ABSTRACT
This study presents the distribution and abundance of key species of the vulnerable marine ecosystems (VMEs) ‘Coral gardens’ and ‘Sea pen and burrowing megafauna’ in Skagerrak and the Norwegian trench. It is based on 543 bycatches from 2017–2021, and 35 ROV dives from 2016-2017. Bycatches were used to indicate distribution and relative abundance of the VME key species while ROV observations provided information on colony densities, associated fauna and damages. Four sea pen species were recorded. Funiculina quadrangularis and Kophobelemnon stelliferum were widely distributed and most abundant below 200 meters. The few records of Pennatula phosphorea were mainly from shallower than 100 meters, and the rare Balticina finmarchica primarily occurred below 200 meters. The ROV videos confirmed the pattern from the bycatches, however, colony densities were much higher, and the sea pen Virgularia mirabilis recorded in high abundances was not present in bycatches. The coral garden key species Isidella lofotensis, endemic to Norway, had a restricted area of occurrence confirmed by both methodologies. The restricted distribution makes it particularly vulnerable. Fishing activities overlap with the VMEs distribution and the observations of sea pen skeletons and the coral predatory anemone Ptychodactis patula are indicative of pressure from bottom trawl fishing.
KEY POLICY HIGHLIGHTS
‘Sea pen and burrowing megafauna’ is a widely distributed VME in Skagerrak and the Norwegian trench, an area with intense bottom trawling.
Recorded sea pen species show different distribution patterns, and vulnerability should be evaluated at species level.
There is an immediate need for protection of the VME ‘Coral Garden’ represented by the bamboo coral Isidella lofotensis and mapping and monitoring is necessary to evaluate ecological status.
Trawl bycatches provide valuable information on VMEs, but the precision on position and abundance of colonies is low compared with visual mapping, and trawling is a threat to the VMEs.
Introduction
Bamboo corals and sea pens are key species of the threatened and vulnerable marine ecosystems (VMEs) ‘Coral gardens’ and ‘Sea pen and burrowing megafauna’, respectively (OSPAR Citation2004; Curd Citation2010; Buhl-Mortensen et al. Citation2015). Sea pens can be found locally in high densities on soft sediment and in relatively warm Atlantic waters and they are common in the Norwegian Exclusive Economic Zone (EEZ) shallower than 700 metres (Buhl-Mortensen et al. Citation2019). Soft bottom gorgonian coral is a category of coral garden that in the Norwegian EEZ is represented by two rare species, Radicipes gracilis and Isidella lofotensis, that locally can form dense stands on sandy soft bottoms. The former is a cold-water deep-sea species known only from northern Norway while the latter occurs from 200–500 metres and has been reported a few times from the shelf but is mainly recorded from Norwegian fjords (Buhl-Mortensen and Buhl-Mortensen Citation2014; Buhl-Mortensen et al. Citation2015). Norway has a national responsibility related to the bamboo coral I. lofotensis because it is viewed as endemic to Norway (Buhl-Mortensen et al. Citation2015, Citation2019).
The main human threats to these habitats are activities that physically disturb the seabed, and in particular the demersal fisheries can impact these VMEs through burial, silting and crushing (Pitcher et al. Citation2022). Commercial fisheries for shrimps and other demersal resources are intense in Skagerrak and the Norwegian trench (Eigaard et al. Citation2017; Amoroso et al. Citation2018; Pitcher et al. Citation2022) and thus represent a potential threat to these habitats (Buhl-Mortensen et al. Citation2019). The objective of this study is to present the distribution and health status of the ‘Coral gardens’ key species I. lofotensis, and the ‘Sea pen and burrowing megafauna’ key species Funiculina quadrangularis, Kophobelemnon stelliferum, Pennatula phosphorea and Balticina finmarchica in Skagerrak and the Norwegian trench.
