 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The North-east Atlantic mackerel stock size increased substantially from 2006–2014 coinciding with high recruitment. This resulted in a pronounced northerly geographic expansion of mackerel, followed by an influx of juvenile mackerel into Norwegian waters. The objective of this work was to study the diet and feeding intensity of juvenile mackerel at the new nursing grounds along the Norwegian coast during the summer. Juvenile mackerel were feeding as far north as 70°N. Stomach content was analysed for the first time from co-occurring juvenile and adult mackerel at the same locations. Almost 80% of all juvenile mackerel had prey in their stomachs, and juveniles had similar stomach fullness as adult mackerel in the same areas. The juveniles preyed on a wide variety of prey species and seemed to utilize both passive filter feeding and active particulate feeding. The most abundant prey group was Appendicularia, accounting for 31% of the stomach content by weight. Juveniles fed on similar prey species as adults, but their diet niche differed somewhat as adult mackerel fed more on krill. Juvenile mackerel can thus successfully survive and feed on various prey in high latitudes and can potentially be a feeding competitor to other planktivorous fish species in the area.
Introduction
The North-east Atlantic (NEA) mackerel (Scomber scombrus) is an example of a highly dynamic migratory fish species that has expanded its distributional range in recent years (Nøttestad et al. Citation2016; Olafsdottir et al. Citation2019). This expansion has potential implications for other species inhabiting the same niche through increased inter-specific trophic interactions. We have also witnessed new fishing opportunities for fishermen from different countries targeting mackerel due to this large-scale spatial expansion, as well as new challenges for international managers in terms of exploitation level and quota sharing (Spijkers and Boonstra Citation2017; ICES Citation2022).
Adult mackerel make extensive long-distance migrations between feeding, overwintering and spawning areas (Nøttestad et al. Citation1999; Iversen Citation2002, Citation2004; Trenkel et al. Citation2014). During summer the Norwegian Sea and adjacent waters serve as major feeding grounds for adult individuals (Nøttestad et al. Citation2016; Nøttestad, Diaz et al. Citation2016), while the traditional nursing grounds have generally been found much further south from the Bay of Biscay, west of Ireland and into the North Sea (Jansen and Gislason Citation2013; Jansen et al. Citation2015; ICES Citation2018), and the Skagerrak area (Iversen Citation2004; ICES Citation2018; Jansen et al. Citation2019). Whereas adult mackerel migrate out of northern areas during winter when food availability is very low, juvenile mackerel do not perform long-distance migrations and are believed to be mostly stationary within relatively limited areas (Jansen et al. Citation2015). There has been an increase in observations and catches of 1-year-old mackerel in the Norwegian Sea and along the entire Norwegian coast since autumn 2016 (Nøttestad et al. Citation2018). This is far north of their traditional nursery area, and we have no information about the feeding and diet of juveniles in this new northern habitat. The longer winters with low light levels in northern waters should prolong the period with low food availability compared with the traditional nursing areas in more southern latitudes, but the productive summers and prolonged days for visual feeding in northern areas, including midnight sun (Suthers and Sundby Citation1996), should on the other hand be beneficial for juvenile mackerel. An increased abundance of juvenile mackerel feeding along the Norwegian coast may however have an impact on the coastal ecosystem, for instance due to increased predation on fish larvae in this region (Skaret et al. Citation2015). A northward expansion of juvenile NEA-mackerel may also be beneficial for the stock’s recruitment as a larger nursery area may be more robust to fluctuating conditions on local scales.
Adult mackerel feed on patchy and seasonally available prey. The diet depends to a large extent on availability (Prokopchuk and Sentyabov Citation2006; Langøy et al. Citation2012; Bachiller et al. Citation2016), and adult mackerel are capable of preying on a wide range of prey species including relatively large prey such as juvenile fish, krill and amphipods (Langøy et al. Citation2012; Bachiller et al. Citation2016; Kvaavik et al. Citation2019, Citation2021). According to Trenkel et al. (Citation2014) the feeding behaviour and diet of the juveniles vary with geographic location, time of day, and size of the individual. In the traditional nursery areas juvenile mackerel are potential competitors with other planktivorous pelagic species, as 1-group mackerel are active predators on larger zooplankton such as euphausiids and amphipods, copepods, and smaller crustacean larvae (Olaso et al. Citation2005). The diet of juvenile mackerel in Norwegian waters has not previously been studied, and the number of studies addressing the diet of juvenile mackerel are in general scarce.
The main objective of this work was to study the stomach fullness and diet of juvenile mackerel in the new nursing grounds far north along the Norwegian coast during the summer feeding season. We also made comparisons between juvenile and co-occurring adult mackerel. We predicted that juvenile mackerel consume a wide range of prey, but have a somewhat different diet than adult mackerel, due to their lower swimming speed and reduced hunting abilities. As prey availability changes further north (Melle et al. Citation2004; ICES Citation2022), we further predicted that the diet would change with increasing latitude. We finally briefly discuss the ecological role of juvenile mackerel within the coastal ecosystem off Norway.
