?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The Arabian carpet shark, Chiloscyllium arabicum is endemic to the Arabian Gulf and the northern Arabian Sea. The aim of this work is to study the reproduction and maturity of this species. A total of 87 males and 139 females, including 19 egg-bearing individuals collected from bottom set longline fisheries in the Saudi Arabian waters of the Arabian Gulf between October 2017 and February 2019, were examined. The size at 50% maturity was estimated as 62.3 and 62.5 cm total length (TL) for males and females, respectively. Changes in the gonadosomatic index (GSI) of males indicated the mating to happen between December and February. The maximum follicle diameter, oviducal gland width and GSI in mature females, suggested the ovulation to extend from September to May with the peak during March–May. Females carried 2–4 egg cases in the uteri, and the egg-laying period was between February and May. The segregation of sexes was observed in the larger (70–80 cm TL) size ranges.
Introduction
The Arabian carpet shark, Chiloscyllium arabicum Gubanov, 1980 (Family Hemiscylliidae) is a shark species endemic to the Arabian Gulf (also known as Persian Gulf, hereafter referred to as the ‘Gulf’) and the northern Arabian Sea, including Iran, Pakistan and India (Carpenter et al. Citation1997; Compagno Citation2001; Moore Citation2017). This small shark species, reaching 80 cm total length (TL), typically occurs at coastal benthic habitats, including coral reefs, lagoons, rocky shores, and mangrove estuaries, and it has been found to feed on squid, other molluscs, crustaceans and snake eels (Compagno Citation2001; Weigmann Citation2016; Falahiehzadeh and Salamat Citation2020). In the Gulf, it is not a species targeted by commercial fisheries, but appears to be a major bycatch or incidental catch of trawl and bottom set longline fisheries (Jabado et al. Citation2015, Citation2017; Hsu et al. Citation2022).
Chiloscyllium arabicum is rare in the landings of the Gulf due to discarding of most specimens after capture (Almojil et al. Citation2015; Jabado et al. Citation2015; Hsu et al. Citation2022). This species may have relatively high post-capture survival rates (Jabado et al. Citation2017). The main threats faced by this species in its distribution range include degradation and loss of habitats, leading to a 20–30% decline in population over the last three generations (Jabado et al. Citation2017; Moore Citation2017). Hence, C. arabicum was addressed as ‘Near Threatened’ on the IUCN (International Union for Conservation of Nature) Red List of Threatened Species in 2009, and the reassessment made in the year 2017 revealed the same status (Moore Citation2009, Citation2017).
Knowledge on the ecology and biology of elasmobranchs, such as size at maturity, fecundity, reproductive season and cycle, is crucial for stock assessment and further ecological risk assessment, and thus enabling appropriate management measures (Chen and Liu Citation2006; Liu et al. Citation2021). However, studies on the biology of this species, in particular reproduction, are scarce. This shark is oviparous and lays up to four egg cases which hatch after 70–80 days of incubation based on observations made in aquaria (Compagno Citation2001). The size at maturity was estimated to vary between 45 and 54 cm TL (Compagno Citation2001). Moore and Peirce (Citation2013) found males to be mature at about 55 cm TL based on 42 specimens from Bahrain waters of the Gulf. An egg-bearing female measuring 52.5 cm TL was collected from the Gujarat waters in India (Dash et al. Citation2013). Abed (Citation2017) reported the length of 35 specimens collected from Iraqi waters, which ranged from 26.2–73.1 cm TL, showing significant differences in some characters between males and females. Porforugh et al. (Citation2018) described the ovarian maturity using histology and steroid hormone assay based on 130 females (mean TL of mature specimens 63.5 ± 13.7 cm) collected from the northwestern waters of the Gulf. The annual reproductive cycle was also investigated based on steroid hormones measurements (Falahiehzadeh and Salamat Citation2020).
In view of the highly variable life history information available, the present study was undertaken to investigate the maturity and reproduction of C. arabicum in the Saudi Arabian waters of the Gulf with the samples collected from the southern waters of the Gulf to supplement sampling gaps and to clarify regional variance.
