Goniolithon elatocarpum Foslie (Citation1900, p. 23; type locality: Cape of Good Hope, coll. W.Tyson; isotype: in BM, the larger part of the actual Tyson specimen in TRH). The isotype was re-examined by Chamberlain and Keats (Citation1995, p. 144–145, figures 52–56, table 1) who documented a non-coaxial hypothallium forming only 10% in a thallus reaching more than 8 mm in thickness. Chamberlain & Keats identified the isotype as carpogonial-carposporangial and concluded that ‘in the absence of multiporate..conceptacles, this..could pertain to the Mastophoroideae … [hence] some doubt must remain’. On the other hand, Foslie (Citation1900) who previously described the same Tyson specimen (in BM) reported the presence of bisporangial conceptacles with ‘elongated tip..soon [to] fall..away and then the conceptacles are hemispherical-conical..’ – one bisporangium 100 × 50 µm was recorded (Foslie l.c.). Foslie associated G. elatocarpum with the genus Neogoniolithon Setchell & Mason (Neogoniolithoideae), and these disagreements on the identity of the conceptacles of the type, along with the lack a coaxial hypothallium dominating at least parts of the thallus (Chamberlain and Keats Citation1995, figures 52–56, table 1), exclude G. elatocarpum from the list of putative synonyms of Phragmope discrepans.
The status of the earlier described Lithothamnion synanablastum Heydrich (Citation1897, pp. 54–55, plate 3, figure 14; type locality: False Bay, South Africa, on granit and shells, coll. Marloth) needs also a comment. The single illustration in the protologue shows an encrusting thallus lacking erect protuberances. Other characters included in the protologue comprise ‘Tetrasporenconceptakel 170–250 µ im Durchmesser, Sporangium 150 µ lang und 35–40 µ breit’ and gametangial ‘Conceptakel..300–350 µ im D … ’. Apart from sporangium size, these measurements may pertain either to external or chamber size – in the latter case agreeing with Ph. discrepans. Heydrich (l.c.) also described the presence of ‘Rhizoiden, welche gegen das Substrat schräg getrichtet sind im flachen Bogen die peripherischen Zellreihen entsenden.’ – ‘Rhizoiden’ most likely referring to the basal layer (hypothallium) forming a bow (coaxial) structure and supporting the ascending (peripheral) perithallium. Although L. synanablastum was not included in the synonymy of ‘Mesophyllum engelhartii’ by Chamberlain and Keats (Citation1995, pp. 134–136), it was commented as being possibly conspecific. Still, ‘..the type..was probably destroyed in Berlin..and crucial diagnostic characters..cannot be confirmed. The only known material identified by Foslie as L. synanablastum..is now identified as Synanrthrophyton schmitzii … ’ (Chamberlain and Keats Citation1995, pp. 143–144). A further detail is that in describing L. synanablastum f. speciosum, Foslie (Citation1900, p.11) simultaneously recognized L. synanablastum f. conspersa Foslie (‘mscr’) citing L. synanablastum in synonymy – and hence he created a superfluous (illeg.) substitute of the autonym (L. synanablastum f. synanablastum). One would expect that Foslie based his conclusions on authentic material, but no collections of the latter taxon have been found in TRH (Woelkerling et al. Citation2005, p. 336), while the name f. conspersa was not mentioned again in Foslie's publications.
Of the remaining four synonyms of ‘Mesophyllum engelhartii’, listed by Woelkerling and Harvey (Citation1993, pp. 582, 597) and/or Chamberlain and Keats (Citation1995, p. 134), none is indigenous to South Africa. In particular, Lithothamnion variabile Foslie (Citation1906, p. 10) was originally described from the Falkland Islands (coll. C. Skottsberg, 23 July 1902). According to the Norwegian protologue, its thallus is [in translation] ‘lamellate composed of roundish to kidney-formed crusts, 0.3–1 mm thick, that grow together; later new crusts develop on top and construct a thallus 1–3 cm thick, with wart-like, sometimes short branch-like or knobby projections. Sporangial conceptacles are weakly convex or slightly prominent, 400–600 µm in external diameter’. Hence L. variabile should differ from Ph. discrepans both in thallus habit and in possessing larger multiporate conceptacles. Printz (Citation1929, p. 53, plate 5, figures 15–17) illustrated the type in TRH, maintaining elements of the protologue including conceptacle size. In transferring the species to Mesophyllum, Adey (Citation1970, p. 26) noted that ‘the epithallium and hypothallium are distinctly Mesophyllum’. On the other hand, Mendoza and Molina (Citation1994, p. 178, footnote) transferred the species to the genus Clathromorphum Foslie, but the original material has not been re-examined in a modern context (Woelkerling et al. Citation2005, p. 424).