Materials and methods
Surveys and biological sampling
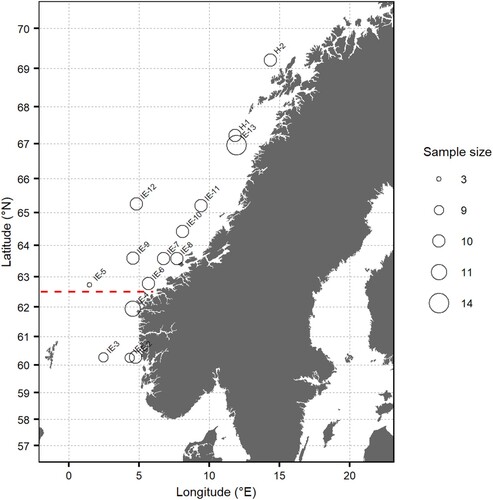
Juvenile (1-year old) and adult (>1-year-old) mackerel were sampled between 60°N and 70°N during the summer 2018 (). A total of 20 juveniles were sampled at two stations during the Norwegian Spring Spawning Herring (NSSH) post-larvae survey on 17 and 23 June, and a total of 126 co-occurring juveniles and 130 adults were sampled at the 13 stations during the International Ecosystem Summer Survey in the Nordic Seas (IESSNS) from 4–14 July. The sample size of juveniles ranged between 3 and 14 individuals (median 10 individuals). Both juvenile and adult fish were sampled with the Multpelt 832 pelagic trawl (ICES Citation2013) with floats at the headline and a vertical opening of 30–35 m towed with a speed of 5 knots for 30 min in the surface. The length and weight of all sampled mackerel were measured on board the vessel. Otoliths were taken out from each individual for age reading and prepared with Entalan, and age determination were conducted by counting the number of winter rings using a microscope (ICES Citation2018). Two individuals from which no otoliths could be retrieved were 19 cm in length and assumed to be 1-group mackerel, based on known age-length relationship (ICES Citation2020). Juveniles were defined as individuals aged 1 year and adult mackerel as individuals aged 2 years or older, although 25–50% of 2-year-old mackerel have not yet matured (ICES Citation2020). To minimize the digestion of stomach content after capture, the stomachs were immediately retrieved from the fish and frozen at sea for later analyses of the stomach content at the lab.
Figure 1. Trawl stations from the IESSNS survey and the NSSH post-larvae survey at which juvenile mackerel (1-year-old) were sampled for diet analysis. Adult mackerel was also sampled at 12 of the 15 stations. Station name was based on the name of the survey (IE = IESSNS and H = NSSH post-larvae survey) with circle size increasing with sample size. The red line at 62.5°N marks the separation between the North Sea and the Norwegian Sea.
Diet analyses
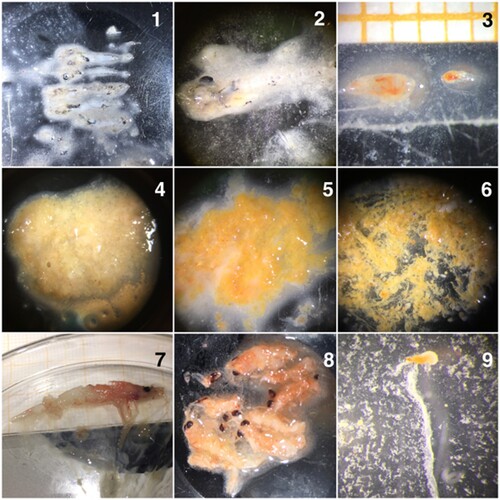
The mackerel stomachs were opened, and the organisms were identified to species level if possible, or to highest possible taxonomic level otherwise (see examples in ). In stomachs where only one taxonomic group was found, the unidentifiable digested material was grouped according to this taxonomic rank. When the stomach content consisted of multiple prey organisms from various taxonomic groups which were difficult to separate, handle or count, the proportion of each prey group in the sample was estimated by subjective evaluation and labelled as a percentage of the total mix. The sorted prey items were dried for 24 h at 70°C, and the dry weight was recorded.
Figure 2. Images of recorded prey items found during stomach analyses of juvenile mackerel. 1 and 2 – Fish larvae, 3 – C. finmarchicus and Temora sp., 4 and 5 – digested prey, 6 – mix of Appenducularia and copepods, 7 and 8 – Euphausiids (krill) and 9 – Appendicularia individuals.
Prey items that could only be identified as crustacean organisms were sorted into the Crustacea category. The taxonomic groups Isopoda and Cladocera and larvae of other crustacean groups (Brachyuran zoea larvae, cirrus larvae of Cirripedia), which were only found as a couple of individuals in few stomachs and accounted for less than 1% of the individual stomach content weight, were grouped together and labelled as ‘Other crustaceans’. The prey category ‘Other copepods’ included digested copepods that could not be identified to species, as well as the three genus’ Microcalanus sp., Psaudocalanus sp. and Temora sp.