Materials and methods
Sample collection and preparation
A total of 226 Arabian carpet sharks, including 87 males (58.0–74.2 cm TL) and 139 females (57.1–77.9 cm TL), was collected from the catches of bottom set longlines in Jubail, Qatif, and Ras Abu Gamys of the Saudi Arabian waters of the Gulf during October 2017–February 2019 (, ). Fishermen set longlines at depths of 10–55 m in Jubail and Qatif, and < 20 m in Ras Abu Gamys, and the main feature of the fishing grounds was sandy bottom with partial seagrass and rocks, and occasionally with scattered coral heads. The precaudal length (PCL) of 130 specimens, TL of 225 specimens, clasper length (for male, CL) of 85 male specimens, total weight (TW) of 225 specimens, and body weight (gutted weight, BW) of 224 specimens were recorded. After dissection, the reproductive tract, liver, oviducal glands, uteri and stomachs were removed for analysis. The sample size in the present study differed as the internal organs of several specimens got damaged during dissection. Therefore, the liver weight of 226 specimens, testes weight of 83 specimens, testis width of 80 specimens, ovary weight of 129 specimens, maximum follicles diameter (MFD) of 125 specimens, oviducal gland width of 85 specimens and uterus width of 116 specimens were recorded.
Figure 1. Study area showing the sampling sites for Chiloscyllium arabicum in the Saudi Arabian waters of the Arabian Gulf.
Table I. Monthly sampling information.
Linear regression was used to describe the relationship between TL and PCL. A potential regression was used to explain the relationship of TW with TL and BW with TL using the following equation:
where a–d are parameters to be estimated.
The relationship of TW-TL and BW-TL among sexes was tested using the chi-square test of maximum likelihood ratios (Kimura Citation1980).
Sex ratio
The sex ratio was calculated in terms of proportion of male: female ratio. The homogeneity of sex ratio was examined by the chi-square test (Chen and Liu Citation2006).
Maturity stage
Sexual maturity of males was assessed using the degree calcification of clasper. Males were considered as mature when: (a) clasper and rhipidion are fully formed and spread open in a fresh specimen; (b) base of the clasper rotates easily so that the clasper can be directed anteriorly; (c) stem cartilages become hardened and calcified; and (d) presence of viable sperm in testes or pouring semen from claspers (Chen and Liu Citation2006).
The following four maturity stages were determined in females: (a) ovaries thin and with no visible follicles, oviducts threadlike, uteri indistinct and no visible oviducal gland; (b) follicles developing but not or less distinct, uteri thickening but not vascularized, and oviducal gland enlarging; (c) ovary with vitellogenic follicles, uteri well developed or egg cases found in oviduct or in uteri, heart-shaped oviducal gland developed; and (d) vitellogenic and developing follicles coexist, uteri developed and vascularized but flaccid. Specimens in stages (a) and (b) were classified as immature, while stages (c) and (d) as mature (Chen and Liu Citation2006; Falahiehzadeh and Salamat Citation2020).
The size at 50% maturity in male and female was determined following the logistic model: Y = [1 + e -(a + bX)]−1, where Y is the binomial maturity data (immature = 0, mature = 1), X is total length (cm), and a and b are parameters to be estimated (Chen and Liu Citation2006). The logistic model was applied to all data and fitted using the statistical programming language R (R Development Core Team; www.r-project.org).
Reproduction
The mating period was estimated based on the monthly gonadosomatic index (GSI, testes weight as percentage of total weight) of mature males. According to Falahiehzadeh and Salamat (Citation2020), the diameter of mature follicle in C. arabicum was around 0.8 cm. The ovulation period was determined based on the monthly GSI (right ovary weight as percentage of total weight), MFD and oviducal gland width of mature females, besides months having the presence of pre-ovulatory follicle. Chiloscyllium spp. lay egg cases over a short period when they move from oviducal glands to the uteri (Chen and Liu Citation2006). Therefore, the egg-laying period was estimated based on the presence of egg cases in uteri in relation to month. The hepatosomatic index (HSI, liver weight as percentage of total weight) was used to observe energy shift during reproduction. The one-way analysis of variance (ANOVA) with post hoc Tukey’s test was used to test the homogeneity of GSI, MFD, HSI and oviducal gland width among months (Chen and Liu Citation2006; Fahmi et al. Citation2021).
