ABSTRACT
Bivalves are known to be infected by filamentous fungi, many of which have pathogenic and toxicogenic properties, and may represent a risk to human health. The present study investigated the mycological characteristics of Perna perna obtained from Guanabara Bay, Brazil. The mussels were sealed individually in sterile bags, weighed and measured, and the internal organs were examined. The organs were grown in different culture media. All but one of the 31 mussels (96.7%) examined contained fungi, and a total of 84 fungal colonies were isolated. The genera identified were Aspergillus (40.5%), Didymella (35.7%), Penicillium (10.7%), Aureobasidium (1.2%), Cladosporium (1.2%) and Phaeoisaria (1.2%). The molecular sequences obtained 99.8 and 100% identity with Didymella sp., Phaeoisaria sp. and Aureobasidium pullulans, and were deposited in GenBank. This is the first record of Didymella and Phaeoisaria in a bivalve. The digestive gland was the organ with the greatest diversity of fungi genera. A number of the fungi identified here, as Aspergillus awamori, Aspergillus japonicus, Aspergillus niger, Aspergillus versicolor and Aspergillus sydowii, are known to produce mycotoxins and others are opportunistic forms, which reinforces the need for the systematic monitoring of the study area to guarantee the safe exploitation of these bivalves for human consumption.
Introduction
The brown mussel, Perna perna (Linnaeus, 1758), has a very ample geographic distribution. It is considered to native to Africa, but can also be found in the Mediterranean Sea, Sri Lanka and southern India. In Europe, P. perna is found only on the southern coast of Portugal. In the Americas, the species has been recorded in the Gulf of Mexico and on some Caribbean islands, in addition to Venezuela, Uruguay and Brazil (Hicks and Tunnell Citation1995; Acosta et al. Citation2006; Resgalla et al. Citation2008; Carranza and Borthagaray Citation2009; Lourenço et al. Citation2012). On the Brazilian coast, P. perna occurs between the states of Espírito Santo and Rio Grande do Sul (Marques Citation1998), where it is considered an important source of income and subsistence, in particular by coastal populations (Resgalla et al. Citation2008; SAP/MAPA Citation2020).
Bivalve mollusks are sessile filter feeders that ingest suspended material, and may retain microorganisms and chemical substances in their tissue (Marques Citation1998; Resgalla et al. Citation2008). The occurrence of filamentous fungi has been investigated in a number of bivalve species, including farmed mussels in France (Mytilus edulis Linnaeus, 1758), Algeria (Mytilus galloprovincialis Lamarck, 1819) and New Zealand (Perna canaliculus (Gmelin, 1791)) (Sallenave-Namont et al. Citation2000; Matallah-Boutiba et al. Citation2012; Li et al. Citation2022). Other species investigated include scallops, Nodipecten nodosus (Linnaeus, 1758), cultivated in southeastern Brazil (Santos et al. Citation2017a), and oysters, Crassostrea gigas (Thunberg, 1793), mussels, Crenomytilus grayanus (Dunker, 1853) and Modiolus modiolus (Linnaeus, 1758), and clams, Anadara broughtonii (Schrenck, 1867), from the Russian waters of the Sea of Japan (Zvereva and Vysotskaya Citation2005; Borzykh and Zvereva Citation2012a, Citation2012b, Citation2014, Citation2015, Citation2018). In the specific case of P. perna, only one mycological study has been published, from southeastern Brazil (Santos et al. Citation2020). These authors found no obvious deleterious effects of fungal infection in these mollusks. Even so, the potential consequences of the infection of bivalves by filamentous fungi are still unclear (Grovel et al. Citation2003; Santos et al. Citation2017a, Citation2020).
Fungi are heterotrophic, eukaryotic microorganisms that are able to act as symbionts, parasites, saprophytes or decomposers, with an extensive distribution in nature, being found in the soil, water, and even suspended in the air (Dube Citation2013). Many species have pathogenic and toxicological properties, which can pose a risk to humans, animals and plants (Grovel et al. Citation2003; Dube Citation2013).
Mussels were selected for this study because bivalves are widely used in national and international environmental monitoring actions, and are also resistant to various environmental conditions (Marques Citation1998; Resgalla et al. Citation2008). The biomonitoring of mussels, especially to determine the presence of filamentous fungi, is necessary for the safe exploitation of these bivalves as a human food, given their economic value and relevance for public health. Understanding the diversity of fungi in marine environments, in particular in areas impacted by human activities, is essential for the evaluation of the quality of these environments and the planning of adequate management strategies. In this context, the present study investigated the occurrence of filamentous fungi in samples of the mussel P. perna obtained from the vicinity of Jurujuba Beach in the state of Rio de Janeiro, in southeastern Brazil.
Materials and methods
Sample collection and processing
The samples of P. perna were collected randomly by local fishers from adjacent rocky shores near Jurujuba Beach in Guanabara Bay (22°55’53’’ S, 43°06’35’’ W), and subsequently were selected specimens with a shell length greater than 50 mm. In this region, the mussels serve as a significant source of income and fishers own consumption, where they are sold primarily to local population and restaurants. The samples were collected in February, March and November 2019, and January 2020, with a total of 31 specimens. These months were based on the availability of the fishers to provide the mussels for this study. The samples were sealed individually in sterile plastic bags to avoid contamination, numbered, packed in a polystyrene cooler containing ice, and transported to the laboratory for processing. The specimens were weighed while still in the bags, and then removed from the bags, placed individually in containers and washed externally with Tween 80 (0.1%) for 5 min and sterile distilled water for 10 min in a BSL-2 biological safety cabinet, to remove any external microbial contamination. After the valves were opened, the sex of each specimen was determined, and the internal organs (foot, gills and digestive gland) were extracted. Each organ was approximately 5 mm in length. The 93 fresh samples were numbered, washed in 0.85% phosphate-buffered saline (PBS) (8 g NaCl, 0.2 g KCl, 1.44 g Na2HPO4, 0.24 g KH2PO4 in 800 ml of distilled water; pH 7.4) and sterile distilled water, centrifuged, and placed in Petri dishes containing 20 mL of potato dextrose agar (PDA) (DifcoTM) and chloramphenicol (Sigma) (dissolve 50 mg of chloramphenicol in 10 ml of 95% alcohol and add to the culture medium) (ANVISA Citation2004) before being incubated for seven days at room temperature (Santos et al. Citation2017a). The shells were then measured.