Samples and information on the occurrence of VME key species come from two sources: (1) bycatch data from an annual bottom trawl survey with 111 fixed stations conducted by the Norwegian Institute of Marine Research (IMR) from the period 2017–2021 (Søvik and Thangstad Citation2021) and (2) video records from ROV dives at 35 localities conducted by OCEANA (a nonprofit ocean conservation organization) in 2016 and 2017 (Álvarez et al. Citation2019). The bycatch data, even though from a gear designed to capture shrimps and demersal fish species, indicate distribution and relative abundance of sea pens and corals while the visual observations provide detailed information from the habitats of local colony densities, associated fauna and fishing related damage to colonies. The distribution of the VME key species is compared with the distribution of the commercial bottom trawl fisheries in the area as an indication of impact from fishing on these habitats.
Materials and methods
Study area
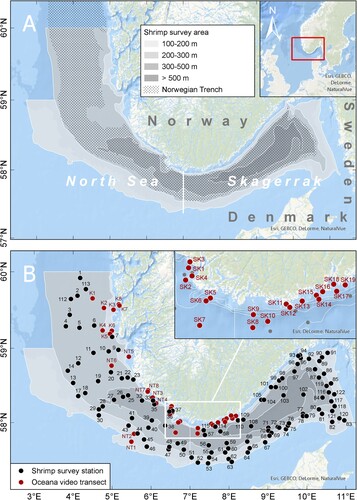
The ocean area Skagerrak is delimited by straight lines from the Lindesnes lighthouse (southernmost tip of Norway) to the Hanstholm lighthouse in Denmark, and from Grenen (northernmost tip of Denmark) to Marstrand in Sweden (A). Skagerrak is linked to the brackish Baltic Sea through Kattegat, has a mixed water column and is less saline and less tidal than the North Sea (ICES Citation2021). The Norwegian trench is a deep channel that cuts into the northeastern part of the shallow North Sea and follows the west and south coast of Norway into Skagerrak, where the deepest part of 600–700 metres depth is located. The bottom sediment of the trench is mud to sandy mud, while the Norwegian coastal areas consist of rocky bottom interspersed with pockets of soft sediment (ICES Citation2021). In this paper, we will use the term Norwegian trench for the part of the channel west of the demarcation line between the North Sea and Skagerrak.
Figure 1. (A) The IMR annual shrimp survey area with depth zones shown in shades of grey. (B) The position of the 111 fixed bottom trawl stations from which bycatch data for the period 2017–2021 were used in this study (black dots), and the 35 localities that was visually mapped by OCEANA in 2016 and 2017 using ROV (Álvarez et al. Citation2019).
Survey data
IMR conducts a yearly bottom trawl survey in January to monitor the stock status of northern shrimp (Pandalus borealis) and demersal fish species in Skagerrak and the Norwegian trench at 111 fixed trawl stations on soft sediments at 111–540 metres depth (B and ) (Søvik and Thangstad Citation2021). With some exceptions, all stations are visited once a year. The sampling gear is a ‘Campelen 1800’ bottom trawl of 3–5 metres in height and with 48–52 metres door spread. The bottom gear is a rock hopper gear. At each station, the trawl is hauled at 3 knots for 30 min, and each haul covers approximately 0.14 km2. Bycatch documentation of sea pens and bamboo corals started in 2017 and from the 5-year period 2017–2021, bycatch information is available from a total of 543 trawl hauls. For each trawl haul, the collected sea pens and corals were displayed at the sorting table in the fish-lab and photographed (). Species identification and quantification was done after the cruise based on the photos. The few and distinct sea pen species present in the area makes it possible to identify the colonies based on photos. Because colonies were often broken into several pieces during trawling the quantification of colonies in each trawl catch is based on counting the peduncles or holdfasts (the part anchored in the sediment) of each colony. For analyses of abundance and distribution of sea pens and coral, we used the average number in trawl catches over the period 2017–2021 at each fixed survey station.
Figure 2. From each trawl catch at the annual shrimp survey the benthos bycatch was displayed on the sorting table in the fish-lab and photographed. Coral and sea pen species were identified, and numbers estimated based on the number of holdfasts or peduncles observed in the bycatch photos.

Table I. Station numbers (Stn.) and geographic position for the 111 fixed bottom trawl stations from which bycatches of sea pens and bamboo corals were recorded in the 5-year period 2017–2021 (see map in B).