Results
Length–weight relationship
The relationship between PCL and TL was linear as shown by the following equation:
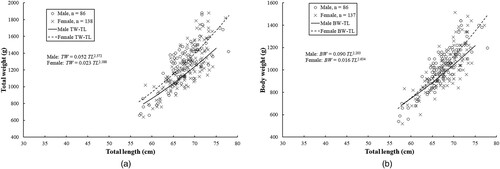
The relationship of TW with TL differed significantly between the sexes (P < 0.01) and the following were the equations derived (A):
The relationship of BW with TL was also significantly different between the sexes (P < 0.01) and the following exponential curves were obtained (B):
Size at maturity
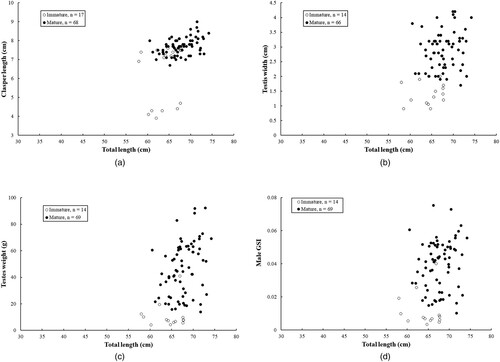
As small-sized sharks were not found due to gear selectivity, gap on early development still remains. Based on clasper development, the smallest mature male measured 60.5 cm TL, and the largest immature male 67.7 cm TL with an average TL of 64.1 cm. All the specimens were mature when the clasper length was > 7.6 cm, testis width > 2.0 cm and testes weight > 41 g, or GSI > 0.04 (). Conversely, specimens were all immature when the clasper length was < 6.7 cm, testis width < 1.7 cm and testes weight < 14 g, or GSI < 0.01 ().
Figure 3. Relationships of (A) clasper length, (B) testis width, (C) testes weight, and (D) gonadosomatic index (GSI) versus total length for male Arabian carpet sharks used in this study.
Among females, the smallest mature individual measured 61.6 cm TL, and the largest immature individual 77.9 cm TL with an average of 69.8 cm TL. In total 19 females ranging from 64.2–73.1 cm TL were found to be egg bearing. All specimens were mature when the oviducal gland width was > 2.3 cm or the uterus width was > 2.5 cm, and specimens were totally immature when the oviducal gland width was smaller than 1.0 cm or the uterus width was smaller than 0.7 cm ().
Figure 4. Relationship of (A) oviducal gland width and (B) uterus width versus total length in female Arabian carpet sharks.
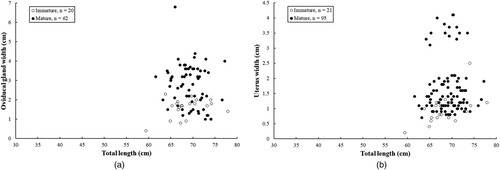
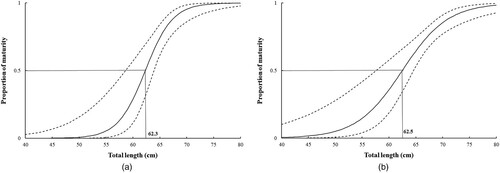
Size at 50% maturity in males and females was calculated as 62.3 and 62.5 cm TL, respectively (A and B). The following equations were obtained relating the per cent maturity with TL:
Seasonality of the reproduction
No mature male was collected during June–October, and significant differences in the HSI (F = 5.71, df = 6, P < 0.01) and the GSI (F = 12.22, df = 6, P < 0.01) were noticed. Significantly higher value of the HSI was observed during November–December (P < 0.05). The GSI was also higher from November, both the values declined after February, and the values in December and February were significantly higher (P < 0.05) than those in March and April, suggesting at least one mating period from December to February (A and B).
Figure 6. Monthly average values of mature Arabian carpet sharks for (A) male and female hepatosomatic index (HSI); (B) male and female gonadosomatic index (GSI); (C) female maximum follicle diameter (MFD); and (D) female oviducal gland width. Vertical bars indicate ± SD.
