ABSTRACT
The structure of canals in multiporate conceptacles of Mesophyllaceae is analysed and several distinct types are recognized. In what appears to be the plesiomorphic condition, canals are straight and bordered by non-differentiated pore cells. In the majority of Mesophyllaceae, pore cells are thinner-wider, starting from basal cells and extending towards the apical opening. This can be coupled with development of triangular (conical) or pyriform canals. Other modifications occur at species or genus level, or across several genera, the most distinctive being formation of cell bars (Phragmope discrepans), elongate basal cells (Melyvonnea), or branched pore filaments composed of fewer cells than adjacent roof filaments and provided with elongate subbasal (or basal and subbasal) cells (Thallis-Perithallis, respectively Printziana-Sunesonia). In Thallis-Perithallis-Sunesonia-Melyvonnea, pore filaments are composed of fewer cells than adjacent roof filaments and terminate below the roof surface. It is postulated that the elongate basal cells in Melyvonnea resulted after loss of basally branched pore cells, an evolutionary step that finds justification in the new genus Sunesonia where homologous cells become reduced or deteriorated. A new thallus organization (aniso-bilateral) characterized by a diminutive ventral perithallium distinguishes Perithallis. Nine new taxa are described, including accounts of their historical record, typifications, comparison of their types to new collections, and the published molecular work that provides further support: Thallis capensis gen. & sp. nov., Perithallis incisa gen. et comb. nov., P. chathamensis (Foslie) nov. comb., Printziana australis gen. & nom. nov. and Sunesonia pseuderubescens gen. et sp. nov.
Introduction
Differentiated pore cells in the coralline algae were first observed by Frans Kjellman (1846–1907), who illustrated and commented their presence in Lithothamnion (Leptophytum) foecundum Kjellm., as ‘en krans med celler, som äro olika takets öfriga kortikalceller’ (Kjellman Citation1883, p. 132, plate 5 figure 18) [‘a ring of cells different from the other cortical cells of the roof’ (Kjellman Citation1885, p. 100)]. This early observation escaped the attention of Mikael Foslie (1855–1909), who in his extensive coralline studies commented just the canal texture as being mucous (‘slimkanaler’; e.g. Foslie Citation1906a, p. 3, 11; Citation1907a, p. 4; Citation1907b, p. 8, 13, 14, 17, 19). No further observations were published for nearly a century, until Woelkerling and Irvine (Citation1986, p. 388, figure 24) reported different pore cells in the unbranched pore filaments of Mesophyllum lichenoides (J. Ellis) Me Lemoine – namely, pore cells ‘shorter than other roof cells’. A few years later, Woelkerling and Harvey (Citation1992, p. 394; Citation1993, table 5) reported pore cells ‘narrower and more elongate than adjacent roof cells’ in Mesophyllum printzianum Woelk. & A.S. Harv. and M. incisum (Foslie) W.H. Adey. Soon after, Verheij (Citation1993, p. 62) observed the presence of elongate basal cells in a species of Mesophyllum Me Lemoine from Indonesia, a character to be included in the description of the new genus Melyvonnea Athanas. & D.L. Ballant. (Athanasiadis and Ballantine Citation2014). More recently, the production of cell bars (closing the function of canals) was documented in Phragmope discrepans (Foslie) Athanas. (Athanasiadis Citation2020). Parallel to these reports, canal shape was also observed to display differentiations in the Mesophyllaceae. Two modifications were reported deviating from the standard (straight) canal type: triangular canals in Synarthrophyton R.A. Towns. (Athanasiadis Citation2018b) and pyriform canals in Mesophyllum engelhartii (Foslie) W.H. Adey (Athanasiadis Citation2017a) and in Carlskottsbergia Athanas. (Athanasiadis Citation2018b).
Since the turn of the century, members of Mesophyllaceae have undergone substantial taxonomic investigations in the northern and southern hemispheres resulting in the recognition of several new species and genera (Athanasiadis Citation1999, Citation2001, Citation2007a, Citation2007b, Citation2008, Citation2016a, Citation2016b, Citation2017a, Citation2017b, Citation2018a, Citation2018b, Citation2019, Citation2020; Adey et al. Citation2001; Athanasiadis and Adey Citation2003, Citation2006; Athanasiadis et al. Citation2004; Athanasiadis and Ballantine Citation2014).
The present study evaluates the various types of pore cell, pore filament and canal shape reported in the Mesophyllaceae. This assessment reveals the great diversity in the family and provides evidence to recognize four new genera in the southern hemisphere. This diversification sets the family apart from all other coralline algae displaying conceptacles with multiporate roofs – a group that also includes the families Lithothamniaceae and Melobesiaceae (formerly ‘Hapalidiaceae’).
Materials and methods
The material examined was borrowed from the herbaria of the Norwegian University of Science and Technology, Trondheim, Norway (TRH), the Royal Botanic Gardens Melbourne (MEL), the Museum of New Zealand Te Papa Tongarewa (WELT) and the British Museum of Natural History (BM). In addition, a collection (D11 – YMC 90/3) made in South Africa by the late Dr Y.M. Chamberlain (YMC) and her co-workers was given to the author as a gift, during his visit to the Marine Laboratory of Portsmouth Polytechnic in October 2008. Specimens were examined under a Zeiss Stemi SV 6 microscope (Zeiss, Jena, Germany). For anatomical observations, transections (30–50 μm thick) were made using a Kryomat 1700 freezing microtome (Leitz, Stuttgart, Germany). Microscopic preparations were made of sectioned fragments that were previously decalcified using acetic acid (20–45%, 5–24 h), stained and hardened in aniline blue (1–5% solution with alcohol 96% for at least 24 h) and sectioned in Hamilton’s freezing solution (1 g gum arabic: 30 g sucrose: one crystal of thymol: 100 ml distilled water). Sections were mounted in 60% Karo® corn syrup (Best Food Division CPC International, Englewood Cliffs, NJ, USA) on microscope slides. Photomicrographs were taken using a scanner (Epson Perfection V700, model J221A, Indonesia) or a camera (Nikon D7000, Nikon Europe B.V.) attached to a Zeiss Axiophot 2 microscope (Zeiss, Jena, Germany). Number of perithallial protuberances was counted in well-developed (ramified) individuals, using an open square (1 × 1 cm) placed over the well-developed parts of thallus. The procedure was repeated several times, moving the square around, until a maximum number of nested protuberances was established (Athanasiadis and Ballantine Citation2014, p. 386). Holotypes of new taxa have been deposited in GB or MEL. Other slides and specimens are in the herbarium of the author (herb.Athanas.). Herbarium abbreviations follow Holmgren et al. (Citation1990), and terminology Bold and Wynne (Citation1978) and Athanasiadis and Ballantine (Citation2014). The following abbreviations are used in the text: breadth (B), diameter (D), height (H), length (L), number of measurements (n), spermatangial mother cells (SMCs), superimposed unattached growth (SUG) and trichocyte (tr).
Comparative material examined
Mastophoropsis canaliculata (Harv.) Woelk. Herbarium BM (BM 001216065): Annotated ‘Algae Muellerianae, Curante J.G. Agardh distributae. Mastophora hypoleuca Harv. Mac Donnells Bay. Recd February 1896’ ‘Mastophoropsis canaliculata (Harvey in Hooker) Woelkerling. For monograph see Br.phycol.J.13:209–225 (1998)’ ‘Signed: Wm. J. Woelkerling Date: 9 December 1980; collected at Mac Donnells Bay (South Australia)’.
Results
At least ten types of pore cells and pore filaments lining canals of multiporate roofs, and three different canal shapes have been reported in members of Mesophyllaceae, including those here recognized or fully appreciated (). They are named after their structure (or shape), or the taxon they characterize. Pore filaments or pore cells are defined as: (a) non-differentiated, (b) producing cell bars (Phragmope), (c) composed of thinner-wider pore cells, (d) composed of smaller cells than adjacent roof cells (Mesophyllum lichenoides), (e) composed of elongate subbasal cells, (f) composed of branched basal cells with elongate subbasal cells, ending below the roof surface and lacking epithallial cells (Thallis), (g) composed of elongate branched basal cells and elongate subbasal cells, terminating at the roof surface or just below/above (Printziana), (h) similar to Printziana except that basal cells are reduced (or deteriorated), and pore filaments terminate below the roof surface (Sunesonia), (i) composed of unbranched elongate basal cells and terminating in epithallial cells below the roof surface (Melyvonnea), and finally pore filaments terminating above the roof surface (Mesophyllum funafutiense – not illustrated in ). Canal shape has been described as being ± straight (in several species and genera of Mesophyllaceae), triangular (e.g. in Synarthrophyton) or pyriform (e.g. in Carlskottsbergia). In particular:
Figure 1. Diagrammatic illustrations (with photographic documentation) of pore filament and canal shape types in Mesophyllaceae: a, non-differentiated pore filaments in straight canals (Athanasiadis and Adey Citation2006, figure 15, Leptophytum tenue, RWM); b, development of cell bars (arrows) from basal cells in Phragmope discrepans (Athanasiadis Citation2020, e); c, thinner-wider pore cells, in transverse (white arrows) or tangential (arrowheads) sections, or in views from the roof interior (black arrows), in Mesophyllum spp. (Athanasiadis Citation2007b, figures 63, 77 Mesophyllum crassiusculum, M. megagastri; RWM); d, thinner-wider pore cells, shorter than adjacent roof cells in Mesophyllum lichenoides (Athanasiadis and Neto Citation2010, figures 25, 29, RWM); e, elongate subbasal cells (shadowed), in certain species of Mesophyllum-Leptophytum (Athanasiadis and Adey Citation2006, figure 47, L. lamellicola, RWM); f, Thallis-type: basally branched pore filaments (supporting a roof filament, black arrows) composed of elongate subbasal cells (shadowed) and two or three smaller cells lacking epithallial cell and terminating below the conceptacle surface (arrowheads) (Keats and Maneveldt 1997, figure 17, as M. incisum, RWM; Figures 4e–h, 9g–i, 12d–e); g, Printziana-type, in P. australis (c–h) and differing from Thallis in having in addition elongate basal cells, and occasionally terminating at the roof surface or just below; h, Sunesonia-type, in S. pseuderubescens (l–p) and differing from Printziana by the development of 4-celled pore filaments terminating below the roof surface, and in having reduced (or deteriorated) basal cells; i, Melyvonnea-type in species of this genus (Jesionek et al. Citation2016, figure 9F, RWM), where pore filaments are unbranched, composed of elongate basal cells (arrows) supporting 2-4 smaller cells and terminating in an epithallial cell below the roof surface; j, triangular (conical) canal with thinner-wider cells along half the canal length (arrows) (Athanasiadis Citation2018b, figure 54, Synarthrophyton patena, RWM); k–m, pyriform canals with thinner-wider basal and subbasal cells (arrows) (k–l: Athanasiadis Citation2018a, figures 30, 31, Carlskottsbergia antarctica, RWM); m, swollen and deeply staining differentiated pore cells (arrows) (Ricker Citation1987, figure 73h, RWM). Abbreviations: canal (c), pore filament (pf), sunken pore filament (spf), reproduced with modifications (RWM); black squares define epithallial cells, and elongate pore cells are shadowed.
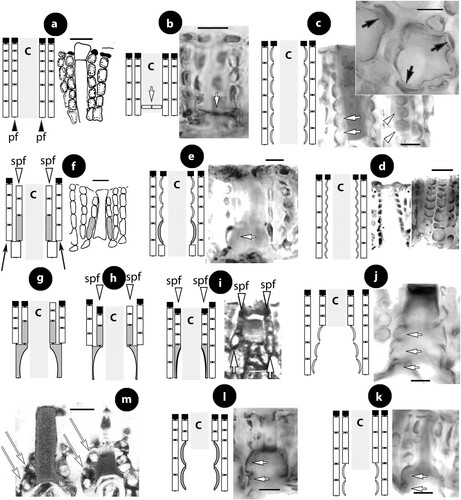
a. Non-differentiated pore filaments, similar to adjacent roof filaments, occur commonly in members of the Arctic and North Pacific genus Clathromorphum Foslie (Lebednik Citation1977a, figures 3D, 14C), Neopolyporolithon W.H. Adey & H.W. Johansen (Lebednik Citation1977a, figure 8F, as Clathromorphum), and Callilithophytum (Setch. & Foslie) P.W. Gabrielson et al. (Lebednik Citation1977a, figure 16D, as Clathromorphum). This type is also found in certain Arctic species of Leptophytum W.H. Adey – including its obligate parasite Kvaleya W.H. Adey & C.P. Sperapani (Adey and Sperapani Citation1971, figure 18), such as in Leptophytum tenue (Kjellm.) Athanas. & W.H. Adey (Athanasiadis and Adey Citation2006, figures 15, 16) and L. flavescens (Kjellm.) Athanas. (Athanasiadis Citation2016a, figures S16, S17). Southern hemisphere Mesophyllaceae with non-differentiated pore cells include Amphithallia crassiuscula (Foslie) Athanas. from South Africa (Athanasiadis Citation2019, figure 5d, e) and Synarthrophyton schielianum Woelk. & M.S. Foster from the Chatham Islands (Woelkerling and Foster Citation1989, figure 23). This type is apparently plesiomorphic, since it also characterizes most other members of the multiporate coralline algae (Chamberlain and Irvine Citation1994, figure 75; Wilks and Woelkerling Citation1995, figure 5), where however studies of canal structure are relatively few (Wilks and Woelkerling Citation1995, figures 10–11; Athanasiadis and Adey Citation2006, figures 142–145; Basso et al. Citation2011, figures 13–24).
b. Phragmope discrepans (Foslie) Athanas. joins the group above, except that in Phragmope we have production of cell bars from basal pore cells that close the canals after spore discharge (Athanasiadis Citation2020, figures 6d–f). A similar development of cell bars has been documented in an undescribed Mesophyllaceae from South Australia, included in the original material of Lithothamnion (Mesophyllum) engelhartii Foslie (Athanasiadis Citation2017a, figure 29).
c. Thinner-wider pore cells have been documented in most members of Mesophyllaceae. Such pore cells appear thinner in transverse section and wider in tangential section (or in views from above or the roof interior). As a rule, thinner-wider pore cells occur at the canal base and spread towards the apical opening, in different extent in the various species or genera. In the majority of cases, thinner-wider pore cells are ± of similar length (in comparison to other pore cells or to adjacent roof cells). Distinctive diminishing or elongation is observed in certain species or genera (below). Typical thinner-wider pore cells occur in most species of the northern hemisphere genus Mesophyllum and in several species of the Arctic-N. Atlantic-N. Pacific genus Leptophytum, namely in Mesophyllum vancouveriense (Foslie) R.S. Steneck & R.T. Paine, M. conchatum (Setch. & Foslie) W.H. Adey, M. lamellatum (Setch. & Foslie) W.H. Adey, M. crassiusculum (Foslie) P.A. Lebednik, M. megagastri Athanas., Leptophytum foecundum (Kjellm.) W.H. Adey, L. laeve (Foslie) W.H. Adey, L. jenneborgii Athanas. and L. helenae Athanas. (Kjellman Citation1883, 1885, plate 5 figure 18; Athanasiadis and Adey Citation2003, figures 15–16, 2006, figures 83–84, 103–104; Athanasiadis et al. Citation2004, figures 43–46, 77–79, 43–46; Athanasiadis Citation2007a, figures 26, 27, Citation2007b, figures 17, 77). Thinner-wider pore cells have also been reported in species from the southern hemisphere, i.e. in Capensia fucorum (Esper) Athanas., Carlskottsbergia antarctica (Hooker fil. & Harv.) Athanas. and in Synarthrophyton patena (Harv.) R.A. Towns. (Athanasiadis Citation2017b, figures 26–29; Citation2018b, figures 29, 30, 54–65).
d. Thinner-wider pore cells, shorter than adjacent roof cells, have been documented in the generitype of Mesophyllum, M. lichenoides (Woelkerling and Irvine Citation1986, figure 24; Woelkerling and Harvey Citation1993, figure 30B; Athanasiadis and Neto Citation2010, figures 25–29). This type is presently considered to have evolved as an autapomorphy for this species within the genus Mesophyllum, whose members generally display thinner-wider pore cells (c).
e. Elongate subbasal pore cells have been reported in Mesophyllum macedonis Athanas. (Athanasiadis Citation1999, figures 11, 14, 15) and also observed in Leptophytum lamellicola Athanas. & W.H. Adey and L. julieae Athanas. & W.H. Adey from the NE Pacific (Athanasiadis and Adey Citation2006, figures 46, 47, 74, 75). Elongate subbasal cells also occur in certain genera of the southern hemisphere, being incorporated in pore filaments showing multiple differentiations (below).
Pore filaments of the above five types are generally unbranched, with a few exceptions of occasionally divided basal cells reported in Mesophyllum lamellatum and M. crassiusculum (Athanasiadis et al. Citation2004, pp. 148, 161). In the following three types, however, basal cells are generally ramified supporting a normal roof filament in addition to the pore filament itself that displays multiple differentiations.
f. In the new genus Thallis, pore filaments are 4-celled (adjacent roof filaments being 5–8-celled), each composed of a normal basal cell, an elongate subbasal cell, and two more cells, lacking an epithallial cell and terminating below the roof surface. In addition, basal cells are ramified supporting a second normal roof filament. This complex organization was originally documented in thalli from South Africa identified as Mesophyllum incisum by Keats and Maneveldt (1997, p. 204, figure 17), and here referred to Thallis capensis gen. & sp. nov. (). This type is identical to that occurring in Perithallis gen. nov. from southern Australia-New Zealand-Chatham (Figures 9g–i, 12d–e), that includes the type of M. incisum, with the only exception that pore filaments in Perithallis can be 4- or 5-celled. This type involves multiple cell differentiations and is recognized as an unmistakable synapomorphy for these two genera, presently restricted to South Africa, respectively southern Australia-New Zealand-Chatham.
Figure 2. Thallis capensis gen. & sp. nov. Vegetative and spermatangial structures; a–b, tetrasporophyte with flattened multiporate conceptacle roofs (holotype); c–d, carposporophyte with conical uniporate conceptacles (syntype); e, thallus section showing a non-coaxial hypothallium (black arrow) with descending hypothallial (long black arrow) and ascending stratified perithallial filaments (white arrow) (isotype); f, thallus section showing a core of at least two or three cell layers (arrows) running parallel to the substratum and giving rise to ascending perithallial (p) and descending (d) hypothallial filaments (isotype); g, surface section showing elongate subepithallial cells (arrows), just divided (white arrowheads), supporting single epithallial cells (black arrowhead) (isotype); h, descending hypothallial filaments ending in wedge-shaped cells (arrowheads). Note the cell fusion (arrow) (isotype); i, spermatangial structures. SMCs (black arrow) cut off elongate spermatangia (arrowhead) which liberate spermatia (white arrow) (Keats and Maneveldt Citation1997, figure 7, reproduced with modifications). Abbreviations: perithallial filaments (p), descending hypothallial filaments (d).
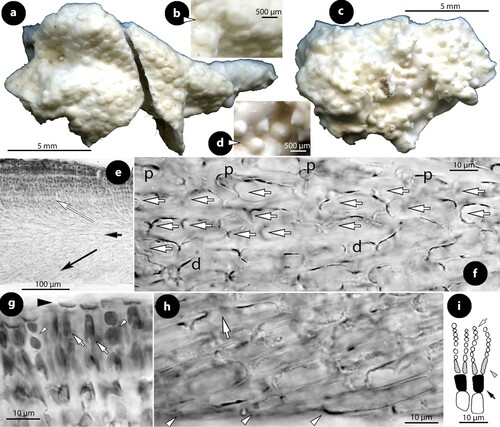
Figure 3. Thallis capensis gen. & sp. nov. Carpogonial, postfertilization stages and carposporangial conceptacle structures (syntype); a, embedded, unfertilized carpogonial conceptacle; b–c, 2- or 3-celled carpogonial branches composed of a carpogonium (ca), a hypogynous (h) and a supporting (s) cell. Note the cell tube (c.t.) connecting the base of the carpogonium with the supporting cell; d–e, postfertilization stages showing the development of a fusion (f) cell that incorporates at least six supporting cells and one hypogynous cell (arrow in figure e), while carpogonia (ca) and basal (b) cells remain intact. Adjacent hypogynous cells develop a contact (broken arrows) to the fusion cell or to gonimoblast filaments (g.f.); f–g, carposporangial conceptacles with a distinctively or slightly raised fertile zone (arrows) and peripheral production of carposporangia (arrowheads); h, older carposporangial conceptacles embedded in the perithallium. Note the lack of a raised fertile zone. Abbreviations: b (basal cell), ca (carpogonium), c.t. (cell tube), f (fusion cell), g.f. (gonimoblast filaments), h (hypogynous cell), s (supporting cell).