ROV observations
In 2016 and 2017, 35 video inspections were conducted by OCEANA (Álvarez et al. Citation2019) at 68–459 metres depth in the study area using ROV equipped with a high-definition (HD) video camera and laser beams as measuring scale (B and ). The relatively few visually mapped localities provide valuable supplementary information to the trawl bycatches on local density, associated fauna and indications of trawl related damage. The sea pens and corals were identified to species (the few and distinct sea pen species present in the area makes it possible to identify the colonies based on video) and counted when present on video. A 5 cm laser scale was used to estimate the local patch density of colonies based on video frames (stills) at a few localities where the occurrence was high.
Table II. Station information for the ROV localities visually mapped by the organization OCEANA (Álvarez et al. Citation2019) between 2016 and 2017, date, geographic position and station depth (m) (see map in B).
Results
In this study the collected or observed sea pen and coral genera are represented with only one species each and for simplicity we are mainly using the genus names for the species in what follows.
Trawl bycatches
From a total of 543 trawl catches collected from the 111 fixed localities (B) the sea pens Funiculina, Kophobelemnon, Balticina and Pennatula were recorded. In addition, the number of the ophiuroid Asteronyx living on Funiculina was noted (). This is one of few associated species that clings so hard to its host that they are captured together. The most common sea pen species in the bycatches were Funiculina and Kophobelemnon; the former was present in more than 50% of the trawl catches and the latter in 10–20%. In total, 1996 and 359 colonies were collected, respectively, with a maximum of 45 and 18 colonies in a single trawl haul.
Table III. Data on bycatch in trawl hauls of sea pens and corals and the common symbiont on Funiculina, the ophiuroid Asteronyx. Number of trawl stations with bycatch, number of collected individuals and colonies, and maximum catch in a single trawl haul per survey year and total for all the years.
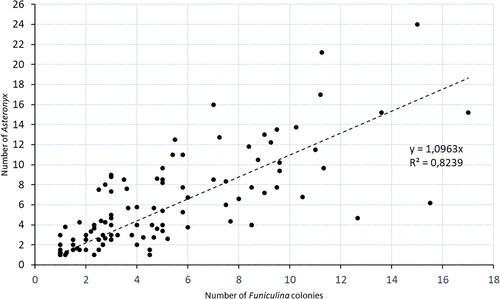
The geographic distribution of Funiculina and Kophobelemnon is shown in . Funiculina is, together with the symbiont ophiuroid Asteronyx (brittle star), widely distributed in the study area with the highest abundance in the Norwegian part of Skagerrak and the Norwegian trench. The largest records of Funiculina were from depths between 200 and 300 metres (A). The abundance of Funiculina and Asteronyx in bycatches is highly correlated (R2 = 0.82) and the ratio is very near to one to one (Y = 1.1X) (). In the catches Asteronyx was normally attached to the host but some detached specimens occurred.
Figure 3. The distribution and abundance of the bamboo coral Isidella and the five sea pen species, Balticina, Funiculina, Kophobelemnon, Pennatula and Virgularia in the study area. For comparison, the bycatch numbers of the brittle star Asteronyx that is closely associated with Funiculina are also provided. Orange-brown circles show average number of colonies sampled per trawl station during the period 2017–2021 provided in three abundance ranges. Blue circles represent numbers of observed colonies at Oceana ROV stations provided in three ranges.
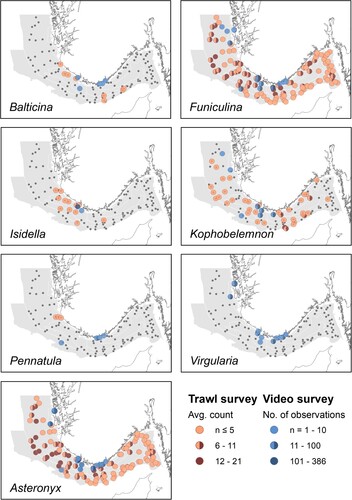
Figure 4. (A) Records of the sea pens Funiculina and Kophobelemnon and (B) Balticina and the bamboo coral Isidella, in different depth zones in the study area. Columns are the largest number observed at a station within the depth zone and lines are average for all stations in a depth zone.