Fungal identification
The fungal colonies that developed on the Petri dishes were transferred to test tubes containing PDA for isolation. The morphology of all the strains that could be identified to genus was examined microscopically and macroscopically. The fungal strains were identified macroscopically using the inoculum point technique (Pitt and Hocking Citation2009) in Petri dishes containing different culture media and incubated in biochemical oxygen demand (BOD) climate chambers, at different temperatures, for 7–21 days, using: malt extract agar (MEA) (DifcoTM) at 25°C; czapek yeast extract agar (CYA) (DifcoTM) at 25°C and 37°C; and CYA with 20% sucrose (CYA20S) at 25°C. The texture, degree of sporulation, production of sclerotia or cleistothecia, mycelial colouration, sporulation, soluble pigments, exudates, and colony reverses were analyzed. The growth of each colony was measured with a Mitutoyo digital calliper (precision of 0.01 mm). The microscopic morphological analyses were based on the microculture technique (Rivalier and Seydel Citation1932), using the same culture media and temperatures described above. Each colony was stained with lactophenol blue-cotton and observed under a Zeiss Axiophot optical microscope. The presence or absence of septa in the hyphae, the formation of fruiting bodies or other types of reproductive structure, the presence or absence of melanized structures, as well as the development pattern and characteristics of dispersal structures, such as spores and conidia, were observed. The morphological features were photographed using an attached Moticam 10 mp camera equipped with the Motic images plus v. 2.0 software (Motic China Group Co.). All measurements are presented in micrometers. The species were identified following the classifications of Raper and Fennell (Citation1965), Ellis (Citation1971, Citation1976), Alexopoulos et al. (Citation1996), Pitt (Citation2000), Klich (Citation2002), Webster and Weber (Citation2007), Seifert et al. (Citation2011) and Bensch et al. (Citation2012).
Genetic analysis
The fungi that were not accurately identified to genus level by their morphological characteristics were separated for molecular analysis. The isolates were transferred to test tubes containing PDA for cultivation. After seven days, the genomic DNA of these fungi was extracted according to Ricci et al. (Citation2011). The 26S rRNA gene was amplified using the NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL4 (5′-GGTCCGTGTTTTTTCAAGACGG-3′) primers (Ricci et al. Citation2011), while the ITS1 (5'-TCCGTAGGTGAACCTGCGG-3’) and ITS4 (5'-TCCTCCTCCGCTTATTATTGATATGC-3’) primers were used to amplify the ITS1, 5.8S and ITS2 rDNA regions (White et al. Citation1990). The 18S rDNA gene was amplified using the primers FF2 (5′-GGTTCTATTTTGTTGGTTTCTA-3′) and FR1 (5′-CTCTCAATCTCTGTCAATCCTTATT-3′) (Zhou et al. Citation2000) and EF4 (5′-GGAAGGG[G/A]TGTATTTATTAG-3′) and EF3 (5′-TCCTCTAAATGACCAAGTTTG-3′) (Gontia-Mishra et al. Citation2014). The PCRs were run in a total of 50 μl, containing 0.5 μl of Taq polymerase, 1.0 μl of each primer with a final concentration of 10 pmol, 5.0 μl of the kit buffer (10x), 5 μl of dNTPs, 3.0 μl of MgCl2, 29.5 μl of ultrapure water, and 5.0 μl of the DNA, with the cycling parameters based on the protocols of White et al. (Citation1990), Zhou et al. (Citation2000), Gontia-Mishra et al. (Citation2014) and Kurtzman and Robnett (Citation1998). A negative control reaction without DNA was included. The PCR products were analyzed by electrophoresis in gels containing 1.5% agarose and Tris-borate Ethylenediamine Tetraacetic Acid (EDTA) stained with SybrGreen DNA gel Stain (Invitrogen, Eugene, Oregon, USA), and photographed under ultraviolet transillumination. The PCR amplicons were purified using the EasyPure® PCR purification kit (TransGen Biotech Co., LTD). The DNA sequencing reactions were based on the Sanger method and the sequences were obtained automatically by the Sequencing Platform of the Oswaldo Cruz Foundation (PDTIS/Fiocruz) in Rio de Janeiro, Brazil. The newly-generated sequences of both strands were verified and edited in the MEGA software, version X (Kumar et al. Citation2018), and the sequences were compared to those available in the GenBank database using the BLAST programme on the National Center for Biotechnology Information (NCBI) server (http://www.ncbi.nlm.nih.gov/BLAST) (Altschul et al. Citation1990).
Results
Total P. perna shell length ranged from 63 to 115 (91.6 ± 11.8) mm, and the weight of the specimens varied from 21.62–113.60 (60.7 ± 20.3) g. The specimens included 17 females and 13 males, while one individual could not be sexed. These organisms provide the local fisher community with a subsistence resource and a source of financial income.
No visible signs of fungi were apparent externally but after culture, the presence of filamentous fungi was detected in 30 of the 31 mussels (96.7%). The only mussel with absence of fungi was a male. A total of 84 fungal colonies were isolated of which 30 were isolated from the foot tissue, 27 from the gills, and 27 from the digestive glands. Eight colonies could not be identified due to the absence of reproductive structures, and were thus classified as Mycelia sterilia.
Overall, 48.4% (45/93) of the examined tissue samples had only one fungal taxon. No more than two taxa were identified in the foot and gill samples, while as many as four taxa were found in the digestive gland samples. No fungi were detected in 30 samples ().
Table 1. The number and the prevalence (%) of different filamentous fungi recorded per organ in the Perna perna mussels collected off Jurujuba Beach in Rio de Janeiro, Brazil.
The fungi were identified by the macroscopic and microscopic examination of their morphological characteristics, except for the genera Aureobasidium Viala & Bayer 1891, Didymella Saccardo, 1880 and Phaeoisaria Höhn, 1909, which could only be diagnosed definitively with a complementary molecular analysis. A total of six sequences were obtained: Didymella – partial 26S rDNA sequence (GenBank accession number: ON246999; 100% identity with Didymella glomerata Q. Chen & L. Cai, 2015, Didymella pedeiae Q. Chen & L. Cai, 2015 and Didymella pomorum Q. Chen & L. Cai, 2015 in a 593 bp) and ITS1, 5.8S and ITS2 (GenBank: ON247031; 99.8% identity with D. pedeiae in a 494 bp); Phaeoisaria – partial 26S rDNA sequence (GenBank: ON246998; 100% identity with Phaeoisaria sp. in a 588 bp) and one partial 18S rDNA sequence (GenBank: ON246995; 99.8% identity with Phaeoisaria fasciculata Réblová & Seifert, 2016 in a 1398 bp) and Aureobasidium – partial 18S rDNA sequence (GenBank: ON246994; 100% identity with Aureobasidium pullulans G. Arnaud 1918 and Aureobasidium namibiae Zalar, Gostincar, Gunde-Cimerman, 2014 in a 406 bp) and ITS1, 5.8S and ITS2 (GenBank: ON247030; 99.8% identity with A. pullulans in a 561 bp).