The prey groups were grouped into the 10 main categories; Amphipoda, Appendicularia, Calanus finmarchicus, Crustacea, Digested, Euphausiacea, Limacina, Other copepods, Other crustaceans and Teleostei. The presence of any prey species belonging to each of the 10 categories were identified for individual mackerel (Stomach occurrence). The proportion of each prey group (Pprey) relative to the total stomach content (in weight) was calculated for each station by summarizing all individuals at a station. The feeding ratio (FR) is an index of stomach fullness and was calculated with the following equation:
where mf is the mass (g) of the mackerel and ms is the mass (g) of the stomach content. The diet overlaps between juvenile and adult mackerel were calculated with Pianka’s index of niche overlap (Pianka Citation1974). The index is given by:
where O is the overlap index between the two species in the range 0–1. pi, j and pi, k are the proportions of the i-th prey group by weight in the diets of species j and k, respectively. If O is 0 there is no diet overlap between the two species, while a value of 1 means a complete overlap. Partly overlapping diets are not necessarily equal to niche overlap, as species using resources independently of each other may still utilize some of the same resources. To test whether the species use the same resources more than what is expected by chance, the overlap index was compared with a null expectation (RA3 algorithm, 1000 repetitions) using the R-package EcoSimR (Gotelli et al. Citation2015).
Statistical analyses
A general linear mixed-effects model (GLMM) was used to investigate if FR of juvenile mackerel sampled in July changed with latitude, and a generalized additive mixed-effect model (GAMM) was used to investigate if FR of juvenile mackerel sampled in July changed with distance from shoreland along the Norwegian coast. Preliminary analyses did not indicate any non-linear effects of latitude on FR, and a GAMM was therefore not necessary to explore the relationship between latitude and FR. The GLMM/GAMM had FR as response variable modelled with a negative binomial distribution and the default log-link due to several completely empty stomachs (∼20%) and a skewed distribution of FR values. Latitude and distance from sampling station to nearest shoreline (km) were included as continuous fixed effects and station number as a random effect. The number of splines applied for distance in the GAMM were restricted to 5 to avoid overfitting of the data. Model residuals were assumed to follow a normal distribution with expected value 0 and variance σ2, and the model performance were assessed for homoscedasticity and normally distributed errors visually using q–q plot and plotting residual variation against the covariates. Furthermore, to test if FR of juvenile mackerel deviated from FR of older mackerel sampled at the same stations, another GLMM was applied. The response variable was FR modelled with a negative binomial distribution and a log-link, ‘juvenile or adult’ as a fixed effect and station number as a random effect. As the negative binomial distribution requires discrete numbers, FR was multiplied with 100 and rounded to nearest discrete number before being applied in the models. Model selection was based on AIC scores and backward elimination of model terms.
All maps and plots were created using the software R, version 3.4.1 (R Development Core Team Citation2017) and R-studio, version 1.0.153 (RStudio Team Citation2016) incorporating the packages ‘ggplot2’ (Wickham Citation2016), ‘ggmaps’ (Kahle and Wickham Citation2013) and ‘gridExtra’ (Auguie Citation2017).
Results
There was geographic variability in juvenile mackerel FR along the Norwegian coast (), but the variability was not significantly linearly related to latitude as the final GLMM did not include this term (). There was however a significant effect of distance from shoreline on FR, as estimated with a GAM, with the highest stomach fullness for 1-year-old mackerel around 20–50 nmi from the shoreline, and the lowest stomach fullness furthest away from the shoreline (, , model diagnostics in supplementary figure ). The feeding ratio (FR) for juveniles (min = 0, median = 0.26, 1. Quartile = 0.03, 3. quartile = 0.57, max = 1.68) and adults (min = 0, median = 0.13, 1. Quartile = 0.04, 3. quartile = 0.36, max = 2.61) caught in the same areas did not show a significant difference, as the final GLMM did not have stage (juvenile/adult) included as a fixed effect ().
Figure 3. Overview of mean feeding ratio of all juvenile individuals at each trawl station, where circle colour indicates feeding rate value. Station name was based on the name of the survey (IE = IESSNS and H = NSSH post-larvae survey). The red line at 62.5°N marks the separation between the North Sea and the Norwegian Sea.
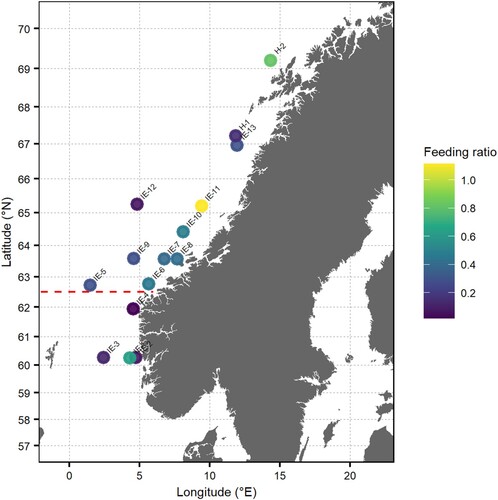
Figure 4. The estimated relationship between feeding ratio (FR) and distance from the shoreline for 1-year-old mackerel along the Norwegian coast.
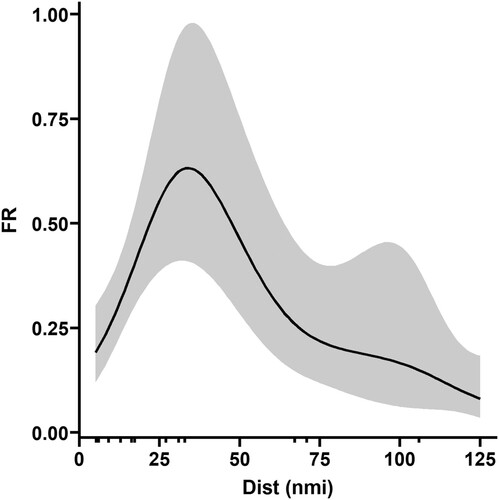
Table I. Comparison of model fit for feeding ratio (FR).