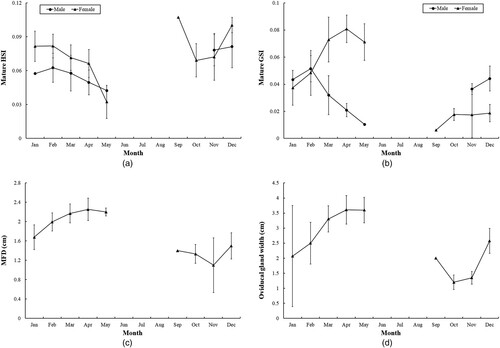
The values of HSI in mature females differed significantly among months (F = 12.98, df = 8, P < 0.01). Significantly higher values (P < 0.05) were found during December–February, and the value in May was significantly lower (P < 0.01) (A). Significant differences were also observed in the values of GSI (F = 22.63, df = 8, P < 0.01), MFD (F = 22.44, df = 8, P < 0.01) and oviducal gland width (F = 6.71, df = 8, P < 0.01) among months. Some mature females collected during September–February contained follicles of > 0.8 cm, indicating that ovulation could take place in September (). Although no adult females were found during June–August, significantly higher average values of GSI (P < 0.01), MFD (P < 0.05) and oviducal gland width (P < 0.05) occurred consistently from March to May, suggesting that the Arabian carpet sharks ovulated mainly from March to May at least (B–D).
Figure 7. Monthly distribution of the maximum follicle diameter in the female Arabian carpet sharks.
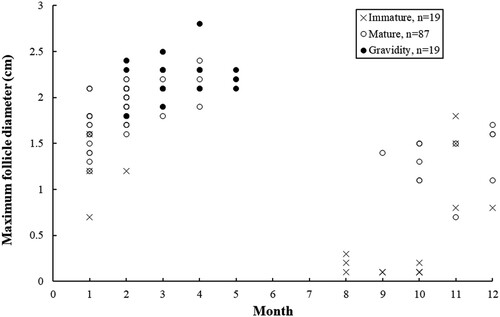
No neonatal shark was observed during the study period. However, egg-bearing females were found during February–May. Among the 19 egg-bearing females observed, 3 specimens were recorded in February, 7 in March, 5 in April and 4 in May. Based on this, the egg-laying period of the C. arabicum was estimated to last from February to May.
In females, only the right ovary was functional. However, both the oviducal glands and uteri were functional. Maternal specimens contained 2–4 egg cases (9 specimens had 2 egg cases, 2 specimens had 3 egg cases, and 8 specimens had 4 egg cases with an average of 2.95 egg cases) in both uteri.
Although no adult males and females were found during June–October and June–August, respectively, males collected during August–October and females collected in August were close or over the size at 50% maturity (males: 58.5–67.7 cm TL, mean 65.3 cm TL; females: 65.3–73.2 cm TL, mean 68.1 cm TL), but still had very low GSI (males: 0.003–0.010 mean 0.007; females: 0.002–0.004, mean 0.003). Either mature male GSI or mature female GSI, MFD and oviducal gland width had only one significant peak during the year. In addition, developing and mature follicles (MFD > 0.8 cm) were found in most of the adult females including egg-bearing specimens, indicating only a very short or no resting period. Therefore, the reproductive cycle classified as annual is more appropriate ( and ).
Sex ratio
The overall sex ratio of 0.63 (87 males and 139 females), differed significantly from the expected 1:1 ratio (χ2 = 11.96, P < 0.01). The monthly sex ratio also differed significantly (χ2 = 21.71, P < 0.01). Females were dominant during January–February and the sex ratio differed significantly from 1:1 (χ2 = 20.05, P < 0.01) and also during October–November (χ2 = 5.54, P < 0.05). Males were dominant in December and the sex ratio deviated significantly from the expected 1:1 ratio (χ2 = 4.26, P < 0.05) (). In terms of size groups (5-cm size classes), the sex ratio differed significantly (χ2 = 7.50, P < 0.05), and also in the larger size ranges of 70–80 cm TL (χ2 = 15.34, P < 0.01) due to the predominance of females (). In terms of fishing grounds also, the sex ratio differed significantly (χ2 = 8.24, P < 0.05), and also in Jubail (χ2 = 18.08, P < 0.01) due to the predominance of females ().