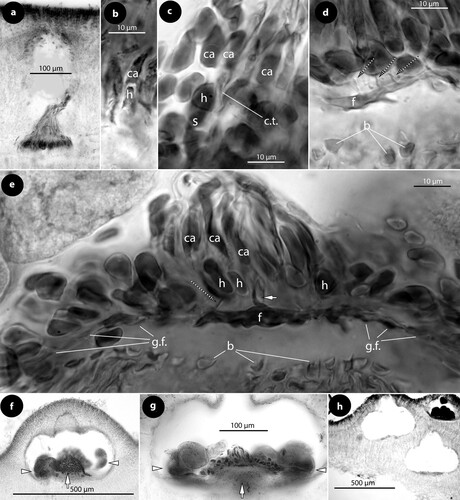
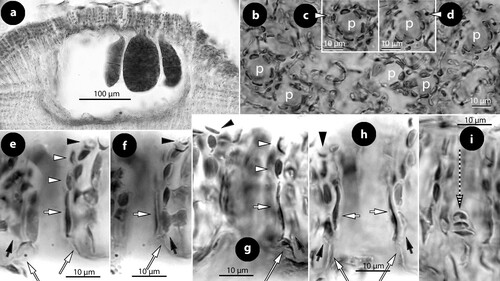
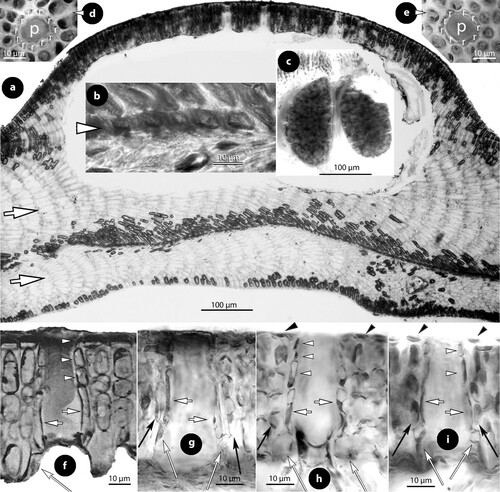
Figure 4. Thallis capensis gen. & sp. nov. Tetrasporangial conceptacle structures (isotype); a, tetrasporangial conceptacle with three tetrasporangia; b–d, views of a multiporate roof from above showing pore (p) canals surrounded by 6–7 pore cells; e–h, sections of canals of multiporate roofs showing filaments lining the canals. Note the branched basal cells (long white arrows), each supporting a normal roof filament (black arrows) and the rest of the lining filament that is composed of an elongate subbasal cell (short white arrows) followed by two cells (arrowheads). Pore filaments lack epithallial cells and terminate below the roof surface, i.e. epithallial cells (black arrowheads) of adjacent roof filaments; i, a diminutive, 3-celled, filament (broken arrow) at the canal base. Abbreviations: p (pore canal).
g. In Printziana gen. nov., pore filaments are basally ramified – supporting a normal roof filament (as in Thallis-Perithallis), but the pore filament itself is provided with elongate basal and subbasal cells. Moreover, pore filaments are 4- or 5- (6-?) celled (fewer than in adjacent roof filaments), terminating at the conceptacle roof surface (or slightly below or above). Top cells can be epithallial cells. This type characterizes species of Printziana gen. nov. from southern Australia-New Zealand-Chatham (Figure 16c–j), a genus based on Mesophyllum printzianum where the presence of ‘narrow, elongate cells bordering the pore canals of tetrasporangial conceptacles’ was already observed (Woelkerling and Harvey Citation1993, p. 593).
h. Sunesonia gen. nov. shares the type of Printziana, except that basal cells become reduced (or deteriorated) and the elongate subbasal cells look like being located in a basal position, resembling those of Melyvonnea (below). In addition, pore filaments are generally 4-celled (fewer than in adjacent roof filaments) and terminate below the roof surface. This type characterizes Sunesonia pseuderubescens gen. & sp. nov. from southern Australia-New Zealand (Figure 17h–p).
i. Species of Melyvonnea display unbranched pore filaments with elongate basal cells, each supporting 2–4 cells (fewer than in adjacent roof filaments) and terminating in epithallial cells below the roof surface (Keats and Chamberlain Citation1994, figure 63, as Mesophyllum erubescens; Harvey et al. Citation2003, figure 12C, D, as Mesophyllum erubescens; Peña et al. Citation2011, table 1, figure 4i, as Mesophyllum canariensis; Athanasiadis and Ballantine Citation2014, p. 392, figures 15–17, 51, 46–48, 80–83, 96–101, 108; Jesionek et al. Citation2016, figure 9F). Regarding the structure of pore filaments, Melyvonnea shares with Thallis-Perithallis and Sunesonia two characters, viz. pore filaments terminating below the roof surface, and composed of fewer cells than in adjacent roof filaments. Considering these characters as synapomorphies, a close relationship between these genera is established, in which case the elongate basal cells in Melyvonnea should be interpreted as a relocation, following the loss of basally ramified pore cells – a condition partially present in Sunesonia where basal cells are reduced or deteriorated (Figure 17l–p; Discussion).
Pore filaments terminating above the roof surface have been reported in Mesophyllum funafutiense (Foslie) E. Verheij (Keats and Chamberlain Citation1994, figure 57, table 1), but this type is not well documented and it is not included in the here diagrammatic illustrations () – see also comments in Athanasiadis and Ballantine (Citation2014, supplementary material, appendix 2). Finally, in Mastophoropsis canaliculata (Harv.) Woelk. from southern Australia, pore cells were said to be similar to adjacent roof cells (Woelkerling Citation1996, p. 175), but the study of a collection from Mac Donnells Bay (South Australia; BM 001216065) showed that basal and subbasal cells are enlarged, as illustrated by Woelkerling (Citation1996, figure 74A). This type clearly requires further examination to elucidate putative variation and relationships to other Mesophyllaceae.Footnote1
Parallel to the above types, canals have undergone changes in shape: being ± straight in the majority of Mesophyllaceae (a–e) to triangular (conical) or pyriform, the former recorded in Synarthrophyton patena (j; Athanasiadis Citation2018b, figures 54–55) and the latter in Mesophyllum engelhartii (Foslie) W.H. Adey and in Carlskottsbergia antarctica (k–l; Athanasiadis Citation2017a, figures 16–17; Citation2018b, figures 30–31). A further difference between the latter two types lies in that the differentiated (thinner-wider) pore cells in Synarthrophyton are at least three or four and occupy at least half the length of the canal, while in M. engelhartii and in C. antarctica homologous cells are usually two (basal and subbasal cells) that can be elongated. We need to add that differentiated (thinner-wider) cells originate as swollen and deeply pigmented cells, before the passage of spores through the canals, as documented in Carlskottsbergia antarctica by Ricker (Citation1987, figure 73h, as Mesophyllum patena; m).
A wider basal opening, interpreted as ‘conical canal’ has also been reported in certain species of Mesophyllum (Athanasiadis et al. Citation2004, table 1), Leptophytum (Athanasiadis and Adey Citation2006, table 1), and it also occurs in species of Melyvonnea (Athanasiadis and Ballantine Citation2014, p. 392, ‘pore canals wider at the base’), Printziana (Figure 16e–g, j), or Sunesonia (Figure 17l–m). This indicates that a wider basal opening (leading to conical canals) most likely occurred independently across members of several genera that also exhibit other distinct types of pore filament differentiation. The hypothesis that canal differentiations took place in the Mesophyllaceae to facilitate the liberation of larger spores is advanced in the Discussion.
Taxonomy
Family Mesophyllaceae Athanasiadis, 2016
Genus Thallis gen. nov.
Type species Thallis capensis sp. nov.
Genus diagnosis
New monotypic genus of Mesophyllaceae (Corallinales), differing from Perithallis (described herein) in possessing dorsiventral thallus organization and a prominent fusion cell in carposporangial conceptacles, and in lacking a predominantly coaxial hypothallium. Thallis and Perithallis differ from other genera of Mesophyllaceae in developing basally branched pore filaments in canals of multiporate conceptacles, the filaments being 4–5-celled, provided with elongate subbasal cells and terminating below the conceptacle surface.
Etymology
The generic name is a new feminine substantive, θαλλις (diminutive of θαλλος, thallus). It is pronounced Thallís (genitive Thalli-dos).
Thallis capensis sp. nov.
()
Species diagnosis
Differing from Perithallis incisa and P. chathamensis by the generic characters described above (Turland et al. Citation2018; Art.38.5).
Etymology
The epithet is the adjective capensis, -ensis, -ense (inhabitant of the Cape).
Type locality
Bird Island (east end of Algoa Bay, Eastern Cape), South Africa.
Holotype
In GB, n°GB-0219096, August 1987, 22 m depth, on geniculate coralline, collector not known (a–b) (tetrasporophyte from the collection D11-YMC 90/3).
Isotypes
Slides made from the holotype specimen (herb.Athanas.) (e–h, 4)
Syntype
D11-YMC 90/3, including slides (herb.Athanas.), August 1987, 22 m depth, on geniculate coralline, collector not known (carposporophyte; c–d, 3).
Misapplied name
Mesophyllum incisum sensu Keats and Maneveldt (Citation1997, Figures 1–21).
Material examined
South Africa: Bird I., east end of Algoa Bay, Eastern Cape: GB n° GB-0219096: holotype, data as above (tetrasporophyte).
D11-YMC 90/3 (herb.Athanas.): syntype, data as above (the collection D11-YMC 90/3 annotated by YMC ‘August 1987’, ‘Mesophyllum engelhartii’, ‘90/3’, ‘at 22 m depth on geniculate coralline’, collector not cited).
Description
Thalli encrusting, to 2 cm in extent and 150–700 µm thick, lacking erect perithallial protuberances or unattached superimposed growth of several layers (a–d), epiphytic on geniculate corallines (possibly adhering to epiphytic hydrozoa). Epizoic thalli encrusting with free margins, weakly adherent, up to 4 cm in extent and 250–550 µm thick, lacking superimposed growth (Keats and Maneveldt Citation1997, figures 1–4). Thallus organization dorsiventral. Hypothallium 40–200 µm thick, non-coaxial (with coaxial patches), composed of a core of at least three cell layers growing parallel to the substratum, and supporting descending hypothallial and ascending perithallial filaments (e–f). Ascending perithallial filaments, 50–500 µm thick, stratified (e). Hypothallial cells 12–32 × 5–10 µm (L × B) (9–22 × 5–13 µm according to Keats and Maneveldt Citation1997). Perithallial cells 7–15 × 5–8 µm (L × B) (6–19 × 4–13 according to Keats and Maneveldt Citation1997). Epithallial cells rectangular, singly borne, 2–3 × 4–5 µm (L × B) (g) (elliptical, domed-shaped to flat topped, 2.5–4 × 2.5–6 µm according to Keats and Maneveldt Citation1997), supported by elongate subepithallial cells 5–12 × 3–5 µm (L × B) (4–18 × 2.5–6 µm according to Keats and Maneveldt Citation1997). Descending hypothallial filaments end in wedge-shaped cells (h). Cell fusions common (h). Trichocytes not seen in the present material (or reported), but see Keats and Maneveldt (Citation1997, figure 8). Secondary pit connections absent.
Gametophytes dioecious. Male conceptacles conical, with chambers 225–400 × 31–62 µm (D × H) (Keats and Maneveldt Citation1997, figure 6). Spermatangial structures simple (unbranched) occurring on the floor, the walls and the roof (i; Keats and Maneveldt Citation1997, figure 7).
Carpogonial conceptacles with chambers c. 150 × 50 µm (D × H; n: 1) (a). Carpogonial branches 2- or 3-celled, composed of a carpogonium, a hypogynous and a supporting cell – all cells staining similarly (b–c). A short cell tube was observed, connecting the base of a carpogonium with the subtending supporting cell (procarpy), and presumably transferring the zygote (c). Following presumed fertilization and zygote transfer, a fusion cell is formed incorporating at least six supporting cells and one hypogynous cell. Subtending basal cells remain connected to the vegetative floor and do not contribute to the radiating gonimoblast filaments. Neighbouring hypogynous cells develop a contact to the fusion cell, or to gonimoblast filaments but remain intact (d, e). A raised fertile zone is formed in fully developed carposporophytes with carposporangia produced from the periphery (f, g), but no ‘pedestal’ was seen in the embedded conceptacles (h). Carposporangial conceptacles, 450–630 × 160–300 µm (D × H), with chambers 340–440 × 135–200 µm (D × H; n:8) (315–380 × 135–165 µm according to Keats and Maneveldt Citation1997). Roof 70–160 µm thick (80–140 µm according to Keats and Maneveldt Citation1997), with a central ostiole 60–130 × 140–160 µm (D × H).
Multiporate (tetrasporangial) conceptacles, 470–700 × 100–200 µm (D × H), with chambers 220–480 × 150–220 µm (D × H; n: 10) (a) [conceptacles 430–550 µm (D) and chambers 225–400 × 115–190 µm (D × H) according to Keats and Maneveldt Citation1997]. Roof convex to flattened, 40–60 µm thick, composed of 5–7-celled filaments (roof 40–62 µm thick, composed of 5–8 cell filaments and perforated by 40–62 canals according to Keats and Maneveldt Citation1997). Canals ± straight, 5–12 µm in diameter, surrounded by 6–7 rosette cells (b–d) which terminate below the roof surface (e). Basal cells of pore filaments branched, supporting a normal roof filament and the lining filament itself that is composed of an elongate subbasal cell followed by two cells, lacking epithallial cell and terminating below the roof (e–h). A diminutive, 3-celled, filament was located at the canal base (i). Tetrasporangia 90–130 × 20–70 µm (L × B: n: 24) (60–170 × 20–100 µm according to Keats and Maneveldt Citation1997). Older conceptacles become embedded in the thallus (Keats and Maneveldt Citation1997, figure 4).
Habitat and distribution
Thalli attached to a geniculate coralline at 22 m depth, growing together with Amphithallia crassiuscula (Kütz.) Athanas., and also reported on dead horny corals, bryozoan skeletons, stony coral skeletons and sponges, between 9 and 14 m depth (Keats and Maneveldt Citation1997, p. 202). Known only from three localities in South Africa: Bird Island (east end of Algoa Bay, Eastern Cape; type locality), Cape Town, and Simonstown, Partridge point, Western Cape Province (Keats and Maneveldt Citation1997, p. 202).
Remarks
Present data indicate that Thallis capensis is an inconspicuous species growing on various marine animals (horny corals, bryozoans, sponges; Keats and Maneveldt Citation1997) and geniculate corallines. Keats and Maneveldt referred their material to Mesophyllum incisum (= Perithallis incisa – below), but following a study of the type, Athanasiadis (Citation1999, p. 249) concluded that the South African specimens belonged to a distinct species, differing in several characters, eight of which remain valid after the present re-evaluation. These include the lack of a predominantly coaxial hypothallium, and the development of an encrusting thallus (lacking regular unattached superimposed growth), smaller tetrasporangial conceptacles (470–700 µm vs 600–1000 µm in the type of M. incisum), smaller tetrasporangial chambers (220–480 × 150–220 µm vs 340–740 × 130–300 µm in the type), thinner multiporate roofs (40–62 µm vs 50–90 µm in the type) perforated by 26–46 pores (vs 40–75 in the type), shorter hypothallial cells (9–32 µm vs 15–49 µm in the type), and a thicker thallus (up to 700 µm vs 310 µm in the type). In addition, conceptacles become embedded in Thallis capensis (h; Keats and Maneveldt Citation1997, figure 4), a condition not reported in the two species of Perithallis (below). The present study confirms most characters described and/or illustrated by Keats and Maneveldt, and furthermore provides for the first time information of carpogonial and postfertilization stages. This supports recognition of Thallis capensis as a new species, possessing an independent triphasic life history and a combination of characters that sets it apart from all other genera of Mesophyllaceae (). Regarding the vegetative characters, Thallis capensis exhibits a strictly dorsiventral organization where descending hypothallial filaments end in wedge-shaped cells (h; Keats and Maneveldt Citation1997, figure 5). This is in line with most species and genera of Mesophyllaceae, but Synarthrophyton, Amphithallia and Carlskottsbergia, which develop a bilateral organization (see Athanasiadis Citation2018b, figure 60). An intermediate condition (aniso-bilateral) is here described in Perithallis, whose two species develop a diminutive ventral perithallium, 1–2 cells thick (below). As to the hypothallial growth, Keats and Maneveldt (Citation1997, p. 201) described ‘a plumose to coaxial ventral layer’ but their illustrations do not show a continuous coaxial formation beyond three cell rows (Keats and Maneveldt Citation1997, figures 4, 5, 9, 10), and the present study failed to locate any coaxial regions (other than patches of coaxial cells). The lack of trichocytes is also questioned, since Keats and Maneveldt’s (Citation1997, figure 8) SEM illustration suggests their rare existence. Sterile thalli of Thallis capensis can be easily distinguished from sympatric Mesophyllaceae, in having a dorsiventral organization (bilateral in Amphithallia), a non-coaxial hypothallium (coaxial in Phragmope), and lack of haustoria or a monostromatic hypothallium which characterize the hemiparasite Capensia fucorum. Young thalli of other South African Mesophyllaceae usually develop a strongly adherent thallus (i.e. species referred to Leptophytum or Synarthrophyton; see Chamberlain and Keats Citation1994, Maneveldt et al. Citation2007), or grow exclusively on certain hosts [e.g. Synarthrophyton eckloniae (Foslie) D.W. Keats & Y.M. Chamb.].
Table I. Comparative data between Thallis, Perithallis, Printziana, Sunesonia and related genera and species of Mesophyllaceae.
Male thalli of Thallis develop simple (unbranched) spermatangial structures, a character that is in line with most genera of Mesophyllaceae. Carpogonial thalli develop 2- or 3-celled carpogonial branches, which is also the standard condition in the Mesophyllaceae – Amphithallia displaying an exception possessing 4-celled carpogonial branches (Athanasiadis Citation2019, figure 4a–d). Following presumed fertilization, the fusion cell of Thallis incorporates at least six supporting and one hypogynous cells (e). This fusion cell is similar in extent to that illustrated in Leptophytum laeve (Foslie) W.H. Adey (Citation1966, figure 80) and Callilithophytum parcum (Setch. & Foslie) P.W. Gabrielson et al. (Lebednik Citation1977a, figures 9, 15, as Clathromorphum) from the Arctic and/or N. Pacific or N. Atlantic, but also in the Antarctic genus Carlskottsbergia (Athanasiadis Citation2018b, figures 19, 20) and in the here described new genus Printziana from Australia-New Zealand-Chatham (d). This fusion cell is definitely smaller than that documented in species of the northern hemisphere genus Mesophyllum, where at least 10 supporting and 10 hypogynous cells coalesce (Suneson Citation1937, figure 40, as Lithothamnion; Lebednik Citation1977b, figures 10, 12, 17; Athanasiadis Citation2017a, 5–7, 11). A smaller fusion cell than that in Thallis capensis, incorporating only a few supporting cells, has been reported in the Arctic Clathromorphum (Lebednik Citation1977a, figures 10, 13, 16), as also in the South African Phragmope (Athanasiadis Citation2020, figure 5e–h). Finally, lack of a fusion cell has been reported in the (hemi-)parasites Kvaleya (Woelkerling Citation1988, figure 186) and Capensia (Athanasiadis Citation2017b, p. 562, ‘non-continuous fusion cell’ = gonimoblast filament), and the epiphytes Amphithallia (Athanasiadis Citation2019, p. 38, figure 4e, f) and in the here described Perithallis (e–f, h). Present data support the view that the degree of development of a fusion cell is a putative synapomorphy for congeneric species, but the direction of evolution in the various genera of Mesophyllaceae is unknown. The lack of a ‘pedestal’ in embedded carposporangial conceptacles (Feature 3h) suggests that the raised fertile zone (f–h) should be considered as a parallelism, not homologous to the pedestal formation in species of Mesophyllum (). Nevertheless, a partial ‘pedestal’ was also observed in Perithallis incisa and Printziana australis (below) indicating that this condition is probably a synapomorphy for several genera from the southern hemisphere.
