 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Some cladobranch sea slugs host algae (Symbiodiniaceae), forming stable or unstable photosymbiotic relationships. Although some benefits from retaining symbionts have been described in stable photosymbioses, unstable photosymbioses remain largely uninvestigated. We examined two potential benefits – nutrition and oxygen produced via photosynthesis – in the unstable cladobranch model species, Berghia stephanieae. To investigate potential nutritive benefits, we conducted transmission electron microscopy and observed both partially digested symbionts and lipid droplets, indicating that B. stephanieae benefits energetically from hosting zooxanthellae through digestion. Since increased temperatures can cause oxygen limitation, any oxygenic benefits B. stephanieae receives from photosynthesis could influence their thermal tolerance, allowing photosynthetic slugs to withstand higher temperatures than specimens where photosynthesis is limited or absent. To assess this, we measured the maximum temperature they can withstand before succumbing to heat-shock under three light intensities (0, 100 and 700 µmol m-2s-1). Oxygen uptake was measured before and after heat-shock to determine whether uptake was affected by thermal stress. Slugs exposed to high light displayed significantly lower thermal limits than those at zero or moderate light, indicating exposure to acute high light negatively impacts thermal tolerance. Lastly, we assessed if and how light affects juvenile development and survival. Juveniles exposed to moderate light survived longest, while both other light intensities reduced survival. These investigations demonstrate that unstable photosymbiosis provides B. stephanieae with nutritive benefits during different ontogenetic stages. Oxygenic benefits are less clear, as slugs exposed to thermal stress in dark and under moderate light did not display different thermal tolerances.
Introduction
Numerous heterotrophic species form symbioses with single-celled algae allowing them to profit energetically from the products of photosynthesis. Amongst metazoan taxa, these relationships, termed photosymbioses, are most often formed with dinoflagellates belonging to the family Symbiodiniaceae (Stanley Citation2006; Rumpho et al. Citation2011). Although corals are perhaps the most well-known photosymbiotic taxon, many other metazoan species also host Symbiodiniaceae (Melo Clavijo et al. Citation2018), commonly referred to as zooxanthellae when in hospite (Kempf Citation1984; Weis et al. Citation2008; Sunagawa et al. Citation2009). To date, photosymbiosis research has predominantly focused on cnidarians like corals and anemones, but lesser known photosymbioses, such as acoelomate flatworms and nudibranchs are garnering increasing attention (Arboleda et al. Citation2018; Rola et al. Citation2022).
Nudibranchia are a carnivorous order of sea slugs feeding on prey such as anemones, corals, sponges, tunicates, and bryozoans (Nybakken and McDonald Citation1981). Some nudibranch species belonging to the suborder Cladobranchia are capable of stealing zooxanthellae from their cnidarian prey. The algae are incorporated into cells of the digestive gland system which runs transversally along the animal’s body and line their dorsal appendages, termed cerata (singular cera) (Kempf Citation1984; Rola et al. Citation2022). Different categories of photosymbiosis are found among cladobranchs, depending on how long the zooxanthellae remain active within their cells, and there is evidence for independent origins of photosymbiosis in these lineages (Rola et al. Citation2022). In unstable photosymbioses, zooxanthellae only remain in the animal for a few days or weeks before they are digested or expelled, while stable photosymbioses hold on to the active zooxanthellae for several months (reviewed in Rola et al. Citation2022).
Since the discovery of the nudibranch and zooxanthellae relationship, researchers have debated whether or not these symbioses are mutualistic and sought to understand potential benefits for each partner. Benefits for the animal host could include sunblock against ultraviolet radiation (Watson et al. Citation2021) and camouflage provided by the photosynthetic pigments (Marín and Ros Citation1991; Di Marzo et al. Citation1993; Wägele Citation2004). The slug could also profit energetically, either via the production and immediate translocation of photosynthates to the animal (Burghardt and Wägele Citation2014) as observed in many cnidarian species (Davy et al. Citation2012), or by digestion of the symbiont itself as food including any photosynthates it has produced and retained, which has been observed in some sacoglossan slugs harboring chloroplasts (Laetz et al. Citation2017; Laetz and Wägele Citation2019). Energetic benefits, through either increased growth and/or reproductive capacity, have been observed in two species that can maintain stable photosymbioses under normal lighting conditions, Melibeengeli Risbec, 1937 and Phyllodesmiumbriareum (Bergh, 1896) (Kempf Citation1984; Burghardt and Wägele Citation2004; Burghardt et al. Citation2005).
While the energetic benefits to stable photosymbioses have been demonstrated in numerous species, including some cladobranch slugs, the energetic benefits of unstable photosymbiosis are unclear. Multiple authors report that some cladobranchs with unstable photosymbioses excrete the symbionts they acquire (Parker Citation1984; Monteiro et al. Citation2019). This suggests that these species do not profit from symbiont incorporation and may even shed the zooxanthellae to avoid potential harm. Therefore, we must also consider if there are potential drawbacks that could explain why zooxanthellae are only retained for limited time spans in some species. Symbiodiniaceae are capable of mixotrophy or heterotrophy if unable to support their metabolism via autotrophy alone (Jeong et al. Citation2012). Their ability to switch modes of energy acquisition could become a burden for the animal host if Symbiodiniaceae can successfully predate its host’s tissues (endoparasitism), which has been observed in cnidarians provided suboptimal light intensities and/or temperatures (Steen Citation1986; Douglas and Smith Citation2019).
Suboptimal light and temperatures could impact other potential photosynthetic benefits that have yet to be examined in cladobranchs capable of stable and unstable photosymbioses. Molecular oxygen (O2, hereafter referred to as oxygen) that is produced as a byproduct of photosynthesis could benefit photosymbiotic slugs, helping them meet their oxygenic demands in order to maintain aerobic respiration, especially for species inhabiting shallow, tropical waters, which already contain lower levels of dissolved oxygen than temperate, polar and deeper waters. Although it is still unknown exactly what determines thermal limits for marine organisms, oxygen availability is increasingly indicated as a key factor for a few reasons. Increasing sea surface temperatures leads to deoxygenation for multiple reasons including, decreased solubility, increased stratification, and changes in water circulation, yet many ectotherms require more oxygen to maintain aerobic respiration at higher temperatures (Woods et al. Citation2022). This can cause a mismatch between the amount of oxygen that is available to the animal and the amount of oxygen it requires for sustaining aerobic metabolism (Pörtner Citation2002; Storch et al. Citation2014; Verberk et al. Citation2016). Therefore, oxygen that is produced as a byproduct of photosynthesis may give photosymbiotic slugs an advantage by reducing the amount of oxygen they need to obtain from their environments in order to maintain aerobic scope, particularly when oxygen becomes more limited as they approach their critical thermal maximum temperatures. These thermal maxima are often defined as the maximum temperature an individual can withstand before it is no longer able to flee a stressor (Cowles and Bogert Citation1944) or the temperature at which it enters a heat-induced coma (Lutterschmidt and Hutchison Citation1997).