Table II. Monthly sex ratio.
Table III. Sex ratio by size range.
Table IV. Sex ratio by fishing ground.
Discussion
Elasmobranch fishes are rather common in the commercial catches of eastern Saudi Arabian waters. However, the Arabian carpet shark was found to be quite rare in the landings and fishery outlets, as this species is commonly discarded at sea (Jabado et al. Citation2015; Hsu et al. Citation2022). Therefore, specimens for the present study were obtained from the fishermen upon our request. However, no specimens were collected during June–July due to it being the non-fishing season within the sampling period. In addition, small-sized individuals could not be collected due to gear selectivity.
Our estimate on size at maturity (62.3–62.5 cm TL) is higher than that of Compagno (Citation2001) (45–54 cm TL) and Moore and Peirce (Citation2013) (55 cm TL for males). The present estimate for females (62.5 cm TL) is close to the estimate (63.5 cm TL) in the northern Gulf (Falahiehzadeh and Salamat Citation2020). However, the average TL of 30 mature females collected by Porforugh et al. (Citation2018) also from the northern Gulf during September–December was 55.6 cm only. A female measuring 52.5 cm TL in the northeastern Arabian Sea was egg-bearing (Dash et al. Citation2013), whereas the smallest mature female collected in this study was 61.6 cm TL, close to the value of 62 cm TL reported by Falahiehzadeh and Salamat (Citation2020), and the average TL of egg-bearing females in the Gulf was 68–69 cm. Geographic variations in size at first maturity and reproduction have been observed in several shark species. The population of brownbanded bamboo shark Chiloscyllium punctatum Müller & Henle, 1838, in the Indo-Malay region was found to be mature at 67–70 cm TL, while in Australia and Papua New Guinea, this species was found to be mature at 84–87 cm TL (Fahmi Citation2021). The whitespotted bamboo shark Chiloscyllium plagiosum (Bennett, 1830) occurring in the Indian Ocean was found to be mature at 50–63 cm TL, while in the northwestern Pacific, it was found to be mature at 64.9–65.6 cm TL (Chen and Liu Citation2006; White et al. Citation2006). Intraspecific difference in size at maturity may be due to many reasons such as water temperature, or higher levels of fishing pressure leading to an earlier maturity (Baje et al. Citation2018). The reason for difference in size at maturity between the populations of Arabian carpet sharks occurring in the southern Gulf and the northern Arabian Sea (same latitude) may possibly be due to geographic variation or segregation of populations, which can be confirmed through further studies using molecular biology and electronic tracking.
Falahiehzadeh and Salamat (Citation2020) reported that only the left uterus and one side of the ovary were functional in C. arabicum. However, the exact side of the functional ovary was not mentioned. But in the same report, both sides of the uterus were shown to carry egg cases (Falahiehzadeh and Salamat Citation2020). In addition, the egg-bearing female caught from India was reported to have two eggs, one on either side of uterus meaning that both are functional (Dash et al. Citation2013). The structure and function of sexual organs in C. arabicum were found to be similar in the congeners such as Chiloscyllium griseum Müller & Henle, 1838, C. punctatum and C. plagiosum (Devadoss Citation1986; Chen and Liu Citation2006; Fahmi et al. Citation2021).
In C. punctatum, Falahiehzadeh and Salamat (Citation2020) deduced the mating period of C. arabicum to extend from April to early June. However, GSI of mature male indicated the mating to take place during December–February. In C. plagiosum occurring in the northwest Pacific, the mating season similarly was found to last during December–January (Chen and Liu Citation2006). Chiloscyllium punctatum occurring in Indonesian coastal waters was also supposed to mate during southern hemisphere winter (July–September) (Fahmi et al. Citation2021).