Lithothamnion fumigatum Foslie (Citation1901, p. 7) was originally described from Port Phillip, Victoria (coll. J. Gabriel, 1899) and its protologue included several characters not in accord with Ph. discrepans. In particular, Foslie (l.c.) described the presence of ‘..densely crowded, small, wartlike or irregular excrescences..1–2 mm in diameter’ and multiporate conceptacles ‘always a little depressed in the central parts’ and the roof ‘traversed by about 20..canals’. The ‘feebly developed hypothallus is composed of up to 22 µ long cells..frequently however shorter.. send[ing] forth perithallic..cells..which are square or rounded..’. Hence, thallus habit, multiporate roof structure and even size of hypothallial and perithallial cells differ significantly from those in Ph. discrepans. Still, there is no modern account of the type material to secure the mismatch (Printz Citation1929, plate 4 figure 2; Adey and Lebednik Citation1967, p. 64; Woelkerling Citation1993, p. 100; Woelkerling et al. Citation2005, p. 315).
Lithothamnion versicolor Foslie (Citation1907a, p. 3) was described from Port Philip Heads, Victoria (coll. J. Gabriel, December 1906). The protologue contained several characters at variance with Ph. discrepans, such as size of tetrasporangial conceptacles, 400–700 µm in external diameter, and tetrasporangia 200–240 µm long – both measurements being twice the size of those in Ph. discrepans. In addition, Foslie (l.c.) described the roof structure as having ‘nedtryckte midtparti’ (depressed middle part). Still, there is no modern account of the type material to confirm these deviations (Printz Citation1929, plate 12, figures 1–2; Adey Citation1970, p. 26; Woelkerling Citation1993, p. 236; Woelkerling et al. Citation2005, p. 461).
Lithothamnion lemniscatum Foslie (Citation1907b, p. 11) was described from Cape Jaffa, South Australia (coll. A. Engelhart, 1899). According to the protologue, the tetrasporangial conceptacles ranged between 500 and 900 µm in external diameter and were weakly depressed in the middle of the roof – both characters not in accord with Ph.discrepans. Still, there is no modern account of the type material to ascertain the differences (Printz Citation1929, plate 7, figure 11; Adey and Lebednik Citation1967, p. 66; Adey Citation1970, p. 25; Woelkerling Citation1993, p. 101; Woelkerling et al. Citation2005, p. 324).
Finally, Lithothamnion aucklandicum (Foslie) Foslie (Citation1907b, p. 18) was originally described as Lithothamnion fumigatum f. aucklandicum from material collected at the Auckland Islands in March 1903 during the British National Antarctic Expedition Discovery (Foslie Citation1905, p. 16, ‘aucklandica’). The holotype has been illustrated by Printz (Citation1929, plate 4 fig. 17) and is a warty crust. According to Foslie (Citation1907b), ‘sporangie’ conceptacles are not prominent (almost flat) and the roof partly sunken. In transferring the species to Mesophyllum, Adey (Citation1970, p. 22) noted the existence of a weakly coaxial hypothallium, but there is no study of the type in a modern context (Woelkerling Citation1993, p. 33; Woelkerling et al. Citation2005, p. 466). It should be added that the last two putative synonyms appeared in the same original publication with Lithothamnion discrepans (Foslie Citation1907b), and do not threaten nomenclaturally the latter species.