Altogether 78% (n = 114) of the juvenile mackerel stomachs contained prey items. Of the 15 taxonomic ranks of prey identified, 12 belonged to various subgroups of crustaceans, two were within classes of Chordata, and one prey group was identified as the Mollusc genus Limacina sp. The diet of juvenile mackerel was not dominated by a specific prey group, as each of the prey groups Appendicularia, ‘Other copepods’, Crustacea spp., ‘Other crustaceans’ and Limacina sp. made up 8–31% of the diet by weight (). In addition, 17.5% of the total diet in weight was the category ‘digested’. Appendicularia contributed the most to the total diet in weight (31%). This prey group occurred in ∼1/3 of all stomachs (). The two categories of copepods (‘Other copepods’ and C. finmarchicus) occurred in 75% and 30% of all stomachs () but only made up 12% and 1.2% of the diet in weight, respectively. Fish larvae were present in stomachs of juveniles at two trawl stations and contributed to 3.3% of the total stomach content weight.
Figure 5. The percentage of individual stomachs containing each prey group relative to all stomachs (stomach occurrence), and the contribution of each prey group in weight relative to the total stomach weight. The top panel is for juvenile individuals while the bottom panel is for adults. The occurrence and relative prey weight was calculated separately for juvenile and adult mackerel.
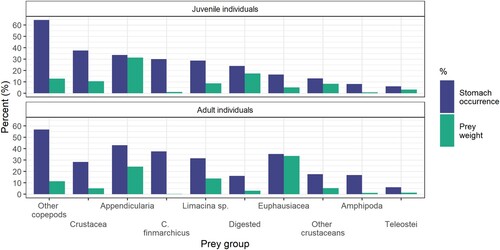
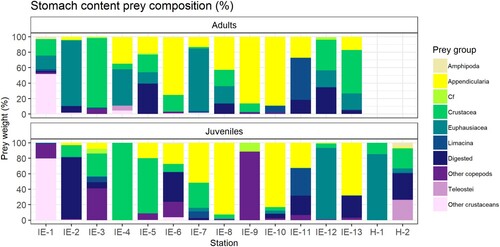
Appendicularia were not consumed in large quantities by juveniles at the southernmost stations but contributed 20% or more to the total stomach content weight at seven of nine stations north of 62°N (IE-5 and onward) sampled in July (). Stomachs from the southernmost station (IE-1) was dominated by Cladocerans (included in the group ‘Other crustaceans’). This prey group was not found in the same quantities at any other stations. The station at 69°N (H-2) was the only station out of the total 15 stations where the juveniles had considerable quantities (∼25%) of fish larvae in the stomachs (). C. finmarchicus was only found in high numbers at station IE-9, at 63.5°N. Prey items grouped together as ‘Other copepods’ were found across multiple latitudes and consisted for the most part of Microcalanus sp. in numbers, and non-identifiable/more digested copepods in weight ().
Figure 6. Diet composition in percentage at each station, separated by the cruise on which the individuals were sampled. The bottom panel is for juvenile mackerel while the top panel is for adult mackerel. Order of the stations for each survey is by increasing latitude with locations mapped in and . The two last stations, H-1-2 were sampled in June during the NSSH post-larvae survey, while all other stations IE-1-13 were sampled in July during the IESSNS.
Juvenile and adult mackerel mainly fed on the same prey organisms as Pianka’s overlap index was 0.73 (see underlying data presented in ), although they did not have the same feeding niche (P = 0.117). Adult mackerel fed more on Euphausiids than juvenile mackerel, with 33% and 4% of the total diet by weight, respectively. For the remaining prey groups there were only minor differences in total diet by weight between juvenile and adult mackerel.
Discussion
This study investigates for the first time the diet of juvenile mackerel at high latitudes during the summer feeding season along the Norwegian coast, far north of the traditional nursery grounds. This is also the first time stomach content was analysed from co-occurring juvenile and adult mackerel caught at the same locations. A high proportion of juvenile mackerel had prey in their stomachs, and the juveniles had as high relative stomach fullness (feeding ratio) as adult mackerel caught in the same areas. A wide assortment of zooplankton groups that varied with latitude were consumed both by juveniles and adults. This demonstrates that juvenile mackerel far north in the North-east Atlantic can locate and successfully feed on various prey.
This study is based on relatively few samples, but it should be enough to draw the general conclusion that the region along the extensive Norwegian coast serves as an adequate feeding ground for juvenile mackerel. However, the spatial differences found should be interpreted with care.