Profiting energetically or oxygenically from photosynthesis requires exposure to light, which depends on a wide variety of abiotic and biotic factors in nature, from depth and turbidity to a slug’s behaviour (i.e. does it demonstrate positive or negative phototaxis) and the location of its prey (detailed in Burgués Palau et al. Citation2024). Light can also be considered a stressor though, when light intensities that exceed an organisms’ photoacclimation state (the amount of light to which it is adapted) inhibit photosynthesis by damaging the photosynthetic machinery leading to photoinhibition and photodamage (Hoegh-Guldberg and Jones Citation1999; Ralph et al. Citation1999; Lesser Citation2006). Some coral hosts expel their symbionts when exposed to intense light exposure, which is amplified when they are subjected to thermal stress (Fitt et al. Citation2001; Bhagooli and Hidaka Citation2004). Increased temperatures can also destabilize the coral/zooxanthellae symbiosis by disrupting their mutually beneficial nutrient exchange (Rädecker et al. Citation2021). Thermal stress can also cause hosts to reach a tipping point at which the presence of their photosynthesizing symbionts becomes more costly for the host than beneficial, shifting a mutualistic interaction toward an endoparasitic interaction (Baker et al. Citation2017). Although it is not known if incorporated zooxanthellae can parasitize cladobranch sea slugs and under which environmental conditions this would occur, the drawbacks described above are plausible in sea slugs and could help explain why some cladobranchs have limited symbiont retention capacities.
To examine potential energetic and oxygenic benefits in a cladobranch slug capable of unstable photosymbiosis, we examined the cladobranch model species, Berghia stephanieae (Valdés, 2005). This species feeds solely on Exaiptasia diaphana (Rapp, 1829) sea anemones that host photosynthetic Symbiodiniaceae as endosymbionts in their endodermal cells (Garrett et al. Citation2013). Berghia stephanieae obtains these algae by feeding on E. diaphana and incorporating the algal cells into its digestive gland epithelial cells, where they can be retained for up to five days in an unstable photosymbiosis (Monteiro et al. Citation2019). While it is clear that photosynthesis does not provide enough energy for B. stephanieae to maintain their biomass, i.e. these slugs are not photoautotrophic (Monteiro et al. Citation2019), it is not clear if B. stephanieae receives any energetic benefits and how or when they receive these benefits. For example, juvenile B. stephanieae have been observed excreting intact symbionts (Monteiro et al. Citation2019) suggesting they do not energetically benefit from acquiring symbionts, yet Melo Clavijo et al. (Citation2022) suggest that symbionts are digested based on the production of proteins related to lysosome maturation in specimens that were fed algae containing symbionts, which indicates that adult B. stephanieae may profit energetically from breaking down their symbionts during digestion, rather than the active translocation of photosynthates observed in some cnidarian photosymbiosis. Additionally, anecdotal observations suggest that B. stephanieae prefers dark environments as it is most commonly found underneath objects or in dark crevices (Quinlan and Katz Citation2022). This could indicate that they avoid light and receive few if any energetic or oxygenic benefits due to a limited photosynthetic capacity, however the relationships between light, oxygen, and nutrient transfer from symbiont to slug have not been thoroughly investigated.
To understand potential nutritive and oxygenic benefits B. stephanieae could receive from incorporated zooxanthellae, we examine photosynthate production and symbiont digestion via transmission electron microscopy. To examine the potential oxygenic benefits of unstable photosymbiosis, we examined how dark (no photosynthesis), optimal light (matching the conditions to which they were photoacclimated) and high light intensity (inducing photoinhibition and photodamage) affect the rate of oxygen uptake and critical thermal limits in this species. We hypothesized that photosynthetic O2 production would offset a slug’s need to acquire oxygen from its environment (i.e. slugs kept in the light would display lower metabolic rates than those kept in darkness). We also expected that photoinhibition and photodamage would decrease photosynthetic oxygen production leading to higher rates of oxygen uptake from the slug’s environment in slugs exposed to high light intensities (700 µmol m−2s−1) when compared to slugs measured at the light intensity to which they were acclimated(100 µmol m−2s−1). Examining their critical thermal limits under each light intensity provided a performance test, allowing us to evaluate if the oxygen produced via photosynthesis could offset oxygen limitation in the environment, which would demonstrate a measurable advantage due to oxygen production. Lastly, we examined the survivorship and development of B. stephanieae juveniles under different light intensities, to determine if potential energetic or oxygenic benefits provided by photosynthesis increase a developing slug’s chance of survival.
Material and methods
Animal husbandry
Exaiptasia diaphana specimens containing Symbiodiniaceae were donated by a local aquarium store (Frits Kuiper Aquarium store, Groningen, The Netherlands) and cultured at a light intensity of 100 µmol m−2 s−1 provided by a full spectrum light (Superfish Slim LED 74) in the lab (the same average conditions they were exposed to in the store) under a 12 h light / 12 h dark cycle each day and acclimated for a minimum of two weeks. They were kept in 25 L glass tanks filled with aerated artificial saltwater (Instant Ocean, Spectrum Brands, USA) with a salinity of 35 ± 1 PPT. All tanks were placed in a climate controlled cabinet that provided a constant temperature of 25 ± 1°C (Clima Temperatur Systeme T600). Exaiptasia diaphana were fed live Artemia sp. brine shrimp (HOBBY, DohseAquaristik GmbH & Co.) twice a week. Every week, one third of the water was replaced with fresh water and full water changes were done monthly.
Berghia stephanieae specimens were also raised under a 12 h light / 12 h dark cycle under 25 µmol m−2s−1 (Daylight Sunrise, Sera, Germany) at 25°C in 35 ml artificial seawater (35 PSU, pH 8.1, ABReef Salt, Aqua Medic, Germany) at the Bergische University in Wuppertal, Germany. Water was changed every other day and the slugs were fed twice a week with E. diaphana. Egg masses and adult specimens were shipped to the University of Groningen, the Netherlands, for the following experiments. Adults were acclimated for at least two weeks according to the same conditions described for E. diaphana. Each tank was provided live E. diaphana and allowed to feed ad libitum. Partial water changes were conducted each week, with full water changes monthly.
Berghia stephanieae egg masses were kept in small glass dishes filled with 50 mL of aerated artificial seawater, and were constantly supplied with either tentacles of or small E. diaphana individuals to facilitate hatching (Carroll and Kempf Citation1990; Monteiro et al. Citation2019). They were maintained at 25 ± 1°C and placed under 100 µmol m−2 s−1 of light (Superfish Slim LED 74) for 12 h each day. Water changes were conducted every two days.
Species names for all taxa used in this study were checked and updated using the World Register of Marine Species (WoRMS Citation2023). Exaiptasia diaphana is often reported in scientific literature under its former name Aiptasia pallida (Agassiz in Verrill, 1864) or under the name Exaiptasia pallida (Agassiz in Verrill, 1864) (see Grajales and Rodríguez Citation2014, Citation2019). Pending the decision on ICZN Case 3790, E. diaphana takes priority over E. pallida and is therefore used here.
Transmission electron microscopy of Symbiodiniaceaein B. stephanieae
To investigate symbiont digestion in B. stephanieae, an adult specimen was fixed in 2.5% glutaraldehyde buffered in 1 M sodium cacodylate at room temperature for an hour and then postfixed in a 1% OsO4 and 1 M sodium cacodylate buffer, dehydrated in an ascending ethanol series, and embedded in Epon (Laetz and Wägele Citation2019). Ultrathin sections (70 nm thick) were stained with 2% uranyl acetate for 3 min, rinsed and then transferred to 3% lead citrate for 3 min and subsequently rinsed again. Imaging was conducted using a CM120 Transmission Electron Microscope (Philips, Global).