The mating period was found to extend from December to February. Although the ovulation period occurred mainly during March–May, the earliest ovulation happened in September. Thus, the time lag implies that remaining sperm might be stored in the oviducal glands for 7 months (from February to September) in C. arabicum. Pratt (Citation1993) defined three patterns of sperm storage and insemination as non-storage/immediate insemination, short-term storage/delayed insemination, and long-term storage/repeated insemination, while long-term storage/repeated insemination pattern could store sperm for months to years. Chiloscyllium arabicum which might store sperm for 7 months was classified as long-term storage. In fact, the duration of sperm storage has been documented in the congeners (Miki Citation1994; Masuda Citation1998; Chen and Liu Citation2006; Bernal et al. Citation2015). The longest period of sperm storage (45 months) among elasmobranchs was observed in C. punctatum (Bernal et al. Citation2015).
Egg-bearing females in the Arabian Sea were found during April (Dash et al. Citation2013), and in the north Arabian Gulf, egg cases in uteri were recorded from April–May only (Falahiehzadeh and Salamat Citation2020). In the southern Gulf, egg cases were present in uteri from February–May. Females which become mature during February–March have the ability to deposit egg cases (Falahiehzadeh and Salamat Citation2020).
In elasmobranchs, geographic segregation is a common type of sex-specific segregation, and sex segregation may lead to disproportionate catches of males or females (Drymon et al. Citation2020). In Chiloscyllium species, C. punctatum occurring in Indonesian waters, and C. hasseltii Bleeker, 1852, and C. plagiosum in the southern South China Sea, sex segregation did not seem to take place (P > 0.05), but in C. indicum (Gmelin, 1789) and C. punctatum occurring in the southern South China Sea, C. griseum in Indian waters, and C. plagiosum in Taiwan waters, segregation of sex was found (P < 0.05) (Devadoss Citation1986; Chen and Liu Citation2006; Arai and Azri Citation2019; Fahmi et al. Citation2021). In specimens of C. arabicum collected from Iraqi waters in the northern Gulf, the sex ratio conformed to the 1:1 ratio (P > 0.05). However, in Jubail waters of Saudi Arabia in the southern Gulf females were dominant, while males were dominant (P < 0.01) in Bahrain waters close to Saudi Arabia (Moore and Peirce Citation2013; Abed Citation2017; this study). Further studies on habitat use by sexes in the southern Gulf are needed to explain the regional variations. Additionally, sex segregation in sharks can vary temporally, as females of many shark species move into discrete areas for parturition (Drymon et al. Citation2020). During June–August, adult female Arabian carpet sharks were not found in the southern Gulf, but they were collected in the northern Gulf during June–July (Porforugh et al. Citation2018; this study). In the northern Gulf, adult C. arabicum females sampled during April–July had mean TL of 73.7 cm, obviously higher than that of 55.6 cm collected during September–December, indicating that larger females moved out of the northern Gulf during autumn (Porforugh et al. Citation2018). In the southern Gulf, females were dominant during October–November and January–February and males during December, indicating sex segregation and suggesting thereby reproductive migration in C. arabicum like that of C. plagiosum (Chen and Liu Citation2006). Further studies involving international collaboration are required on C. arabicum to complement and strengthen the findings on the distribution besides temporal and spatial variations in this shark species occurring in the Gulf waters.
Development of follicles indicated an annual reproductive cycle like that of the northern Gulf stock (Falahiehzadeh and Salamat Citation2020). Similar results have also been reported for other species belonging to the same genus such as C. griseum, C. plagiosum and captive C. punctatum (Miki Citation1994; Masuda Citation1998; Jagadis and Ignatius Citation2003; Chen and Liu Citation2006; Harahush et al. Citation2007), although wild C. punctatum could be classified as having a partially defined annual reproductive cycle (Fahmi et al. Citation2021).
Data on batch fecundity vary in Chiloscyllium species. As per captive records, C. plagiosum deposited an average of 8 egg cases, C. griseum 27 egg cases, C. arabicum 33 egg cases and C. punctatum 58 egg cases per egg-laying season (Jagadis and Ignatius Citation2003; Chen and Liu Citation2006; Harahush et al. Citation2007). Further studies on reproductive cycle and batch fecundity on C. arabicum will be of help in comprehending the reproductive strategy of this species in the Gulf.
Animal ethics statement
Sample of specimens for this study was obtained from the catches of licensed fishermen as per The Law of Fisheries and Aquaculture Resources Investment and Protection in KSA Territorial Waters. This study complied with all ethical requirements in Saudi Arabian animal welfare laws and guidelines. No experiments were conducted on live animals.