Multiporate conceptacles of Thallis capensis develop highly specialized pore filaments lining the canals. These filaments are 4-celled (adjacent roof filaments being 5–8 cells long), basally branched (supporting a normal filament in addition to the lining filament), and provided with an elongate subbasal cell that bears two more cells (terminating below the roof surface and lacking an epithallial cell). This complex organization was originally documented by Keats and Maneveldt (Citation1997, p. 204, figure 17), and it is here confirmed (e–h) and named Thallis-type (f). This character sets the genus apart from all Mesophyllaceae, except Perithallis (from southern Australia-New Zealand-Chatham) where a similar organization is here demonstrated, differring only in that pore filaments can be composed of 4 or 5 cells (below). The Thallis-type of pore filament is here recognized as an unmistakable synapomorphy, on account of which Thallis-Perithallis are here considered to be sister-taxa. The fact that they deviate in so many other characters suggests a long time isolation, placing their common ancestor at a time when South Africa, Australia and New Zealand were closely located so that dispersal of biota was feasible (see Discussion). Characters that have evolved in a different direction in these two allopatric sister-genera are discussed under Perithallis.
Genus Perithallis gen. nov.
Type species Perithallis incisa (Foslie) comb. nov.
Genus diagnosis
New genus of Mesophyllaceae (Corallinales), differing from Thallis (described herein) in possessing a ventral diminutive perithallium (aniso-bilateral thallus organization) and a predominantly coaxial hypothallium, and in lacking a prominent fusion cell in carposporangial conceptacles and embedded conceptacles. Thallis and Perithallis differ from other genera of Mesophyllaceae in developing basally branched pore filaments in canals of multiporate conceptacles, the filaments being 4–5-celled, provided with elongate sub-basal cells, lacking epithallial cells and terminating below the roof surface.
Etymology
The generic name is a new compound substantive, after the prefix peri (round) and the genus Thallis. It is pronounced Perithallís (genitive Perithalli-dos) and denotes to the development of a perithallium around the thallus (in contrast to Thallis).
Remarks
Perithallis here includes the generitype P. incisa and P. chathamensis (Foslie) comb. nov. These two species display a new thallus organization, the aniso-bilateral, intermediate between the strictly dorsiventral (e.g. in Thallis) and the bilateral (e.g. in Synarthrophyton) – see diagrammatic illustrations in Athanasiadis (Citation2018b, figure 60). This new thallus organization displays a limited (1–2 cells thick) ventral perithallium (f–g, 11i, j) that may also support ventral trichocytes (as further evidence of its existence). This condition most likely evolved due to a partly unattached thallus growth, that allows descending hypothallial filaments to develop meristematic activity, when in most other Mesophyllaceae (with dorsiventral organization) descending hypothallial filaments end in wedge-shaped cells facing the substratum. It should be added though that this new organization shows facultative development in the type species of Perithallis (that may also possess a strictly dorsiventral thallus). Ventral epithallial cells (but not a ventral perithallium) have also been recorded in Mesophyllum lichenoides (Suneson Citation1937, figure 37, as Lithothamnion; Woelkerling and Irvine Citation1986, figure 16) and in M. conchatum (unpubl. observ.) – the latter two species also exhibiting a partly unattached growth on geniculate corallines. Perithallis displays the pore filament structure of multiporate conceptacles of Thallis (f). This complex, multicellular organization is here recognized as an unmistakable synapomorphy for the two genera that, on account of their disjunctive present distribution, must share a common ancestor dating long back in time when South Africa, Australia and New Zealand were closely located (and dispersal of biota feasible). Characters that evolved in a different direction in members of these two genera since their separation are several, which also provides evidence of a long time isolation. In terms of vegetative structure, Perithallis demonstrates a predominantly coaxial hypothallium with aniso-bilateral organization, while the thallus organization of Thallis is strictly dorsiventral displaying coaxial hypothallial patches. Trichocytes, which are common in Perithallis, have not been recorded with certainty in Thallis, and embedded conceptacles have only been recorded in the latter genus. Reproductively, Perithallis incisa lacks a fusion cell, which is present in Thallis capensis. As discussed under Thallis, the lack of a fusion cell also characterizes the hemiparasites Kvaleya and Capensia (from the Arctic respectively South Africa), and the epiphyte Amphithallia from South Africa. These four taxa, however, do not share any other apparent synapomorphy, which suggests that the lack of a fusion cell either occurred several times (as a loss), or that it represents a plesiomorphic condition retained in the above four genera. A closer comparison between Thallis, Perithallis and related genera of Mesophyllaceae appears in .
Perithallis incisa (Foslie) comb. nov.
()
Figure 5. Perithallis incisa gen. & comb. nov. Original material in TRH (B17-2551, B17-2552, B17-2553); a, the lectotype collection (B17-2551) that includes materials in a box (A) holding a Setchell label (B), remains of the collection in small fragments (C), two Foslie slides (n°1175; D, E), and a smaller box (F) that includes the here selected lectotype specimen (G); b, the here selected lectotype, a conglomerate including two thalli with multiporate conceptacles (top) and gametangial thalli underneath, mostly carposporophytes (arrowhead); c, thalli with multiporate and a few uniporate (male; arrowhead) conceptacles (syntype B17-2552); d, thallus belonging to a different species provided with smaller carpogonial-carposporangial conceptacles (syntype B17-2553); e, side view of a broken unattached thallus of the lectotype showing coaxial hypothallial growth; f, view of the underside of a carposporangial thallus, showing zonate texture (lectotype); g, view of the underside of a male thallus, showing smooth texture (lectotype).

Figure 6. Perithallis incisa gen. & comb. nov. Vegetative structures; a, section at the margin showing a non-coaxial patch (black arrow) between two coaxial ones (white arrows) (iso-lectotype); b, section of a carposporophyte with predominantly coaxial growth (iso-lectotype); c, surface view of a decalcified lamella showing a striated thallus (due to coaxial hypothallial growth) (syntype, Setchell n°6354 in TRH, B17-2551); d, thallus section showing a core of up to four cell layers of hypothallial filaments running parallel to the substratum, supporting ascending perithallial (p) and descending (d) hypothallial filaments (LTB 14763). Abbreviations: p (perithallial filaments), d (descending hypothallial filaments).
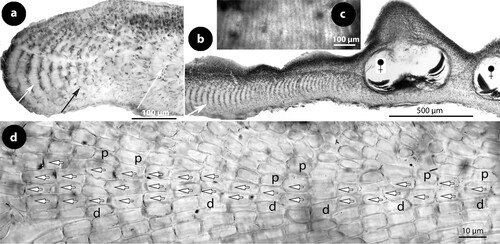
Figure 7. Perithallis incisa gen. & comb. nov. Vegetative structures; a, terminal meristematic cells (arrows) protected by a cuticle (c) undergoing synchronous anticlinal divisions (LTB 14763); b, section at the margin showing terminal meristematic cells (white arrows) progressively displaced dorsally to become epithallial cells (arrowheads), at the end of the protective cuticle (c). Note the subdichotomous hypothallial divisions (black arrows) that cause the thallus vertical expansion (syntype B17-2551); c, section at the dorsal surface showing elongate subepithallial cells (white arrows) and a layer of flattened epithallial cells (arrowheads) (LTB 14763); d, terminal meristematic cells (arrow) progressively displaced ventrally to become epithallial cells (arrowheads) (syntype, B17-2551); e, section at the ventral side showing descending (d) hypothallial filaments ending in wedge-shaped cells (arrows) (iso-lectotype); f, section at the ventral side of a tetrasporangial specimen, showing descending filaments (d) terminating into perithallial cells (p) supporting single flattened epithallial cells (arrowheads). Note the trichocyte (tr) and the distinctive angle between perithallial (p) and descending (d) hypothallial cells (LTB 14763); g, section at the ventral side showing descending filaments (d) to support one layer of perithallial cells (arrows) with terminal epithallial cells (arrowheads) (iso-lectotype); h–i, sections at the surface showing flattened epithallial cells (black arrowheads), an elongate subepithallial cell (arrow), a trichocyte (tr), and the presence of two epithallial cells in sequence (white arrowheads) (LTB 14763). Abreviations: c (cuticle), d (descending hypothallial filament), p (perithallial cell), tr (trichocyte).
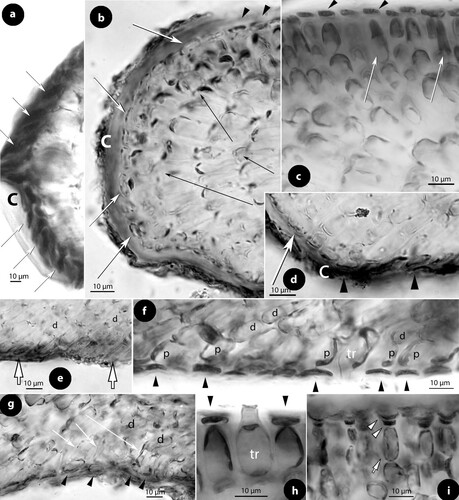
Figure 8. Perithallis incisa gen. & comb. nov. Male, carpogonial and postfertilization structures (LTB 14763); a, section at the chamber floor of a mature male conceptacle, showing lunate SMCs (arrows) cutting off spermatangia (arrowheads) that release roundish spermatia; b, section at the chamber floor of an old male conceptacle, showing remains of SMCs (arrows) supporting elongate or inflated, empty spermatangia (arrowheads); c–d, carposporangial conceptacles showing peripheral development of carpospores (arrows) from a centrally raised (black arrowhead) or nearly flattened (white arrowhead) fertile zone; e, section at the carposporangial floor showing peripheral production of carposporangia (c) from a gonimoblast filament (black arrow), and the presence of gonimoblast filaments (white arrows). Note the remains of carpogonia (ca) and hypogynous cells (h), which remain intact in contrast to basal (b) cells that are incorporated in the gonimoblast filaments; f–g, section at the carposporangial floor showing remains of carpogonia (ca) and hypogynous cells (h) that remain intact. Gonimoblast filaments (long white arrows) form a chain of smaller fusions cells, each including one or two supporting cells and their basal cells. Carposporangia (c) are produced from the periphery (black arrow). Gonimoblast filaments bend down (black arrow) from an apparently raised fertile zone. The connection (normal pit-plug?) between a hypogynous cell and the gonimoblast filament (broken line) is magnified in figure g; h, view of the carposporangial floor from above, at the level of carposporangia (c), showing their peripheral production and the apparent lack of a fusion cell. Abbreviations: b (basal cell), c (carposporangium), ca (carpogonium), h (hypogynous cell).
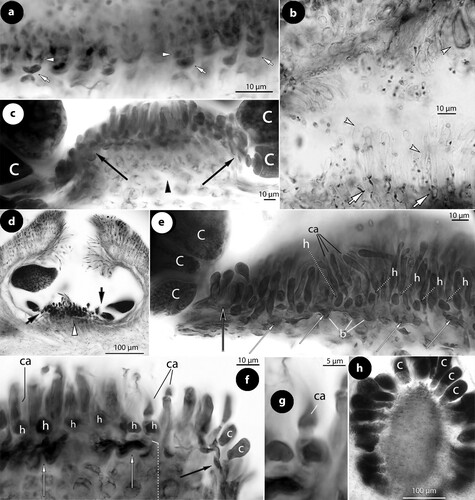
Figure 9. Perithallis incisa gen. & comb. nov. Multiporate conceptacle structures; a–b, sections showing two thalli (arrows) growing back-to-back, one provided with a multiporate conceptacle. The presence of ventral epithallial cells in the upper thallus is magnified in figure b (arrowhead) (Foslie slide n°1175 ‘Tegning’, syntype B17-2551); c, two tetrasporangia (LTB 14763); d–e, surface views of pore canals of a multiporate roof, surrounded by 11 respectively nine rosette (r) cells (syntype B17-2551); f–i, sections of canals of multiporate roofs, showing pore filaments lining the canals. Basal cells (white long arrows) are branched supporting a normal roof filament (long black arrows) and the rest of the lining filament that includes an elongate subbasal cell (white arrows) and two or three top cells (white arrowheads) lacking epithallial cells (that occur in adjacent roof filaments; black arrowheads) and terminating below the roof surface (f: Woelkerling and Harvey Citation1993, figure 14D ‘Lectotype collection’, reproduced with modifications; g: syntype B17-2551; h–i: LTB 14763). Abbreviations: r (rosette cell).
Basionym
Lithothamnion patena f. incisum Foslie (Citation1906a, p. 6, ‘incisa’).
Homotypic synonyms
Lithothamnion incisum (Foslie) Foslie (Citation1907b, p. 12),
Mesophyllum incisum (Foslie) W.H. Adey (Citation1970, p. 24).
Polyporolithon patena var. incisum (Foslie) Chapman and Parkinson (Citation1974, p. 202, ‘incisa’).
Type locality
Island Bay, near Wellington (North Island, New Zealand); upper sublittoral zone, on Corallina and Amphiroa.
Lectotype
A conglomerate of at least three thalli in superimposition, in the box annotated ‘L. incisum Cyst. Konc … . n° 6354 Foto nr. 67B’ (TRH B17-2551, in part), June 1904, coll. W.A. Setchell n°6354, annotated ‘On Corallina and Amphiroa’; selected and illustrated herein (b, e–g).
Iso-lectotypes
Slides in herb.Athanas. (a–b, 7e, g)
Syntypes
TRH (B17-2551, in part), June 1904, coll. W.A.Setchell n°6354 (c, 7b, d, 9a–b, d–e, g); TRH (B17-2552), June 1904, coll. W.A. Setchell n°6353 (c); TRH (B17-2553) a mixture of Setchell n°6353 & 6354 (d); TRH (B17-5150); TRH (B17-2543).
Material examined
Australia: Victoria: Otway Plain: Port Fairy, 2nd breakwater W of Port Fairy (38°22′S, 142°15′E): MEL 2263459 (LTB 14763): on Haliptilon, 3.5 m depth, 18 March 1985, coll. D. May n°14763; annotated ‘ … There are 75 … slides with this collection. Spirit 10998B … ’, ‘M. erubescens’, ‘Syn. M. incisum’ [tetrasporangial and gametangial thalli on Haliptilon (Decne) Lindl.; collection cited under M. incisum in Woelkerling and Harvey (Citation1992, p. 384; Citation1993, p. 199)].
New Zealand: North Island: Island Bay, near Wellington: TRH (B17-2551): lectotype and syntypes, data as above (male, carposporangial and tetrasporangial thalli); TRH (B17-2552): syntypes, data as above (multiporate and uniporate thalli); TRH (B17-2553): syntypes, data as above (heterogeneous collection with carposporangial thalli).
Historical background and lectotypification
Perithallis incisa was originally described as a form of Lithothamnion patena Hook.fil. & Harv., a species later selected as the generitype of the genus Synarthrophyton (Townsend Citation1979). Foslie’s (Citation1906a) protologue reads in the most essential parts (in translation from the Norwegian): ‘Lithothamnion Patena (Hook.fil. & Harv.) Heydr. Melob. in Ber.d.deutsch.Bot. Gesellsch.1897, p. 413; Melobesia Patena Hook. f. & Harv. Ner. austr. (1847) p. 111.
f. incisa Fosl. mscr. Thallus generally smaller than in the typical form, 1–1.5 cm in diameter, with the underside loosely attached to the host, usually vaguely proliferated and more or less incised with undulate margin; [multiporate] sporangial conceptacles 500 (400)–800 (900) µm in diameter.
… The form incisa differs from the main form, apart from the above mentioned characters, by its smaller conceptacles and the somewhat smaller hypothallial cells. It occurs on Corallina, Amphiroa and Lenormandia. This form is found in Island Bay, near Wellington, New Zealand (Setchell, nr. 6353 and 6354). In addition, a small crust occurs amongst Lithoth. cystocarpideum from the Chatham Islands.’
Of the three cited collections, Adey and Lebednik (Citation1967, p. 68) selected the Setchell collections (n°6353 & 6354) as type, and subsequently Woelkerling and Harvey (Citation1992, p. 382, figure 1) restricted ‘ … no 6354 () … as lectotype of the species. This collection contains gametangial and tetrasporangial plants mixed with fragmented bits of the host … All morphological and anatomical features evident in the lectotype also are evident in southern Australian material.’ Yet, Woelkerling and Harvey’s (Citation1992) illustration of the lectotype ‘collection’ shows no ‘gametangial and tetrasporangial plants’ but a mixture of fragments (beyond identification), together with the box (holding the material), two Foslie slides (both labelled n°1175), and Setchell’s original label, while a smaller box and its content (included in Setchell n°6354; F–G) is missing. A year later, Woelkerling (Citation1993, p. 123) specified that the ‘lectotype’ was illustrated by Printz (Citation1929, plate 10 figures 10–13). Nevertheless, Printz (l.c.) illustrated four specimens without indicating a Setchell number, and it is not possible to match Printz's illustrations with the remains of the original material. In the same year, Woelkerling and Harvey (Citation1993, p. 618, figures 12–14) published a series of illustrations of fragments from the ‘Lectotype collection in TRH’ without selecting a particular specimen as lectotype, and we have to conclude that even this act is an invalid typification – since no particular specimen was selected (Art. 9.2; Turland et al. Citation2018).
The original material (in TRH) was re-examined by Athanasiadis (Citation1999, p. 247), who noted that the relevant Setchell collections were placed in three larger and four smaller boxes that also included four Foslie slides. The three larger boxes were annotated: ‘6353 og 6354 … ’, ‘6353 … ’, respectively ‘6354, North Isl., New Zealand, Island Bay, Setchell 1904 juni, Lith. Patena f. incisa … Prep. 1175’. The four smaller boxes were annotated: ‘ … 6353, 6354 … [with gametangial specimens, to 1 cm in greatest extent]’, ‘ … 6353 … [a tetrasporic specimen, ca. 1, 2 cm in diameter]’, ‘ … 6354 … [a gametangial specimen]’, respectively ‘L. incisum anth.? Konc … ’ – the last box lacking a Setchell number. Of the four slides two were annotated ‘ … Setchell 1904 nr 6354’ and the other two ‘ … Setchell 1904 nr 6353.’
The re-arrangement of Foslie's herbarium in TRH (Woelkerling et al. Citation2005, p. 342) resulted in placing the above collections (as cited by Athanasiadis Citation1999) in three new boxes: (1) B17-2551 (including all elements of the ‘Lectotype collection’ Setchell n°6354), (2) B17-2552 (including all elements of Setchell n°6353), and (3) B17-2553 (including the mixture of Setchell n°6353 and 6354, and the single smaller box lacking collection number).
The entire material of the ‘Lectotype collection’ (Setchell n°6354; B17-2551) is here illustrated (a). The only element that qualifies to be a specimen (identifiable for comparative studies) is indicated (a–G, b) and is here selected as the lectotype. The lectotype is missing in Woelkerling and Harvey’s (Citation1992, figure 1; 1993, figure 12A) illustrations. The largest multiporate specimen in Setchell's collection n°6353 (B17-2552) bears also a minor thallus with uniporate conceptacles on its side (c). A carposporangial specimen (d) from the mixture of Setchell's n°6353 & 6354 (B17-2553) apparently belongs to a different taxon (below).
The two other collections cited in the protologue (Foslie Citation1906a), i.e. thalli on Lenormandia, and a small crust amongst Lithothamnion cystocarpideum Foslie from the Chatham Islands, have also been located in TRH – the former filed under B17-5150 (Setchell n°6052a; Woelkerling et al. Citation2005, p. 342) and the latter under B17-2543 (Woelkerling et al. Citation2005, p. 341). These two syntype collections have not been examined in the present investigation.
The study of the lectotype showed that it is a conglomerate of several individuals. The top two thalli bear multiporate conceptacles, while the lower thalli bear either a few male or plenty of carposporangial conceptacles (b). It is here assumed that all thalli belong to the same species (judging from size of conceptacles that is in agreement with those of the here recognized species). A fragment with carposporangial conceptacles from the lectotype was sectioned (a, b), and its structure was found to be in agreement with other thalli and fragments in B17-2551 and B17-2552, the illustrations published by Woelkerling and Harvey (Citation1993, figures 12–14), and several other thalli of a recent collection (LTB 14763) from Victoria (southern Australia). The material in B17-2553 includes three gametangial thalli placed in the smaller box ‘Anth.? Konc … # 6353 6354 … ’ and one gametangial thallus placed in the box ‘L. incisum anth.? Konc … ’. The study of a thallus from the former box (‘Anth.? Konc … # 6353 6354 … ’), showed that it is a carposporophyte with non-coaxial hypothallium and densely grouped conceptacles (d), distinctively smaller than those of the lectotype, and therefore this syntype is here considered to represent a different species (with uncertain generic assignment).