The name ‘Fam.Mesophylleae Dum.’ Heeg (Citation1891)
‘Mesophyllaceae’ as a family name was used a few times before in the literature of Marchantiophyta (Leverworts). It first appeared as ‘Fam. Mesophylleae Dum.’, subordinate the widely accepted Jungermanniaceae (Heeg Citation1891, p. 568). In the introduction of his work, Heeg made clear that ‘In der nachfolgenden Zusammenstellung sind nur alle jene Arten und bemerkenswerthen Formen unserer Lebermoose aufgenommen, welche in der im Jahre 1887 von dem Herrn Custos Dr. G. R. v. Beck veröffentlichten … nicht verzeichnet erscheinen.’ (‘in the here following arrangement are only those species and worth mentioning forms of our recorded Leverworts, which in Beck’s publication … were not in writing recognized.’). Heeg presented the source of his materials and concluded that after his observations, the number of species and varieties in ‘Niederösterreichische’ (Lower Austria, one of the nine states of Austria) should be 118. Beck (Citation1887, p. 351) had previously included some 104 species and varieties within the widely accepted families Jungermaniaceae, Marchantiaceae, Anthocerotaceae, and Ricciaceae, making also clear that his classification followed Dumortier (Citation1874). Three of these family names were also adopted by Heeg, in a similar manner, except that Heeg further divided the Jungermaniaceae into 3 unranked groups (A. Foliosae, B. Sub-Frondosae, C. Frondosae) each including one to several tribes (or subtribes) preceded by the term ‘Fam.’ and followed by the species and varieties which he recognized. Most of these tribes (or subtribes) were correctly ascribed to Dumortier, or to others authorities such as Nees or Trevisan, or to well known publications.
Apart from the term ‘Fam.’ that precedes each tribal (or subtribal) name, there is no other evidence in Heeg’s work that he intended to provide a new classification of Marchantiophyta, adding eight new families to Beck’s (Citation1887) or Dumortier’s (Citation1874) system. On the contrast, Heeg was explicit that his scope was just to recognize those species and worth mentioning forms of Leverworts, which in Beck’s publication were missing. Therefore the term ‘Fam.’ that precedes well known tribes or subtribes of Marchantiophyta in Heeg’s paper must be regarded as a misplacement (conscious or not). Under Art. 5 & 37.6 (Turland et al. Citation2018), unordered classification ‘denoted by a misplaced term’ is considered to be invalid and hence ‘Fam. Mesophylleae Dum.’ or ‘Fam. Jungermannieae Dum.’, both cited below Jungermaniaceae, are to be treated as tribe Mesophylleae Dum. and tribe Jungermannieae Dum. in accordance to Dumortier’s (Citation1874) classification. The same applies to all other well known tribes (or subtribes) in Heeg’s (Citation1891) publication cited as ‘Fam.Saccogyneae Dum.’, ‘Fam. Platyphylleae Syn.Hep.’, ‘Fam. Jubuleae Nees.’, ‘Fam. Fossombronieae Trevis.’, ‘Fam. Haplolaeneae Nees.’, ‘Fam. Aneureae Nees.’, ‘Fam. Marchantieae Dum.’, ‘Fam. Sphaerocarpeae Dum.’, and ‘Fam. Riccieae Dum.’.
Nearly 100 years later, Grolle (Citation1972) reviewed the nomenclature of Leverworts and interpreted differently Heeg's citations. In particular, Grolle (Citation1972, p. 14) recognized the ‘Mesophyllaceae (Dum.) Heeg, … 1891, ‘Mesophylleae’..’, but on page 34 Grolle cited this name as a synonym of Jungermanniaceae Reichenb. Hence, neither ‘Mesophyllaceae Heeg ex Grolle Citation1972’ was validly published being ‘merely cited as a synonym’ (Art.36.1). I have recorded one more citation of ‘Mesophyllaceae Heeg’ in the literature (Shaw et al. Citation2015, p. 39), but even in that case the name was accepted as a synonym of Gymnomitriaceae H.Klinggr.
Conclusions
Phragmope discrepans (Foslie) comb.nov. is recognized as the correct name of the South African species previously known with the misapplied name ‘Mesophyllum engelhartii’ (Chamberlain and Keats Citation1995). Phragmope is remotely related to the Northern Hemisphere genus Mesophyllum, as shown by both morpho-anatomic () and molecular () data. Phragmope exhibits three apomorphies, namely: (1) frequent replacement of the hypothallial meristem, (2) cell bars that close the pore canals of multiporate roofs, and (3) a secondarily formed compact, imperforate roof above older multiporate conceptacles. Phragmope shares most of its other characters with taxa occurring either sympatrically (Melyvonnea) or in the Australian-New Zealand-Chatham region (species of the former Mesophyllum complex). Like other endemic coralline genera from South Africa (Amphithallia, Capensia), the sister-taxon of Phragmope remains unknown suggesting that the new genus has been isolated for a long period of time.