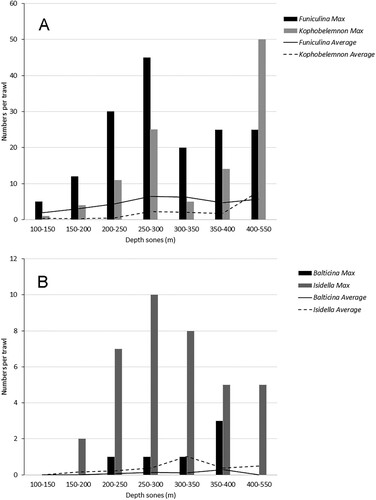
Figure 5. The relation between bycatch numbers for Asteronyx and its host Funiculina. R2 and the y value for the linear equation are provided. The equation shows that the relation between the brittle star and its host is close to one to one. The slightly larger value (1.1) indicates that a single colony can have more than one specimen attached, which is in line with ROV observations (see A).
The distribution of Kophobelemnon is more restricted and the highest numbers are from deeper parts of Skagerrak and the Norwegian trench, from depths deeper than 400 metres ( and A). Records of the sea pens Balticina and Pennatula were few. Pennatula has a shallower distribution than most of the trawl survey stations in the study area, while Balticina is rare in Norwegian waters and mainly occurred deeper than 200 metres and in the Norwegian trench. The bamboo coral Isidella occurred in low numbers and in total 82 individuals (colonies) were reported based on holdfasts. The largest occurrences were 10 colonies collected at trawl station number 35 close to the coast in the Norwegian trench (). The main occurrence was deeper than 200 metres (B).
ROV observations
Soft bottom sea pens and corals were observed at 17 out of the 35 ROV stations (). In general, the numbers of sea pens and corals recorded on the video transects were much higher than in the trawl bycatches. The most abundant species, Virgularia, was not recorded as bycatch. The largest density of Virgularia was observed by ROV from an outer fjord station on the west coast of Norway (K7), which can explain why this small and delicate species was not recorded from the bottom trawl survey that is conducted mainly offshore. The ROV records also had more observations of Pennatula compared with the trawl bycatches, mainly related to a station at 68 metres depth close to the coast. Funiculina and Kophobelemnon were amongst the more common species observed by ROV which is in line with the trawl data, and both occurred in particularly high numbers below 200 metres (). At station SK6, the density of Funiculina was ∼3 colonies per m2 and the anemone Ptychodactis patula was found feeding on its tissue (). The ROV transects documented the bamboo coral Isidella in high numbers at the station SK2 close to the coast, at 317 metres depth (). This observation was near the largest trawl bycatches of this coral (station 35) and indicates that the bamboo coral occurs in a rather well-defined and restricted region in the study area. As in the trawl bycatches, the large sea pen Balticina was observed only in low numbers.
Figure 6. Funiculina was the most common sea pen in the trawl bycatches. (A) Photo from ROV transects conducted at station SK 6 showed high densities (∼3 colonies per m2) with the symbiont brittle star Asteronyx present on all colonies. (B) The sea pen predator anemone Ptychodactis patula was found eating tissue of the sea pen skeleton.

Figure 7. The bamboo coral Isidella lofotensis was documented in densities of several colonies per m2 on video from the ROV station SK2. As do many other gorgonians (laser scale (red dots) is 5 cm), Isidella provides a habitat that allows for other organisms, here a pandalid shrimp, to hide amongst the branches away from predators (photo from ROV station SK6).

Table IV. The 17 ROV stations (out of 35) where observations of sea pens and corals were made.