The most prevalent genus was Aspergillus Micheli, 1729, with a total of 34 colonies, observed in all the organs analyzed (12 in the foot, 13 in the gills, and nine in the digestive glands). Didymella was represented by 30 colonies, followed by Penicillium Link 1809 with nine colonies. These two genera were also found in all three organs examined in P. perna. Aureobasidium, Cladosporium Link, 1816 and Phaeoisaria were present in one colony each in the digestive gland ().
Table 2. The number and the prevalence (%) of filamentous fungal colonies (per genus) isolated from the organs of the Perna perna mussels collected off Jurujuba Beach in Rio de Janeiro, Brazil.
The fungi identified to the species level were: Aspergillus awamori Nakaz. 1907, Aspergillus caespitosus Raper & Thom 1944, Aspergillus carbonarius Thom 1916, Aspergillus flavipes Thom & Church 1926, Aspergillus japonicus Saito 1906, Aspergillus niger Tiegh. 1867, Aspergillus sydowii Thom & Church 1926, Aspergillus versicolor Tirab. 1908, Penicillium raistrickii G. Sm. 1933, and A pullulans. The occurrence and the macroscopic and microscopic morphological characteristics of these species and the genera are reviewed in .
Table 3. Occurrence and macroscopic and microscopic morphological characteristics of identified filamentous fungi that were isolated from the organs of the Perna perna mussels collected off Jurujuba Beach in Rio de Janeiro, Brazil.
Figure 1. Morphological characteristics of Aspergillus awamori after seven days of incubation. A- Macroscopic view of the colony surface. B- Macroscopic view of the colony reverse. C- Microscopic view of the conidiophores with brown conidia and vesicle, lactophenol staining on CYA at 25°C. Abbreviations: CYA-1 = czapek yeast extract agar at 25°C; CYA-2 = czapek yeast extract agar at 37°C; CYA20S = czapek yeast extract agar with 20% sucrose; MEA = malt extract agar; C = conidia; S = stipe; V = vesicle. Scale bar = 20 µm.
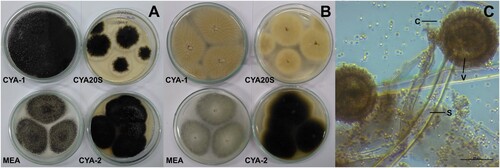
Figure 2. Morphological characteristics of Aspergillus caespitosus after seven days of incubation. A- Macroscopic view of the colony surface. B- Macroscopic view of the colony reverse. C- Microscopic view of conidiophores with greenish conidia, lactophenol blue-cotton staining on CYA at 25°C. Abbreviations: CYA-1 = czapek yeast extract agar at 25°C; CYA-2 = czapek yeast extract agar at 37°C; CYA20S = czapek yeast extract agar with 20% sucrose; MEA = malt extract agar; C = conidia; S = stipe; V = vesicle. Scale bar = 50 µm.
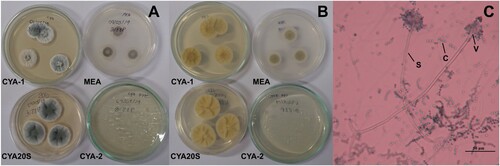
Figure 3. Morphological characteristics of Aspergillus carbonarius after seven days of incubation. A- Macroscopic view of the colony surface of colonies. B- Macroscopic view of the colony reverse. C- Microscopic view of conidiophores with brown and black conidia, lactophenol blue-cotton staining on CYA at 25°C. Abbreviations: CYA-1 = czapek yeast extract agar at 25°C; CYA-2 = czapek yeast extract agar at 37°C; CYA20S = czapek yeast extract agar with 20% sucrose; MEA = malt extract agar; C = conidia; S = stipe. Scale bar = 50 µm.
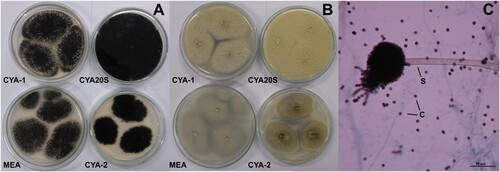
Figure 4. Morphological characteristics of Aspergillus flavipes after seven days of incubation. A- Macroscopic view of the colony surface. B- Macroscopic view of the colony reverse. C- Microscopic view of conidiophore with greenish conidia, lactophenol blue-cotton staining on CYA at 25°C. Abbreviations: CYA-1 = czapek yeast extract agar at 25°C; CYA-2 = czapek yeast extract agar at 37°C; CYA20S = czapek yeast extract agar with 20% sucrose; MEA = malt extract agar; C = conidia; S = stipe. Scale bar = 20 µm.
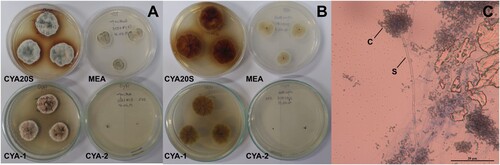
Figure 5. Morphological characteristics of Aspergillus japonicus after seven days of incubation. A- Macroscopic view of the colony surface. B- Macroscopic view of the colony reverse. C- Microscopic view of conidiophores with light brown conidia, lactophenol staining on CYA at 25°C. Abbreviations: CYA20S = czapek yeast extract agar with 20% sucrose; C = conidia; S = stipe; V = vesicle. Scale bar = 20 µm.
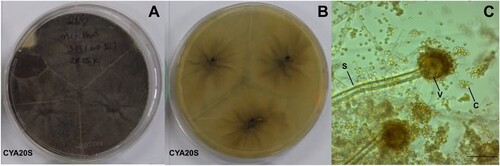
Figure 6. Morphological characteristics of Aspergillus niger after seven days of incubation. A- Macroscopic view of the colony surface. B- Macroscopic view of the colony reverse. C- Microscopic view of conidiophores with dark brown to black conidia, lactophenol staining on CYA at 25°C. Abbreviations: CYA-1 = czapek yeast extract agar at 25°C; CYA-2 = czapek yeast extract agar at 37°C; CYA20S = czapek yeast extract agar with 20% sucrose; MEA = malt extract agar; C = conidia; S = stipe; V = vesicle. Scale bar = 20 µm.