Description
Thalli irregularly lamellate to orbicular, to 1.2 cm in diameter and 100–310 µm thick (excluding conceptacles), with roundish margins and superimposed unattached growth (a–c). Thallus surface glossy. Underside either striated (reflecting a coaxial hypothallial growth; f) or glossy (reflecting the existence of epithallial cells; g). A coaxial hypothallial growth is visible in broken lamellae (e) or in views of decalcified lamellae from above (c). Thallus organization dorsiventral to aniso-bilateral. Individual lamellae composed of a predominantly coaxial, arching hypothallium 80–200 µm thick, supporting an ascending perithallium to 50 µm thick and descending hypothallial filaments (a, b, d). Patches of non-coaxial, hypothallial cells (up to ten rows in sequence) may occur (a). A core of up to four cell layers of hypothallial filaments runs parallel to the substratum, supporting ascending (perithallial) and descending (hypothallial) filaments (d). Hypothallial cells 10–49 × 8–12 µm (L × B) and perithallial cells 5–25 × 3–5 µm (L × B). Hypothallial filaments grow via terminal meristematic cells that undergo anticlinal, synchronous divisions and elongations, protected by a cuticle and contributing to the thallus elongation (a, b). Subdichotomous divisions (b) add to the thallus thickness, and gradually displace hypothallial filaments dorsally or ventrally. Displaced terminal meristematic cells become epithallial cells, either dorsally (b), or ventrally (d). Descending hypothallial filaments end in wedge-shaped cells (e), or produce a ventral perithallium, one or two (?) cells thick, that supports epithallial cells and occasionally trichocytes (f, g). Both dorsal and ventral perithallial filaments grow by subepithallial divisions. Dorsal subepithallial meristematic cells attain a length of 20 µm, being distinctively longer than subtending cells during division (c), while ventral subepithallial meristematic cells are distinguished by their short length and angle to the descending hypothallial filaments (f, g). Dorsal and ventral epithallial cells differ in size or shape, the former being usually flattened (to isodiametric or concave) 1–4 × 4–8 µm (L × B) (c), and the latter usually concave to 15 µm in diameter (f, g). Terminal trichocytes, up to 20 × 10 µm (L × B) occur singly, amongst epithallial cells, both on the dorsal (h) and ventral side (f). Dorsal perithallial filaments attain a length of 50 µm and eventually produce conceptacles, whereas the ventral perithallium remains limited and may be entirely lacking (being replaced by wedge-shaped terminal hypothallial cells; e). Dorsal subepithallial cells may support one or two epithallial cells (i). Back-to-back growth was observed in the Foslie slide (n°1175 ‘Tegnig’; a) and also in one of the specimens in B17-2553. The two lamellae (clearly dissimilar in size) grew in the same direction, attached ventrally without merging into a single thallus (cf. P. chathamensis) and with one layer of epithallial cells between (the epithallial cells ending descending hypothallial filaments of the upper lamella; b).
Gametophytes dioecious. The ontogeny of male conceptacles has been described and illustrated by Woelkerling and Harvey (Citation1992, figures 18–24). Male conceptacles 450–530 × 100–150 µm (D × H) with chambers 160–350 × 50–80 µm (D × H; n: 4) (chambers 190–420 µm in diameter according to Woelkerling and Harvey Citation1992, p. 382). Roof 35 to 140 µm thick with a central ostiole 45–70 × 65–140 µm (D × H; n: 4). Spermatangial structures simple (unbranched) with lunate SMCs occurring on the floor (a), the walls and the roof. Older structures show elongate or inflated cell walls of spermatangia attached to elongate SMCs (b). Carpogonial conceptacles have been described and illustrated by Woelkerling and Harvey (Citation1992, figures 25–27). Carpogonial branches 2-celled, composed of the carpogonium and a hypogynous cells. Supporting cells of carpogonial branches make a part of the chamber wall, when they do not support carpogonial branches (Woelkerling and Harvey Citation1992, figure 27), and hence do not belong to the carpogonial branch. The presence of a sterile cell attached to the hypogynous cell has been documented (Woelkerling and Harvey Citation1992, figure 27, ‘second undeveloped carpogonium’).
Carposporangial conceptacles 430–650 × 180–270 µm (D × H) with chambers 270–470 × 130–200 µm (D × H) and a central ostiole 50–100 × 130–170 µm (D × H; n: 5). The fertile zone is flattened (d) or raised (c, f). Following presumed fertilization and zygote transfer (not seen), no fusion cell is formed and gonimoblast filaments establish a sequence of minor fusions at the level of the supporting cells (e, f). These small fusions are laterally connected, forming a chain, each fusion incorporating 2–3 supporting cells and the underlying basal cell(s), while carpogonia and hypogynous cells remain intact (e–g). In those chambers having a distinctively raised fertile zone, gonimoblast filaments bend down to fill the gap (f). Carposporangia develop from the periphery of the fertile zone (h).
Multiporate (tetrasporangial) conceptacles (c and a), 600–1000 × 200–260 µm (D × H) with chambers 580–740 × 250–300 µm (D × H; n: 2) (chambers 340–655 × 130–265 µm according to Woelkerling and Harvey Citation1992, p. 382). Tetrasporangia (c) 110–170 × 35–90 µm (L × B; n: 5) (130–220 × 55–130 µm according to Woelkerling and Harvey Citation1992, p. 382). Roof 50–60 µm thick, composed of 5–6-celled filaments, and perforated by up to 75 pores. Canals ± straight, 10–12 µm in diameter at the apical opening, surrounded by 9–11 rosette cells which are sunken and thinner than adjacent epithallial cells (d, e, h). Pore filaments lining the canals 4- or 5-celled lacking epithallial cells, and composed of branched basal cells, each supporting a normal roof filament and the pore filament lining the canal. Subbasal cells lining the canal are distinctively elongate, supporting two or three cells (f–i). Embedded gametangial or tetrasporangial conceptacles do not occur in the thallus.
Habitat and distribution
The species is reported to be a common epiphyte on geniculate corallines such as Haliptilon (Decne) Lindley, Corallina L., Amphiroa J.V. Lamour., and also on Lenormandia Sond. (Rhodomelaceae, Ceramiales). Mesophyllum incisum has been widely reported from southern, western and eastern Australia, including Tasmania, and also from New Zealand (North Island), Auckland, Snares & Chatham Islands (being occasionally merged within the broad concept of ‘Mesophyllum erubescens’); but here confirmed only from Victoria and the type locality (Island Bay, near Wellington). A record from South Africa (Keats and Maneveldt Citation1997) is here referred to Thallis capensis.
Remarks
The broad concept of Mesophyllum incisum held by Woelkerling and Harvey (Citation1992, pp. 381–399, figures 1–36; Citation1993, pp. 587–590, figures 12–16) and Woelkerling (Citation1996, p. 197, figures 84–85) was criticized by Athanasiadis (Citation1999, p. 247). In particular, our Australian colleagues concluded that M. incisum encompass ‘encrusting to discoid, layered, foliose, warty or lumpy’ thalli (Woelkerling Citation1996, p. 197), and this polymorphism was coupled with considerable variation of anatomical features including a coaxial to non-coaxial hypothallium and embedded conceptacles or not. The concept of the species was further expanded in a later study of eastern Australian populations, where Harvey et al. (Citation2003, p. 664) recognized M. incisum to be a synonym of Mesophyllum erubescens (Foslie) W.H. Adey – a species originally described from Brazil and more recently referred to the new genus Melyvonnea, a genus not recorded in Australia-New Zealand (Athanasiadis and Ballantine Citation2014, figure 2; Jesionek et al. Citation2016).
The present study shows that the concept of Perithallis incisa should be limited to the here emended description that accommodates all three phases in the life history of the species. This was also supported by the molecular study of Broom et al. (Citation2008, p. 971, figures 1, 5) who showed that ‘Mesophyllum erubescens’ sensu Harvey et al. (Citation2003, Citation2005) is a group of genera with sequence divergence up to 60 nucleotide substitutions. An isolate from the type locality of Perithallis incisa was also included in the study of Broom et al. [Citation2008, table 1, WELT A026956-DQ167884-EF628223, ‘on geniculate coralline’ (Harvey et al. Citation2005, p. 111)], but given that the original material was here found to be heterogeneous, the identity of this isolate is uncertain (albeit flagged ‘Gen.nov.sp.’ in WELT – data online) and is here referred to the species with question mark (Figure 18a–d; see also P. chathamensis).
Two other collections growing on rock and referred to Mesophyllum incisum by Woelkerling and Harvey (Citation1993, p. 588, LTB 14744 & LTB 1353) are here shown to belong to Sunesonia pseuderubescens gen. & sp. nov. (below). Similarly, the here illustrated syntype (d; B17-2553) is excluded, because it lacks a coaxial hypothallium and possesses smaller, densely aggregated carposporangial conceptacles. Although the identity of the latter specimen remains unknown, it clearly indicates that several different taxa share the habitat preference of Perithallis incisa on geniculate corallines.
The report of ‘one or possibly several independent fusion cells … (figure 28, 29)’ by Woelkerling and Harvey (Citation1992, p. 393, as M. incisum) or ‘an apparently discontinuous fusion cell’ (Woelkerling and Harvey Citation1992, figure 31, as M. incisum) was not confirmed – although the relevant collection LTB 14763 was re-examined. No fusion cell was detected in the present study, that instead demonstrated the presence of a sequence of small fusions during the development of gonimoblast filaments (each fusion involving one or two supporting cells and the underlying basal cells; e, f). The lack of a fusion cell, as demonstrated in both transverse sections (e, f) and in surface view (h), should be compared with the postfertilization stages in Mesophyllum, where similar illustrations have shown the presence of a distinctive fusion cell incorporating up to 10 hypogynous and 10 supporting cells (Suneson Citation1937, figure 40, as Lithothamnion; Lebednik Citation1977b, figures 10, 12, 17; Athanasiadis Citation2018a, figures 5–7, 9–11; ). The occasional development of a raised fertile zone in carposporangial conceptacles (b, c, d, f) indicates variability in the expression of this character that should not be considered homologous to the pedestal formation in species of Mesophyllum (). Nevertheless, a similar variability was observed in Thallis capensis and Printziana australis (below), indicating that this character is probably a synapomorphy for these three southern hemisphere genera. Perithallis presently accommodates one more species with disparate habit and habitat, restricted to the Chatham Islands.
Perithallis chathamensis (Foslie) comb. nov.
()
Figure 10. Perithallis chathamensis (Foslie) comb. nov. The original material in TRH (B18-2594) that comprises materials placed in a box (a) and including: four paper sheets (b, c, d, e), three slides (f, g, h), two smaller boxes (i, j) with minute algal fragments, and a single large specimen (a), the here selected lectotype (scale bars: a–j: 2 cm; k: 1 cm). Two magnifications of the lectotype (l, m) show multiporate conceptacles (arrows).

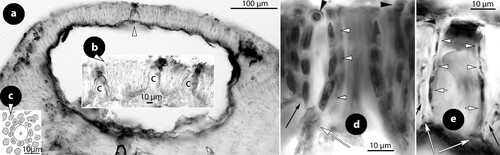
Figure 11. Perithallis chathamensis (Foslie) comb. nov. Vegetative structures; a, section showing a predominantly coaxial hypothallium (short white arrow) supporting ascending perithallial filaments (black arrow) and descending hypothallial filaments (long white arrow). Note the multiporate conceptacle with slightly sunken roof (arrowhead) (NZC0745); b, view of a decalcified thallus from above showing the predominantly coaxial hypothallium (NZC0745); c, section at the margin showing terminal synchronous, anticlinal divisions (black arrows) below the cuticle (white arrows) (NZC0745); d, section at the surface where terminal meristematic cells (arrows) become epithallial cells (arrowheads). The cuticle (white arrows) ceases to exist (protect) the meristematic cells at the transition zone (NZC0745); e, section showing coaxial hypothallial growth (arrow) (iso-lectotype); f, section of two lamellae (arrows) growing back-to-back (iso-lectotype); g, conglomerate of several lamellae (arrows) anastomosing. Not clear if all lamellae belong to the same individual/species (Foslie slide n°302, B18-2594); h, section showing two thalli growing back-to-back (arrows) and gradually becoming confluent (NZC0745); i–j, sections at the ventral side showing isodiametric to flattened epithallial cells (arrowheads) terminating 1- or 2-celled perithallial (p) filaments. Note the trichocytes (tr) and the angle between descending hypothallial (h) and perithallial (p) cells (i: iso-lectotype; j: NZC0745); k–m, sections at the surface showing epithallial cells (arrowheads) and trichocytes (tr). Note that subepithallial cells are longer than cells below (k: iso-lectotype; l–m: NZC0745).
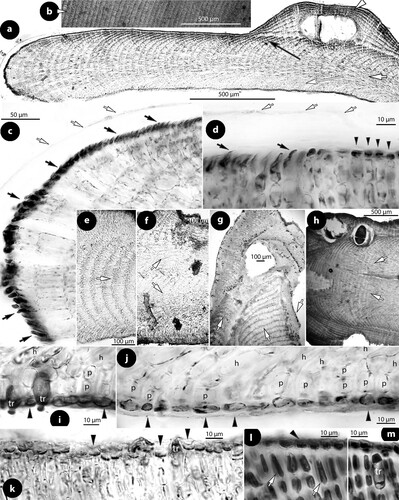
Figure 12. Perithallis chathamensis (Foslie) comb. nov. Multiporate conceptacle structures; a–b, sections of multiporate conceptacles showing the roof with one canal (arrowhead) and a second roof (Figure b) with three canals (c) (iso-lectotype); c, surface view of a canal showing nine rosette (r) cells surrounding the pore (p) (iso-lectotype; Keats and Chamberlain Citation1997, figure 10, redrawn with modifications); d–e, sections of two canals showing 4-celled lining filaments, with branched basal cells (long white arrows) supporting a roof filament (black arrows), and the rest of the lining filament that is composed of elongate subbasal cells (white arrows), and two more cells (arrowheads) that lack epithallial cells ending below the conceptacle surface. Note that adjacent roof filaments are provided with epithallial cells (black arrowheads) (d: NZC0745; e: iso-lectotype). Abbreviations: c (canal), r (rosette cell).
Basionym
Lithothamnion chathamense Foslie (Citation1906b, p. 18, reprint p. 2, ‘chatamense’).
Homotypic synonym
Mesophyllum chathamense (Foslie) W.H. Adey (Citation1970, p. 23, ‘chatamense’).
Type locality
Chatham Islands.
Lectotype
TRH, B18-2594, in part, including slides n°301 & 302, coll. Schauinsland, no date; illustrated by Printz (Citation1929, plate 9 figure 10); designated herein (k–m).
Iso-lectotypes
Slides in herb.Athanas. (d–e, i–k; a–c, e).
Syntypes
In TRH, B18-2594, ‘Reinbold nr. C.’, including slide n°546.
Material examined
Chatham Islands: TRH (B18-2594): lectotype (data as described above; locality not specified).
Point Durham, north side of point (44°0′.00″S–176°40′.50″E): WELT (A027264/A-NZC0745-DQ167929-EF628222): annotated ‘24 February 2004’, coll. ‘PD2. W.A. Nelson, T. Farr & K. Neill’ [includes 2 slides annotated ‘multi tetrasp. mounded, long cells lining pore … ’, ‘Mesophyllum erubescens’].
Observations on the protologue, original material, and lectotypification
Foslie (Citation1900, p. 14) originally referred a Schauinsland collection from Chatham Islands to Lithothamnion lichenoides f. heterophyllum Foslie (Citation1900, p. 13, ‘heterophylla’), considering L. agariciforme f. decussatum (J.Ellis & Sol.) Foslie (Citation1897, p. 5) to be a synonym and rendering f. heterophyllum a superfluous (illegitimate) name (Woelkerling Citation1993, p. 117). Foslie’s (Citation1900) conclusion was based on the following observations: ‘A specimen that I have seen from the Chatam islands1) [sic] is to be referred to the same form. It is nearly hemispherical, about 6 cm in diameter by a thickness of about 3 cm. in the thickest part. The lamels [sic] are smaller than in Mediterranean specimens [i.e. L. agariciforme f. decussatum] but of about the same thickness, partly rather depressed and irregularly formed over each other partly with more or less cup-shaped or cupulate [sic], now nearly free now here and there anastomosing lamels. This specimen fully coincides in structure with Mediterranean ones, and also as regards the conceptacles of sporangia, except that the latter occasionally are slightly smaller. …
1)Cp. Reinbold, Ergebnisse einer Reise nach dem Pacific (Prof. Dr. Schauinsland 1896–1897). Meeresalgen. – Abh. Nat. Ver. Bremen 1899, Bd.XVI.Pag.300.’
A few years later Foslie recognized the same material as a new species, Lithothamnion chat[h]amense Foslie (Citation1906b, p. 18), commenting [in translation from the Norwegian] that ‘This alga was described in New or crit. Calc. Alg. (1899), p. 14 [1900, p. 14] as somehow deviating from Lithoth. lichenoides f. heterophylla. It has though to be considered as a separate species, although slightly different. It looks like L. lichenoides in structure, but the hypothallial cells are partly little smaller. The conceptacles are 400–700 µm in diameter.- Chatam [sic] Islands (Schauinsland).’
The original material of Lithothamnion chathamense exists in a single box (a) annotated on the lid: ‘Prep. 301–302 og 546’, ‘L. chatamense’ ‘Chatam-öarna’ ‘leg. Dr. Schauinsland’ ‘comm. Reinbold /12 1898 B’. ‘Lithoth.Monogr.pl.9, figure 10’. and on the back: ‘Prep. 301–302 og 546’, ‘L. lichenoides f. heterophylla chatamense’ ‘Chatam-öarna’ ‘leg. Dr. Schauinsland’ ‘comm. Reinbold /12 1898 B’. The box includes: two smaller boxes annotated ‘Lith. chatamense Sp. konc. Foto nr. 79A’. respectively ‘Prep. 546 Lith. chatam. paa [on] Lithoph. carpophylli … ’ (i–j), four paper sheets (b–e), three slides (f–h), and one specimen matching the size described by Foslie (Citation1900) (k). Of the four paper sheets, the 1st reads: ‘Prep. 301 Chatamöern Perith. 13 × 7 11 × 7 14 × 7 11 × 6 Hypoth.43 × 11 29 × 11 36 × 9 25 × 7 22 × 7 Skorpens tykkelse [Crust thickness] 400–600. 700 µ’ (b), the 2nd: ‘Prep. 302 Chatamöerne Perith. 13 × 7 11 × 6 11 × 7 14 × 7 16 × 7 11 × 8 14 × 6 (9 × 7) Hypth. 36 × 6 48 × 11 29 × 7 47 × 11 22 × 7 25 × 7’ (c), the 3rd: ‘L. agaricif.[orme] f. decussata Dersom ej strukt. forskj’. [… if not in structure different], ‘A.B.C.’, ‘Chatam-öerne leg. Schauinsland comm. Reinbold /12 1898. 40–50 kanaler konc. ca. 5–600 µ B.’, ‘Prep. Anderss. 301–302’ (d), and the 4th: ‘400–700 µ 40–50 µm’ (e).
Of the three slides, those numbered ‘301’ and ‘302’ (the only numbers noted on the paper sheets) are further annotated: ‘L. chataemense’ ‘Chatamöerne leg. Schauinsland comm. Reinbold 301’ and ‘L. chataemense’ ‘302 Chatamöerne leg. Schauinsland comm. Reinbold 302 Teqning!’ [drawing]. These annotations link the material to Foslie's original identification as ‘Lithothamnion lichenoides f. heterophylla’ (Foslie Citation1900, pp. 13–14). The 3rd slide is identified as ‘Lithophyllum carpophylli Heydr. og Lithoth’. ‘546. Chatam-öerne leg. Schauinsland comm. Reinbold nr. C.’., and is apparently the product of material kept in one of the smaller boxes (‘Prep. 546 Lith. chatam. paa Lithoph. Carpophylli … ’). The annotation on this slide ‘Reinbold nr. C.’ indicates that it is a subsample of another collection, the largest part existing under the material referred to Lithophyllum carpophylli (Heydr.) Heydr. (Woelkerling et al. Citation2005, p. 168) – Foslie recognized Lithophyllum carpophylli as early as in 1900 (Foslie Citation1900, p. 20), before transferring this species to Dermatolithon (Foslie Citation1909, p. 58).