Oxygen consumption and heat shock thresholds
To examine potential oxygenic benefits to having an unstable photosymbiosis, eight random B. stephanieae individuals were first massed by gently blotting each individual on a piece of aluminum foil and then using a fine glass pipette to remove as much water as possible around the individual before placing it in a pre-tared petri dish filled with sea water on a scale. They were then transferred to 2 mL vials (respiratory chambers) containing freshly aerated and filtered seawater at 100% O2 saturation and a PSt3 oxygen sensor spot (PreSens Precision Sensing GmbH, Germany, detection range: 0–45 mg/L, precision: ±0.04 mg/L at 9.1 mg/L as reported by the manufacturer), to determine their rates of oxygen consumption (MO2). These vials were secured in a water bath to maintain the desired temperature (25°C). A light source was fixed above the water bath to provide either 100 µmol m−2s−1 or acute high light (700 µmol m−2s−1) to examine if light intensities far above the level to which our specimens were photoacclimated would induce light stress. A moderate light intensity of 100 µmol m−2 s−1 was chosen for the slugs since it matches the light intensity at which the anemones and their zooxanthellae were cultured and therefore photoacclimated. The high light treatment (700 µmol m−2 s−1) was chosen as it is much higher than the light intensity to which these specimens were acclimated so it could induce photodamage in the zooxanthellae. Furthermore, 700 µmol m−2 s−1falls within the range of light intensities to which these species are likely exposed in nature (e.g. Grajales and Rodríguez Citation2014; Durán-Fuentes et al. Citation2022), which ranges from 600 to >2000 µmol m−2s−1 at 0–5 m depth (Mantelatto et al. Citation2020; Burgués Palau et al. Citation2024). The lamp was turned off for specimens to induce acute darkness (0 µmol m−2s−1) and a black-out box around the set up prevented exposure to ambient light. A Fibox 4 trace (PreSens Precision Sensing GmbH, Germany) with a polymer optical fibre was used to measure changes in oxygen saturation over time by holding the sensor on the oxygen sensor spot. Before the experiment, the Fibox was calibrated via two-point calibration (aerated seawater was used to define 100% O2 saturation and nitrogen gas was used to define 0% O2 saturation). The oxygen saturation values were recorded every 10 min. Each specimen was measured for a total of 1 h or until the oxygen saturation was recorded below ∼70% to avoid stressing the slugs with hypoxic conditions. Each respiratory chamber was manually rotated after each measurement to stir the water and ensure that oxygen gradients did not develop. A blank respirometry chamber containing the same seawater was also measured throughout every measurement cycle to control for background oxygen consumption by microbes. Respiratory chambers were sterilized with ethanol, and then washed and dried after each use.
Directly following the oxygen saturation experiment, each specimen’s tolerance to acute hyperthermal stress was measured to examine if photosynthetic activity affects a stressed slug’s physiological performance, i.e. if the oxygen produced via photosynthesis helps them withstand environmentally stressful conditions where oxygen is limited. Specimens were placed inindividual 1 L transparent glass bottles filled with aerated and filtered seawater and kept under the same light intensity they were provided with while their metabolic rates were measured. The temperature was increased by 1°C every 15 min and each specimen’s ability to move around the bottle and climb its walls was closely monitored as outlined in Armstrong et al. (Citation2019). The temperature at which a specimen was no longer able to freely move around its bottle, meaning it had lost voluntary muscle control, was recorded as CTfirst. The temperature increases continued and specimens were lightly prodded with a probe every few minutes until they no longer exhibited movement in response to this stimulus, a sign that they had lost involuntary muscle control (recorded as CTlast). To prevent further stress or mortality, the temperature was then decreased to 34°C (the average CTfirst) before each specimen was transferred back to a sterilized respirometry chamber. Oxygen consumption was measured again following recovery from heat shock to reveal the change in metabolic rate after exposure to hyperthermal and/or light stress (ΔMO2) (See Table SI, supplementary material). All experiments were performed one time for each specimen.
For each individual, the pre-heat shock and post-heat shock metabolic rates (MO2 in mgO2 g−1 h−1) were calculated using the following formula:
where c is the amount of oxygen consumed in mgO2, v is the volume of the respirometry chamber in litres (minus the slug’s volume), w is the wet mass of the individual in grams, and t is the total time (duration) of the experiment in hours. The wet mass of the slug was also used to estimate their density, as their small size made it impossible to directly measure volume of B. stephanieae. The average density of a larger, and therefore more accurately measurable sea slug species, Elysia crispata Mörch, 1863 (n = 10), was computed by massing them and measuring their volume in a graduated cylinder, resulting in a density of 1.25 g/ml. This value was then used to approximate the density of B. stephanieae, which allowed us to convert each specimen’s wet mass to its respective volume. This calculated volume was then subtracted from the total volume of each respiratory chamber, to determine how much water (and therefore dissolved O2) was in the respirometry chamber when the experiment began. Oxygen saturation was then converted to concentration (mgO2 L−1) via an online lookup table from the United States Geological Survey (USGS Citation2011) for each test temperature. Then the amount of O2 in each chamber could be calculated using the actual amount of water (total volume minus slug volume) at the beginning and end of the experiment. The difference in these concentrations reveals the amount of O2 (in mg) that was taken up by the slug during the experiment (i.e. it’s oxygen consumption).
Juvenile survivorship under different light intensities
Egg masses and freshly hatched veligers of B. stephanieae were observed every other day until they metamorphosed into the slug phase of the life cycle and lost their veliger shell ((C)). Once metamorphosed, each slug was individually placed into a small glass dish filled with 50 mL of aerated artificial seawater, randomly placed under either zero light (0 µmol m−2 s−1), moderate light (∼100 µmol m−2 s−1), or high light (∼400 µmol m−2 s−1) and given E. diaphana tentacles on which to feed. Twenty-eight individuals for each light treatment were followed through development. Partial water changes were done every 2–3 days.
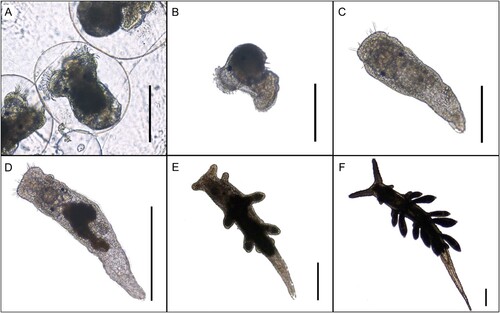
Figure 1. Life stages of Berghia stephanieae. (A) Close-up of egg mass strand. Early veliger stage larvae within egg capsules. (B) Hatched larvae with veliger shell. (C) Early vermiform juvenile that has lost its veliger shell but has not yet fed on Exaiptasia diaphana, 4 days post metamorphosis. (D) Young juvenile with digested Symbiodiniaceae from Exaiptasia diaphana, 7 days post metamorphosis. (E) First few cerata begin to form, 14 days post metamorphosis. (F) Late juvenile stage nearly reached with additional sets of cerata forming, 18 days post metamorphosis. Scale bar A–C: 400 µm, D–F: 800 µm.
Berghia stephanieae juveniles that originated from the cultivated egg masses and followed through development were photographed every 2–3 days using a Nikon Eclipse e800 epi-fluorescence microscope (Nikon, Ltd.) (). Light microscope images were taken to track developmental progress and fluorescent images were used to monitor chlorophyll concentrations within the slug’s digestive tract (detected via a custom filter cube with 400–440 nm peak excitation, a 515 nm dichroic and 610 nm long-pass emission (Chroma, USA)). The images containing chlorophyll were used to monitor symbiont acquisition and abundance. Juvenile longevity was measured by recording the number of days each post-metamorphic specimen survived or until it reached the late juvenile stage (as defined by Kristof and Klussmann-Kolb Citation2010) (Table SIII). Whether a post-metamorphic individual had fed or not was also noted in Table SIII, and the percentage of fed individuals under each light intensity was calculated. A two proportion z-test (under the stats (ver. 3.6.2) package (R Core Team Citation2022)) was then used to assess differences between the proportions of fed individuals under the three light intensities. Only the fed individuals were used for the later statistical analysis on juvenile survivorship described below.