The name ‘Fam.Mesophylleae Dum.’ Heeg (Citation1891) is invalid since it was not Heeg’s intent to revise Beck’s (Citation1887) or Dumortier’s (Citation1874) classification adding eight new families to Marchantiophyta. The family name Mesophyllaceae Athanasiadis (Citation2016) is therefore available, and Mesophyllumaceae Schneider & Wynne (Citation2019) becomes a later synonym. In proposing the latter name as a substitute, Schneider & Wynne apparently overlooked the fact that Heeg's (Citation1891) citation of ‘Fam.Mesophylleae Dum.’ was contradicting his explicit intent to add several species and forms (but not new families) to Beck's (Citation1887) publication.
Acknowledgements
Particular thanks to Dr Jennifer Dalen for arranging two loans (nos 501697 & 501779) from WELT to GB. Two reviewers and Daniella Basso made helpful comments on the manuscript. The study is dedicated to the memory of Yvonne Chamberlain (1933-2018) for inspiring and sharing her interest on the coralline algae.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Adey WH. 1966. The genera Lithothamnium, Leptophytum (nov. gen.) and Phymatolithon in the Gulf of Maine. Hydrobiologia 28: 321–370. doi:10.1007/BF00130389
- Adey WH. 1970. A revision of the Foslie crustose coralline herbarium. Det Kongelige Norske Videnskabers Selskabs Skrifter. 1970(1):1–46. https://www.ntnu.no/ojs/index.php/DKNVS_skrifter/issue/archive
- Adey WH, Hernandez-Kantun JJ, Johnson G, Gabrielson PW. 2015. DNA sequencing, anatomy, and calcification patterns support a monophyletic, subarctic, carbonate reef-forming Clathromorphum (Hapalidiaceae, Corallinales, Rhodophyta). Journal of Phycology. 51:189–203. doi:10.1111/jpy.12266
- Adey WH, Lebednik PA. 1967. Catalog of the Foslie Herbarium. Det Kongelige Norske Videnskabers Selskab Museet, Trondheim, Norway. 92 pages.
- Athanasiadis A. 2016. Phycologia Europaea Rhodophyta, Vols 1 & 2. Published by the author, Gothenburg. xlviii + 1504 pages.
- Athanasiadis A. 2017a. A study of the original material of Lithothamnion engelhartii Foslie (Corallinales, Rhodophyta). Botanica Marina. 60:67–78. doi:10.1515/bot-2016-0110
- Athanasiadis A. 2017b. Capensia fucorum (Esper) gen. et comb. nov. (Mesophyllaceae, Corallinales, Rhodophyta), a hemiparasite on Gelidium from South Africa. Botanica Marina. 60:555–565. doi:10.1515/bot-2017-0027
- Athanasiadis A. 2018a. Zygote transfer and post-fertilization stages in Mesophyllum (Mesophyllaceae, Corallinales, Rhodophyta). Nova Hedwigia. 107(3-4):519–529. doi:10.1127/nova_hedwigia/2018/0489
- Athanasiadis A. 2018b. Carlskottsbergia antarctica (Hooker fil. & Harv.) gen. & comb.nov., with a re-assessment of Synarthrophyton (Mesophyllaceae, Corallinales, Rhodophyta). Nova Hedwigia. 108(2019):291–320. doi:10.1127/novahedwigia/2018/0506
- Athanasiadis A. 2019. Amphithallia, a genus with four-celled carpogonial branches and connecting filaments in the Corallinales (Rhodophyta). Marine Biology Research. 15:13–25. doi:10.1080/17451000.2019.1598559
- Athanasiadis A, Adey WH. 2006. The genus Leptophytum (Melobesioideae, Corallinales, Rhodophyta) on the Pacific coast of North America. Phycologia. 45:71–115. doi:10.2216/04-38.1
- Athanasiadis A, Ballantine DL. 2014. The genera Melyvonnea gen. nov. and Mesophyllum s.s. (Melobesioideae, Corallinales, Rhodophyta) particularly from the central Atlantic Ocean. Nordic Journal of Botany. 32:385–436. doi:10.1111/njb.00265
- Athanasiadis A, Lebednik PA, Adey WH. 2004. The genus Mesophyllum (Melobesioideae, Corallinales, Rhodophyta) on the Northern Pacific coast of North America. Phycologia. 43:126–165. doi:10.2216/i0031-8884-43-2-126.1
- Athanasiadis A, Neto I. 2010. On the occurrence of Mesophyllum expansum (Philippi) Cabioch et Mendoza (Melobesioideae, Corallinales, Rhodophyta) in the Mediterranean Sea, the Canary Isles and the Azores. Botanica Marina. 53:333–341. doi:10.1515/BOT.2010.042
- Bailey JC, Chapman RL. 1998. A phylogenetic study of the Corallinales (Rhodophyta) based on nuclear small-subunit rRNA gene sequences. Journal of Phycology. 34:692–705. doi:10.1046/j.1529-8817.1998.340692.x
- Beck G. 1887. Uebersieht der bisher bekannten Kryptogamen Niederösterreichs. Verhandl. der k.k. zool.-botan. Gesellsch. in Wien 37:253–378. http://www.biologiezentrum.at/
- Bittner L, Payri CE, Maneveldt GW, Couloux A, Cruaud C, de Reviers B, Le Gall L. 2011. Evolutionary history of the Corallinales (Corallinophycidae, Rhodophyta) inferred from nuclear, plastidial and mitochondrial genomes. Molecular Phylogenetics and Evolution. 61:697–713. doi:10.1016/j.ympev.2011.07.019
- Bold HC, Wynne MJ. 1978. Introduction to the algae structure and reproduction. Englewood Cliffs, NJ: Prentice-Hall. 706 pages.