Hence the original material of Lithothamnion chathamense included two elements (collections), Reinbold ‘A.B.C’ and ‘Reinbold nr. C.’, and therefore restriction to a single specimen is required. Since ‘Reinbold nr. C.’ consists of tiny fragments only (in one of the smaller boxes) and a section on slide n°546, the larger specimen illustrated by Printz (Citation1929, plate 9 figure 10) and previously examined by Keats and Chamberlain (Citation1997, pp. 73–75, as ‘holotype’) is here selected as the lectotype (k). Whether a third element (Reinbold ‘A’) exists in Foslie's herbarium in TRH has not been clarified.
Description
Thalli saxicolous (?), at least 7.5 cm in extent and 3 cm high, forming a conglomerate mass of interwoven-anastomosing, lamellae, with roundish-undulate margins (k). Individual lamellae, 220–570 µm thick (except conceptacles), occasionally growing back-to-back and becoming confluent (below). Thallus organization dorsiventral to aniso-bilateral. Individual lamellae composed of a predominantly coaxial hypothallium, 250–300 µm thick, supporting an ascending stratified perithallium, to 150 µm thick, and descending hypothallial filaments (a, e). Lamellae may grow back-to-back (f), or form a conglomerate variously orientated (g), or even coalesce reaching at least 1.2 mm in thickness (h). Hypothallial filaments dominate the margin and grow via terminal synchronous anticlinal divisions and elongations protected by a cuticle (c). Terminal meristematic cells gradually become displaced either dorsally or ventrally to form epithallial cells (c–d). The cuticle that protects the terminal meristem ceases to exist at the border where meristematic cells become epithallial cells (d). Descending hypothallial filaments produce a 2-celled ventral perithallium with a distinctive angle between descending hypothallial and perithallial cells (i, j). Hypothallial cells 20–34 × 4–9 µm (L × B) and perithallial cells 6–23 × 4–7 µm (L × B). Dorsal and ventral perithallial filaments support single isodiametric to flattened epithallial cells, 2–8 × 5–9 µm (L × B) (d, k–m), ventral epithallial cells being slightly wider, to 12 µm in diameter (i, j). Dorsal subepithallial cells reach 20 µm long and 5–9 µm broad (l) (Keats and Chamberlain Citation1997, table 3). Terminal trichocytes, 20–25 × 7–10 µm (L × B) occur both dorsally and ventrally amongst epithallial cells (i–k, m), and occasionally in groups (i). Cell fusions between contiguous cells common (Keats and Chamberlain Citation1997, figure 93). Secondary pit connections absent.
Gametophytes not seen. Multiporate (tetrasporangial) conceptacles 350–1000 × 100–200 µm (D × H) with chambers 200–400 × 100–200 µm (D × H; n: 2) (figure 79). Roof convex to flattened (or slightly sunken), 50–60 µm thick, composed of 5-cells (including the epithallial cell) (a, a, d), and perforated by 35–39 pores each surrounded by 8–10 rosette cells (c). Canals ranging about 10 µm in diameter at the apex and 20 µm near the base. Filaments lining the canals are 4-celled, lacking epithallial cells and terminating below adjacent roof filaments. Each pore filament is composed of a basal cell that also supports a normal roof filament (d, e). One tetrasporangium 150 × 80 µm (L × B) was seen. Older conceptacles do not become embedded in the thallus.
Habitat and distribution
Data for the lectotype and two recently collected specimens unknown. The species is reported only from the Chatham Islands.
Remarks
Perithallis chathamensis is presently known from two collections comprising specimens with multiporate conceptacles, and its habitat preferences are unknown. The lectotype was apparently communicated as unattached specimen (rhodolith?), while the other two thalli here studied were also unattached lacking habitat data. Apart from a larger thallus (at least 7.5 cm in extent) that precludes epiphytic growth, and the development of back-to-back growing lamellae eventually becoming confluent, all other anatomical characteristics of P. chathamensis agree with those of P. incisa. Both species exhibit an aniso-bilateral organization, a predominantly coaxial hypothallium, elongate subepithallial initials which support flattened to isodiametric single epithallial cells. Both species lack embedded conceptacles. Their multiporate conceptacles lack a rim and are provided with a Thallis-type of pore filaments (f). The here examined collection A027264/A (NZC0745) in WELT included two thalli (7.5 cm and 3 cm in extent), and a subsample was apparently included in the molecular studies of Broom et al. (Citation2008, p. 960, table 1, as ‘Mesophyllum erubescens’, ‘A027264 -DQ167929-EF628222’), Peña et al. (Citation2011, figure 3, as ‘Mesophyllum erubescens’, ‘EF628222’), and Sissini et al. (Citation2014, figure 5, as ‘Mesophyllum erubescens’, ‘DQ167929’, New Zealand group 3). In the study of Broom et al., this isolate resolved as sister-taxon either to Printziana (nSSU data) or to (?) Perithallis incisa (psbA data) (see a, b) and is presently flagged as ‘Gen.nov.sp.’ in WELT (information online). The unstable gene topology suggests that Perithallis chathamensis is genetically remotely related to the above sister-taxa and may deserve recognition as a separate genus, providing that new taxonomic investigations will reveal further differences from P. incisa (than just habit and habitat preference). Sissini et al. (Citation2014, table 2) also calculated the sequence divergence for the psbA gene between isolates here referred to Perithallis chathamensis and Sunesonia pseuderubescens, Printziana australis and Melyvonnea, showing a variation between 8.1% and 8.6%, 6.3%, respectively 12.9%–13.1%, which complies with the genetic distance between other genera of Mesophyllaceae (Jesionek et al. Citation2020, p. 110). The divergence between Perithallis chathamensis and the putative isolate of Perithallis incisa (Broom et al. Citation2008, table 1, WELT A026956-DQ167884-EF628223) was estimated to 2.7%–3.0% and 12–13 bp (Sissini et al. Citation2014, table 2, ‘New Zealand 3 vs New Zealand 4’). The original spelling ‘chatamense’ (Foslie Citation1906b) is here corrected.
Genus Printziana gen. nov.
Type species Printziana australis nom. nov.
Genus diagnosis
New monotypic genus of Mesophyllaceae, allied to Sunesonia (described herein) from which differs by the development of trichocytes and a predominantly coaxial hypothallium, and the lack of reduced basal cells of pore filaments lining canals of multiporate conceptacles. Printziana and Sunesonia differ from other genera of Mesophyllaceae in developing elongate basal and subbasal cells in pore filaments lining the canals of multiporate conceptacles.
Etymology
The new genus commemorates the Norwegian phycologist Henrik Printz (1888–1978).
Material examined
New Zealand: North Island: Kapiti Island, between Kapiti Island and mainland (40°52′.20″S–174°55′.25″E): WELT (A028096-NZC0858): annotated ‘Mesophyllum erubescens (Foslie) M.Lemoine’, ‘1 June 2000’, coll. ‘K13; D. Stotter’ [includes two slides annotated ‘1.1’, ‘1.3’, ‘small multi’ with multiporate conceptacles; bisporangial (?) thalli, 5-celled pore filaments].
Northland, Doubtless Bay east end of Cable Bay beach (34°59′.40″S–173°29′.22″E): WELT (A029315-NZC2141): annotated ‘Mesophyllum printzianum Woelk. et A.S. Harv.’, ‘22 September 2005’, coll. ‘NCB; K.Neill & T.Farr’ (includes thalli with tetrasporangial conceptacles, and pore filaments at least 6-celled).
Bay of Islands, western side of Okahu I. (35°12′.08″S–174°12′.30″E): WELT (A029331-NZC2269): annotated ‘Mesophyllum erubescens (Foslie) M.Lemoine’, ‘2 March 2006’, coll. ‘NOI; S. Miller & J.S. Forman’ (bisporangial thalli with ‘multi-rim’ outgrowths around the sunken pore plates).
South Island: Tasman Bay, Cable Bay, ‘Cable Bay Site 1’ (41°9.40′S, 173°24.05′E): WELT (A027076-NZC0441): annotated ‘Mesophyllum printzianum Woelk. et A.S. Harv.’, ‘20 March 2003’, coll. ‘ZE; R. Cole & S. Brown’, annotated ‘on shells’ by Harvey et al. (Citation2005, p. 115) (includes one slide annotated ‘multis’, ‘1.2’; bisporangial thalli, 4-celled pore filaments).
Remarks
The collections above comprise thalli deviating from the generitype Printziana australis, both in reproductive and vegetative characters, indicating that the genus comprises several species. These differences are discussed below in comparison to P. australis. The molecular divergence of Printziana to Perithallis, Sunesonia and Melyvonnea is considered in the light of Sissini et al.’s (Citation2014, table 2) study of the psbA gene, and appears in the Discussion.
Printziana australis nom. nov.
(a–h).
Figure 13. Printziana australis gen. & nom.nov. Vegetative structures; a, thalli in view from above and the side (arrows) showing the prominent erect perithallial protuberances (isotype); b, transverse section of a thallus encircling an object (arrowhead) and producing a perithallial protuberance (arrow). Note the embedded tetrasporangial conceptacles (isotype); c, transverse section of a perithallial protuberance with embedded tetrasporangial conceptacles (LTB 12850); d, section at the margin showing synchronous anticlinal divisions in terminal hypothallial cells (white arrows) protected by a cuticle (c). Subdichotomous divisions (black arrow) expand the thallus and terminal meristematic cells become displaced developing to epithallial cells (arrowheads) – lower part magnified in figure f (NZC2508); e, section showing a predominantly coaxial, arching hypothallium (arrow) supporting an ascending stratified (black arrow) perithallium (isotype); f, section near the ventral side showing a subdichotomous division (black arrow) and descending hypothallial filaments ending in wedge-shaped cells (arrowheads) (NZC2508); g–j, sections at the surface showing elongate (long white arrows) or just divided (white arrows) subepithallial cells, supporting single epithallial cells; the latter spherical to flattened or even elongate (black arrowheads). Note two terminal trichocytes (black arrows) and the fusions between contiguous (arrowheads) cells (g–h: isotype; i: LTB 12850; j: NZC0621). Abbreviations: c (cuticle).
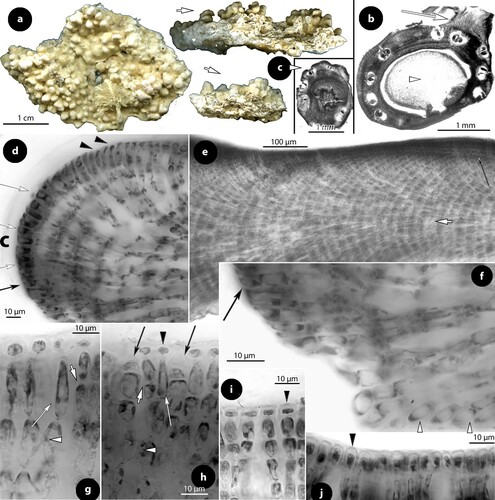
Figure 14. Printziana australis gen.& nom. nov. Male structures (isotypes); a–b, early stages of simple (unbranched) spermatangial structures restricted to the floor with lunate SMCs (arrows) (Woelkerling and Harvey Citation1993, figure 29B, reproduced with modifications); c–d, male conceptacle at late maturity with elongate SMCs (broken arrows) supporting spermatangia in pairs (arrowheads) that release spermatia (black arrowheads).
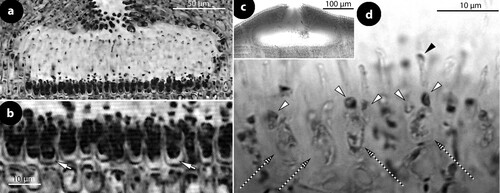
Figure 15. Printziana australis gen.& nom. nov. Carpogonial, postfertilization and carposporangial structures (NZC0621); a–b, carpogonial conceptacle with unfertilized carpogonial branches extending across the entire floor. Carpogonial branches composed of carpogonia (ca) and hypogynous (h) cells, attached to supporting (s) cells. Note that supporting cells support the chamber walls (arrows) when they do not support carpogonial branches; c, a carpogonial branch composed of a carpogonium (ca) and a hypogynous (h) cell, attached to a supporting (s) cell. A sterile cell may occur attached to the hypogynous cell (broken line); d, postfertilization stage showing a distinctive fusion cell (f), composed of at least 6 or 7 supporting cells and incorporating at least 2 hypogynous (h) cells, producing the gonimoblast filaments (g.f.). Remains of carpogonia (ca), hypogynous (h) and basal (b) cells remain intact and do not merge with the fusion cell or the gonimoblast filaments; e–f, peripheral production (arrows) of carposporangia (c) from a slightly or distinctively raised fertile zone (pe). Abbreviations: b (basal cell), c (carposporangia), ca (carpogonium), g.f. (gonimoblast filament), h (hypogynous cell), pe (pedestal), s (supporting cell).
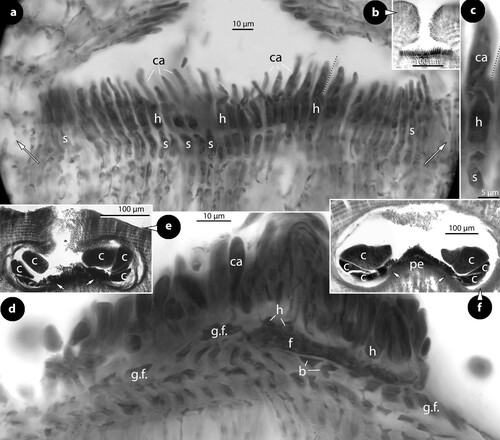
Figure 16. Printziana australis gen.& nom. nov. Tetrasporangial structures (a–h) and Printziana spp. (i–k); a, section of a tetrasporangial conceptacle with sunken roof (isotype); b, surface view of canals (p) of a multiporate roof surrounded by seven-nine rosette cells (NZC0621); c–h, sections through canals of multiporate roofs showing elongate basal (long white arrows) and subbasal (short white arrows) cells, each supporting two (occasionally three) cells (arrowheads) the last being occasionally an epithallial cell (black arrowheads). Note that the branched basal cells also support a normal roof filament (black arrows) (c–e: isotype: f–h: NZC0621); i, bisporangial thallus with 5-celled pore filaments composed of elongate basal and subbasal cells (arrows) and three smaller cells (arrowheads) (NZC0858); j, tetrasporangial thallus with at least 6-celled pore filaments composed of elongate basal and subbasal cells (arrows) and four smaller cells (arrowheads) (NZC2141); k, bisporangial thallus with ‘multi-rim’ outgrowths (arrowheads) around the sunken pore plate (NZC2269). Abbreviations: p (pore canal).
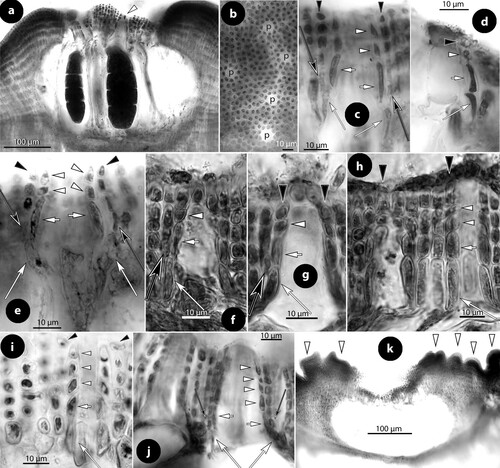
Etymology
The epithet denotes the southern hemisphere distribution of the species and is a new name to avoid the tautonym (Turland et al. Citation2018; Art. 6.11 & 23.4).
Homotypic synonym
Mesophyllum printzianum Woelkerling and Harvey (Citation1993, p. 593).
Type locality
Blanket Bay, Otway National Park (38°49′S, 143° 34′E), Victoria, southern Australia.
Holotype
In MEL, 2271675 (LTB 15249), 14 November 1985, coll. S. Campbell n°15249.
Isotypes
Slides in herb.Athanas. (a, b, e, g–h; ; a, c–e).
Material examined
Australia: Victoria: isotype (data as for the holotype above; tetrasporangial and male thalli).
Tasmania: East coast, North Shore, Safety Cove, Port Arthur (43°10′S, 147°51′E): MEL 2269664 (LTB 12850), annotated ‘0–3 metres deep or rock. Large white conceptacles … ’, ‘25 February 1983’, coll. ‘Platt, S.’, ‘Mesophyllum printzianum Woelk. and F.L. Harv.’ (tetrasporangial specimen cited in Woelkerling and Harvey Citation1993, p. 594).
New Zealand:
North Island: Bay of Islands, western side of Okahu I. (35°12′.08″S, 174°12′.30″E): WELT (A029261-NZC2272): annotated ‘Mesophyllum erubescens (Foslie) M.Lemoine’, ‘2 March 2006’, coll. ‘NOI; S. Miller & J.S. Forman’ (tetrasporangial thalli).
Far North, Henderson Point (34°44′.45″S, 173°7′.08″E): WELT (A029323-NZC2508): annotated ‘Mesophyllum printzianum Woelk. et A.S. Harv.’, ‘19 March 2007’, coll. ‘NHP; K. Neill & T. Farr’ (tetrasporangial thalli; includes a Lithophyllaceae with uniporate tetrasporangial conceptacles).
South Island: Christchurch, Banks Peninsula, Taylors Mistake, South end of beach (43°35.10′S, 172° 46.43′E): WELT (A028173-NZC0621-DQ168004): annotated ‘Mesophyllum printzianum Woelk. et A.S. Harv.’, ‘28 November 2003’, coll. ‘TYS; N. Gust & M. Flanagan’ (includes two slides, ‘1.3’, ‘1.4’ with multiporate conceptacles; heterogeneous collection including tetrasporangial and carposporangial specimens of Printziana australis, males of a Lithophyllaceae, and tetrasporangial specimens of Mesophyllum macroblastum sensu Woelkerling and Harvey Citation1993).
Description
Thalli encrusting, closely adhering to the substratum (rock), to 5 cm in extent and at least 7 mm thick (excluding protuberances) (to 9.1 cm in extent and 25 mm thick according to the protologue), producing superimposed lamellae and erect perithallial protuberances. Protuberances, up to 18 borne per square cm (n: 6), each 1.6 to 6 mm in diameter and up to 5 mm high (1.4–11 mm in diameter and up to 14 mm high according to the protologue) (a–c). Individual lamellae composed of a predominantly coaxial hypothallium, 150–310 µm thick, supporting an ascending stratified perithallium, at least 800 µm thick (b, e), and descending hypothallial filaments ending in wedge-shaped cells (f). Hypothallial filaments dominate at the margin and grow via terminal synchronous, anticlinal divisions and elongations protected by a cuticle (d). Meristematic cells also divide subdichotomously, adding to the thallus thickness, and gradually displace hypothallial filaments dorsally or ventrally, the former to become perithallium (d) and the latter descending hypothallial filaments (f). Terminal meristematic cells displaced dorsally become epithallial cells (d). Hypothallial cells 18–35 × 4–6 µm (L × B) (16–42 × 5–12 µm according to the protologue) and perithallial cells 6–15 × 4–6 µm (L × B) (6–14 × 4–7 µm according to the protologue). Subepithallial cells distinctively elongate during division (g, h), supporting a single layer of isodiametric (spherical), flattened or even elongate epithallial cells, 4–10 × 4–7 µm (L × B) (4–6 × 3–5 µm according to the protologue) (g–j). Trichocytes, 12–15 × 8–9 µm (L × B), occur terminally amongst epithallial cells, either solitary or occasionally in small fields (h). Cell fusions between contiguous cells common (g, h). Secondary pit connections absent.