Statistical analyses
To analyse differences in the metabolic rates calculated for each individual in each treatment, a linear mixed-effects model was generated in R Studio (ver. 1.3; R Studio Team Citation2020). Light intensity and heat-shock were used as predictor variables, the log of the body mass was considered a covariate, specimen identity was treated as a random factor since each specimen was measured pre- and post-heat shock, and the log of MO2 was the response variable. To assess normality, a Shapiro–Wilk test was performed (p = 0.088). Bartlett’s test was then performed to examine heterogeneity of variances (p = 0.45). Both tests were from the stats package. A linear mixed-effects interaction model was created and refitted with restricted maximum likelihood (REML) using the lme4 package (Bates et al. Citation2015). Pairwise comparisons were done using the emmeans package (Lenth Citation2021).
Critical thermal limits were analysed using linear models with the predictors described above, but with CTfirst and CTlast as response variables. Specimen was excluded as a random factor since no repeat measurements were made. One-way ANOVA assumptions were also tested via a Shapiro–Wilk normality test (p = 0.10) and Bartlett’s test (p = 0.81). Finally, a Tukey HSD test was used to determine differences amongst treatments. Heat-shock thresholds and critical thermal limits were visualized via violin plots in R Studio using the ggplot2 package (Wickham Citation2016) to show distribution.
Juvenile survivorship of the fed slugs under each light intensity was analysed using a Shapiro–Wilk normality test, which revealed that the data was not normally distributed (p = 0.0033). Therefore, a Kruskal–Wallis rank sum test from the stats package was used to assess if one or more treatment differed from the others (p = 0.0036), and Dunn’s post-hoc testing (dunn.test package (ver. 1.3.5) (Dinno Citation2017)) was used to assess which light intensities differed significantly. Violin plots were created in order to visualize the data and show the distribution.
Results
Energetic benefits to unstable photosymbiosis in B. stephanieae
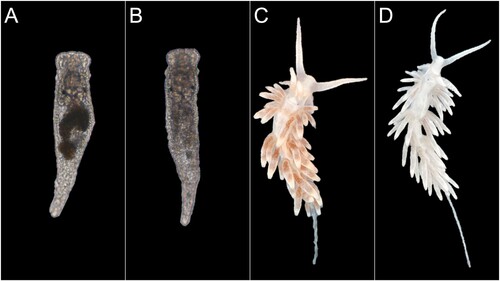
Intact zooxanthellae containing large lipid reserves, as well as partially degraded symbionts, were observed in the ultrastructure images of unstarved adult B. stephanieae (). We also observed that both juvenile and adult B. stephanieae ‘bleached’ quickly upon entering starvation, i.e. lost the brownish colouration they had from their incorporated zooxanthellae within 2–3 days after they were prevented from feeding on E. pallida ().
Figure 2. TEM images of intact and digested zooxanthellae in Berghia stephanieae. The large black structures inside these zooxanthellae are lipid droplets, made electron dense via OsO4 staining after fixation. (A) An intact zooxanthella located in Berghia stephanieae digestive cells. (B) Two partially degraded zooxanthellae in the same tissue.
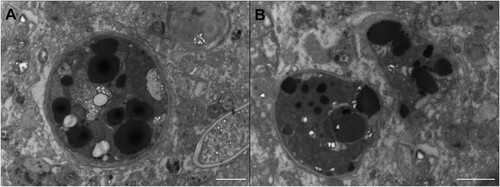
Figure 3. Comparison between starved/bleached and fed/unbleached Berghia stephanieae juveniles and adults. (A) A fed juvenile and thus full of symbionts as indicated by dark brown colouration in its digestive tract. Approximate total length: 1.22 mm. (B) A nearly bleached juvenile. Approximate total length: 1.24 mm. (C) A fed adult full of symbionts in its cerata. Approximate total length: 17.00 mm. (D) A nearly bleached adult. Approximate total length: 19.00 mm. White arrows point to cerata to accentuate the visual difference between fed and bleached individuals.
Oxygenic benefits to unstable photosymbiosis (MO2 and CT)
Mature B. stephanieae specimens had a significantly lower metabolic rate after heat shock than before, at all light intensities (zero light p = 0.0037, moderate light p = 0.010, and high light p = 0.0004). Furthermore, specimens that were exposed to high light conditions had significantly lower metabolic rates than those exposed to moderate light after the heat shock (p = 0.0096, Table SII). Specimens kept in darkness did not differ significantly from those kept under moderate light (p = 0.0722) ((A)). The mean log metabolic rates before heat shock and after heat shock can be found in .
Figure 4. (A) Violin plot of log(MO2) of Berghia stephanieae individuals before and after heat shocking at the three different light intensities. Significant differences in A include moderate light to high light after heat shock (p = 0.0096) and when comparing each light treatment before and after heat shock (p = 0.0037 for zero light, 0.010 for moderate light, and 0.00040 for high light). (B) Violin plot of the mean heat tolerance (CTlast) (in °C) of all three light intensities (zero light, moderate light, high light). The significant differences in B between zero light and high light, and moderate light and high light are 0.0012 and 0.0041, respectively.
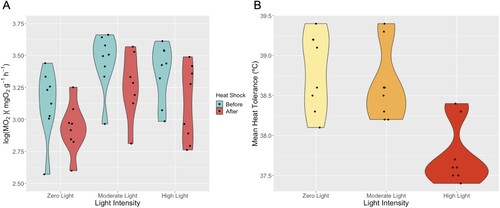
Table I. The mean log metabolic rates in µmolO2g−1h−1 before heat-shock and after heat-shock and the CTfirst and CTlast values in °C for each light intensity.
The mean critical thermal limit at which specimens lost voluntary muscle control (CTfirst) did not differ across light intensities ((B)). Specimens exposed to the high light intensity treatment lost involuntary muscle control (CTlast) at a lower temperature than specimens exposed to zero light (p = 0.0012) and moderate light (p = 0.0041) (, (B)). We also observed that a majority of the B. stephanieae individuals autotomized their cerata when exposed to acute heat stress, regardless of light intensity.
Effects of light stress on juvenile survivorship
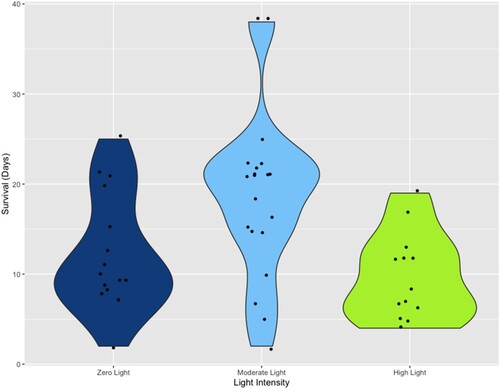
Of the juvenile B. stephanieae kept under a moderate light intensity, 20/28 ± 2.39 (71.43%) individuals fed on anemones during these experiments, compared to those kept under zero light (16/28 ± 2.62 (57.14%)) and high light intensities (13/27 ± 2.64 (48.15%)), although these three groups did not differ significantly when assessed with a two-proportion z-test (each p > 0.05). Fed juveniles exposed to moderate light also had the longest mean survival duration (mean ± std. dev. = 18.75 ± 9.10 days), which differed significantly (p = 0.0020) from specimens exposed to high light (9.77 ± 4.78 days) (). Juvenile survival when exposed to zero light had a mean of 12.19 ± 6.42 days and also differed significantly (p = 0.020) from specimens exposed to moderate light. Survivorship of juveniles kept under high light when compared to zero light were not significantly different (p = 0.14) ().