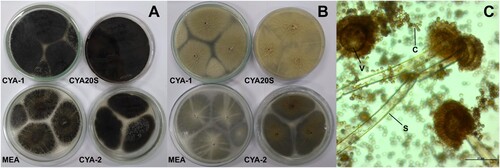
Figure 7. Morphological characteristics of Aspergillus sydowii after seven days of incubation. A- Macroscopic view of the colony surface. B- Macroscopic view of the colony reverse. C- Microscopic view of conidiophores with greenish conidia, lactophenol blue-cotton staining on CYA at 25°C. Abbreviations: CYA-1 = czapek yeast extract agar at 25°C; CYA-2 = czapek yeast extract agar at 37°C; CYA20S = czapek yeast extract agar with 20% sucrose; MEA = malt extract agar; C = conidia; S = stipe. Scale bar = 20 µm.
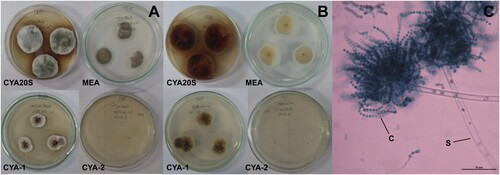
Figure 8. Morphological characteristics of Aspergillus versicolor after seven days of incubation. A- Macroscopic view of the colony surface. B- Macroscopic view of the colony reverse. C- Microscopic view of conidiophores with greenish conidia, lactophenol blue-cotton staining on CYA at 25°C. Abbreviations: CYA-1 = czapek yeast extract agar at 25°C; CYA-2 = czapek yeast extract agar at 37°C; CYA20S = czapek yeast extract agar with 20% sucrose; MEA = malt extract agar; C = conidia; S = stipe; FC = foot cell. Scale bar = 50 µm.
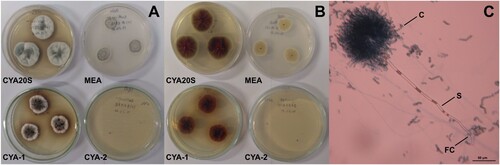
Figure 9. Morphological characteristics of Didymella sp. after seven days of incubation. A- Macroscopic view of the colony surface. B- Macroscopic view of the colony reverse. C- Microscopic view of conidiophores with brown perithecia, lactophenol staining on CYA at 25°C. Abbreviations: CYA-1 = czapek yeast extract agar at 25°C; CYA-2 = czapek yeast extract agar at 37°C; CYA20S = czapek yeast extract agar with 20% sucrose; MEA = malt extract agar; P = pycnidia. Scale bar = 20 µm.
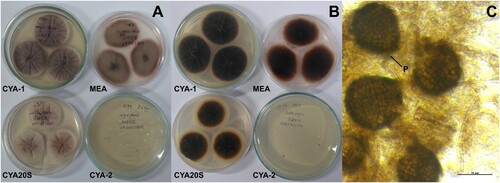
Figure 10. Morphological characteristics of Penicillium raistrickii after seven days of incubation. A- Macroscopic view of the colony surface. B- Macroscopic view of the colony reverse. C- Microscopic view of conidiophores, lactophenol staining on CYA at 25°C. Abbreviations: CYA-1 = czapek yeast extract agar at 25°C; CYA-2 = czapek yeast extract agar at 37°C; CYA20S = czapek yeast extract agar with 20% sucrose; MEA = malt extract agar; C = conidia; M = metulae; P = phial. Scale bar = 20 µm.
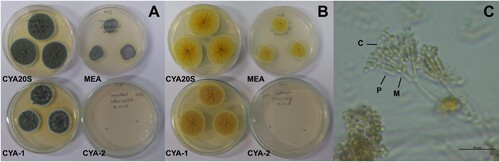
Figure 11. Morphological characteristics of Cladosporium sp. after seven days of incubation. A- Macroscopic view of the colony surface. B- Macroscopic view of the colony reverse. C- Microscopic view of conidiophores, lactophenol blue-cotton staining on CYA at 25°C. Abbreviations: CYA-1 = czapek yeast extract agar at 25°C; CYA-2 = czapek yeast extract agar at 37°C; CYA20S = czapek yeast extract agar with 20% sucrose; MEA = malt extract agar; C = conidia. Scale bar = 20 µm.
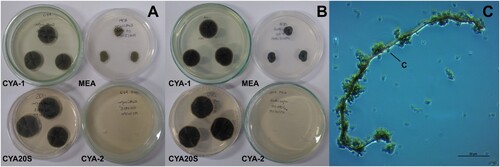
Figure 12. Morphological characteristics of Phaeoisaria sp. after seven days of incubation. A- Macroscopic view of the colony surface. B- Macroscopic view of the colony reverse. C- Microscopic view of conidia and septate hyphae, lactophenol blue-cotton staining on CYA at 25°C. Abbreviations: CYA-1 = czapek yeast extract agar at 25°C; CYA-2 = czapek yeast extract agar at 37°C; CYA20S = czapek yeast extract agar with 20% sucrose; MEA = malt extract agar; C = conidia; CL = chlamydospore; H = hyphae. Scale bar = 20 µm.
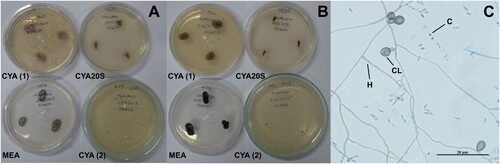
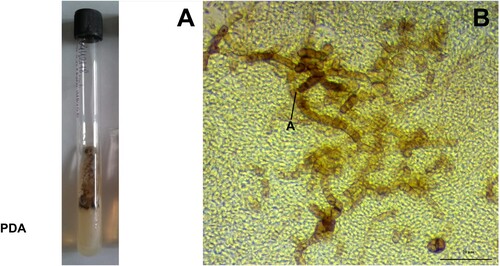
Figure 13. Morphological characteristics of Aureobasidium pullulans after seven days of incubation. A- Fungal growth in the test tube containing potato dextrose agar. B- Microscopic view of black arthroconidia, lactophenol staining in potato dextrose agar at 25°C. Abbreviations: PDA = potato dextrose agar; A = arthroconidia. Scale bar = 20 µm.
Discussion
The gonads in the mantle of P. perna show a characteristic colouration during the reproductive period, which make it possible to determine the sex: female mussels display a orange mantle colour, and males, creamy white (Lunetta Citation1969; Resgalla et al. Citation2008). One specimen sampled was classified as an indeterminate sex due to a transparent mantle, probably associated with the emptying stage of the gonadal follicles (Lunetta Citation1969).