Gametophytes dioecious. Male conceptacles c. 480 × 70 µm (D × H) with chambers c. 280 × 80 µm (D × H; n: 1) and a central ostiole 45–70 × 60 µm (D × L) (chambers 175–243 × 48–60 µm with ostioles 27–40 µm in diameter according to the protologue). Spermatangial structures simple (unbranched), with lunate SMCs (a, b). At the end of maturity, SMCs become elongate with spermatangia borne terminally in pairs releasing spermatia in the chamber (c, d). Carpogonial conceptacles provided with 2-celled carpogonial branches, that extend along the entire fertile floor (a, b). Carpogonial branches are composed of a carpogonium and a hypogynous cell (a, c). Supporting cells are not part of the carpogonial branches because they integrate with the walls of the conceptacle when they do not support carpogonial branches (a, arrows). A sterile cell may occur attached to the hypogynous cell, next to the carpogonium (a, c). Following fertilization and zygote transfer (not seen), a fusion cell is formed composed of at least six supporting cells and at least two hypogynous cells, while carpogonia, adjacent hypogynous cells, and basal cells, remain intact and do not participate in the fusion (d). Proliferations from the fusion cell form gonimoblast filaments which radiate along the fertile zone producing carposporangia from the periphery (e, f). A raised fertile zone with low inclination may occasionally develop, and gonimoblast filaments follow the slope (f, arrows). Carposporangial conceptacles, 550–750 × 70–160 µm (D × H) with chambers 370–580 × 180–200 µm (D × H) and a central ostiole 50–70 × 80 µm (D × H; n: 5).
Tetrasporangial conceptacles, 440–800 × 0–150 µm (D × H; n: 16), generally rimmed (a), and occasionally with flattened roof (Woelkerling and Harvey Citation1993, figure 27A). Rimmed conceptacles with pore plates, 130–210 µm in diameter, sunken 40–50 µm below the roof edges and perforated by at least 28 pores, each pore surrounded by 7–10 rosette cells (b). Roof 40–50 µm thick across the pore plate and up to 110 µm thick at the roof edges. Roof filaments of the pore plate composed of 5–7 cells. Canals 8–15 µm in diameter, straight or wider at the base, lined by 4- or 5-celled filaments that end at the roof surface or slightly below/above (c–h). Basal cells of lining filaments branched, supporting a normal roof filament and the rest of the filament lining the canal. Basal and subbasal cells of pore filaments distinctively elongate (c–h). Chambers of tetrasporangial conceptacles 300–390 × 150–190 µm (D × H; n: 2) (185–420 × 175–190 µm according to the protologue), with tetrasporangia 140–150 × 40–75–95 µm (L × B; n: 8) (121–150 × 54–122 µm according to the protologue). Older gametangial and tetrasporangial conceptacles become embedded in the perithallium.
Habitat and distribution
The species grows in the upper sublittoral, 0–6 m depth, on rock and on holdfasts of Phyllospora comosa (Labill.) C.Ag. (Woelkerling and Harvey Citation1993, p. 594). It is widely reported as Mesophyllum printzianum from southern Australia to Tasmania (Woelkerling Citation1996, p. 204), eastern Australia (Harvey et al. Citation2003), New Zealand and Chatham Islands (Harvey et al. Citation2005, Farr et al. Citation2009) – but here confirmed only from collections in Victoria, Tasmania, and New Zealand.
Remarks Printziana australis is a common species in southern Australia and New Zealand, being recognized as Mesophyllum printzianum since its original description (Woelkerling and Harvey Citation1993; Woelkerling Citation1996; Harvey et al. Citation2005; Farr et al. Citation2009). Despite the several taxonomic accounts, carpogonial and postfertilization stages, are here documented for the first time, while the structure of the pore filaments lining canals of multiporate conceptacles is here fully appreciated (g). Printziana shares the presence of elongate basal and subbasal pore cells with Sunesonia (h), and these sympatric genera are here considered to be sister-taxa. A full comparison betwen them is given under Sunesonia (below) and in .
Several New Zealand collections identified as ‘Mesophyllum printzianum’ (in schedula) were here referred to the genus Printziana pending further studies. These include: (1) bisporangial thalli with 4- or 5-celled pore filaments (i), (2) tetrasporangial thalli provided with at least 6-celled pore filaments (j), and (3) bisporangial thalli with multiporate conceptacles having ‘multi-rim’ outgrowths around pore plates (k). It could be added that bisporangial thalli, not known from southern Australia (Woelkerling and Harvey Citation1993, p. 593; Woelkerling Citation1996, p. 204; present study), have also been recorded from eastern Australia being included in the form range of Mesophyllum printzianum by Harvey et al. (Citation2003, p. 674, figures 18B, D). The present observations, however, support the view that Printziana comprises several species, which questions the identity of the isolate of ‘Mesophyllum printzianum’ from the Chatham Islands, included in the molecular studies of Broom et al. (Citation2008, table 1, A027270-DQ167935-EF628224), Peña et al. (Citation2011) and Bittner et al. (Citation2011). The latter isolate is here referred to the genus (a–d), not being immediately related to another isolate from South Island (Sissini et al. Citation2014, figure 5, WELT A028173-NZC0621-DQ168004) identified as Printziana australis in the present study.
The partial development of a ‘pedestal’ in carpsoporangial conceptacles is in accord with the present observations in Thallis capensis (f–h) and Perithallis incisa (c, d, f), indicating that this character is probably a synapomorphy for these three southern hemisphere genera. Printziana shows the closest taxonomic affinity to the northern hemisphere genus Mesophyllum, in possessing a predominantly coaxial hypothallium, 2-celled carpogonial branches, a partially raised fertile zone (‘pedestal’) in carposporangial conceptacles, and a prominent fusion cell. At least one molecular study (Bittner et al. Citation2011) has supported a close relationship, clustering the generitype of Mesophyllum (M. lichenoides) next to Printziana-Perithallis (d). Indeed, Printziana australis has also been reported from the European fossil record, namely in material collected at the Carpathian Foredeep (Czech Republic) and dating since the Lower Badenian (Early Langhian; 15 MYA) (Hrabovský et al. Citation2015, figures 11, 18H, as Mesophyllum printzianum, with reservation).
The examined isotype material of M. printzianum was further annotated ‘ … There are 38 microscope slides associated with this collection. The dry component of this specimen includes resin blocks, resin chips and ten SEM stubs. Spirit 10991 D Slide 10991 Sheet 1 of 2 (MEL 2271676)’.
Genus Sunesonia gen. nov.
Type species Sunesonia pseuderubescens sp. nov.
Genus diagnosis
New monotypic genus of Mesophyllaceae (Corallinales), differing from Printziana (described herein) in lacking trichocytes and a predominantly coaxial hypothallium, and in possessing 4-celled pore filaments with reduced (or deteriorated) basal cells lining canals of multiporate roofs. Printziana and Sunesonia differ from other genera of Mesophyllaceae in developing pore filaments (lining canals of multiporate conceptacles) with elongate basal and subbasal cells.
Etymology
The new genus commemorates the Swedish corallinologist Svante Suneson (1904–1997).
Remarks
Of the four new genera here recognized, Sunesonia is the least known, lacking a historical record, and direct connection of its Australian type to molecular data. The association of the generitype S. pseuderubescens with previous herbarium records of ‘Mesophyllum erubescens’ or ‘Mesophyllum incisum’ from South Australia-New Zealand is however certain on the basis of morphological and anatomical characters. Unfortunately, none of the three isolates of ‘Mesophyllum erubescens’ used in the molecular studies of Broom et al. (Citation2008, table 1), Peña et al. (Citation2011, figure 3) or Bittner et al. (Citation2011, table 2, figure 1) was available for examination, although material of ‘Mesophyllum erubescens’ from one of the localities (Golden Bay, Tauro point, New Zealand) included in the above studies is here identified as Sunesonia pseuderubescens.
As a result, published molecular records of ‘Mesophyllum erubescens’ from New Zealand-Chatham are here referred to Sunesonia ‘?’, with reservation, except of the epiphytic material from Island Bay (Wellington) which is referred to Perithallis incisa ‘?’ (since the type collection was found to be heterogeneous) and the Chatham collection that was identified as Perithallis chathamensis. Externally, sterile thalli of Sunesonia and Printziana are easily confused, as both grow on rock and develop erect perithallial protuberances. From the material examined, it would appear that Printziana australis may develop a higher number of protuberances per square cm (up to 18 vs 8 in Sunesonia pseuderubescens), but more collections need to be studied to confirm this distinction. Anatomically, the two genera differ in the presence/absence of trichocytes and a predominantly coaxial hypothallial growth (both features lacking in Sunesonia). Moreover, the hypothallium in S. pseuderubescens appears to be less developed in comparison to P. australis (up to 170 µm vs 310 µm). Comparing their multiporate conceptacles, those of P. australis are generally rimmed – occasionally with flattened roof (Woelkerling and Harvey Citation1993, figure 27A), while those of S. pseuderubescens generally convex. Pore filaments lining canals of multiporate roofs show also differences, since in Printziana they are 4- or 5- (6- ?) celled ending at the conceptacle surface or slightly below/above (c–j), while in Sunesonia they are generally 4-celled terminating below the conceptacle roof (l–p). In addition, basal pore cells in Sunesonia become reduced and may finally deteriorate (p), in which case they strongly resemble those of species of Melyvonnea (i). In both Sunesonia and Printziana, the nature of top cells of pore filaments ending below the roof surface was not clarified in several cases (c, e, j, m) – these cells differing in shape and/or not staining similarly like other epithallial cells (d, l). The molecular divergence of the monotypic Sunesonia to Melyvonnea, Printziana and Perithallis is considered in the light of Sissini et al.’s (Citation2014, table 2) study of the psbA gene, and appears in the Discussion.
Sunesonia pseuderubescens sp. nov.
(a–p).
Figure 17. Sunesonia pseuderubescens, gen. & sp. nov. Vegetative and multiporate conceptacle structures; a, habit of the holotype from above showing several erect perithallial protuberances (arrows); b, section at the margin showing hypothallial meristematic cells (black arrows) producing a non-coaxial hypothallium (white arrow) (isotype); c, section showing descending hypothallial filaments ending in wedge-shaped cells (arrowhead)(isotype); d, section at the surface showing ± stratified perithallium (isotype); e–f, sections at the thallus surface showing isodiametric to flattened epithallial cells (arrowheads), supported by elongate (arrows) subepithallial cells (isotype); g, section of a tetrasporangial conceptacle (NZC0061); h–i, transverse sections through an erect perithallial protuberance showing superficial and embedded (arrows) conceptacles with bisporangial remains (isotype; scale bar: 500 µm); j, an empty conceptacle with distinctive convex roof (isotype); k, surface view of a multiporate roof showing canals (p) surrounded by 7–9 rosette cells (LTB 14744); l–p, sections through canals of multiporate roofs showing 4-celled lining filaments composed of elongate basal (long white arrows) and subbasal (short white arrows) cells, followed by two smaller cells (white arrowheads). Top cells can be epithallial cells (double arrowhead) and generally end below epithallial cells (black arrowheads) of adjacent roof filaments. Basal cells are divided supporting a roof filament (black arrows) and the rest of the pore filament. Note that basal cells are ± reduced. Figures o–p are tangential sections (facing pore filaments) (l–m, p: isotype; n–o: NZC0835). Abbreviations: p (pore canal).
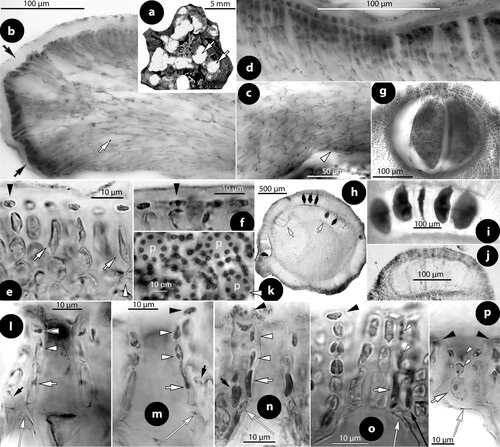
Etymology
The species name is a new compound word after the prefix pseudo (false) and the epithet erubescens (reddening), pointing to the resemblance to Melyvonnea erubescens.
Species diagnosis
New species differing from its closest relative Printziana australis by the generic characters described above (Turland et al. Citation2018; Art.38.5).
Type locality
Ingoldsby Reef, Anglesea, Victoria, southern Australia.
Holotype
In MEL, 2269644 (LTB 13531), collected 7 March 1983, coll. W.R. Beanland n°13531 (a).
Isotypes
Slides in herb.Athanas. (b–f, h–j, l, m, p).
Syntypes
In MEL, including one slide (10982 D – 10982), herbarium specimens on two sheets (both MEL 2269644), and material in alcohol (MEL 2269445); data of collection as for the holotype.
Misapplied name
Mesophyllum incisum sensu Woelkerling and Harvey (Citation1993, p. 588, LTB 13531 & LTB 14744).
Material examined
Australia: Victoria: Ingoldsby Reef, Anglesea (38°25′S, 144°10′E): MEL 2269644 (LTB 13531): holotype, annotated ‘Mesophyllum erubescens (Foslie) Me.Lemoine’, ‘Vic.Grid Ref.: P19’, ‘ … on rock 3 m, seaward side, 7 March 1983, coll. W.R. Beanland n°13531’ [three fragments 0,5 to 4 cm in extent, with multiporate conceptacles and bisporangial remains; material cited under Mesophyllum incisum in Woelkerling and Harvey (Citation1993, p. 588, no figure)].
Londsdale Bommie: Atoll 1 km offshore, south of Point Lonsdale (38°18′S, 144°37′E): MEL 226946 (LTB 14744): annotated ‘Vic.Grid Ref.: P. 13’, ‘20 m deep on rock’, 24 February 1985, coll. D. May n°14744’, ‘M. erubescens (incisum)’ [bisporangial remains; material cited under Mesophyllum incisum in Woelkerling and Harvey (Citation1993, p. 588, no figure)].
New Zealand: North Island: Golden Bay, Tauro Point, Tauro Rocks north (40°47′03″S, 172°57′68″E): WELT (A028017-NZC0456): annotated ‘Mesophyllum erubescens (Foslie) M.Lemoine’, ‘18 March 2003’, coll. ‘ZA: R. Cole & N. Alcock’, ‘most of collection, multiporate & uniporate fragments, 2 slides multis’ (bisporangial thalli; the collection includes a Lithophyllaceae with uniporate conceptacles).
Waiheke Isl., south side, Rocky Bay, towards Te Wjau Point (36°49′12″S, 175°3′20″E): WELT (A029268-NZC2035-FJ361670): annotated ‘Mesophyllum erubescens (Foslie) M.Lemoine’, ‘23 July 2005’, coll. ‘NRB: W.A. Nelson, T. Farr, K. Neill, M. Lee & S. Heesch’ (tetrasporangial thalli).
Wellington, Palmer Head (41°20′70″S, 174°49′30″E): WELT (A027070/A-NZC0061): annotated ‘Mesophyllum erubescens (Foslie) M.Lemoine’, ‘11 September 2002’, coll. ‘PH2: S. Brown & P. Marriott’, ‘whole collection, multiporate & uniporate fragments, 2 slides multis’ [tetrasporangial thalli; the uniporate conceptacles belong to a species of Lithophyllaceae; collection annotated ‘on rock’ by Harvey et al. (Citation2005, p. 111)].
Kapiti Island, between Kapiti Island and the mainland (40°52′18″S,174°55′27″E): WELT (A028046/A-NZC0835-DQ167996): annotated ‘Mesophyllum erubescens (Foslie) M.Lemoine’, ‘1 June 2000’, coll ‘KI1: J.S. Forman’, ‘whole collection, multiporate & uniporate fragments, 2 slides multis’ (tetrasporangial thalli).
Southern Hawkes Bay, Pourerere, Tuingara point (40°7′.23″S, 176°52′.48″E): WELT (A028090-NZC0794): annotated ‘Mesophyllum erubescens (Foslie) M.Lemoine’, ‘26 January 2004’, coll. ‘TIS; D. Stotter & J.S. Forman’ (includes one slide annotated ‘multis … M.incisum’, ‘1.2’, bisporangial thalli).
South Island: West coast: Cape Foulwind, Three Steeples (41°43′.88″S, 171°28′.18″E): WELT (A028055-NZC0606): annotated ‘Mesophyllum erubescens (Foslie) M.Lemoine’, ‘most of collection, multiporate + uniporate fragments + 2 slides … multis’, ‘16 April 2003’, coll. ‘SP1; D.M. Neele & M. Martini’ (bisporangial thalli).
Description
Thalli encrusting, at least 4 cm in extent and 1 mm thick, adhering to rock and producing erect perithallial protuberances, 2–5 mm in diameter and up to 5 mm high (a). One to eight protuberances may develop per square cm (n: 10). Thallus organization dorsiventral, composed of a non-coaxial hypothallium (b), 50–170 µm thick, producing an ascending, ± stratified perithallium at least 800 µm thick (b, d). Descending hypothallial filaments end in wedge-shaped cells (c). Hypothallial cells 8–22 × 5–10 µm (L × B) and perithallial cells 5–10 × 3–5 µm (L × B), with subepithallial cells to 12 µm long supporting single isodiametric to rectangular epithallial cells, 2–3 × 2–5 µm (L × B) (b–f). Cell fusions between contiguous cells common (e). Trichocytes and secondary pit connections not observed.
Gametophytes not observed. Tetrasporangial and bisporangial thalli provided with multiporate conceptacles, 270–450 × 50–80 µm (D × H; n: 7) with chambers 210–260 × 140–200 µm (D × H; n: 5), borne on the encrusting thallus or the perithallial protuberances (h). Roof convex, 40–60 µm thick, composed of 5–7 celled filaments. Canals of multiporate conceptacles, 8–12 µm in diameter, wider at the base, and surrounded by 7–9 rosette cells (k). Pore filaments lining the canals 4-celled, usually lacking epithallial cells and generally terminating below the roof surface (l–p). Basal and subbasal cells elongate, to 13 µm long, usually dissimilar in size. Basal cells branched, supporting a normal roof filament and the pore filament itself. Basal cells becoming gradually reduced, and may deteriorate, so that pore filaments appear to be composed of a single elongate ‘basal’ cell (i.e. the subbasal cell) followed by two top cells (m, p). Tetrasporangia 65–180 × 30–80 µm (L × B) (n: 13). Bisporangial conceptacles 340–430 × 90–120 µm (D × H; n: 4) with chambers 200–450 × 150–190 µm (D × H; n: 5). Bisporangia 130–180 × 50–90 µm (L × B; n: 7). Older conceptacles disintegrate or become embedded in the perithallium (h).
Habitat and distribution
The species grows on rock in the sublittoral zone, between 3 and at least 20 m depth. It is recorded from Victoria, (?) eastern Australia (Harvey et al. Citation2003, as M. erubescens in part), and New Zealand (North & South Island).
Remarks
Sunesonia pseuderubescens occurs in southern Australia (Victoria), western New Zealand (present study), and most likely in eastern Australia being included in the wide concept of ‘Mesophyllum erubescens’ held by Harvey et al. (Citation2003, Citation2005) and Farr et al. (Citation2009). In particular, six New Zealand collections referred to Mesophyllum erubescens (= Melyvonnea erubescens) and two southern Australian collections [originally referred to M. incisum (= Perithallis incisa) and later annotated ‘Mesophyllum erubescens’ (in schedula)] are here included in the circumscription of the new species.
On the other hand, the original material of Mesophyllum erubescens was described from Chaloup Bay, Fernando de Noronha (Brazil) (Foslie Citation1900, p. 9–10, as Lithothamnion) and more recently referred to the genus Melyvonnea (Athanasiadis and Ballantine Citation2014; Jesionek et al. Citation2016), which accommodates five species in the tropics and subtropics. The study of 15 collections referred to ‘Mesophyllum erubescens’ from southern Australia-New Zealand failed to confirm the presence of Melyvonnea in this region (Athanasiadis and Ballantine Citation2014, appendix 3), and three molecular records from Vanuatu and Fiji were considered to be the nearest representatives (Athanasiadis and Ballantine Citation2014, p. 389, figure 2D). Comparing a large number of DNA sequences referred to ‘Mesophyllum erubescens’ from localities across the tropics and subtropics, Sissini et al. (Citation2014, figure 5) reached the same result.