Figure 5. Violin plot of the longevity of juvenile Berghia stephanieae individuals, recorded as the number of days each post-metamorphic specimen survived at the three light intensities (zero light, moderate light, high light). Significant differences are found between individuals kept under zero light and moderate like (p = 0.020) and between those kept under moderate light and high light (p = 0.0020).
Discussion
Energetic benefits to having an unstable photosymbiosis
The presence of large lipid droplets in the intact symbionts and partially degraded symbionts indicates that adult B. stephanieae do gain access to photosynthetically produced energy reserves, but the bulk of this energy is likely made available upon symbiont digestion. This was predicted by Melo Clavijo et al. (Citation2022), based on the high recruitment of Rab7, a key protein involved in lysosome maturation, that was observed in symbiotic B. stephanieae. The microscopical data presented here now confirms that adult B. stephanieae digest at least some of their incorporated zooxanthellae ((B)), receiving some energetic benefits from their unstable photosymbiosis, although these benefits are very short lived (). This contrasts juvenile B. stephanieae, which have been observed excreting their symbionts and therefore, probably do not benefit energetically from the symbionts they acquire (Monteiro et al. Citation2019). Whether or not some photosynthetically-derived energy molecules are translocated to the slug before symbiont digestion will require further study. The eventual digestion of the symbionts makes this relationship more of a predator/prey interaction rather than a commensal or mutualistic symbiosis.
Oxygenic benefits to unstable photosymbiosis under light and temperature stress
Light and temperature are abiotic conditions that can become stressors when organisms are exposed to amounts that exceed the limits to which they are acclimated and able to plastically withstand (Stenseng et al. Citation2005; Cereja Citation2020). Photosymbioses are particularly susceptible to increased light intensities because excess light can induce photoinhibition and photodamage, which impairs photosynthetic activity and can destabilize the symbiosis, impacting symbiont and host function (see review by Cruz et al. Citation2013; Christa et al. Citation2018). Exposure to light stress (our high light treatment) decreased critical thermal tolerance in B. stephanieae by about 1°C when compared to the specimens measured under the same light intensities to which they were acclimated (100 µmol m−2s−1), meaning high light has a negative effect on thermal tolerance. We cannot, however, conclude that this effect is due to a reduced photosynthetic capacity from photodamage as we initially predicted, since slugs exposed to high light had a slightly lower rate of oxygen uptake from the seawater, meaning either more oxygen was produced via photosynthesis under high light conditions (suggesting there is no photodamage), or the slug reduced its aerobic demands when subjected to high light.
The high light value we used in this study (700 µmol m−2s−1) is toward the lower end of the maximum light intensities to which these specimens are exposed in nature, which regularly ranges from 600–2000 µmol m−2s−1 in the shallow (0–5 m deep) tropical waters where these species naturally occur (Mantelatto et al. Citation2020; Burgués Palau et al. Citation2024). Light intensities have been reported to be even higher depending on season, weather, depth, location, etc. (Edmunds et al. Citation2018; EMJL unpublished data). The decrease in thermal tolerance indicates that light stress can affect physiological function and thermal resistance in B. stephanieae. This is probably not due to photosynthesis, as there was no significant difference between the critical thermal limit for specimens exposed to acute darkness and the specimens allowed to photosynthesize under the light intensity to which they were photoacclimated, although this may also be due to the relatively small sample sizes used in this study (n = 8/light intensity). Therefore, we conclude that the ability to photosynthesize does not lead to a higher thermal tolerance in B. stephanieae. While oxygen production due to photosynthesis still benefits this species by contributing oxygen to aerobic respiration, we cannot infer that photosynthetically-derived oxygen helped these slugs overcome acute thermal stress and its accompanying oxygen limitation.
Since exposure to a high light intensity negatively impacts thermal tolerance in B. stephanieae, we must examine other reasons why these slugs were compromised under high light conditions. Exposure to light intensities above those to which they have been acclimated (as in our high light treatment) is stressful for photosynthetic organisms. Numerous indicators of cellular stress such as reactive oxygen species have been observed in algae exposed to acute high light (Ledford and Niyogi Citation2005; Pospíšil Citation2016). Stress in the algae could certainly affect the slug cells in which they are housed, causing a decrease in thermal tolerance for the slug, but this will require further study. Decreased thermal tolerance could also be explained by factors unrelated to the algae. For example, exposure to high light could be stressful to B. stephanieae specimens because their increased visibility could lead to increased risk of predation in nature. Some anecdotal evidence suggests that B. stephanieae is nocturnal and only emerges at night to feed (Mies et al. Citation2017; Monteiro et al. Citation2019), but this contrasts the observations we made during this study. Our B. stephanieae were observed feeding throughout the day when exposed to moderate light (100 µmol m−2s−1), indicating that our population is not nocturnal.
We observed many B. stephanieae specimens autotomizing their cerata when exposed to acute heat stress. The cerata play an important role in increasing the surface area of both the slugs’ respiratory and digestive tracts (Clark Citation1975). While not all species of nudibranch are capable of autotomizing their cerata, autotomy can be a useful defensive strategy to distract predators when under stress in species that are capable of autotomy (Furfaro and Mariottini Citation2020). Discarding their cerata causes a significant decrease in mass and surface area for gas exchange, both of which decrease a slug’s metabolic rate (Clark Citation1975). It also means they lose most of their algal symbionts, and reduce the energetic benefits they could get from digesting these symbionts. Therefore, autotomy could explain a decrease in metabolic rate for some cerata-bearing nudibranchs following exposure to acute thermal stress, including the specimens observed in this study. Another explanation for the lower metabolic rate observed in B. stephanieae following exposure to hyperthermal stress is that the slug enters metabolic depression, i.e. the suppression of non-critical metabolic processes to conserve energy. Metabolic depression has been observed in numerous gastropod species following exposure to extreme temperatures (Sokolova and Pörtner Citation2001, Citation2003; Marshall et al. Citation2011; Marshall and McQuaid Citation2011).
Although B. stephanieae had a lower metabolic rate after exposure to heat shock, multiple previous studies showed an increase in metabolic rate for adult (non-photosymbiotic) sea slugs when exposed to increased temperature (Potts Citation1983; Smith and Sebens Citation1983; Havenhand and Todd Citation1988). Armstrong et al. (Citation2019) found that some species of nudibranchs had a lower metabolic rate after heat exposure (such as Doriopsillaalbopunctata and Doris odhneri), but the three nudibranch species they surveyed that are most closely related to B. stephanieae (Hermissendaopalescens, Phidiana hiltoni, and Dironapicta) all showed an increase in metabolic rate. None of these species are known to host zooxanthellae though, so photosymbiosis could explain these differing responses to thermal stress. Since this study is the first to examine oxygen dynamics in a photosymbiotic nudibranch under heat stress, it can therefore serve as a reference for future studies that compare photosymbiotic nudibranch species.