- Broom JES, Farr DR, Nelson WA, Neill KF, Harvey AS, Woelkerling WJ. 2008. Utility of psbA and nSSU for phylogenetic reconstruction in the Corallinales based on New Zealand taxa. Molecular Phylogenetics and Evolution. 46:958–973. doi:10.1016/j.ympev.2007.12.016
- Chamberlain YM, Irvine LM. 1994. Melobesioideae Bizzozero. In: Irvine LM, Chamberlain YM, editors. Seaweeds of the British Isles. Volume 1 Rhodophyta, Part 2B Corallinales, Hildenbrandiales. London: Natural London: Natural History Museum, Pelagic Publishing; p. 159–234.
- Chamberlain YM, Keats D. 1994. Three melobesioid crustose coralline red algae from South Africa: Leptophytum acervatum (Foslie) nov. comb., L. foveatum sp. nov., and L. ferox (Foslie) nov. comb. Phycologia. 33:111–133. doi:10.2216/i0031-8884-33-2-111.1
- Chamberlain YM, Keats D. 1995. The melobesioid alga Mesophyllum engelhartii (Rhodophyta, Corallinaceae) in South Africa. South African Journal of Botany. 61:134–146. doi:10.1016/S0254-6299(15)30499-3
- Dumortier BC. 1874. Hepaticae Europae. Jungermannideae Europae post semiseculum recensitae, adjunctis Hepaticis. Bulletin de la Société Royale de Botanique de Belgique. 13:5–203. https://www.jstor.org/stable/20793209
- Farr T, Broom J, Hart D, Neill K, Nelson W. 2009. Common coralline algae of northern New Zealand. An identification guide. NIWA Information Series 70, Wellington. 125 pages. https://niwa.co.nz/sites/niwa.co.nz/files/common_coralline_algae_-_northern_nz_-_niwa_information_series_no_70.pdf.
- Foslie M. 1900. New or critical calcareous algae. Det kongelige Norske Videnskabers Selskabs Skrifter. 1899(5):1–34. https://www.ntnu.no/ojs/index.php/DKNVS_skrifter/issue/archive
- Foslie M. 1901. New melobesieae. Det kongelige Norske Videnskabers Selskabs Skrifter. 1900-6:1–24. https://www.ntnu.no/ojs/index.php/DKNVS_skrifter/issue/archive
- Foslie M. 1905. Den botaniske samling. Det kongelige Norske Videnskabers Selskabs Aarsberetning. 1904:15–18. https://www.ntnu.no/ojs/index.php/DKNVS_skrifter/issue/archive
- Foslie M. 1906. Algologiske notiser. II. Det kongelike Norske Videnskabers Selskabers Skrifter. 2:1–28.
- Foslie M. 1907a. Algologiske notiser. III. Det kongelige Norske Videnskabers Selskabs Skrifter. 1906(8):1–34.
- Foslie M. 1907b. Algologiske notiser. IV. Det kongelike Norske Videnskabers Selskabers Skrifter. 1907(6):1–30.
- Grolle R. 1972. Die Namen der Familien und Unterfamilien der Lebermoose (Hepaticopsida). Journal of Bryology. 7:201–236. doi:10.1179/jbr.1972.7.2.201
- Harvey AS, Woelkerling W, Farr T, Nelson W. 2005. Coralline algae of central New Zealand. Niwa Information Series. 57:1–145. https://niwa.co.nz/sites/niwa.co.nz/files/import/attachments/CentralNZ_IDCor_NIS57.pdf.