Authors’ contributions
Conceptualization: AHA, HHH, YAA and ASA; methodology: HHH, AHA and ZN; validation: YAA and ASA; formal analysis: HHH, AHA and ZN; writing-original draft: AHA and HHH; writing-review and editing: YAA, ASA and SAK; supervision: YAA and ASA; funding acquisition: ASA. All authors read and approved the final manuscript.
Acknowledgments
We deeply appreciate the great help of fishermen during sample collection. We acknowledge the support of the College of Agriculture and Food Sciences, King Faisal University, Saudi Arabia in providing resources for the lab work. We also thank Syed Azher Hussain of the Center for Environment and Marine Studies, King Fahd University of Petroleum and Minerals, Saudi Arabia for the GIS assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support this study will be shared upon reasonable request to the corresponding author.
Additional information
Funding
References
- Abed JM. 2017. Biometry of Arabian carpetshark, Chiloscyllium arabicum Gubanov, 1980 (Elasmobranchii: Hemiscyllidae) from Iraqi marine water. Journal of King Abdulaziz University. 26:1–9. doi:10.4197/Mar.26-2.1
- Almojil DK, Moore ABM, White WT. 2015. Sharks & Rays of the Arabian/Persian Gulf. London: MBG (INT) Ltd.
- Arai T, Azri A. 2019. Diversity, occurrence and conservation of sharks in the southern South China Sea. PLoS One. 14:e0213864. doi:10.1371/journal.pone.0213864
- Baje L, Smart JJ, Chin A, White WT, Simpfendorfer CA. 2018. Age, growth and maturity of the Australian sharpnose shark Rhizoprionodon taylori from the Gulf of Papua. PLoS One. 13:e0206581. doi:10.1371/journal.pone.0206581
- Bernal MA, Sinai NL, Rocha C, Gaither MR, Dunker F, Rocha LA. 2015. Long-term sperm storage in the brownbanded bamboo shark Chiloscyllium punctatum. Journal of Fish Biology. 86:1171–1176. doi:10.1111/jfb.12606
- Carpenter KE, Krupp F, Jones DA, Zajonz U. 1997. FAO species identification field guide for fishery purposes- living marine resources of Kuwait, Eastern Saudi Arabia, Bahrain, Qatar, and the United Arab Emirates. FAO, Rome.
- Chen WK, Liu KM. 2006. Reproductive biology of whitespotted bamboo shark Chiloscyllium plagiosum in northern waters off Taiwan. Fisheries Science. 72:1215–1224. doi:10.1111/j.1444-2906.2006.01279.x
- Compagno LJV. 2001. Sharks of the world: an annotated and illustrated catalogue of shark species know to date - FAO species catalogue for fishery purposes No. 1, Vol. 2. - Bullhead, mackerel and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes). U. N. Dev. Prog., Rome.
- Dash SS, Bharadiya Sangita A, Kamalia Kiran R, Zala MS. 2013. On the egg case of Arabian carpet shark, Chiloscyllium arabicum from Gujarat. Marine Fisheries Information Service; Technical & Extension Series. 218:14–15.
- Devadoss P. 1986. Studies on the catshark Chiloscyllium griseum from Indian waters. Journal of the Marine Biological Association of India. 28:192–198.
- Drymon JM, Dedman S, Froeschke JT, Seubert EA, Jefferson AE, Kroetz AM, Mareska JF, Powers SP. 2020. Defining sex-specific habitat suitability for a northern Gulf of Mexico shark assemblage. Frontiers in Marine Science. 7:35. doi:10.3389/fmars.2020.00035
- Fahmi. 2021. Biology and population structure of the brown-banded bamboo shark Chiloscyllium punctatum. Dissertation, University of Queensland.