Discussion
Sampling methodology
Bottom trawls are not designed to collect benthic organisms and depending on substratum and rigging they will collect only a portion of the soft bottom epifauna during a haul. Bycatch records from trawls must therefore be viewed as minimum estimates of densities, and some common species may not even be present in the trawl. Our comparison with videos from the ROV survey conducted by OCEANA showed that all the species present as bycatch in the trawl were recorded in much higher numbers on the ROV stations where they occurred ( and ). This difference is not surprising and in a recent study Mendoca and Metaxa (Citation2021) compared epifauna data from ROV and drop camera with trawl sampling at two stations. They showed that the image-based methods are more efficient in general and particularly in relation to Pennatulacean recruits, in addition the fine-scale distributions could not be elucidated with a trawl. This demonstrates that in addition to being non-destructive, visual mapping is more suitable for monitoring the populations of vulnerable species.
Also, the small and delicate sea pen Virgularia, not present in trawl bycatches, occurred in large numbers on the videos. This can be explained by its small size and its ability to withdraw into the sediment but could also be due to its association with watery fjord sediments that was documented by the ROV transects but not well covered by the trawl survey stations.
The trawl bycatches were documented using photos and detailed inspection of colonies for identification was not possible, however, the sea pens and bamboo coral collected or observed were easy to recognize based on their large-scale morphology. Quantification was sometimes difficult when bycatches were large, and many colonies broken. This problem was solved by counting only holdfasts or peduncles of colonies, however, the risk of missing out colonies when bycatches were large is substantial and did probably cause a bias towards smaller counts. Thus, we did not use the trawl data to estimate densities of the colonies on the seafloor but instead compared abundances between survey stations based on average numbers in trawl catches from each sampling station from the survey period 2017–2021.
Distribution and vulnerability
Commercial fisheries for demersal species are intense in Skagerrak and the Norwegian trench and represent a potential threat to VMEs in the area. and show the distribution of fisheries with demersal trawl during the last 5-year period. Trawling is most intense on the slope in Skagerrak and the Norwegian trench, and the central deeper part of Skagerrak has little trawling. Still the overlap with sea pen habitats is large. shows that the highest numbers of sea pens are related to areas that are trawled less than 200 h per grid cell. This relation could be explained by removal of sea pens by trawling, alternatively the best sea pen habitats are not co-occurring with areas where trawling is most intense. The ROV videos from areas with high trawling intensity show damaged sea pens and trawl marks ( and ).
Figure 8. Occurrences of sea pens (all species combined, whole area) and Isidella (inserted figure) in relation to bottom trawling intensity in the study area. Fisheries intensity is shown as the number of hours trawled per 5 × 5 km2 grid cell, using fisheries statistics for 2016–2020 provided by the Norwegian Fisheries Directorate, Open data: electronic reporting (ERS) (fiskeridir.no).
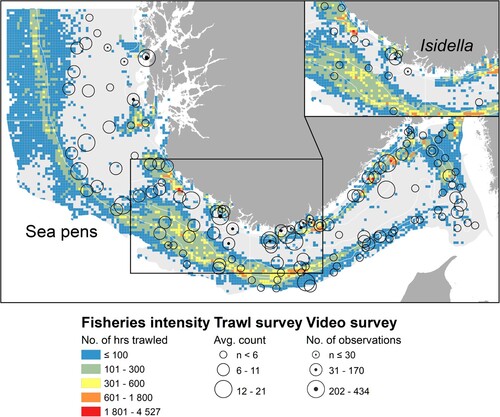
Figure 9. Relationship between trawling intensity (hours per grid cell) and total numbers of sea pens and Isidella sampled as trawl bycatch from the IMR shrimp survey from 2017–2021, and sea pens recorded on the OCEANA ROV stations in 2016 and 2017. Recorded colonies are provided as number per grid cell where trawl activity was also recorded (see ). The dotted trend line shows the relation between recorded number of colonies and fishing intensity.
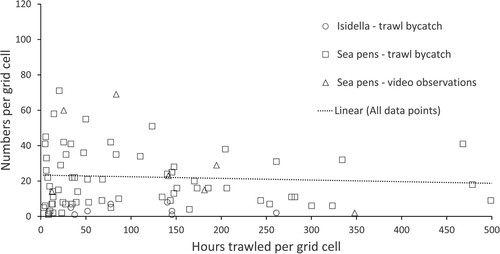
Figure 10. Damage to sea pens presumably from trawling at station SK2. At the top is a Funiculina skeleton and to the left below a colony with damaged tip of colony. At the bottom right are two Kophobelemnon colonies, the one in the back with damaged tip of colony.