Melyvonnea resembles both Sunesonia and Printziana in habit and habitat. Members of these genera grow on rock in the sublittoral and develop an encrusting thallus with erect perithallial protuberances. In Melyvonnea, the protuberances ramify and gradually dominate over the encrusting base, becoming even detached to form rhodoliths. Anatomically, Sunesonia differs from these genera in lacking a predominantly coaxial hypothallium or trichocytes. Multiporate conceptacles of Melyvonnea exhibit canals bordered by unbranched pore filaments with elongate basal cells (i), while in Sunesonia and Printziana the pore filaments are basally branched and provided with elongate basal and subbasal cells (g–h). Moreover, the basal cells in Sunesonia become reduced, so that pore filaments seem to be composed of a single elongate ‘basal’ cell and two smaller cells (p), as described in Melyvonnea madagascariensis (Foslie) Athanas. & D.L. Ballant. (Athanasiadis and Ballantine Citation2014, Figure 108). This resemblance explains the confusion in adopting the name ‘Mesophyllum erubescens’ for Australian-New Zealand specimens. More significantly though, it suggests that Sunesonia might be an evolutionary step between Printziana and Melyvonnea (Discussion).
Lack of gametophytes in Sunesonia pseuderubescens may well be the common condition, in which case the present-day populations should be regarded as apomeiotic, recycling the parental (tetra- or bisporangial) phase. Lack of sexual reproduction could thereby favour random mutations reflecting polymorphisms.
The holotype of Sunesonia pseuderubescens is the largest specimen (a) amongst those borrowed from MEL (via LTB) – (MEL loan 2005/10 to GB) – the other fragments reaching less than 12 mm in extent. The rest of the original material is here recognized as ‘syntypes’ because it includes several elements (as described above) mounted on two separate sheets (according to the information on the loan), suggesting that it was the product of several gatherings.
Discussion
We can safely conclude that the broad concept of ‘Mesophyllum erubescens’ held by Australian and New Zealand colleagues has been falsified in a series of studies. In particular, Broom et al. (Citation2008, p. 971) found considerable genetic variation between members of this group, ranging up to 60 nucleotide substitutions, and the five isolates of ‘M. erubescens’ from New Zealand-Chatham (i.e. A027246, A027266, A027263, A027264, A026956) presently stand as putative new genera (WELT, information online). Broom et al. (Citation2008, p. 969), recognized two lineages, one including Mesophyllum printzianum (= Printziana australis) and ‘Mesophyllum erubescens’ from Chatham (= Perithallis chathamensis) and Wellington (= Perithallis incisa ?), and the second one including three isolates of ‘Mesophyllum erubescens’ from Golden Bay (1 & 2) and Wharariki (= Sunesonia ?) – with an isolate of Mesophyllum (Melyvonnea) erubescens from Brazil nested between (a). In two subsequent molecular studies that also included several other taxa, the five isolates from New Zealand-Chatham resolved either near the European Mesophyllum lichenoides and distantly to the Brazilian Melyvonnea erubescens (Bittner et al. Citation2011, figure 1) or vice versa (Peña et al. Citation2011, figure 1). This unstable topology, rather indicates a remote genetic distance between European, Brazilian and New-Zealand-Chatham isolates/taxa.
Figure 18. a–d, relationships between species and genera of Mesophyllaceae as indicated by nSSU (a, c, d) and psbA (b) gene sequences, using maximum likelihood (a, d) and Bayesian inference (b, c); a–b, after Broom et al. (Citation2008, table 2, figure 5, figure 1); c, after Peña et al. (Citation2011, figure 3); d, after Bittner et al. (Citation2011, table 2, figure 1). Unidentified taxa in the cited papers have been excluded in the here shown reproductions. Ingroup members polarized by the closest outgroup taxon: Synarthrophyton spp. (a, b), Clathromorphum compactum (c), and ‘Leptophytum’ spp. (d). Phragmope replaces a sequence of ‘Mesophyllum engelhartii’ from South Africa (Athanasiadis Citation2020, figure 8); Melyvonnea replaces sequences of Mesophyllum erubescens from Brazil, Hawaii, Fiji and Vanuatu (Athanasiadis & Ballantine Citation2014, figure 2D). New taxa proposed in this study are indicated by asterisks and replace sequences of Mesophyllum printzianum (= Printziana) or ‘Mesophyllum erubescens’ (= Perithallis chathamensis, P. incisa ?, Sunesonia ?) from New Zealand-Chatham. Accession numbers (in alphabetic order): Clathromorphum compactum Canada (U60742); ‘Leptophytum’ spp. SAF (U62119 & U62120); ‘Leptophytum’ repandum NZ (EF628216); ‘Leptophytum’ repandum Chatham (EF628215); Melyvonnea Brazil (U61257); Melyvonnea Hawaii (DQ629011, DQ629012); Melyvonnea Fiji-Vanuatu (GQ917390, GQ917392, GQ917411); Mesophyllum lichenoides Spain, France (CH13992, GQ917384); ‘Mesophyllum’ sphaericum Spain (CH1814, CH1891); ‘Mesophyllum’ sp. Chatham (EF628218); Perithallis chathamensis Chatham (EF628222); Perithallis incisa ? Wellington (EF628223); Phragmope SAF (U61256); Printziana Chatham (EF628224); Sunesonia ? Wharariki Beach, Golden Bay 1, 2 (EF628219, EF628220, EF628221); Synarthrophyton patena SA (U61255); ‘Synarthrophyton’ schielianum Stewart Island (EF628217). e, hypothetical evolution of pore filaments in Thallis, Perithallis, Printziana, Sunesonia and Melyvonnea, based on parsimony. The ancestral condition, Thallis-type (white arrow), persisted in Thallis-Perithallis. A derivative condition (black arrow), involving the acquisition of elongate basal cells, is the ancestor of the present-day Printziana-, Sunesonia- and Melyvonnea-types. It assumes that the Melyvonnea-type evolved via loss of basally branched pore cells, the latter persisted in Printziana and reduced in Sunesonia. Black squares indicate epithallial cells and arrowheads sunken pore filaments (below the conceptacle roof). Abbreviations: c (canal), SA (southern Australia), NZ (New Zealand), Ch (Chatham), SAF (South Africa), S.-C.Atl. (South & Central Atlantic), J (Japan), H (Hawaii), P (Polynesia).
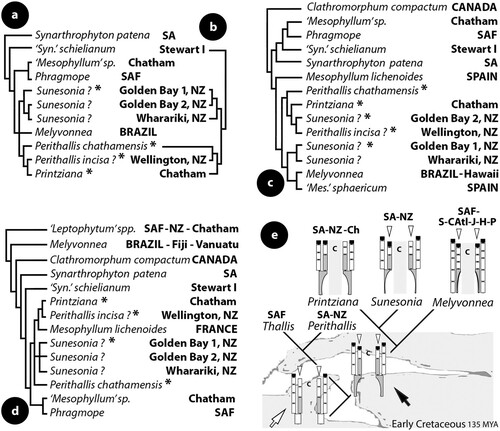
The phylogenetic analysis of the genus Mesophyllum (Athanasiadis and Ballantine Citation2014, figure 1), based on morphological and anatomical characters, recognized an exclusively northern hemisphere distribution for this genus and its 11 species, excluding all Australian-New Zealand members (e.g. Mesophyllum printzianum and M. incisum) and the Brazilian Mesophyllum erubescens. The latter species was referred to the new genus Melyvonnea, while the Australian-New Zealand species resolved between Melyvonnea and Mesophyllum. Moreover, a study of 15 collections referred to ‘Mesophyllum erubescens’ from Australia-New Zealand confirmed the absence of this species (or the genus Melyvonnea) in this region (Athanasiadis and Ballantine Citation2014, appendix 3), and three molecular records of ‘M. erubescens’ from Vanuatu and Fiji were accepted as the nearest generic representatives (Athanasiadis and Ballantine Citation2014, p. 389, figure 2D). The latter conclusion was also supported by the molecular study of Sissini et al. (Citation2014, figure 5). More significantly, Sissini et al. (Citation2014) included in their study four isolates of the here described new taxa, namely Sunesonia pseuderubescens (WELT A029268-NZC2035-FJ361670), Sunesonia pseuderubescens (WELT A028046/A-NZC0835-DQ167996), Printziana australis (WELT A028173-NZC0621-DQ168004) and Perithallis chathamensis (WELT A027264/A-NZC0745-DQ167929-EF628222). These four isolates resolved within three (of the five) New Zealand-Chatham groups recognized by Sissini et al. (Citation2014, table 2), with the following sequence divergence for the psbA gene: Sunesonia pseuderubescens (group 1) vs Perithallis chathamensis (group 3): 8.1–8.6% and 36–38 bp; Sunesonia pseuderubescens (group 1) vs Printziana australis (group 5): 11.7–12% and 52–53 bp; Perithallis chathamensis (group 3) vs Printziana australis (group 5): 6.3% and 28 bp. Sissini et al. (Citation2014, p. 313) concluded that these New Zealand-Chatham isolates ‘clearly do not belong to M. erubescens’. Sissini et al. (Citation2014, table 2) also calculated the sequence divergence between topotypes of Melyvonnea erubescens and the above three New Zealand – Chatham species, that was: Melyvonnea erubescens vs Sunesonia pseuderubescens (group 1): 8.6–8.8% and 38–39 bp; Melyvonnea erubescens vs Perithallis chathamensis (group 3): 12.9–13.1% and 57–58 bp; Melyvonnea erubescens vs Printziana australis (group 5): 11.7–12.0% and 52–53 bp. This psbA sequence divergence is indeed higher than that found between other genera of Mesophyllaceae and which ranges from 4.1–8.2% (Jesionek et al. Citation2020, p. 110).
The present study clarifies the morphological and anatomical diversity in the southern Australian and/or South African species previously referred to Mesophyllum printzianum and/or M. incisum, and in the ‘Mesophyllum erubescens’ group from southern Australia-New Zealand-Chatham, recognizing a diversification into four new genera. Indeed, apart from similarities in pore canal structure, conservative characters such as thallus organization and postfertilization events show remarkable divergence in three of these genera, matching their significant genetic distance (Broom et al. Citation2008, p. 971; Sissini et al. Citation2014, table 2). Thereby, the relevant taxon names on the molecular trees have been replaced accordingly (a–d). Still, we need to bear in mind the questioned IDs for Perithallis incisa (?) and Sunesonia (?), since the available data indicate heterogeneity in the type material of the former taxon, or rely upon other collections than topotypes. Moreover, the finding that Printziana, based on a type (P. australis) from Victoria (South Australia), most likely includes several other species in New Zealand-Chatham, questions the identity of Broom et al.’s (2008, table 1, A027270-DQ167935-EF628224) Chatham isolate that is here referred to the genus (a–d) – being distantly clustered to the here studied isolate (WELT A028173-NZC0621-DQ168004; Sissini et al. Citation2014, figure 5).
The psbA gene topology (Broom et al. Citation2008, figure 2; b; Sissini et al. Citation2014, ) fits best the present results, clustering the two species of Perithallis in a sister-taxon relationship, with immediate connection to Printziana, and grouping Broom et al.'s three isolates of Sunesonia ‘?’ in a second clade – as also indicated by Sissini et al. (Citation2014, figure 5).
The four new genera here recognized (including five species), exhibit a highly specialized canal structure of multiporate conceptacles, which sets them apart from all other Mesophyllaceae. This structure involves at least four differentiations, namely: (1) basally branched pore filaments, (2) composed of 4 or 5 (−6?) cells, (3) possession of elongate subbasal cells, and (4), with the exception of Printziana australis (where this character is variable), termination of pore filaments below the roof surface. Collectively, these characters are here recognized as an unmistakable synapomorphy, indicating an origin in a common ancestor that most likely existed at a time when South Africa, Australia and New Zealand-Chatham were closely located so that dispersal of biota was feasible – i.e. between Jurassic and early Cretaceous, 165–150 to 135 MYA (Sanmartin and Ronquist Citation2004, p. 218). The four genera form pairs: Thallis-Perithallis and Printziana-Sunesonia, the latter pair being further distinguished by the development of elongate basal cells. In Sunesonia the basal cells gradually become reduced or deteriorate, so that pore filaments look like being composed of a single elongate ‘basal’ (formerly subbasal) cell and two top cells (p) – as described in Melyvonnea madagascariensis (Athanasiadis and Ballantine Citation2014, Figure 108). This particular condition in Sunesonia, along with habitat preferences (thalli attached to rock in the sublittoral), presence of conceptacles with convex roof, and a thallus forming erect perithallial protuberances, explains its previous confusion with Melyvonnea (Mesophyllum) erubescens. Most significantly though, it allows reconsidering the evolution of pore canals in Melyvonnea (i), relating this genus to Thallis-Perithallis and Printziana-Sunesonia (f–h).
Melyvonnea shares with these genera at least two characters: the presence of fewer cells in pore filaments (than in adjacent roof filaments), and the termination of pore filaments below the conceptacle roof surface. The matter comes to the interpretation of the elongate (unbranched) basal cells in this genus. Could the ‘basal’ cells of Melyvonnea be a relocation, following the loss of basally branched pore cells? There is no report of ‘rare’ or ‘occasionally’ basally branched pore filaments in Melyvonnea, which suggests that if such an event took place it occurred long ago (leaving no traits in the present-day populations). The only support we presently have for such a hypothesis is the deteriorating basally divided pore cells in Sunesonia, which genus could be the closest relative. Assuming that loss of basally branched pore cells did occur in the ancestor of Melyvonnea, causing relocation of the subbasal cells to a basal position in the present-day populations, it would lead to a natural position of this genus next to Printziana-Sunesonia (e). After all, in the molecular studies of Broom et al. (Citation2008) and Peña et al. (Citation2011), Melyvonnea nested next to the New Zealand-Chatham isolates of ‘Mesophyllum erubescens’ (the latter name here replaced with Sunesonia ‘?’ – a–d), while in the study of Sissini et al. (Citation2014, figure 5), Melyvonnea appearred as the sister taxon to Sunesonia-(Perithallis-Printziana).
A new type of thallus of organization (aniso-bilateral) is described in the two species of Perithallis, intermediate between the dorsiventral (e.g. in Thallis) and the bilateral (e.g. in Synarthrophyton; Athanasiadis Citation2018b, figure 60). Perithallis develops a diminutive, 1–2 cells thick, ventral perithallium that also supports trichocytes. This thallus organization is presently recognized as a unique synapomorphy for the two species of Perithallis.
Comparing the Mesophyllaceae to the multiporate families Lithothamniaceae and Melobesiaceae, several other differentiations have occurred along its evolution, the most conspicuous being the development of: (1) trichocytes, (2) a coaxial hypothallium, (3) a monostromatic hypothallium (with bilateral subdichotomous ramification), (4) sympodial branching, (5) bilateral (or aniso-bilateral) thallus organization, (6) apical meristem relocation to subepithallial position, (7) haustoria, (8) triangular or pyriform canals in multiporate roofs, (9) a cell tube (or connecting filament) involved in post-fertilization stages, (10) a fusion cell (following putative zygote transfer), and (11) a pedestal in carposporangial conceptacles. Some of these characters distinguish particular taxa, or groups of taxa, and presently stand as (syn)apomorphies – as for example the presence of a bilateral thallus organization (Synarthrophyton, Amphithallia, Carlskottsbergia). In several characters, however, the degree of expression or the direction of evolution is ambiguous, awaiting further taxonomic studies to clarify the circumscription of little known (or new) members, and a phylogenetic analysis to point out which of the characters have been acquired, reduced or lost in time.
Regarding the development of a pedestal in species of Mesophyllum, it has been appreciated that this was achieved through dissolution (decalcification) of the peripheral tissue of the chamber to make space for the carposporangia. It was further concluded that such a formation ‘has evolutionary value in allowing the development of larger carpospores. It requires a specialized dissolution of previously formed cellular, high-magnesium calcite. Such dissolution is common in coralline algae; it occurs in the formation of the ubiquitous ‘cell fusions’ in some genera and during the ontogeny of conceptacles of most genera. However, as a circumferential depression in conceptacles … , it appears to be unique to species of Mesophyllum … ’ (Athanasiadis et al. Citation2004, p. 163).
Development of larger tetrasporangia or bisporangia would similarly affect the architecture of canals in multiporate conceptacles, requiring modifications to solve the ‘problem’ of larger spores passing through the canals. Such a connection is apparent in taxa displaying triangular or pyriform canals (j–m), but even the development of thinner-wider pore cells (c–d), or reduction of the canal length (f, h, i) would affect positively the discharge of larger spores. Tetra-(bi)sporangia in Mesophyllaceae show considerable variation in size, reaching a breadth of 100–180 µm in several species of Mesophyllum, e.g. in M. lichenoides (Woelkerling and Irvine Citation1986, p. 388), M. aleuticum P.A. Lebednik, M. vancouveriense, M. conchatum, M. lamellatum (Athanasiadis et al. Citation2004, table 1), M. crassiusculum (Athanasiadis Citation2007b, table 2), M. expansum (Philippi) Cabioch & M.L. Mendoza (Athanasiadis and Neto Citation2010, p. 336), but are significantly thinner (25–120 µm) in most species of Leptophytum and Clathromorphum which display non-differentiated pore cells in straight canals, as e.g. in Leptophytum elatum Y.M. Chamb. (Chamberlain and Irvine Citation1994, p. 172), L. tenue, L. foecundum, L. julieae, L. adeyi R.S. Steneck & R.T. Paine, and L. microsporum (Foslie) Athanas. & W.H. Adey (Athanasiadis and Adey Citation2006, table 1), Clathromorphum circumscriptum (Strömf.) Foslie (Strömfelt Citation1886, p. 20; Foslie Citation1905, p. 88–95; Kjellman Citation1889, p. 22, as C. durum), and in C. compactum (Kjellm.) Foslie (Masaki Citation1968, p. 20).
Larger carpospores, tetraspores or bispores may have resulted via polyploidy which is common in the plant and algal kingdoms, but if polyploidy is the driving force leading to the here recognized canal differentiations is uncertain, since cytological studies are rare in the coralline algae and are totally lacking in members of the multiporate families (Cole Citation1990, p. 82, table 4–1).
Taxonomic conclusions
The present analysis and evaluation of pore cell, pore filament, and canal shape differentiations in the Mesophyllaceae led to the full appreciation of several unique types in several members from the southern hemisphere. This, however, alone cannot support taxonomic recognition, requiring the investigation of several other reproductive and vegetative characters to examine the status of these members as putative new species or genera. The study of little-known species, including the examination of their types, is a further necessity that requires access to historical collections – a need that becomes difficult to satisfy when several international herbaria, such as L, PC, S no longer provide collections on loan. Given, however, the information that guarantees higher taxon assignment, and an independent life history that stands for continuity in time, the finding of new characters or character combinations does support recognition of unique life forms. In the four new genera and the two new species of Mesophyllaceae here described we have all the prerequisites.
Acknowledgements
Sincere thanks are due to Tommy Prestø (TRH), Jennifer Dalen (WELT), Joanna Wilbraham (BM), and William Woelkerling and Adele Harvey (LTB) for arranging the loans of collections to GB. Two reviewers and the subject editor, made comments on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Notes
1 The same applies to the recently described ‘Crustaphytym’ (Liu et al. Citation2018, figures 3d, e, 4d–f) and ‘Tectolithon’ (Jesionek et al. Citation2020, p. 12, ‘no diagnostic morpho-anatomical features … observed’) in order to be validated (Art.44.2). These two putative new genera possess a combination of characters accommodated within the concept of Leptophytum (Athanasiadis and Adey Citation2006, p. 72) that includes species with ‘ … regular or rare patches of coaxial cells arranged in series of 2–15 arching rows’, ‘few branched (dendroid) SMCs, … centrally or in other places on the chamber floor’, and conceptacle overgrowth by peripheral filaments (Athanasiadis and Adey l.c., figures 105, 140) – even if the latter character is not evident in the generitype. Moreover, the postulation of ‘basal [pore] cells [in multiporate conceptacles of ‘Crustaphytym’] … smaller than the upper ones (figure 3g, h)’ (Liu et al. Citation2018, p. 3461) requires documentation, while ‘tetrasporangial conceptacle chambers relatively smaller (130–155 μm high by 115–200 μm wide)’ (Liu et al. Citation2018, p. 3467), have also been described in Leptophytum microsporum (Foslie) Athanasiadis and Adey (Citation2006, table 1, 70–110 × 110–220 µm). Current data suggest that specimens referred to ‘Crustaphytym’ and ‘Tectolithon’ display the plesiomorphic (non-differentiated) type of canals in multiporate conceptacles.
References
- Adey WH. 1966. The genera Lithothamnium, Leptophytum (nov. gen.) and Phymatolithon in the Gulf of Maine. Hydrobiologia. 28:321–370. doi:https://doi.org/10.1007/BF00130389
- Adey WH. 1970. A revision of the Foslie crustose coralline herbarium. Det Kongelige Norske Videnskabers Selskabs Skrifter. 1:1–46. https://www.ntnu.no/ojs/index.php/DKNVS_skrifter/issue/archive.