All the mussels analyzed in the present study were over 50 mm in length, and were therefore considered to be adults, based on the criteria of the Brazilian Institute of the Environment and Renewable Natural Resources (IBAMA Citation2006), which has established that P. perna from natural stocks with a shell length of at least 50 mm are adults and are suitable for sale and human consumption. Despite previous studies shown differences in metabolism between juvenile and adult bivalves (Xião et al. Citation2014; Oliveira et al. Citation2023), it is not yet known whether this could influence in the presence of different fungi for each group. Nonetheless, the results found here represent the fungi associated with adult mussels.
Environmental pollution may influence the quantity and diversity of fungi (Wellbaum et al. Citation2007), and as the mussels examined here were obtained from an area with clear signs of pollution (Fries et al. Citation2019; Oliveira et al. Citation2022), there is a clear need for the investigation of the pattern of occurrence of fungi in P. perna. Borzykh and Zvereva (Citation2014) reported that the species richness of the genera Aspergillus, Penicillium, Cladosporium, and Chaetomium Kunze 1817 in scallops increased in polluted coastal waters. According to Sarquis and Borba (Citation1997), the human intervention may lead to an increase in organic matter, facilitating the development of fungal flora. At Jurujuba Sound, which includes Jurujuba Beach, there are non-treated industrial and domestic effluents and urban runoff, as well as nautical activities and atmospheric depositions (Baptista Neto et al. Citation2005). Therefore, the Jurujuba Beach is under the influence of anthropogenic activities, which make this area relevant for investigation of filamentous fungi in P. perna.
In the present study, it was possible to verify a diversity of filamentous fungi in the internal organs of P. perna collected off the coast of Rio de Janeiro. The highest percentage of fungi was found in the foot of the molluscs, indicating the specificity of the area where they settle since the foot into contact with the rocky shores. After filtering and ingesting their food, fungi in the gills and digestive glands occurred in lower percentages. It is important to consider that the fact that no colonies were obtained in some organs does not mean that the sample contained no fungi. There are fungi that are not easily cultivated and may require different culturing conditions for each species (Meletiadis et al. Citation2001). The absence of visible signs of fungi in the specimens before culture was expected. This was similar in other studies (Grovel et al. Citation2003; Marrouchi et al. Citation2013; Santos et al. Citation2017a, Citation2020). Nevertheless, it is important to better understand the role of fungi in bivalve metabolism. Thus, opens up a new area of research that needs to be explored in subsequent studies.
The genera identified here are all members of the Ascomycota, the largest phylum of the Fungi kingdom. Ascomycetes include fungi of both ecological and economic importance, some are edible, while others act as pathogens (Watkinson et al. Citation2015). The current taxonomy recognizes 339 species of Aspergillus, 46 species of Aureobasidium, 218 species of Cladosporium, 495 species of Didymella, 354 species of Penicillium, and 39 species of Phaeoisaria (Samson et al. Citation2014; Visagie et al. Citation2014; Bensch et al. Citation2018; Index Fungorum Citation2022).
Cladosporium, Didymella and Phaeoisaria are darkly-pigmented or dematiaceous fungi. In particular, the last two genera, given the morphological similarities of many members of the families Didymellaceae and Pleurotheciaceae, the taxa could not be identified reliably based on the macroscopic and microscopic examination of their characteristics alone. Recent phylogenetic studies have sought to better define the arrangements within these families (Dong et al. Citation2021; Magaña-Dueñas et al. Citation2021). In the present study, the complementary molecular analyses permitted a more conclusive identification of Didymella and Phaeoisaria.
For the molecular analyses in this study, the 18S rDNA gene was chosen because it represents a conserved region of DNA, and by using universal primers specific for fungi, makes it possible to obtain PCR products from most of these microorganisms (Gontia-Mishra et al. Citation2013). On the other hand, the 28S rDNA and ITS regions provide for certain fungi a better separation of closely related species (Smit et al. Citation1999). Therefore, it is interesting to explore more than one region of DNA.
Aspergillus, Didymella and Penicillium were observed in all the different organs of P. perna analyzed here, which appears to indicate that these fungi have no preference for a specific organ. Overall, a greater diversity of genera was found in the digestive gland, which was the only organ infected with up to four different fungal taxa. This may be related to the accumulation of nutritional residues in this gland (Magalhães Citation1998). Li et al. (Citation2022) also found a slightly higher diversity of fungal species in the digestive gland of P. canaliculus in comparison with the gills, stomach, hemolymph and seawater. Sallenave et al. (Citation1999) and Marrouchi et al. (Citation2013) identified that the digestive gland was the part of the mussels with the most mycotoxins. The characterizing the microbiota by host parts/organs can help in understanding host-microbiota interactions and associations (Li et al. Citation2022).
Santos et al. (Citation2020) isolated Aspergillus sp. and Penicillium sp., as well as Fusarium Link, 1809 and Pestalotiopsis Steyaert, 1949, from P. perna specimens obtained from Tarituba in the municipality of Paraty, in western Rio de Janeiro state but the prevalence of these fungi was not reported in this study. This means that it is not possible to identify the predominant fungal genus in this region. Studies of other bivalves have identified the same genera found here, with the exception of Didymella and Phaeoisaria (Zvereva and Vysotskaya Citation2005; Borzykh and Zvereva Citation2014; Santos et al. Citation2017a). Santos et al. (Citation2017a) found dematiaceous fungi in scallops (N. nodosus) from the coast of Rio de Janeiro, although the taxon was not identified, and only two colonies were recorded. Aspergillus and Penicillium were among the most abundant genera identified in surveys of sandy beach substrates on the Brazilian coast (Sarquis and Oliveira Citation1996; Gomes et al. Citation2008), which confirms the presence of these fungi in the marine environment. As Aspergillus, Cladosporium and Penicillium are among the most frequent genera in many of the mycological studies, Santos et al. (Citation2017a) inferred that the interaction between bivalves and fungi may be symbiotic or that the absence of detrimental effects may be due to the short period of infection. Salazar-Vallejo and González (Citation1986) identified a symbiotic relationship between the marine gastropod Collisella Dall, 1871 (considered to be a synonym of Lottia Gray, 1833 by WoRMS (Citation2021)) and Didymella conchae Bonar 1936, which punctured the shell of the gastropod frequently. In the present study, puncture marks were observed in the inner portion of some of P. perna shells, although it is not possible to confirm with certainty that these marks are related to the presence of Didymella in these bivalves. This further reinforces the importance of continuing research to ensure a better understanding of this interaction.