Figure 11. Trawl marks at station NT2. At the top is a Funiculina colony in front of lines from chain or bobbins. Below, is a squat lobster Nephrops norvegicus in between trawl marks.

Sea pen VME
Funiculina is widely distributed and occurs in large numbers locally in the study area () but is still under pressure from bottom trawling in most parts of its distribution area (). Greathead et al. (Citation2015) has reported this species as of the greatest conservation importance within the Greater North Sea and Celtic Sea. Unlike the other sea pen species, Funiculina is unable to withdraw into the sediment (Greathead et al. Citation2015) and is therefore probably more susceptible to damage from bottom trawling.
Skeletons of dead Funiculina colonies were observed at three ROV transects and this, together with the large numbers recorded in the trawl bycatches, indicates that it is vulnerable to bottom trawling (). The rare anemone Ptychodactis patula was found on Funiculina at stations SK6 and SK10 (B). This anemone that has been reported to be associated with octocorals (Appelöf Citation1893) has a morphology adapted to preying on the sea pens (Cappola and Fautin Citation2000). Tissue damage and exposed skeleton are common on corals, resulting from fishing gear, and this was observed at several ROV stations (). Mortensen et al. (Citation2005) observed that anemones can colonize exposed coral skeleton and then prey on the live parts of the colony. In B we can see dead tissue near the anemone where it likely has foraged on the sea pen. The presence of P. patula could be related to damage to Funiculina from the intense bottom trawling in the area.
Funiculina constitutes an essential habitat to Asteronyx (Fujita and Ohta Citation1988) and provides the brittle star with an elevated position for preying on small pelagic animals, mainly copepods. Catches of Asteronyx showed a clear correlation with catches of its host (), and they appear to occur in a one-to-one ratio as has been reported by De Clippele et al. (Citation2015) from Norwegian waters. In trawl bycatches the brittle star can lose its grip and the sea pens are often broken. Thus, they may not be evenly distributed amongst the host colonies collected in a trawl. The video records (A) show that a single colony can have more than one specimen attached.
Kophobelemnon was like Funiculina common and widely distributed in the study area but with a deeper maximum of occurrence ( and ). Finer sediments with a higher water content are probably more suitable for this smaller sea pen that can fully withdraw into the sediment. Even though it occurs in areas with large trawling intensity () its ability to withdraw into the sediment might make it less susceptible to bottom trawling. Even so, the occurrence in the bycatches indicates that it is dislodged by trawl but to a lesser extent than Funiculina. ROV observations also documented damage to this species (). The squat lobster Munida sarsi that is very common in the study area is known to associate with Kophobelemnon (De Clippele et al. Citation2015).
Balticina, a large sea pen with a very limited distribution in the study area, might also be at risk from fisheries.
Coral gardens VME
Isidella lofotensis is the key species of an OSPAR habitat that is at special risk because it occurred only in a very small and well-defined part of our study area. In 2018, ‘Bamboo coral forest’ was added to the Norwegian Red List for Ecosystems and Habitat Types, under the category of Endangered (i.e. very high risk of becoming extinct). The aim of this list is to give decision-makers better knowledge for biodiversity management, although it does not mandate legal protection. Our observations show that this Red Listed habitat with a very limited distribution in the study area is in an area that is frequently trawled ( and ) and thus is at high risk of local extinction. However, the ROV transect at SK2 showed that Isidella can occur in dense populations locally (). This species is viewed as endemic to Norway (Buhl-Mortensen et al. Citation2015) and outside the Skagerrak area it has been reported mainly from fjords (Buhl-Mortensen and Buhl-Mortensen Citation2014). Isidella colonies offer a habitat for shrimps that were observed hiding amongst the branches of colonies (). Corals are known to provide valuable habitat for crustaceans (Buhl-Mortensen and Mortensen Citation2005; Buhl-Mortensen et al. Citation2010; De Clippele et al. Citation2015).