- Fahmi OS, Bennett MB, Dudgeon CL, Tibbetts IR. 2021. Reproductive biology of a bamboo shark as a framework for better fisheries management. Marine and Freshwater Research. 72:964–977. doi:10.1071/MF20189
- Falahiehzadeh N, Salamat N. 2020. Histomorphological and endocrine assessment of female Arabian carpetshark, Chiloscyllium arabicum (Elasmobranchii: Hemiscylliidae) from the Persian Gulf during annual reproductive cycle. Journal of Fish Biology. 97:938–952. doi:10.1111/jfb.14419
- Harahush BK, Fischer ABP, Collin SP. 2007. Captive breeding and embryonic development of Chiloscyllium punctatum Muller & Henle, 1838 (Elasmobranchii: Hemiscyllidae). Journal of Fish Biology. 71:1007–1022. doi:10.1111/j.1095-8649.2007.01569.x
- Hsu HH, Yacoubi L, Lin Y-J, Le Loc'h F, Katsanevakis S, Giovos I, Qurban MA, et al. 2022. Elasmobranchs of the western Arabian Gulf: Diversity, status, and implications for conservation. Regional Studies in Marine Science. 56:102637. doi:10.1016/j.rsma.2022.102637
- Jabado RW, Al-Ghais SM, Hamza W, Shivji MS, Henderson AC. 2015. Shark diversity in the Arabian/Persian Gulf higher than previously thought: insights based on species composition of shark landings in the United Arab Emirates. Marine Biodiversity. 45:719–731. doi:10.1007/s12526-014-0275-7
- Jabado RW, Kyne PM, Pollom RA, Ebert DA, Simpfendorfer CA, Ralph GM, Dulvy NK. 2017. The conservation status of sharks, rays, and chimaeras in the Arabian Sea and adjacent waters. environment agency - Abu Dhabi and IUCN species survival commission shark specialist group, Abu Dhabi.
- Jagadis I, Ignatius B. 2003. Captive breeding and rearing of grey bamboo shark, Chiloscyllium griseum (Muller1839). Indian Journal of Fisheries. 50:539–542.
- Kimura DK. 1980. Likelihood methods for the von Bertalanffy growth curve. Fishery Bulletin. 77:765–776.
- Liu K-M, Huang L-H, Su K-Y, Joung S-J. 2021. Vulnerability assessment of pelagic sharks in the western North Pacific by using an integrated ecological risk assessment. Animals. 11:2161. doi:10.3390/ani11082161
- Masuda M. 1998. Mating, spawning and hatching of the whitespotted bamboo shark in an aquarium. Journal of Ichthyology. 45:29–35. doi:10.1007/BF02678572
- Miki T. 1994. Spawning, hatching, and growth of the whitespotted bamboo shark, Chiloscyllium plagiosum. J Jpn Assoc Zoos Aquar. 36:10–19.
- Moore A. 2009. Chiloscyllium arabicum. The IUCN Red List of Threatened Species 2009: e.T161426A5421166. doi:10.2305/IUCN.UK.2009-2.RLTS.T161426A5421166.en.
- Moore A. 2017. Chiloscyllium arabicum. The IUCN Red List of Threatened Species 2017: e.T161426A109902537. doi:10.2305/IUCN.UK.2017-2.RLTS.T161426A109902537.en.
- Moore A, Peirce R. 2013. Composition of elasmobranch landings in Bahrain. African Journal of Marine Science. 35:593–596. doi:10.2989/1814232X.2013.866160
- Porforugh F, Salamat N, Movahedinia A. 2018. Ovarian maturatıon stages in Arabian carpet shark, Chiloscyllium arabicum Gubanov, 1980 (Orectolobiformes, Hemiscylliidae) during spring and autumn in the Persian Gulf. International Journal of Aquatic Biology. 6:321–329. doi:10.22034/ijab.v6i6.540
- Pratt HL. 1993. The storage of spermatozoa in the oviducal glands of western North Atlantic sharks. Environ Biol Fish. 38:139–149. doi:10.1007/BF00842910
- Weigmann S. 2016. Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity. Journal of Fish Biology. 88:837–1037. doi:10.1111/jfb.12874
- White WT, Last PR, Stevens JD, Yearsley GK, Fahmi D. 2006. Economically important sharks and rays of Indonesia. [Hiu dan pari yang bernilai ekonomis penting di Indonesia]. Canberra, Australia: Australian Centre for International Agricultural Research.