- Adey WH, Athanasiadis A, Lebednik PA. 2001. Re-instatement of Leptophytum and its type Leptophytum laeve: taxonomy and biogeography of the genera Leptophytum and Phymatolithon (Corallinales, Rhodophyta). European Journal of Phycology. 36:191–203. doi:https://doi.org/10.1080/09670260110001735338
- Adey WH, Lebednik PA. 1967. Catalog of the Foslie herbarium. Trondheim: Det Kongelige Norske Videnskabers Selskabs Museet. p. 92.
- Adey WH, Sperapani CP. 1971. The biology of Kvaleya epilaeve, a new parasitic genus and species of Corallinaceae. Phycologia. 10:29–42. doi:https://doi.org/10.2216/i0031-8884-10-1-29.1
- Athanasiadis A. 1999. Mesophyllum macedonis nov. sp. (Rhodophyta, Corallinales), a putative Tethyan relic in the North Aegean Sea. European Journal of Phycology. 34:239–252. doi:https://doi.org/10.1080/09670269910001736302
- Athanasiadis A. 2001. Lectotypification of Lithophyllum arcticum Kjellman (Corallinales, Rhodophyta) and a study of its relationships within the Melobesioideae. Nordic Journal of Botany. 21:93–112. doi:https://doi.org/10.1111/j.1756-1051.2001.tb01343.x
- Athanasiadis A. 2007a. The genus Leptophytum (Melobesioideae, Corallinales, Rhodophyta) in NW Spitsbergen. Nordic Journal of Botany. 24:469–499. doi:https://doi.org/10.1111/j.1756-1051.2004.tb00851.x
- Athanasiadis A. 2007b. Revision of Dawson’s collections referred to Lithothamnion lamellatum (Melobesioideae, Corallinales, Rhodophyta). Nova Hedwigia. 85:195–242. doi:https://doi.org/10.1127/0029-5035/2007/0085-0195
- Athanasiadis A. 2008. Two endemic coralline red algae on the northern limits of the latest glaciation. Taxon. 57:223–230. doi:https://doi.org/10.2307/25065962
- Athanasiadis A. 2016a. Leptophytum flavescens comb. nov. (Corallinales, Rhodophyta), an Arctic endemic from the sublittoral of NW Spitsbergen, North Norway, and western Novaya Zemlya, with epitypification of L. laeve. Marine Biology Research. 12:551–558. doi:https://doi.org/10.1080/17451000.2016.1164320
- Athanasiadis A. 2016b. Phycologia europaea Rhodophyta, Vols 1 & 2. Gothenburg: Published by the author. p. xlviii + 1504.
- Athanasiadis A. 2017a. A study of the original material of Lithothamnion engelhartii Foslie (Corallinales, Rhodophyta). Botanica Marina. 60:67–78. doi:https://doi.org/10.1515/bot-2016-0110
- Athanasiadis A. 2017b. Capensia fucorum (Esper) gen. et comb. nov. (Mesophyllaceae, Corallinales, Rhodophyta), a hemiparasite on Gelidium from South Africa. Botanica Marina. 60:555–565. doi:https://doi.org/10.1515/bot-2017-0027
- Athanasiadis A. 2018a. Zygote transfer and post-fertilization stages in Mesophyllum (Mesophyllaceae, Corallinales, Rhodophyta). Nova Hedwigia. 107:519–529. doi:https://doi.org/10.1127/nova_hedwigia/2018/0489
- Athanasiadis A. 2018b. Carlskottsbergia antarctica (Hooker fil. & Harv.) gen. & comb.nov., with a re-assessment of Synarthrophyton (Mesophyllaceae, Corallinales, Rhodophyta). Nova Hedwigia. 108(2019):291–320. doi:https://doi.org/10.1127/novahedwigia/2018/0506
- Athanasiadis A. 2019. Amphithallia, a genus with four-celled carpogonial branches and connecting filaments in the Corallinales (Rhodophyta). Marine Biology Research. 15:13–25. doi:https://doi.org/10.1080/17451000.2019.1598559
- Athanasiadis A. 2020. Phragmope discrepans, gen. & comb. nov. (Mesophyllaceae, Corallinales, Rhodophyta), the species known as ‘Mesophyllum engelhartii’ from South Africa. Marine Biology Research. 16:532–549. doi:https://doi.org/10.1080/17451000.2020.1831543
- Athanasiadis A, Adey WH. 2003. Proposal to conserve the name Leptophytum laeve Strömfelt against L. laeve Kützing (Corallinales, Rhodophyta) with a conserved type. Taxon. 52:342–350. doi:https://doi.org/10.2307/3647411
- Athanasiadis A, Adey WH. 2006. The genus Leptophytum (Melobesioideae, Corallinales, Rhodophyta) on the Pacific coast of North America. Phycologia. 45:71–115. doi:https://doi.org/10.2216/04-38.1
- Athanasiadis A, Ballantine DL. 2014. The genera Melyvonnea nov.gen. and Mesophyllum sensu stricto (Melobesioideae, Corallinales), especially in the central Atlantic Ocean. Nordic Journal of Botany. 32:385–436. doi:https://doi.org/10.1111/njb.00265
- Athanasiadis A, Lebednik PA, Adey WH. 2004. The genus Mesophyllum (Melobesioideae, Corallinales, Rhodophyta) on the Northern Pacific coast of North America. Phycologia. 43:126–165. doi:https://doi.org/10.2216/i0031-8884-43-2-126.1
- Athanasiadis A, Neto AI. 2010. On the occurrence of Mesophyllum expansum (Philippi) Cabioch et Mendoza (Melobesioideae, Corallinales, Rhodophyta) in the Mediterranean Sea, the Canary Isles and the Azores. Botanica Marina. 53:33–341. doi:https://doi.org/10.1515/BOT.2010.042
- Basso D, Rodondi G, Bressan G. 2011. A re-description of Lithothamnion crispatum and the status of Lithothamnion superpositum (Rhodophyta, Corallinales). Phycologia. 50:144–155. doi:https://doi.org/10.2216/10-20.1
- Bittner L, Payri CE, Maneveldt GW, Couloux A, Cruaud C, de Reviers B, Le Gall L. 2011. Evolutionary history of the Corallinales (Corallinophycidae, Rhodophyta) inferred from nuclear, plastidial and mitochondrial genomes. Molecular Phylogenetics and Evolution. 61:697–713. https://doi.org/https://doi.org/10.1016/j.ympev.2011.07.019
- Bold HC, Wynne MJ. 1978. Introduction to the algae structure and reproduction. Englewood Cliffs, NJ: Prentice-Hall. p. 706.
- Broom JES, Farr DR, Nelson WA, Neill KF, Harvey AS, Woelkerling WJ. 2008. Utility of psbA and nSSU for phylogenetic reconstruction in the Corallinales based on New Zealand taxa. Molecular Phylogenetics and Evolution. 46:958–973. doi:https://doi.org/10.1016/j.ympev.2007.12.016
- Chamberlain YM, Irvine LM. 1994. Melobesioideae Bizzozero. In: Irvine LM, Chamberlain YM, editors. Seaweeds of the British Isles. Volume 1 Rhodophyta, Part 2B Corallinales, Hildenbrandiales. London: Natural History Museum, Pelagic Publishing; p. 159–234.
- Chamberlain YM, Keats D. 1994. Three melobesioid crustose coralline red algae from South Africa: Leptophytum acervatum (Foslie) nov. comb., L. foveatum sp. nov., and L. ferox (Foslie) nov. comb. Phycologia. 33:111–133. doi:https://doi.org/10.2216/i0031-8884-33-2-111.1
- Chapman VJ, Parkinson PG. 1974. Cryptonemiales. In: Chapman VJ, editor. The Marine Algae of New Zealand. Part III: Rhodophyceae. Lehre: J. Cramer; p. 155–278, 51–94 plates.
- Cole KM. 1990. Chromosomes. In: Cole KM, Sheath RG, editors. Biology of red algae. New York: Cambridge University Press; p. 73–102.
- Farr T, Broom J, Hart D, Neill K, Nelson W. 2009. Common coralline algae of northern New Zealand. An identification guide. NIWA Information Series 70, Wellington. p. 125. https://niwa.co.nz/sites/niwa.co.nz/files/common_coralline_algae_-_northern_nz_-_niwa_information_series_no_70.pdf.
- Foslie M. 1897. On some Lithothamnia. Det kongelige Norske Videnskabers Selskabs Skrifter. 1:1–20. https://www.ntnu.no/ojs/index.php/DKNVS_skrifter/issue/archive.
- Foslie M. 1900. New or critical calcareous algae. Det kongelige Norske Videnskabers Selskabs Skrifter. 1899(5):1–34. https://www.ntnu.no/ojs/index.php/DKNVS_skrifter/issue/archive.
- Foslie M. 1905. Remarks on northern Lithothamnia. Det kongelige Norske Videnskabers Selskabs Skrifter. 3:1–138. https://www.ntnu.no/ojs/index.php/DKNVS_skrifter/issue/archive.
- Foslie M. 1906a. Algologiske notiser. II. Det kongelige Norske Videnskabers Selskabs Skrifter. 2:1–28. https://www.ntnu.no/ojs/index.php/DKNVS_skrifter/issue/archive.
- Foslie M. 1906b. Den botaniske samling. Det kongelige Norske Videnskabers Selskabs Aarsberetning. 1905:17–24. https://www.ntnu.no/ojs/index.php/DKNVS_skrifter/issue/archive.
- Foslie M. 1907a. Algologiske notiser. III. Det kongelige Norske Videnskabers Selskabs Skrifter. 1906(8):1–34. https://www.ntnu.no/ojs/index.php/DKNVS_skrifter/issue/archive.
- Foslie M. 1907b. Algologiske notiser. IV. Det kongelige Norske Videnskabers Selskabs Skrifter. 1907(6):1–30. https://www.ntnu.no/ojs/index.php/DKNVS_skrifter/issue/archive.
- Foslie M. 1909. Algologiske notiser. VI. Det kongelige Norske Videnskabers Selskabs Skrifter. 2:1–63. https://www.ntnu.no/ojs/index.php/DKNVS_skrifter/issue/archive.
- Harvey AS, Woelkerling W, Farr T, Nelson W. 2005. Coralline algae of central New Zealand. Niwa Information Series. 57:1–145. https://niwa.co.nz/sites/niwa.co.nz/files/import/attachments/CentralNZ_IDCor_NIS57.pdf.
- Harvey AS, Woelkerling WJ, Millar AJK. 2003. An account of the Hapalidiaceae (Corallinales, Rhodophyta) in south-eastern Australia. Australian Systematic Botany. 16:647–698. doi:https://doi.org/10.1071/SB03008
- Holmgren PK, Holmgren NH, Barnett LC. 1990. Index herbariorum, part I. The herbaria of the world. Regnum Vegetabile. 120:1–693.
- Hrabovský J, Basso D, Doláková N. 2015. Diagnostic characters in fossil coralline algae (Corallinophycidae: Rhodophyta) from the Miocene of southern Moravia (Carpathian Foredeep, Czech Republic). Journal of Systematic Palaeontology. 14:499–525. doi:https://doi.org/10.1080/14772019.2015.1071501
- Jesionek MB, Bahia RG, Hernández-Kantún JJ, Adey WH, Yoneshigue-Valentin Y, Longo LL, Amado-Filho GM. 2016. A taxonomic account of non-geniculate coralline algae (Corallinophycidae, Rhodophyta) from shallow reefs of the Abrolhos Bank, Brazil. Algae. 31:317–340. doi:https://doi.org/10.4490/algae.2016.31.11.16
- Jesionek MB, Bahia RG, Lyra MB, Leão LAB, Oliveira MC, Amado-Filho GM. 2020. Newly discovered coralline algae in Southeast Brazil: Tectolithon fluminense gen.et sp.nov. and Crustaphytum atlanticum sp.nov. (Hapalidiales, Rhodophyta). Phycologia. 59:101–115. doi:https://doi.org/10.1080/00318884.2019.1702320
- Keats DW, Chamberlain YM. 1994. Two melobesioid coralline algae (Rhodophyta, Corallinales), Mesophyllum erubescens (Foslie) Lemoine and Mesophyllum funafutiense (Foslie) Verheij from Sodwana Bay, South Africa. South African Journal of Botany. 60:175–190. doi:https://doi.org/10.1016/S0254-6299(16)30630-5
- Keats DW, Chamberlain YM. 1997. The non-geniculate coralline algae Synarthrophyton eckloniae (Foslie) comb. nov., and S. magellanicum (Foslie) comb. nov. in South Africa including a comparison with the types of Lithophyllum schmitzii Hariot, Lithothamnion magellanicum, L. muelleri f. neglecta Foslie, L. lamellatum Setchell & Foslie, and L. chatamense Foslie. European Journal of Phycology. 32:55–79. doi:https://doi.org/10.1080/09541449710001719375
- Keats DW, Maneveldt G. 1997. First report of the melobesioid alga (Corallinales, Rhodophyta) Mesophyllum incisum (Foslie) Adey in South Africa. South African Journal of Botany. 63:201–209. doi:https://doi.org/10.1016/S0254-6299(15)30745-6
- Kjellman FR. 1883. Norra Ishafvets Algflora. Vega-Expeditionens Vetenskapliga Arbeten. 3:1–431. 31 plates.
- Kjellman FR. 1885. The Algae of the Arctic Sea. Öfversigt af Kongliga Vetenskaps-Akademiens Förhandlingar. 20(5):1–350 + 1 + 31 plates.
- Kjellman FR. 1889. Om Beringhafvets algflora. Kongliga Svenska Vetenskaps-Akademiens Handlingar. N.F. 23:1–58 + 7 plates.
- Lebednik PA. 1977a. The Corallinaceae of northwestern North America. I. Clathromorphum Foslie emend. Adey Syesis. 9:59–112.
- Lebednik PA. 1977b. Postfertilization development in Clathromorphum, Melobesia and Mesophyllum with comments on the evolution of the Corallinaceae and the Cryptonemiales (Rhodophyta). Phycologia. 16:379–406. doi:https://doi.org/10.2216/i0031-8884-16-4-379.1
- Liu L-C, Lin S-M, Caragnano A, Payri C. 2018. Species diversity and molecular phylogeny of non-geniculate coralline algae (Corallinophycidae, Rhodophyta) from Taoyuan algal reefs in northern Taiwan, including Crustaphytum gen.nov. and three new species. Journal of Applied Phycology. 30:3455–3469. doi:https://doi.org/10.1007/s10811-018-1620-1
- Maneveldt GW, Keats DW, Chamberlain YM. 2007. Synarthrophyton papillatum sp.nov.: A new species of non-geniculate coralline algae (Rhodophyta, Corallinales, Hapalidiaceae) from South Africa and Namibia. South African Journal of Botany. 73:570–582. doi:https://doi.org/10.1016/j.sajb.2007.05.003
- Masaki T. 1968. Studies on the Melobesioideae of Japan. Memoirs of the Faculty of Fisheries, Hokkaido University. 16:1–80. plates 1–79.
- May DI, Woelkerling WJ. 1988. Studies on the genus Synarthrophyton (Corallinaceae, Rhodophyta) and its type species, S. patena (J.D. Hooker et W.H. Harvey) Townsend. Phycologia. 26:50–71. doi:https://doi.org/10.2216/i0031-8884-27-1-50.1
- Peña V, Adey WH, Riosmena-Rodríguez R, Jung M-Y, Afonso Carrillo J, Coi H-G, Bárbara I. 2011. Mesophyllum sphaericum sp. nov. (Corallinales, Rhodophyta): a new maërl-forming species from the northeast Atlantic. Journal of Phycology. 47:911–927. doi:https://doi.org/10.1111/j.1529-8817.2011.01015.x
- Printz H. 1929. M. Foslie: contributions to a monograph of the Lithothamnia. Trondheim: Det kongelike Norske Videnskabers Selskabers Museet. p. 60, 75 plates.
- Ricker RW. 1987. Taxonomy and biogeography of Macquarie Island seaweeds. London: British Museum (Natural History). p. viii + 344.
- Sanmartin I, Ronquist F. 2004. Southern Hemisphere biogeography inferred by event-based models: plant versus animal patterns. Systematic Biology. 53:216–243. doi:https://doi.org/10.1080/10635150490423430
- Sissini MN, Oliveira MC, Gabrielson PW, Robinson NM, Okolodkov YB, Riosmena-Rodriguez R, Horta PA. 2014. Mesophyllum erubescens (Corallinales, Rhodophyta) – so many species in one epithet. Phytotaxa. 190:299–319. doi:https://doi.org/10.11646/phytotaxa.190.1.18
- Strömfelt HFG. 1886. Om Algvegetationen vid Islands Kuster. Doctoral Thesis. Gothenburg: Uppsala University. p. 89. (in Swedish)
- Suneson S. 1937. Studien über die Entwicklungsgeschichte der Corallinaceen. Lund: Akademische Abhandlung, H. Ohlssons Bochdruckerei. p. 102, 4 plates.
- Townsend RA. 1979. Synarthrophyton, a new genus of Corallinaceae (Cryptonemiales, Rhodophyta) from the southern Hemisphere. Journal of Phycology. 15:251–259. doi:https://doi.org/10.1111/j.1529-8817.1979.tb02634.x
- Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, et al. 2018. International code of nomenclature for algae, fungi, and plants (Shenzhen code). Regn Veg. 159:xxxviii + 253. https://www.iapt-taxon.org/nomen/main.php.
- Verheij E. 1993. Marine plants on the reefs of the Spermonde Archipelago, southwest Sulawesi, Indonesia: aspects of taxonomy, floristics, and ecology [PhD thesis]. Nieuw-Lekkerland: University of Leiden, Offsetdrukkerij Van der Perk BV. p. 320.
- Wilks KM, Woelkerling WJ. 1995. An account of southern Australian species of Lithothamnion (Corallinaceae, Rhodophyta). Australian Systematic Botany. 8:549–583. doi:https://doi.org/10.1071/SB9950549
- Woelkerling WJ. 1988. The coralline red algae: an analysis of the genera and subfamilies of nongeniculate corallinaceae. London: British Museum (Natural History), Oxford University Press. p. xi + 268.
- Woelkerling WJ. 1993. Type collections of Corallinales (Rhodophyta) in the Foslie Herbarium (TRH). Gunneria. 67:1–289. https://www.ntnu.no/c/document_library/get_file?uuid=929a1e6d-f505-4239-b1ef-71143bb439da&grou pId=10476.
- Woelkerling WJ. 1996. Subfamily Melobesioideae Bizzozero. In: Womersley HBS, editor. The marine benthic flora of southern Australia. Rhodophyta – Part IIB Gracilariales, Rhodymeniales, Corallinales and Bonnemaisoniales. Canberra: Australian Biological Resaerch Study & State Herbarium of South Australia; p. 164–210.
- Woelkerling WJ, Foster MS. 1989. A systematic and ecographic account of Synarthrophyton schielianum sp.nov. (Corallinaceae, Rhodophyta) from the Chatham Islands. Phycologia. 28:39–60. doi:https://doi.org/10.2216/i0031-8884-28-1-39.1
- Woelkerling WJ, Harvey A. 1992. Mesophyllum incisum (Corallinaceae, Rhodophyta) in Southern Australia: implications for generic and specific delimitation in the Melobesioideae. British Phycological Journal. 27:381–399. doi:https://doi.org/10.1080/00071619200650321
- Woelkerling WJ, Harvey A. 1993. An account of southern Australian species of Mesophyllum (Corallinaceae, Rhodophyta). Australian Systematic Botany. 6:571–637. doi:https://doi.org/10.1071/SB9930571
- Woelkerling WJ, Gustavsen G, Myklebost HE, Prestø T, Såstad SM. 2005. The coralline red algal herbarium of Mikael Foslie: revised catalogue with analyses. Gunneria. 77:1–625. https://www.ntnu.no/c/document_library/get_file?uuid=6350bf35-5dd1-45c2-9003-2c85ea91d520&groupId=10476.
- Woelkerling WJ, Irvine LM. 1986. The neotypification and status of Mesophyllum (Corallinaceae, Rhodophyta). Phycologia. 25:379–396. doi:https://doi.org/10.2216/i0031-8884-25-3-379.1