Visual stomach content analyses, as used in this study, provide a snapshot of the weight composition of the stomach content. Furthermore, if the consumed prey is not too digested, this method can provide information on weight and size/stages of individual prey, compared with using occurrence-based methods. One weakness with the visual stomach content method is that digestion times vary for different prey groups, and that the method only gives a snapshot of the diet which may not be representative for a longer time period. Furthermore, the samples presented in this study were taken within a short time period in late June/July, and the diet composition and mackerel stomach fullness may change rapidly during the summer and autumn. The method has nevertheless been used in several diet studies from the Norwegian Sea (Dalpadado et al. Citation2000; Prokopchuk and Sentyabov Citation2006; Langøy et al. Citation2012; Skaret et al. Citation2015; Bachiller et al. Citation2016). We have used the term ‘feeding behaviour’, but it is important to realize that the stomach contents just provide a snapshot of what the fish ate prior to sampling, and not ‘how’ they are feeding. A relatively large copepod such as C. finmarchicus may presumably be eaten both by filter feeding and active feeding. To get a better description of the food web in that ecosystem and potential inter- and intraspecific trophic interactions future studies could apply a multi-proxy approach combining stomach content characterization with isotope analyses (Jennings et al. Citation2002) and genetic analysis (e.g. DNA-metabarcoding; Allan et al. Citation2021; Günther et al. Citation2021).
Plankton sampling related to food availability is available from survey reports, time series from ICES reports and previous publications (Langøy et al. Citation2012; Nøttestad, Diaz et al. Citation2016; Ólafsdóttir et al. Citation2018; ICES Citation2022). The geographic distribution of zooplankton standing biomass, as measured from WP2 net hauls with 180 µm mesh size from 0–200 m depth, is not correlated to mackerel abundance (Langøy et al. Citation2012; Olafsdottir et al. Citation2019). Furthermore, results obtained from WP2 nets most probably underestimate larger zooplankton such as krill and amphipods due to its small sampling diameter (Melle et al. Citation2004). This study has not attempted to directly link mackerel stomach content to prey availability due to the above-mentioned reasons and few sampled stations of zooplankton along the coast in the study presented here.
Since we catch both juvenile and adult mackerel in the same trawl hauls and thus in the same areas along the coast, this may suggest some food competition between juveniles and adults in these areas. There was considerable diet overlap between juvenile and adult mackerel in our study, but our results also suggest an opportunistic feeding behaviour and diet plasticity for both juvenile and adult mackerel, which may reduce the direct prey competition along the Norwegian coast. Juveniles furthest away from the shoreline had significantly lower FR, although the analyses are sensitive to the few stations in the dataset. This finding may be due to food availability as well as competition with adult mackerel. Adult mackerel predominantly feed further offshore in the Norwegian Sea (Prokopchuk and Sentyabov Citation2006; Langøy et al. Citation2012; Nøttestad, Diaz et al. Citation2016), which will limit the direct intraspecific feeding competition along the coast during the summer.
Stomach fullness and diet composition
Juvenile and co-occurring adult mackerel did not have significantly different feeding ratios. The variability in feeding ratio of the juveniles was not related to latitude, and the juveniles seemed to feed as successfully at the northernmost stations as further south in the North Sea.
As predicted, juvenile mackerel along the Norwegian coast feed on similar prey groups as co-occurring adult mackerel. The precocious digestive system could allow the juveniles to feed on prey items that also are preferred by adult mackerel (Prokopchuk and Sentyabov Citation2006; Langøy et al. Citation2012; Jansen Citation2016). However, krill were a more important prey for the adults, resulting in different diet niches. The diet of juveniles from our study seems to be different from the diet of juveniles in the traditional nursery areas around the British Isles and within the North Sea basin (Jansen Citation2016). Juveniles from the traditional nursery areas in the Cantabrian Sea fed primarily on euphasiids, hyperiid amphipods, decapod larvae and copepods (Olaso et al. Citation2005), while juveniles in the new northern nursery areas mostly feed on Appendicularia, various Crustaceans and copepods. The difference in diet for juvenile mackerel at the traditional and new northern nursery area is most likely due to spatial differences in prey availability, although temporal differences in sampling may also have impacted the results.
The occurrence of the calanoid copepod species Calanus finmarchicus in the stomachs of the juveniles was low when considering it is the most common and preferred prey of adult mackerel in other studies (Prokopchuk and Sentyabov Citation2006; Langøy et al. Citation2012; Bachiller et al. Citation2016), as well as its role as a key prey potentially regulating juvenile survival in the traditional nursery areas (Jansen Citation2016). In our study, C. finmarchicus only accounted for 1.2% of the total stomach content weight. Some C. finmarchicus individuals in early copepodite stages may have been misclassified as Pseudocalanus, and if the digested copepods could have been identified to species, it is likely that the total weight and number of C. finmarchicus found in the juvenile stomachs would be higher. Juveniles fed on C. finmarchicus and one or more individuals were present in ∼30% of the stomachs. These findings indicate that there were low concentrations of C. finmarchicus where the juveniles were caught, which is supported by the low biomass of this prey species in adult mackerel stomachs from the same areas (). The first seasonal bloom of C. finmarchicus had likely occurred earlier with the onset of the second seasonal bloom occurring later, resulting in a low abundance of this prey during late June through mid-July (see also Melle et al. Citation2004).
As predicted, the diet of juvenile mackerel changed with increasing latitude. For instance, Appendicularia that contributed to 20% or more to the total stomach content weight at most stations north of 62°N was considerably less frequent at the southernmost stations.