The genera Aspergillus and Penicillium are opportunistic fungi found in a variety of substrates and environments, including the marine one. These fungi have been isolated from marine animals, plants, seawater and sediments (Zuluaga-Montero et al. Citation2010; Gonçalves et al. Citation2019; Li et al. Citation2023). The occurrence of both genera in the foot, gills and digestive gland may be related to the direct interaction of P. perna specimens with seawater, sediments and marine plants. Didymella is known as a worldwide fungus that often takes advantage of specific conditions to colonize terrestrial plants and is associated with gummy stem blight disease in cucurbits, such as watermelon and cantaloupe, and with respiratory infections in humans, albeit very rarely (Paret et al. Citation2018; Salehi et al. Citation2019; Chen et al. Citation2023). Brazilian studies have also confirmed the role of this genus as a phytopathogen (Santos et al. Citation2009; Santos et al. Citation2017b). Didymella spp. have been also reported as abundant in aquatic plants in China (Chen et al. Citation2023). The occurrence of Didymella sp. on foot, gills and digestive glands of P. perna could be due to an interaction with nearby aquatic plants. There are few published reports the occurrence of Didymella in mollusks, however, and the fact that this genus was the second most common in the P. perna samples analyzed here reinforces the need for further research, given the potential for public health problems related to the consumption of infected mussels. To our knowledge this is the first record of Didymella in bivalves. Phaeoisaria is also recorded here for the first time in bivalves, although it was present in only one colony.
The genera Aspergillus, Cladosporium and Penicillium are a group of opportunistic filamentous fungi that produce secondary metabolites known as mycotoxins (Borzykh and Zvereva Citation2015; Girisham et al. Citation2016). The level of toxicity of these compounds varies considerably among the different genera, however. Previous studies have shown that the highest levels of toxicity were found in Penicillium species isolated from bivalves, followed by Aspergillus (Sallenave-Namont et al. Citation2000; Matallah-Boutiba et al. Citation2012).
Some fungal secondary metabolites have therapeutic properties, and are used in industrial pharmacology for the production of substances such as immunosuppressive drugs, antibiotics and antioxidants (Frisvad et al. Citation2018). Not all these metabolites are beneficial, however. The effects of a mycotoxin will depend on the dose and level of exposure, in addition to the susceptibility of the individual, which may vary from acute to chronic (Knechtges Citation2012). Three of the fungi identified in the present study, A. awamori, A. japonicus and A. niger, which all belong to the Nigri section (black Aspergillus), are known to produce Ochratoxin A, or OTA (Oliveri et al. Citation2008; Zouhair et al. Citation2017). This mycotoxin is believed to be associated with degenerative effects in the kidneys and tumours of the urinary tract in humans (Petkova-Bocharova et al. Citation1988; Wafa et al. Citation1998). In addition to OTA, A. niger can synthesize two other types of mycotoxin – oxalic acid and fumonisins (B2, B4 and B6). The principal effects of oxalic acid are pulmonary and renal failure, and fumonisins may cause renal and hepatic toxicity (Kimmerling et al. Citation1992; Edrington et al. Citation1995; Botha et al. Citation2009; Månsson et al. Citation2010; Mogensen et al. Citation2010). Many Aspergillus species, including A. versicolor and A. sydowii, produce sterigmatocystin or similar compounds (Purchase and Van der Watt Citation1973; Davis Citation1981). Sterigmatocystin is considered to be carcinogenic and hepatotoxic. In addition to liver damage, it can also cause damage to the lungs, kidneys, pancreas and stomach (Sumi et al. Citation1987; Tongxin et al. Citation1991). This emphasizes the need for further research to monitor mycotoxins in edible shellfish.
Mussels are typically consumed fresh or even raw (Ferreira and Magalhães Citation2004), and it is important to note that it is extremely difficult to eliminate mycotoxins completely from food, even by cooking (Knechtges Citation2012). The Brazilian Health Regulatory Agency (ANVISA) has established the maximum tolerable limits for some mycotoxins in foods marketed in Brazil (Brasil Citation2011, Citation2017). These mycotoxins include OTA and fumonisins, although no limits have been established for animal foods, such as bivalve mollusks. In fact, no specific legislation has been established for many different types of food. This is not restricted to Brazil. Virtually all countries and organizations have not established monitoring programmes or maximum limits for mycotoxins in seafood. In general, the legislations are directed at mycotoxins in animal feed and vegetable food matrices (Smaoui et al. Citation2023). Considering the economic importance of P. perna and their human consumption, especially in coastal areas, the absence of legal mycotoxin limits in bivalves represents a public health concern. Thus, further studies should be conducted in this regard to discuss and determine the specific limits.
Due to insufficient data on the presence of fungi in P. perna, it was not possible to compare which fungi would be the same and different in areas with and without anthropogenic activities. Eventually, more studies will be needed with this mussel to identify possible patterns of fungal diversity that would help characterize the environmental and the disease-causing fungi.
Conclusions
Although the brown mussel, P. perna, is common on the coast of southern and southeastern Brazil where it is widely cultivated, few data are available on the association of fungi with in this mussel. The present study identified a number of different fungi in the P. perna samples analyzed, including opportunistic taxa and fungi that produce mycotoxins. These findings indicate potential risks to public health, and emphasize the need for the systematic monitoring of the study area to ensure the safe exploitation of these bivalves for human consumption. Didymella and Phaeoisaria are reported here for the first time in bivalves, together with morphological and genetic data. New gene sequences were deposited in GenBank and will support the identification of species in future studies.
Ethics statement
The study was authorized by the Brazilian Institute for the Environment and Renewable Natural Resources (IBAMA, license no. 68263–1) and the National Genetic Heritage and Associated Traditional Knowledge Management System (SISGEN no. A20BC45), and was approved by the Animal Ethics Committee of the Oswaldo Cruz Foundation (CEUA, Fiocruz no. L-008/2018) in accordance with the guidelines of the Brazilian National Council for the Control of Animal Experimentation (CONCEA).
Availability of data and materials
All sequence data generated for this study can be accessed via GenBank: https://www.ncbi.nlm.nih.gov/genbank/.
Acknowledgements
The authors would like to thank Alessandra Rodrigues von Randow for technical support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Acosta V, Prieto A, Lodeiros C. 2006. Índice de condición de los mejillones Perna perna y Perna viridis (Bivalvia: Mytilidae) bajo un sistema suspendido de cultivo en la Ensenada de Turpialito, Golfo de Cariaco, Venezuela. Zootecnia Trop. 24(2):177–192.
- Alexopoulos CJ, Mims CW, Blackwell M. 1996. Introductory mycology. New York (NY): John Wiley & Sons Inc.
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. Journal of Molecular Biology. 215:403–410. doi:10.1016/S0022-2836(05)80360-2.
- ANVISA. 2004. Detecção e Identificação dos fungos de importância médica. Módulo VII. Brasil.