Light stress affecting juvenile survivorship
Light exposure after hatching had a demonstrable effect on the survival rate of B. stephanieae (). The complete absence of light as well as exposure to a high light intensity resulted in a reduced feeding potential as well as survivorship when compared to juveniles kept under moderate lighting conditions. Although the percentages of individuals that fed were not significantly different from one another, a trend is still visible in favour of individuals kept under moderate light intensity. Out of all the fed juveniles observed during development, only two individuals survived into the late juvenile/adult stage, both of which were kept under moderate light. The overall high mortality rate is unsurprising considering sea slugs can lay hundreds of eggs at a time and multiple egg masses per week to make up for the low percentage of individuals that successfully reach adulthood (Carroll and Kempf Citation1990).
High light intensity has shown to cause a higher mortality rate in egg masses of multiple molluscan species (Biermann et al. Citation1992), and some have even observed particular species of nudibranchs exclusively laying their eggs in shaded habitats as if to avoid direct solar radiation, although this could be for reasons other than light avoidance, i.e. reduced predation. Egg masses laid in the shade also had a slower hatch rate (and therefore developmental rate) when compared to egg masses laid in the light (Przeslawski et al. Citation2004). In our study, exposure to high light as well as darkness demonstrated a trend of decreased feeding potential of young B. stephanieae. Considering the slugs are not born with their symbionts but rather acquire them after feeding, it is probable that light intensity plays a role on the survivorship of the animal itself. After feeding and thus acquiring symbionts, light continues to show an effect, but this time on the animal host as well as its symbiont. When kept in darkness, fed B. stephanieae juveniles can no longer obtain photosynthetically-derived energetic benefits, which may explain why those juveniles also had a lower survivorship than the juveniles kept in moderate light. The lower mean survival duration of fed juveniles kept in darkness could be due to the zooxanthellae switching to heterotrophy or to other fitness consequences caused by continued exposure to darkness.
This study has shown that B. stephanieae can be an ideal model species when looking into the potential benefits of unstable photosymbiosis and for providing possible insight into the reasoning behind the evolutionary drive for unstable photosymbiosis in Cladobranchia.
Author statement
NMB: Investigation, Methodology, Visualization, Formal analysis, Writing – original draft.
SETvdM: Supervision, Writing – review & editing.
GC: Resources, Writing – review & editing.
EMJL: Conceptualization, Methodology, Visualization, Formal analysis, Writing – original draft.
Supplemental Material
Download Zip (28.3 KB)Acknowledgements
We would like to thank Can Kahyaoglu, Michiel Merkx, Maja Bošnjaković and Rianne Pap for their help throughout this project, our technicians Jan Veldsink and Stella Boele-Bos for help in the laboratory (all University of Groningen) and the staff at Frits Kuiper Aquarium store in Groningen, The Netherlands, for supplying us with anemones. We would also like to thank Jenny Melo Clavijo and Elena Garcia Galera (University of Wuppertal) for providing and fixing the tissue for TEM imaging. We also greatly appreciate Klemens Eriksson (University of Groningen) for lending us lab space and supplies. Finally, we would like to thank our editor, Dr. Carolin Löscher and an anonymous reviewer for their suggestions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Arboleda E, Hartenstein V, Martinez P, Reichert H, Sen S, Sprecher S, Bailly X. 2018. An emerging system to study photosymbiosis, brain regeneration, chronobiology, and behavior: the marine acoel Symsagittifera roscoffensis. BioEssays. 40(10). doi:10.1002/bies.201800107.
- Armstrong EJ, Tanner RL, Stillman JH. 2019. High heat tolerance is negatively correlated with heat tolerance plasticity in nudibranch mollusks. Physiological and Biochemical Zoology. 92(4):430–444. doi:10.1086/704519.
- Baker AC, Correa AMS, Cunning R. 2017. Diversity, distribution and stability of symbiodinium in reef corals of the eastern tropical pacific. In: Glynn PW, Manzello DP, Enochs IC, editors. Coral reefs of the eastern tropical pacific. Vol. 8. Dordrecht, Netherlands: Springer; p. 405–420. doi:10.1007/978-94-017-7499-4_13.
- Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 67(1):1–48. doi:10.18637/jss.v067.i01.
- Bhagooli R, Hidaka M. 2004. Photoinhibition, bleaching susceptibility and mortality in two scleractinian corals, Platygyra ryukyuensis and Stylophora pistillata, in response to thermal and light stresses. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 137(3):547–555. doi:10.1016/j.cbpb.2003.11.008.
- Biermann CH, Schinner GO, Strathmann RR. 1992. Influence of solar radiation, microalgal fouling, and current on deposition site and survival of embryos of a dorid nudibranch gastropod. Marine Ecology Progress Series. 86(3):205–215. http://www.jstor.org/stable/24830479.
- Burghardt I, Evertsen J, Johnsen G, Wägele H. 2005. Solar powered seaslugs-mutualistic symbiosis of aeolid nudibranchia (Mollusca, Gastropoda, Opisthobranchia) with Symbiodinium. Symbiosis. 38(3):227–250. https://dalspace.library.dal.ca/bitstream/handle/10222/78144/VOLUME%2038-NUMBER%203-2005-PAGE%20227.pdf?sequence=1
- Burghardt I, Wägele H. 2004. A new solar powered species of the genus Phyllodesmium Ehrenberg, 1831 (Mollusca: Nudibranchia: Aeolidoidea) from Indonesia with analysis of its photosynthetic activity and notes on biology. Zootaxa. 596(1):1–18. doi:10.11646/zootaxa.596.1.1.
- Burghardt I, Wägele H. 2014. The symbiosis between the ‘solar-powered’ nudibranch Melibeengeli Risbec, 1937 (Dendronotoidea) and Symbiodinium sp. (Dinophyceae). Journal of Molluscan Studies. 80(5):508–517. doi:10.1093/mollus/eyu043.
- Burgués Palau L, Senna G, Laetz EMJ. 2024. Crawl away from the light! Assessing behavioral and physiological photoprotective mechanisms in tropical solar-powered sea slugs exposed to natural light intensities. Marine Biology. 171:50. doi:10.1007/s00227-023-04350-w.
- Carroll DJ, Kempf SC. 1990. Laboratory culture of the Aeolid Nudibranch Berghia verrucicornis (Mollusca, Opisthobranchia): some aspects of its development and life history. The Biological Bulletin. 179(3):243–253. doi:10.2307/1542315.
- Cereja R. 2020. Critical thermal maxima in aquatic ectotherms. Ecological Indicators. 119:106856. doi:10.1016/j.ecolind.2020.106856.
- Christa G, Pütz L, Sickinger C, Melo Clavijo J, Laetz EM, Greve C, Serôdio J. 2018. Photoprotective non-photochemical quenching does not prevent kleptoplasts from net photoinactivation. Frontiers in Ecology and Evolution. 6:121. doi:10.3389/fevo.2018.00121.
- Clark KB. 1975. Nudibranch life cycles in the Northwest Atlantic and their relationship to the ecology of fouling communities. Helgoländer Wissenschaftliche Meeresuntersuchungen. 27(1):28–69. doi:10.1007/BF01611686.
- Cowles RB, Bogert CM. 1944. A preliminary study of the thermal requirements of desert reptiles. Bulletin of the AMNH. 83(5):261–296.
- Cruz S, Calado R, Serôdio J, Cartaxana P. 2013. Crawling leaves: photosynthesis in sacoglossan sea slugs. Journal of Experimental Botany. 64(13):3999–4009. doi:10.1093/jxb/ert197.
- Davy SK, Allemand D, Weis VM. 2012. Cell biology of cnidarian-dinoflagellate symbiosis. Microbiology and Molecular Biology Reviews. 76(2):229–261. doi:10.1128/MMBR.05014-11.