Feeding strategy
Mackerel is predominantly a visual predator potentially feeding during all hours of the day at high northern latitudes during summer with the midnight sun likely being highly beneficial for both juvenile and adult mackerel. As there were many types of prey in the stomachs and the composition varied between stations and across latitudes the results presented in this study indicate that both juveniles and adults seem to be opportunistic when selecting prey and to have a kind of an ‘eat-what-is-present’ behaviour. The sizes of the prey across all stations ranged between 1 mm (Microcalanus sp. and Temora sp.) and 3–4 cm (krill and fish larvae) (Melle et al. Citation2004). Thus, juveniles along the Norwegian coast seem to switch between passive filtering of smaller plankton through the gill rakes and active feeding on larger prey types in the same way as adults (Bachiller et al. Citation2016; Kvaavik et al. Citation2019, Citation2021).
Ecological consequences of the shifts in juvenile distribution
The ecological effects of the northern expansion of juvenile mackerel are yet unknown. Mackerel is a fast swimming, schooling species that feeds with high intensity (Iversen Citation2004; Nøttestad, Diaz et al. Citation2016), and increasing numbers of juvenile mackerel along the coast creating an additional predation pressure on the zooplankton community may affect species feeding on plankton through increased interspecific competition (Huse et al. Citation2012). The results presented in this study showed that juvenile mackerel along the Norwegian coast prey on species that are also exploited by adult mackerel. Juvenile mackerel can thus successfully survive and feed on various prey in high latitudes and can potentially be a feeding competitor to other planktivorous fish species in the area, such as Norwegian spring-spawning herring (Langøy et al. Citation2012). Density-dependent growth has been documented for mackerel in the North-east Atlantic and with increasing mackerel abundance after year 2003 the length and weight at age decreased gradually for adult mackerel (Olafsdottir et al. Citation2016). This density-dependent growth suggests that mackerel can deplete the abundance of their main prey at least on a local scale. Overall, there has been a reduction of zooplankton (mainly C. finmarchicus) along the Norwegian coast since 2004 (Dupont et al. Citation2017; Toresen et al. Citation2019). Reduced zooplankton abundance in general, and the presence of juvenile mackerel along the Norwegian coast during the summer increase the probability of competition for prey within the mackerel stock along the Norwegian coast. However, adult mackerel predominantly feed further offshore in the Norwegian Sea (Nøttestad et al. Citation2016). Hence, adult mackerel have the possibility to focus their feeding in offshore areas if the competition for prey is too high in coastal waters.
Fish larvae in the stomachs of juvenile mackerel demonstrates that juveniles along the Norwegian coast also target on this type of prey. However, only 6% of the sampled stomachs contained fish larvae, and fish larvae only accounted for 3.3% of the total stomach content weight. The consumed fish larvae are assumed to be herring larvae. Adult mackerel have been found to feed opportunistically on herring larvae (Skaret et al. Citation2015), and this could also be the case for juvenile mackerel, as their distribution during spring and early summer along the Norwegian coast overlaps with the distribution of herring larvae drifting northwards (Dragesund et al. Citation1997). Predation on herring larvae by juvenile mackerel along the Norwegian coast is at present not likely to affect the total herring population but may have local and regional effects (Skaret et al. Citation2015).
If an increasing number of adult mackerel make more use of Norwegian waters also as spawning areas, and the nursery areas continue to advance northwards, the impact on local fjord and coastal ecosystems by juvenile mackerel will increase in the future. There is probably no such thing as the ‘optimum’ conditions, rather ‘acceptable’ conditions, but given that both the spawning and feeding of adult mackerel has expanded and shifted to the north, it may be expected that eggs, larvae and juveniles to a larger extent will stay, survive and feed in more northern waters throughout the year. To further understand the ecological role of juvenile mackerel, the use of newly developed methods such as isotope analyses and DNA metabarcoding can be helpful to determine the effects of egg and/or larvae predation and potential inter- and intraspecific trophic interactions.
Supplemental Material
Download MS Word (273.5 KB)Supplemental Material
Download MS Word (202.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Allan BJM, Ray JL, Tiedemann M, Komyakova V, Vikebø F, Skaar KS, Stiasny MH, Folkvord A, Nash RDM, Stenevik EK, Kjesbu OS. 2021. Quantitative molecular detection of larval Atlantic herring (Clupea harengus) in stomach contents of Atlantic mackerel (Scomber scombrus) marks regions of predation pressure. Scientific Reports. 11(1). doi:10.1038/s41598-021-84545-7
- Auguie B. 2017. gridExtra: miscellaneous functions for “grid” graphics. R package version 2.3.