- Baptista Neto JA, Smith BJ, McAllister JJ, Silva MAM. 2005. Fontes e transporte de metais pesados para a Enseada de Jurujuba (Baía de Guanabara) SE – Brasil. Rev Tamoios. 2:11–21.
- Bensch K, Braun U, Groenewald JZ, Crous PW. 2012. The genus Cladosporium. Studies in Mycology. 72:1–401. doi:10.3114/sim0003.
- Bensch K, Groenewald JZ, Meijer M, Dijksterhuis J, Jurjević Ž, Andersen B, Houbraken J, Crous PW, Samson RA. 2018. Cladosporium species in indoor environments. Studies in Mycology. 89:177–301. doi:10.1016/j.simyco.2018.03.002.
- Borzykh OG, Zvereva LV. 2012a. Filamentous fungi in the epibiosis of the scallop Mizuhopecten yessoensis (Bivalvia) in Peter the Great Bay, Sea of Japan. Russian Journal of Marine Biology. 38(6):454–455. doi:10.1134/S1063074012060041.
- Borzykh OG, Zvereva LV. 2012b. Mycobiota of the giant oyster Crassostrea gigas (Thunberg, 1787) (Bivalvia) from the Peter the Great Bay of the Sea of Japan. Microbiology. 81(1):109–111. doi:10.1134/S0026261712010031.
- Borzykh OG, Zvereva LV. 2014. Comparison of fungal complexes of japanese scallop Mizuhopecten yessoensis (Jay, 1856) from different areas in the Peter the Great Bay of the sea of Japan. Microbiology. 83(5):684–689. doi:10.1134/S0026261714050075.
- Borzykh OG, Zvereva LV. 2015. Mycobiota of the bivalve mollusk Anadara broughtoni (Schrenck, 1867) from various parts of Peter the Great Bay, Sea of Japan. Russian Journal of Marine Biology. 41(4):321–323. doi:10.1134/S1063074015040033.
- Borzykh OG, Zvereva LV. 2018. Fungal assemblages associated with commercial bivalve species in coastal waters of the Sea of Japan, Russia. Botanica Marina. 61(4):355–363. doi:10.1515/bot-2017-0088.
- Botha CJ, Truter M, Bredell T, Lange L, Mülders MSG. 2009. Putative Aspergillus niger-induced oxalate nephrosis in sheep. Journal of the South African Veterinary Association. 80(1):50–53. doi:10.4102/jsava.v80i1.169.
- Brasil. 2011. Resolução da Diretoria Colegiada - RDC no 7, de 18 de fevereiro de 2011. Dispõe sobre limites máximos tolerados (LMT) para micotoxinas em alimentos. Diário Oficial da União.
- Brasil. 2017. Resolução da Diretoria Colegiada - RDC no 138, de 8 de fevereiro de 2017. Altera a Resolução da Diretoria Colegiada - RDC n° 7, de 18 de fevereiro de 2011, que dispõe sobre limites máximos tolerados (LMT) para micotoxinas em alimentos, para alterar os LMT da micotoxina deoxinivalenol (DON) em trigo e produtos de trigo prontos para oferta ao consumidor e os prazos para sua aplicação. Diário Oficial da União.
- Carranza A, Borthagaray AI. 2009. The brown mussel Perna perna in the native mussel beds of Cerro Verde (Uruguay). Marine Biodiversity Records. 2:e76. doi:10.1017/S1755267209000608.
- Chen T, Wang S, Jiang X, Huang Y, Mo M, Yu Z. 2023. New species of Didymellaceae within aquatic plants from southwestern China. Journal of Fungi. 9:761. doi:10.3390/jof9070761.
- Davis ND. 1981. Sterigmatocystin and other mycotoxins produced by Aspergillus species. Journal of Food Protection. 44(9):711–714. doi:10.4315/0362-028X-44.9.711.
- Dong W, Jeewon R, Hyde KD, Yang E, Zhang H, Yu X, Wang G, Suwannarach N, Doilom M, Dong Z. 2021. Five novel taxa from freshwater habitats and new taxonomic insights of pleurotheciales and savoryellomycetidae. Journal of Fungi. 7(9):711. doi:10.3390/jof7090711.
- Dube HC. 2013. An introduction to fungi. Jodhpur: Scientific Publishers.
- Edrington TS, Kamps-Holtzapple CA, Harvey RB, Kubena LF, Elissalde MH, Rottinghaus GE. 1995. Acute hepatic and renal toxicity in lambs dosed with fumonisin-containing culture material. Journal of Animal Science. 73:508–515. doi:10.2527/1995.732508x.
- Ellis MB. 1971. Dematiaceous hyphomycetes. London: Commonwealth Mycological Institute.
- Ellis MB. 1976. More dematiaceous hyphomycetes. London: CABI.
- Ferreira JF, Magalhães ARM. 2004. Cultivo de mexilhões. In: Poli CR, Poli ATB, Andreatta E, Beltrame E A, editor. Aquicultura: experiências brasileiras. Florianópolis: Multitarefa; p. 221–250.
- Fries AS, Coimbra JP, Nemazie DA, Summers RM, Azevedo JPS, Filoso S, Newton M, Gelli G, Oliveira RCN, Pessoa MAR, Dennison WC. 2019. Guanabara Bay ecosystem health report card: science, management, and governance implications. Regional Studies in Marine Science. 25:100474. doi:10.1016/j.rsma.2018.100474.
- Frisvad JC, Møller LLH, Larsen TO, Kumar R, Arnau J. 2018. Safety of the fungal workhorses of industrial biotechnology: update on the mycotoxin and secondary metabolite potential of Aspergillus niger, Aspergillus oryzae, and Trichoderma reesei. Applied Microbiology and Biotechnology. 102:9481–9515. doi:10.1007/s00253-018-9354-1.
- Girisham S, Rao VK, Reddy SM. 2016. Taxonomy of mycotoxigenic fungi. Warangal: Scientific Publishers.
- Gomes DNF, Cavalcanti MAQ, Fernandes MJS, Lima DMM, Passavante JZO. 2008. Filamentous fungi isolated from sand and water of “Bairro Novo” and “Casa Caiada” beaches, Olinda, Pernambuco, Brazil. Brazilian Journal of Biology. 68(3):577–582. doi:10.1590/S1519-69842008000300016.
- Gonçalves MFM, Santos L, Silva BMV, Abreu AC, Vicente TFL, Esteves AC, Alves A. 2019. Biodiversity of Penicillium species from marine environments in Portugal and description of Penicillium lusitanum sp. nov., a novel species isolated from sea water. International Journal of Systematic and Evolutionary Microbiology. 69:3014–3021. doi:10.1099/ijsem.0.003535.