- Di Marzo V, Marin A, Vardaro RR, De Petrocellis L, Villani G, Cimino G. 1993. Histological and biochemical bases of defense mechanisms in four species of Polybranchioidea ascoglossan molluscs. Marine Biology. 117(3):367–380. doi:10.1007/BF00349312.
- Dinno A. 2017. dunn.test: Dunn's test of multiple comparisons using rank sums. R package version 1.3.5. https://CRAN.R-project.org/package=dunn.test.
- Douglas AE, Smith DC. 2019. Intracellular space as oligogenetic ecosystem. In: Schenk HEA, Schwemmler W, editors. Proceedings: Second International Colloquium on Endocytobiology; April 10–15, 1983; Tübingen, Germany, 2. De Gruyter. p. 631–648. doi:10.1515/9783110841237-067.
- Durán-Fuentes J, Gracia A, González-Muñoz R. 2022. Sea anemones (cnidaria, anthozoa, actiniaria) in high sedimentation environments influenced by the Magdalena river (Colombian Caribbean). Anais da Academia Brasileira de Ciências. 94. doi:10.1590/0001-3765202120190862.
- Edmunds PJ, Tsounis G, Boulon R, Bramanti L. 2018. Long-term variation in light intensity on a coral reef. Coral Reefs. 37:955–965. doi:10.1007/s00338-018-1721-y.
- Fitt W, Brown B, Warner M, Dunne R. 2001. Coral bleaching: Interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs. 20(1):51–65. doi:10.1007/s003380100146.
- Furfaro G, Mariottini P. 2020. A new Dondice Marcus Er. 1958 (Gastropoda: Nudibranchia) from the Mediterranean Sea reveals interesting insights into the phylogenetic history of a group of Facelinidae taxa . Zootaxa. 4731(1):001–022. doi:10.11646/zootaxa.4731.1.1.
- Garrett TA, Schmeitzel JL, Klein JA, Hwang JJ, Schwarz JA. 2013. Comparative lipid profiling of the cnidarian aiptasia pallida and its dinoflagellate symbiont. PLoS One. 8(3):e57975. doi:10.1371/journal.pone.0057975.
- Grajales A, Rodríguez E. 2014. Morphological revision of the genus Aiptasia and the family Aiptasiidae (Cnidaria, Actiniaria, Metridioidea). Zootaxa. 3826(1):55–100. doi:10.11646/zootaxa.3826.1.2.
- Grajales A, Rodríguez E. 2019. Case 3790 – proposed review of opinion 2404 and reconsideration of Case 3633: Dysactis pallida Agassiz in Verrill, 1864 (currently Exaiptasia pallida; Cnidaria, Anthozoa, Hexacorallia, Actiniaria): Proposed precedence over Exaiptasia diaphana (Rapp, 1829). The Bulletin of Zoological Nomenclature. 76(1):127–131. doi:10.21805/bzn.v76.a036.
- Havenhand JN, Todd CD. 1988. Physiological ecology of Adalariaproxima (Alder et Hancock) and Onchidoris muricata (Mtiller) (Gastropoda: Nudibranchia). I. Feeding, growth, and respiration. Journal of Experimental Marine Biology and Ecology. 118:151–172. doi:10.1016/0022-0981(88)90237-7.
- Hoegh-Guldberg O, Jones R. 1999. Photoinhibition and photoprotection in symbiotic dinoflagellates from reef-building corals. Marine Ecology Progress Series. 183:73–86. doi:10.3354/meps183073.
- Jeong HJ, Yoo YD, Kang NS, Lim AS, Seong KA, Lee SY, Lee MJ, Lee KH, Kim HS, Shin W, et al. 2012. Heterotrophic feeding as a newly identified survival strategy of the dinoflagellate symbiodinium. Proceedings of the National Academy of Sciences. 109(31):12604–12609. doi:10.1073/pnas.1204302109.
- Kempf SC. 1984. Symbiosis between the zooxanthella symbiodinium (=gymnodinium) microadriaticum (freudenthal) and four species of nudibranchs. The Biological Bulletin. 166(1):110–126. doi:10.2307/1541435.
- Kristof A, Klussmann-Kolb A. 2010. Neuromuscular development of aeolidiella stephanieae valdez, 2005 (mollusca, gastropoda, nudibranchia). Frontiers in Zoology. 7:5. doi:10.1186/1742-9994-7-5.
- Laetz EM, Moris VC, Moritz L, Haubrich AN, Wägele H. 2017. Photosynthate accumulation in solar-powered sea slugs – starving slugs survive due to accumulated starch reserves. Frontiers in Zoology. 14(4):1–9. doi:10.1186/s12983-016-0186-5.
- Laetz EMJ, Wägele H. 2019. Comparing amylose production in two solar-powered sea slugs: the sister taxa Elysia timida and E. cornigera (Heterobranchia: Sacoglossa). Journal of Molluscan Studies. 85(1):166–171. doi:10.1093/mollus/eyy047.
- Ledford HK, Niyogi KK. 2005. Singlet oxygen and photo-oxidative stress management in plants and algae. Plant, Cell & Environment. 28(8):1037–1045. doi:10.1111/j.1365-3040.2005.01374.x.
- Lenth RV. 2021. emmeans: estimated marginal means, aka least-squares means. R package version 1.6.0. https://CRAN.R-project.org/package=emmeans.
- Lesser MP. 2006. Oxidative stress in marine environments: biochemistry and physiological ecology. Annual Review of Physiology. 68(1):253–278. doi:10.1146/annurev.physiol.68.040104.110001.
- Lutterschmidt WI, Hutchison VH. 1997. The critical thermal maximum: history and critique. Canadian Journal of Zoology. 75(10):1561–1574. doi:10.1139/z97-783.
- Mantelatto MC, Oliveira AESD, Menegola C, Casares FA, Creed JC. 2020. Depth and grazing intensity are the main drivers of subtidal hardground benthic community structure on tropical south Atlantic reefs. Marine Ecology. 41(3):1–13. doi:10.1111/maec.12586.
- Marín A, Ros J. 1991. Presence of intracellular zooxanthellae in Mediterranean nudibranchs. Journal of Molluscan Studies. 57(4):87–101. doi:10.1093/mollus/57.Supplement_Part_4.87.
- Marshall DJ, Dong Y, McQuaid CD, Williams GA. 2011. Thermal adaptation in the intertidal snail Echinolittorina malaccana contradicts current theory by revealing the crucial roles of resting metabolism. Journal of Experimental Biology. 214(21):3649–3657. doi:10.1242/jeb.059899.
- Marshall DJ, McQuaid CD. 2011. Warming reduces metabolic rate in marine snails: adaptation to fluctuating high temperatures challenges the metabolic theory of ecology. Proceedings of the Royal Society B: Biological Sciences. 278(1703):281–288. doi:10.1098/rspb.2010.1414.
- Melo Clavijo J, Donath A, Serôdio J, Christa G. 2018. Polymorphic adaptations in metazoans to establish and maintain photosymbioses. Biological Reviews. 93(4):2006–2020. doi:10.1111/brv.12430.
- Melo Clavijo J, Sickinger C, Bleidißel S, Gasparoni G, Tierling S, Preisfeld A, Christa G. 2022. The nudibranch Berghia stephanieae (Valdés, 2005) is not able to initiate a functional symbiosome-like environment to maintain Breviolum minutum (JE Parkinson & LaJeunesse 2018). Frontiers in Marine Science. 9. doi:10.22028/D291-38136.