- Harvey AS, Woelkerling W, Millar AJK. 2003. An account of the Hapalidiaceae (Corallinales, Rhodophyta) in south-eastern Australia. Australian Systematic Botany. 16:647–698. doi:10.1071/SB03008
- Heeg M. 1891. Niederösterreiehische Lebermoose.- Verhandl. der k.k. zool.-botan. Gesellsch. in Wien 41: 567-573. http://www.biologiezentrum.at/
- Heydrich F. 1897. Corallinaceae, inbesondere Melobesieae. Berichte der Deutschen Botanischen Gesellschaft 15:34–71, pl. 3.
- Holmgren PK, Holmgren NH, Barnett LC. 1990. Index Herbariorum, part I. The herbaria of the world. Regnum Vegetabile. 120:1–693.
- Kaleb S, Falace A, Sartoni G, Woelkerling W. 2011. Morphology–anatomy of Mesophyllum macroblastum (Hapalidiaceae, Corallinales, Rhodophyta) in the northern Adriatic Sea and a key to Mediterranean species of the genus. Cryptogamie, Algologie. 32:223–242. doi:10.7872/crya.v32.iss3.2011.223
- Kato A, Baba M, Suda S. 2011. Revision of the Mastophoroideae (Corallinales, Rhodophyta) and polyphyly in nongeniculate species widely distributed on Pacific coral reefs. Journal of Phycology. 47:662–672. doi:10.1111/j.1529-8817.2011.00996.x
- Keats DW, Chamberlain YM. 1994. Two melobesioid coralline algae (Rhodophyta, Corallinales), Mesophyllum erubescens (Foslie) Lemoine and Mesophyllum funafutiense (Foslie) Verheij from Sodwana Bay, South Africa. South African Journal of Botany. 60:175–190. doi:10.1016/S0254-6299(16)30630-5
- Keats DW, Maneveldt G. 1997a. First report of the melobesioid alga (Corallinales, Rhodophyta) Mesophyllum incisum (Foslie) Adey in South Africa. South African Journal of Botany. 63:201–209. doi:10.1016/S0254-6299(15)30745-6
- Keats DW, Maneveldt G. 1997b. Two new melobesioid algae (Corallinales, Rhodophyta), Synarthrophyton robbenense sp. nov. and S. munimentum sp. nov., in South Africa and Namibia. Phycologia. 36:447–467. doi:10.2216/i0031-8884-36-6-447.1
- Lebednik PA. 1977. Postfertilization development in Clathromorphum, Melobesia and Mesophyllum with comments on the evolution of the Corallinaceae and the Cryptonemiales (Rhodophyta). Phycologia. 16:379–406. doi:10.2216/i0031-8884-16-4-379.1
- Lemoine M. 1928. Un nouveau genre de Mélobésiées: Mesophyllum. Bulletin de la Société Botanique de France, Sér. 75(2):251–254. doi:10.1080/00378941.1928.10836268
- Maneveldt GW, Chamberlain YM, Keats DW. 2008. A catalogue with keys to the non-geniculate coralline algae (Corallinales, Rhodophyta) of South Africa. South African Journal of Botany. 74:555–566. doi:10.1016/j.sajb.2008.02.002
- Maneveldt GW, Keats DW, Chamberlain YM. 2007. Synarthrophyton papillatum sp.nov.: A new species of non-geniculate coralline algae (Rhodophyta, Corallinales, Hapalidiaceae) from South Africa and Namibia. South African Journal of Botany. 73:570–582. doi:10.1016/j.sajb.2007.05.003
- May DI, Woelkerling WJ. 1988. Studies on the genus Synarthrophyton (Corallinaceae, Rhodophyta) and its type species, S. patena (J.D. Hooker et W.H. Harvey) Townsend. Phycologia. 27:50–71. doi:10.2216/i0031-8884-27-1-50.1
- Mendoza ML, Molina S. 1994. Corallinales of Malvinas Islands: Biological characteristics.- Cryptogamie, Algologie. 15:175–182.