Future needs
We have shown that trawl bycatches can provide valuable information on the distribution and abundance of VMEs, but the recorded colonies in bycatches can only provide minimum estimates and come from a sampling method that is a threat to most VMEs. Actual density and health status for VMEs can only be gained by conducting detailed visual mapping in this understudied area. Even so, our results show an immediate need for protection of the ‘Bamboo coral forest’ in the study area followed by visual mapping and monitoring to allow for evaluation of ecological status and to be able to suggest relevant protection areas based on firm knowledge.
Acknowledgements
We are indebted to the scientists and technicians for their great work photo documenting bycatch of corals on board the IMR research vessel during the shrimp surveys in 2017–2021. OCEANA kindly lent us their videos from their Skagerrak missions in 2016–2017 for a detailed analysis of coral occurrences and environmental settings.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Álvarez H, Perry AL, Blanco J, Conlon S, Petersen HC, Aguilar R. 2019. Protecting the North Sea: Norway. Oceana, Madrid. 96pp.
- Amoroso RO, Buhl-Mortensen L, Pitcher CR, Rijnsdorp AD, McConnaughey RA, Parma AM, Suuronen P, Eigaard OR, Bastardie F, Hintzen NT, Althaus F, et al. 2018. Bottom trawl fishing footprints on the world’s continental shelves. Proceedings of the National Academy of Sciences. 115(43):201802379. doi:10.1073/pnas.1802379115.
- Appelöf A. 1893. Ptychodactis patula n.g. & sp. der Repräsentant einer neuen Hexactinien-Familie. Bergen Museums Aarbog. 4:3–22.
- Buhl-Mortensen L, Aglen A, Breen M, Buhl-Mortensen P, Ervik A, Husa V, Løkkeborg S, Røttingen I, Stockhausen HH. 2013. Impacts of fisheries and aquaculture on sediments and benthic fauna: suggestions for new management approaches. Fisken og Havet 2, 69pp.
- Buhl-Mortensen L, Burgos JM, Steingrund P, Buhl-Mortensen P, Ólafsdóttir SH, Ragnarsson SA. 2019. Vulnerable marine ecosystems (VME) Coral and sponge VMEs in Arctic and sub-Arctic waters – Distribution and threats, TemaNord, 519: 144pp, ISSN 0908-6692.
- Buhl-Mortensen L, Ellingsen KE, Buhl-Mortensen P, Skaar KL, Gonzalez-Mirelis G. 2016. Trawling disturbance on megabenthos and sediment in the Barents Sea: chronic effects on density, diversity, and composition. ICES Journal of Marine Science. 73(Supplement 1):98–114. doi:10.1093/icesjms/fsv200.
- Buhl-Mortensen L, Mortensen PB. 2005. Distribution and diversity of species Associated with Deep-sea gorgonian corals off Atlantic Canada. In: Freiwald A, Roberts JM, editors. Cold-water corals and ecosystems. Berlin, Heidelberg: Springer-Verlag; p. 849–879.
- Buhl-Mortensen L, Olafsdottir SH, Buhl-Mortensen P, Burgos JM, Ragnarsson SA. 2015. Distribution of nine cold-water coral species (Scleractinia and Gorgonacea) in the cold temperate North Atlantic: effects of bathymetry and hydrography. Hydrobiologia. 759:39–61. doi:10.1007/s10750-014-2116-x.
- Buhl-Mortensen L, Vanreusel A, Gooday AJ, Levin LA, Priede IG, Buhl-Mortensen P, Gheerardyn H, King NJ, Raes M. 2010. Biological structures as a source of habitat heterogeneity and biodiversity on the deep ocean margins. Marine Ecology. 31(1):21–50. doi:10.1111/j.1439-0485.2010.00359.x.
- Buhl-Mortensen P, Buhl-Mortensen L. 2014. Diverse and vulnerable deep-water biotopes in the Hardangerfjord. Marine Biology Research. 10(3):253–267. doi:10.1080/17451000.2013.810759.