- Mies M, Voolstra CR, Castro CB, Pires DO, Calderon EN, Sumida PY. 2017. Expression of a symbiosis-specific gene in Symbiodinium type A1 associated with coral, nudibranch and giant clam larvae. Royal Society Open Science. 4(5):170253. doi:10.1098/rsos.170253.
- Monteiro EA, Güth AZ, Banha TNS, Sumida PYG, Mies M. 2019. Evidence against mutualism in an aeolid nudibranch associated with symbiodiniaceae dinoflagellates. Symbiosis. 79(2):183–189. doi:10.1007/s13199-019-00632-4.
- Nybakken J, McDonald G. 1981. Feed mechanisms of West American nudibranchs feeding on Byrozoa, Cnidaria and Ascidiacea, with special respect to the radula. Malacologia. 20(2):439–449.
- Parker GM. 1984. Dispersal of zooxanthellae on coral reefs by predators on cnidarians. The Biological Bulletin. 167(1):159–167. doi:10.2307/1541344.
- Pörtner HO. 2002. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 132(4):739–761. doi:10.1016/S1095-6433(02)00045-4.
- Pospíšil P. 2016. Production of reactive oxygen species by photosystem II as a response to light and temperature stress. Frontiers in Plant Science. 7. doi:10.3389/fpls.2016.01950.
- Potts GW. 1983. The respiration of Onchidorisbilamellata and Archidorispseudoargus (Doridacea). Journal of the Marine Biological Association of the United Kingdom. 63(2):399–407. doi:10.1017/S0025315400070752.
- Przeslawski R, Davis A, Benkendorff K. 2004. Effects of ultraviolet radiation and visible light on the development of encapsulated molluscan embryos. Marine Ecology Progress Series. 268:151–160. doi:10.3354/meps268151.
- Quinlan PD, Katz PS. 2022. State-dependent, visually-guided behaviors in the nudibranch Berghia stephanieae. bioRxiv. doi:10.1101/2022.10.24.513581.
- Rädecker N, Pogoreutz C, Gegner HM, Cárdenas A, Roth F, Bougoure J, Guagliardo P, Wild C, Pernice M, Raina J-B, et al. 2021. Heat stress destabilizes symbiotic nutrient cycling in corals. Proceedings of the National Academy of Sciences. 118(5):e2022653118. doi:10.1073/pnas.2022653118.
- Ralph P, Gademann R, Larkum A, Schreiber U. 1999. In situ underwater measurements of photosynthetic activity of coral zooxanthellae and other reef-dwelling dinoflagellate endosymbionts. Marine Ecology Progress Series. 180:139–147. doi:10.3354/meps180139.
- R Core Team. 2022. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. https://www.R-project.org/.
- Rola M, Frankenbach S, Bleidissel S, Sickinger C, Donath A, Frommlet JC, Greve C, Serôdio J, Preisfeld A, Melo Clavijo J, Christa G. 2022. Cladobranchia (gastropoda, nudibranchia) as a promising model to understand the molecular evolution of photosymbiosis in animals. Frontiers in Marine Science. 8. doi:10.3389/fmars.2021.745644.
- R Studio Team. 2020. Rstudio: integrated development environment for R. Boston (MA): RStudio, Inc. http://www.rstudio.com.
- Rumpho ME, Pelletreau KN, Moustafa A, Bhattacharya D. 2011. The making of a photosynthetic animal. Journal of Experimental Biology. 214(2):303–311. doi:10.1242/jeb.046540.
- Smith DA, Sebens P. 1983. The physiological ecology of growth and reproduction in Onchidoris aspera (Alder & Hancock) (Gastropoda: Nudibranchia). Journal of Experimental Marine Biology and Ecology. 72:287–304. doi:10.1016/0022-0981(83)90112-0.
- Sokolova IM, Pörtner HO. 2001. Physiological adaptations to high intertidal life involve improved water conservation abilities and metabolic rate depression in Littorina saxatilis. Marine Ecology Progress Series. 224:171–186. doi:10.3354/meps224171.
- Sokolova IM, Pörtner H-O. 2003. Metabolic plasticity and critical temperatures for aerobic scope in a eurythermal marine invertebrate (Littorina saxatilis, Gastropoda: Littorinidae) from different latitudes. Journal of Experimental Biology. 206(1):195–207. doi:10.1242/jeb.00054.
- Stanley GD. 2006. Ecology: photosymbiosis and the evolution of modern coral reefs. Science. 312(5775):857–858. doi:10.1126/science.1123701.
- Steen RG. 1986. Evidence for heterotrophy by zooxanthellae in symbiosis with Aiptasia pulchella. The Biological Bulletin. 170(2):267–278. doi:10.2307/1541808.
- Stenseng E, Braby CE, Somero GN. 2005. Evolutionary and acclimation-induced variation in the thermal limits of heart function in congeneric Marine Snails (Genus Tegula): implications for vertical zonation. The Biological Bulletin. 208(2):138–144. doi:10.2307/3593122.
- Storch D, Menzel L, Frickenhaus S, Pörtner H-O. 2014. Climate sensitivity across marine domains of life: limits to evolutionary adaptation shape species interactions. Global Change Biology. 20(10):3059–3067. doi:10.1111/gcb.12645.
- Sunagawa S, Wilson EC, Thaler M, Smith ML, Caruso C, Pringle JR, Weis VM, Medina M, Schwarz JA. 2009. Generation and analysis of transcriptomic resources for a model system on the rise: the sea anemone Aiptasia pallida and its dinoflagellate endosymbiont. BMC Genomics. 10(1):258. doi:10.1186/1471-2164-10-258.
- USGS. 2011. Dissolved oxygen solubility tables. Washington, DC, USA: US Department of the Interior. [accessed throughout 202] https://water.usgs.gov/water-resources/software/DOTABLES/
- Verberk WCEP, Overgaard J, Ern R, Bayley M, Wang T, Boardman L, Terblanche JS. 2016. Does oxygen limit thermal tolerance in arthropods? A critical review of current evidence. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 192:64–78. doi:10.1016/j.cbpa.2015.10.020.
- Wägele H. 2004. Potential key characters in Opisthobranchia (Gastropoda, Mollusca) enhancing adaptive radiation. Organisms Diversity & Evolution. 4(3):175–188. doi:10.1016/j.ode.2004.03.002.
- Watson WH, Bourque KMF, Sullivan JR, Miller M, Buell A, Kallins MG, Curtis NE, Pierce SK, Blackman E, Urato S, Newcomb JM. 2021. The digestive diverticula in the carnivorous nudibranch, melibe leonina, do not contain photosynthetic symbionts. Integrative Organismal Biology. 3(1):1–11. doi:10.1093/iob/obab015.
- Weis VM, Davy SK, Hoegh-Guldberg O, Rodriguez-Lanetty M, Pringle JR. 2008. Cell biology in model systems as the key to understanding corals. Trends in Ecology & Evolution. 23(7):369–376. doi:10.1016/j.tree.2008.03.004.
- Wickham H. 2016. Ggplot2: elegant graphics for data analysis. New York: Springer-Verlag. ISBN 978-3-319-24277-4, https://ggplot2.tidyverse.org.
- Woods HA, Moran AL, Atkinson D, Audzijonyte A, Berenbrink M, Borges FO, Burnett KG, Burnett LE, Coates CJ, Collin R, et al. 2022. Integrative approaches to understanding organismal responses to aquatic deoxygenation. The Biological Bulletin. 243(2):85–103. doi:10.1086/722899.
- WoRMS Editorial Board. 2023. World register of Marine species. https://www.marinespecies.org at VLIZ. [accessed 2023 December 19].