- Peña V, Adey WH, Riosmena-Rodríguez R, Jung M-Y, Afonso Carrillo J, Coi H-G, Bárbara I. 2011. Mesophyllum sphaericum sp. nov. (Corallinales, Rhodophyta): a new maërl-forming species from the northeast Atlantic. Journal of Phycology. 47:911–927. doi:10.1111/j.1529-8817.2011.01015.x
- Peña V, De Clerck O, Afonso Carrillo J, Ballesteros E, Bárbara I, Barreiro R, Le Gall L. 2014. An integrative systematic approach to species diversity and distribution in the genus Mesophyllum (Corallinales, Rhodophyta) in Atlantic and Mediterranean Europe. European Jurnal of Phycology. 50(2015):20–36. doi:10.1080/09670262.2014.981294
- Printz H. 1929. M. Foslie: contributions to a monograph of the Lithothamnia. Trondheim: Det kongelike Norske Videnskabers Selskabers Museet. 60 pages 75 plates.
- Schneider CW, Wynne MJ. 2019. Fourth addendum to the synoptic review of red algal genera. Botanica Marina. 62:355–367. doi:10.1515/bot-2019-0003
- Shaw B, Crandall-Stotler B, Vana J, Stotler RE, von Konrat M, Engel JJ, Davis ED, Long DG, Sova P, Shaw AJ. 2015. Phylogenetic Relationships and Morphological Evolution in a Major Clade of Leafy Liverworts (Phylum Marchantiophyta, Order Jungermanniales): Suborder Jungermanniineae. Systematic Botany. 40:27–45. doi:10.1600/036364415X686314
- Suneson S. 1937. Studien über die Entwicklungsgeschichte der Corallinaceen. Akademische Abhandlung, H. Ohlssons Bochdruckerei, Lund. 102 pages, 4 plates.
- Townsend RA. 1979. Synarthrophyton, a new genus of Corallinaceae (Cryptonemiales, Rhodophyta) from the southern Hemisphere. Journal of Phycology. 15:251–259. doi:10.1111/j.1529-8817.1979.tb02634.x
- Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, et al. 2018. International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code). Regnum Vegetabile. 159: xxxviii + 253 pages. https://www.iapt-taxon.org/nomen/main.php.
- Van der Merwe E, Maneveldt GW. 2014. The genus Phymatolithon (Hapalidiaceae, Corallinales, Rhodophyta) in South Africa, including species previously ascribed to Leptophytum. South African Journal of Botany. 90:170–192. doi:10.1016/j.sajb.2013.11.004
- Wilks KM, Woelkerling WJ. 1994. An account of southern Australian species of (Corallinaceae, Rhodophyta) with comments on. Australian Systematic Botany. 7:183–223. doi:10.1071/SB9940183
- Woelkerling WJ. 1993. Type collections of Corallinales (Rhodophyta) in the Foslie Herbarium (TRH). Gunneria. 67:1–289. https://www.ntnu.no/c/document_library/get_file?uuid=929a1e6d-f505-4239-b1ef-71143bb439da&groupId=10476.
- Woelkerling WJ. 1996. Subfamily Melobesioideae Bizzozero. In: Womersley HBS, editor. The marine benthic flora of suthern Australia. Rhodphyta – Part IIB Gracilariales, Rhodymeniales, Corallinales and Bonnemaisoniales. Canberra: Australian Biological Resaerch Study & State Herbarium of South Australia; p. 164–210.
- Woelkerling WJ, Foster MS. 1989. A systematic and ecographic account of Synarthrophyton schielianum sp.nov. (Corallinaceae, Rhodophyta) from the Chatham Islands. Phycologia. 28:39–60. doi:10.2216/i0031-8884-28-1-39.1
- Woelkerling WJ, Gustavsen G, Myklebost HE, Prestø T, Såstad SM. 2005. The coralline red algal herbarium of Mikael Foslie: revised catalogue with analyses. Gunneria. 77:1–625. https://www.ntnu.no/c/document_library/get_file?uuid=6350bf35-5dd1-45c2-9003-2c85ea91d520&groupId=10476.
- Woelkerling WJ, Harvey A. 1992. Mesophyllum incisum (Corallinaceae, Rhodophyta) in Southern Australia: Implications for generic and specific delimitation in the Melobesioideae. British Phycological Journal. 27:381–399. doi:10.1080/00071619200650321
- Woelkerling WJ, Harvey A. 1993. An account of southern Australian species of Mesophyllum (Corallinaceae, Rhodophyta). Australian Systematic Botany. 6:571–637. doi:10.1071/SB9930571