- Bachiller E, Skaret G, Nøttestad L, Slotte A. 2016. Feeding ecology of Northeast Atlantic mackerel, Norwegian Spring-Spawning herring and blue whiting in the Norwegian Sea. PLoS One. 11(2):e0149238. doi:10.1371/journal.pone.0149238
- Dalpadado P, Ellertsen B, Melle W, Dommasnes A. 2000. Food and feeding conditions of Norwegian spring-spawning herring (Clupea harengus) through its feeding migrations. ICES Journal of Marine Science. 57(4):843–857. doi:10.1006/jmsc.2000.0573
- Dragesund O, Johannessen A, Ulltang Ø. 1997. Varlation in migration and abundance of Norwegian spring spawning herring (Clupea harengus L.). Sarsia. 82(2):97–105. doi:10.1080/00364827.1997.10413643
- Dupont N, Bagøien E, Melle W. 2017. Inter-annual variability in spring abundance of adult Calanus finmarchicus from the overwintering population in the southeastern Norwegian Sea. Progress in Oceanography. 152:75–85. doi:10.1016/j.pocean.2017.02.004
- Gotelli NJ, Hart EM, Ellison AM. 2015. EcoSimR: null model analysis for ecological data. R package version 0.1.0. doi:10.5281/zenodo.16522
- Günther B, Fromentin JM, Metral L, Arnaud-Haond S. 2021. Metabarcoding confirms the opportunistic foraging behaviour of Atlantic Bluefin Tuna and reveals the importance of gelatinous prey. PeerJ. 9:e11757. doi:10.7717/peerj.11757
- Huse G, Holst JC, Utne K, Nøttestad L, Melle W, Slotte A, Ottersen G, Fenchel T, Uiblein F. 2012. Effects of interactions between fish populations on ecosystem dynamics in the Norwegian Sea – results of the INFERNO project. Marine Biology Research. 8(5-6):415–419. doi:10.1080/17451000.2011.653372
- ICES. 2013. Report of the workshop on Northeast Atlantic mackerel monitoring and methodologies including science and industry involvement (WKNAMMM). ICES CM 2013/SSGESST:18.
- ICES. 2018. Report of the workshop on age estimation of Atlantic mackerel (Scomber scombrus) (WKARMAC2); October 22–26; San Sebastian. ICES CM 2018/EOSG:32. p. 96.
- ICES. 2020. Working group on widely distributed stocks (WGWIDE). ICES Scientific Reports. 2(82):1019. doi:10.17895/ices.pub.7475
- ICES. 2022. Working group on the integrated assessments of the Norwegian Sea (WGINOR; outputs from 2021 meeting). ICES Scientific Reports. 4(35):48. doi:10.17895/ices.pub.19643271
- Iversen SA. 2002. Changes in the perception of the migration pattern of Northeast Atlantic mackerel during the last 100 years. ICES Marine Science Symposia. 215:382–390.
- Iversen SA. 2004. Mackerel and horse mackerel. In: Skjoldal HR, editor. The Norwegian Sea ecosystem. Trondheim: Tapir Academic Press.
- Jansen T. 2016. First-year survival of North East Atlantic mackerel (Scomber scombrus) from 1998 to 2012 appears to be driven by availability of Calanus, a preferred copepod prey. Fisheries Oceanography. 25(4):457–469. doi:10.1111/fog.12165
- Jansen T, Gislason H. 2013. Population structure of Atlantic mackerel (Scomber scombrus). PLoS One. 8(5):e64744. doi:10.1371/journal.pone.0064744
- Jansen T, Kristensen K, van der Kooij J, Post S, Campbell A, Utne KR, Carrera P, Jacobsen JA, Gudmundssdottir A, Roel BA, Hatfield EMC. 2015. Nursery areas and recruitment variation of Northeast Atlantic mackerel (Scomber scombrus). ICES Journal of Marine Science. 72(6):1779–1789.
- Jansen T, Post S, Olafsdottir AH, Reynisson P, Óskarsson GJ, Arendt KE. 2019. Diel vertical feeding behaviour of Atlantic mackerel (Scomber scombrus) in the Irminger current. Fisheries Research. 214:25–34. doi:10.1016/j.fishres.2019.01.020
- Jennings S, Warr KJ, Mackinson S. 2002. Use of size-based production and stable isotope analyses to predict trophic transfer efficiencies and predator-prey body mass ratios in food webs. Marine Ecology Progress Series. 240:11–20. doi:10.3354/MEPS240011
- Kahle D, Wickham H. 2013. Ggmap: spatial visualization with ggplot2. The R Journal. 5(1):144–161.
- Kvaavik C, Óskarsson GJ, Daníelsdóttir AK, Marteinsdóttir G. 2019. Diet and feeding strategy of Northeast Atlantic mackerel (Scomber scombrus) in Icelandic waters. PLoS One. 14(12):e0225552. doi:10.1371/journal.pone.0225552
- Kvaavik C, Óskarsson GJ, Petursdóttir H, Marteinsdóttir G, Daníelsdottir AK. 2021. New insight into trophic niche partitioning and diet of mackerel (Scomber scombrus) and herring (Clupea harengus) in Icelandic waters. ICES Journal of Marine Science. 78:1485–1499. doi:10.1093/icesjms/fsaa100
- Langøy H, Nøttestad L, Skaret G, Broms C, Fernö A. 2012. Overlap in distribution and diets of Atlantic mackerel (Scomber scombrus), Norwegian spring-spawning herring (Clupea harengus) and blue whiting (Micromesistius poutassou) in the Norwegian Sea during late summer. Marine Biology Research. 8(5-6):442–460. doi:10.1080/17451000.2011.642803
- Melle W, Ellertsen B, Skjoldal HR. 2004. Zooplankton: the link to higher trophic levels. In: Skjoldal HR, editor. The Norwegian Sea ecosystem. Trondheim: Tapir Academic Press. p. 137–202.