- Cappola V, Fautin D. 2000. All three species of Ptychodactiaria belong to order Actiniaria (Cnidaria: Anthozoa). Journal of the Marine Biological Association of the United Kingdom. 80(6):995–1005. doi:10.1017/S0025315400003064.
- Clark MR, Tittensor DP. 2010. An index to assess the risk to stony corals from bottom trawling on seamounts. Marine Ecology. 31(September):200–211. doi:10.1111/j.1439-0485.2010.00392.x.
- Curd A. 2010. Background Document for Seapen and Burrowing megafauna communities. ISBN 978-1-907390-22-7. Publication Number: 481/2010, 26pp.
- De Clippele LH, Buhl-Mortensen P, Buhl-Mortensen L. 2015. Fauna associated with cold water gorgonians and sea pens. Continental Shelf Research. 105:67–78. doi:10.1016/j.csr.2015.06.007.
- Eigaard OR, Bastardie F, Hintzen NT, Buhl-Mortensen L, Buhl-Mortensen P, Catarino R, Dinesen GE, Egekvis J, Fock HO, Geitner K, et al. 2017. The footprint of bottom trawling in European waters: distribution, intensity, and seabed integrity. ICES Journal of Marine Science. 74:847–865. doi:10.1093/icesjms/fsw194.
- Fujita T, Ohta S. 1988. Photographic observations of the life style of a deep-sea ophiuroid Asteronyx loveni (Echinodermata). Deep Sea Research Part A. Oceanographic Research Papers. 35(12):2029–2043. doi:10.1016/0198-0149(88)90123-9.
- Greathead C, Gonzalez-Irusta JM, Clarke J, Boulcott P, Blackadder L, Weetman A, Wright PJ. 2015. Environmental requirements for three sea pen species: relevance to distribution and conservation. ICES Journal of Marine Science. 72:576–586. doi:10.1093/icesjms/fsu129.
- ICES. 2021. Greater north Sea ecoregion – ecosystem overview. In Report of the ICES Advisory Committee, 2021. ICES Advice 2021, Section 9.1. doi:10.17895/ices.advice.9434.
- Mendoca SN, Metaxa A. 2021. Comparing the performance of a remotely operated vehicle, a drop camera, and a trawl in capturing deep-Sea epifaunal abundance and diversity, Frontiers in Marine Science. 8. doi:10.3389/fmars.2021.631354.
- Mortensen PB, Buhl-Mortensen L, Gordon DC Jr, Fader GB, McKeown DM, Fenton DG. 2005. Evidence of fisheries damage to deep-water gorgonians in the Northeast Channel, Nova Scotia. In: Thomas J, Barnes P, editors. Proceeding from the symposium on the effects of fishing activities on benthic habitats: linking geology, biology, socioeconomics and management. American fisheries society symposium, November 12–14, 2002, Florida, USA.
- OSPAR. 2004. Descriptions of Habitats on the Initial OSPAR List of Threatened and or Declining Species and Habitats. OSPAR Convention for the Protection of the Marine Environment of the North–East Atlantic 7, p. 7.
- Pitcher RC, Hiddink JG, Jennings S, Collie J, Parma AM, Amoroso R, Mazor T, Sciberras M, McConnaughey RA, Rijnsdorp AD, Kaiser MJ, Suuronen P, Hilborn R. 2022. Trawl impacts on the relative status of biotic communities of seabed sedimentary habitats in 24 regions worldwide. Proceedings of the National Academy of Sciences. 119(2):e2109449119. doi:10.1073/pnas.2109449119.
- Søvik G, Thangstad TH. 2021. Results of the Norwegian Bottom Trawl Survey for Northern Shrimp (Pandalus borealis) in Skagerrak and the Norwegian Deep (ICES Divisions 3.a and 4.a east) in 2021. NAFO SCR Doc. 21/001, Serial No. N7157. 38pp. https://www.nafo.int/Portals/0/PDFs/sc/2021/scr21-001.pdf.