- Nøttestad L, Diaz J, Penã H, Søiland H, Huse G, Fernö A. 2016. Feeding strategy of mackerel in the Norwegian Sea relative to currents, temperature, and prey. ICES Journal of Marine Science. 73(4):1127–1137. doi:10.1093/icesjms/fsv239
- Nøttestad L, Giske J, Holst JC, Huse G. 1999. A length-based hypothesis for feeding migrations in pelagic fish. Canadian Journal of Fisheries and Aquatic Sciences. 56:26–34. doi:10.1139/cjfas-56-S1-26
- Nøttestad L, Utne KR, Óskarsson GJ, Jónsson SÞ, Jacobsen JA, Tangen Ø, Anthonypillai V, Aanes S, Vølstad JH, Bernasconi M, et al. 2016. Quantifying changes in abundance, biomass, and spatial distribution of Northeast Atlantic mackerel (Scomber scombrus) in the Nordic seas from 2007 to 2014. ICES Journal of Marine Science. 73(2):359–373. doi:10.1093/icesjms/fsv218
- Nøttestad L, Utne KR, Sandvik A, Skålevik A, Slotte A, Huse G. 2018. Historical distribution of juvenile mackerel northwards along the Norwegian coast and offshore following the 2016 mackerel spawning. Working Document to ICES Working Group on Widely distributed stocks (WGWIDE). Havstovan, Tórshavn, Faroe Islands, August 28 – September 3, p. 25.
- Olafsdottir AH, Slotte A, Jacobsen JA, Oskarsson GJ, Utne KR, Nøttestad L. 2016. Changes in weight-at-length and size-at-age of mature Northeast Atlantic mackerel (Scomber scombrus) from 1984 to 2013: effects of mackerel stock size and herring (Clupea harengus) stock size. ICES Journal of Marine Science. 73:1225–1235.
- Ólafsdóttir AH, Nøttestad L, Anthonypillai V, Jacobsen JA, Jansen T, Wieland K, et al. 2018. Cruise report from the international ecosystem summer survey in the Nordic Seas (IESSNS) 30th of June – 6th of August 2018. ICES Working Group on Widely Distributed Stocks (WGWIDE, No. 05), Havstovan, Tórshavn, Faroe Islands, August 28 – September 3. p. 39.
- Olafsdottir AH, Utne KR, Jacobsen JA, Jansen T, Óskarsson GJ, Nøttestad L, Elvarsson BÞ, Broms C, Slotte A. 2019. Geographical expansion of Northeast Atlantic mackerel (Scomber scombrus) in the Nordic Seas from 2007 to 2016 was primarily driven by stock size and constrained by low temperatures. Deep Sea Research Part II: Topical Studies in Oceanography. 159:152–168. doi:10.1016/j.dsr2.2018.05.023
- Olaso I, Gutiérrez J, Villamor B, Carrera P, Valdés L, Abaunza P. 2005. Seasonal changes in the north-eastern Atlantic mackerel diet (Scomber scombrus) in the north of Spain (ICES DivisionVIIIc). Journal of the Marine Biological Association of the United Kingdom. 85:415–418. doi:10.1017/S0025315405011343h
- Pianka E. 1974. Niche overlap and diffuse competition. Proceedings of the National Academy of Sciences. 71:2141–2145. doi:10.1073/pnas.71.5.2141
- Prokopchuk I, Sentyabov E. 2006. Diets of herring, mackerel, and blue whiting in the Norwegian Sea in relation to Calanus finmarchicus distribution and temperature conditions. ICES Journal of Marine Science. 63:117–127. doi:10.1016/j.icesjms.2005.08.005
- R Development Core Team. 2017. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
- RStudio Team. 2016. RStudio: integrated development for R. Boston (MA): RStudio, Inc. http://www.rstudio.com/.
- Skaret G, Bachiller E, Langøy H, Stenevik EK. 2015. Mackerel predation on herring larvae during summer feeding in the Norwegian Sea. ICES Journal of Marine Science. 72(8):2313–2321. doi:10.1093/icesjms/fsv087
- Spijkers J, Boonstra WJ. 2017. Environmental change and social conflict: the northeast Atlantic mackerel dispute. Regional Environmental change. 17:1835–1851.
- Suthers IM, Sundby S. 1996. Role of the midnight sun: comparative growth of pelagic juvenile cod (Gadus morhua) from the Arcto-Norwegian and a Nova Scotian stock. ICES Journal of Marine Science. 53:827–836. doi:10.1006/jmsc.1996.0104
- Toresen R, Skjoldal HR, Vikebø F, Martinussen MB. 2019. Sudden change in long-term ocean climate fluctuations corresponds with ecosystem alterations and reduced recruitment in Norwegian spring-spawning herring (Clupea harengus, Clupeidae). Fish Fish. 20:686–696. doi:10.1111/faf.12369
- Trenkel VM, Huse G, MacKenzie BR, Alvarez P, Arrizabalaga H, Castonguay M, Goñi N, Grégoire F, Hátún H, Jansen T, et al. 2014. Comparative ecology of widely distributed pelagic fish species in the North Atlantic: implications for modelling climate and fisheries impacts. Progress in Oceanography. 129:219–243. doi:10.1016/j.pocean.2014.04.030
- Wickham H. 2016. Ggplot2: elegant graphics for data analysis. New York: Springer-Verlag.