- Gontia-Mishra I, Deshmukh D, Tripathi N, Bardiya-Bhurat K, Tantwai K, Tiwari S. 2013. Isolation, morphological and molecular characterization of phytate-hydrolysing fungi by 18S rDNA sequence analysis. Brazilian Journal of Microbiology. 44(1):317–323. doi:10.1590/S1517-83822013005000021.
- Gontia-Mishra I, Tripathi N, Tiwari S. 2014. A simple and rapid DNA extraction protocol for filamentous fungi efficient for molecular studies. Indian Journal of Biotechnology. 13:536–539.
- Grovel O, Pouchus YF, Verbist J. 2003. Accumulation of gliotoxin, a cytotoxic mycotoxin from Aspergillus fumigatus, in blue mussel (Mytilus edulis). Toxicon. 42:297–300. doi:10.1016/S0041-0101(03)00146-6.
- Hicks DW, Tunnell JW Jr. 1995. Ecological notes and patterns of dispersal in the recently introduced mussel, Perna perna (Linne, 1758), in the Gulf of Mexico. American Malacological Bulletin. 11(2):203–206.
- IBAMA. 2006. Instrução Normativa n° 105, de 20 de julho de 2006. Diário Oficial da União.
- Index Fungorum. 2022. Search Index Fungorum. Royal Botanic Gardens Kew; [accessed 2022 Feb 22]. http://www.indexfungorum.org/Names/Names.asp.
- Kimmerling EA, Fedrick JA, Tenholder MF. 1992. Invasive Aspergillus niger with fatal pulmonary oxalosis in chronic obstructive pulmonary disease. Chest. 101:810–872. doi:10.1378/chest.101.3.870.
- Klich MA. 2002. Identification of common Aspergillus species. Utrecht: Centraalbureau voor Schimmelcultures.
- Knechtges P. 2012. Food safety: theory and practice. Burlington: Jones & Bartlett.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA x: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution. 35(6):1547–1549. doi:10.1093/molbev/msy096.
- Kurtzman CP, Robnett CJ. 1998. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie van Leeuwenhoek. 73:331–371. doi:10.1023/A:1001761008817.
- Li H, Fu Y, Song F. 2023. Marine Aspergillus: a treasure trove of antimicrobial compounds. Marine Drugs. 21:277. doi:10.3390/md21050277.
- Li S, Young T, Archer S, Lee K, Sharma S, Alfaro AC. 2022. Mapping the green–lipped mussel (Perna canaliculus) microbiome: a multi–tissue analysis of bacterial and fungal diversity. Current Microbiology. 76. doi:10.1007/s00284-021-02758-5.
- Lourenço CR, Nicastro KR, Serrão EA, Zardi GI. 2012. First record of the brown mussel (Perna perna) from the European Atlantic coast. Marine Biodiversity Records. 5:e39. doi:10.1017/S1755267212000280.
- Lunetta JE. 1969. Fisiologia da reprodução dos mexilhões (Mytilus perna -Mollusca lamellibranchia). Boletim Zoologia e Biol Marinha. 26:33–111. doi:10.11606/bffcluspzoobm.v26i26.121175.
- Magalhães ARM. 1998. Efeito da parasitose por Trematoda Bucephalidae na reprodução, composição bioquímica e índice de condição de mexilhões Perna perna (L.) [thesis]. São Paulo (SP/Brasil): Instituto de Biociências da Universidade de São Paulo.
- Magaña-Dueñas V, Cano-Lira JF, Stchigel AM. 2021. New Dothideomycetes from freshwater habitats in Spain. Journal of Fungi. 7(12):1102. doi:10.3390/jof7121102.
- Månsson M, Klejnstrup ML, Phipps RK, Nielsen KF, Frisvad JC, Gotfredsen CH, Larsen TO. 2010. Isolation and NMR characterization of fumonisin B2 and a new fumonisin B6 from Aspergillus niger. Journal of Agricultural and Food Chemistry. 58:949–953. doi:10.1021/jf902834g.
- Marques HLA. 1998. Criação comercial de mexilhões. São Paulo: Nobel.
- Marrouchi R, Benoit E, Caer JL, Belayouni N, Belghith H, Molgó J, Kharrat R. 2013. Toxic C17-Sphinganine analogue mycotoxin, contaminating Tunisian mussels, causes flaccid paralysis in rodents. Marine Drugs. 11:4724–4740. doi:10.3390/md11124724.
- Matallah-Boutiba A, Ruiz N, Sallenave-Namont C, Grovel O, Amiard J, Pouchus YF, Boutiba Z. 2012. Screening for toxigenic marine-derived fungi in Algerian mussels and their immediate environment. Aquaculture. 342:75–79. doi:10.1016/j.aquaculture.2012.02.016.
- Meletiadis J, Meis JFGM, Mouton JW, Verweij PE. 2001. Analysis of growth characteristics of filamentous fungi in different nutrient media. Journal of Clinical Microbiology. 39(2):478–484. doi:10.1128/jcm.39.2.478-484.2001.
- Mogensen JM, Frisvad JC, Thrane U, Nielsen KF. 2010. Production of fumonisin B2 and B4 by Aspergillus niger on grapes and raisins. Journal of Agricultural and Food Chemistry. 58:954–958. doi:10.1021/jf903116q.
- Oliveira AGL, Rocha RCC, Saint’Pierre TD, Hauser-Davis RA, Mello-Silva CC, Santos CP. 2022. Elemental contamination in brown mussels (Perna perna) marketed in Southeastern Brazil. Biological Trace Element Research. 200:402–412. doi:10.1007/s12011-021-02644-y.
- Oliveira AGL, Valladares V, Santos CP, Mello-Silva CC. 2023. Near infrared spectroscopy: a method for the monitoring and management of the commercial exploitation of the brown mussel (Perna perna) in southeastern Brazil. Frontiers in Marine Science. 10:1192024. doi:10.3389/fmars.2023.1192024.
- Oliveri C, Torta L, Catara V. 2008. A polyphasic approach to the identification of ochratoxin A-producing black Aspergillus isolates from vineyards in Sicily. International Journal of Food Microbiology. 127:147–154. doi:10.1016/j.ijfoodmicro.2008.06.021.
- Paret ML, Dufault NS, Newark M, Freeman JH. 2018. Management of Gummy Stem Blight (Black Rot) on Cucurbits in Florida. UF/IFAS Extension Service, University of Florida. PP280.
- Petkova-Bocharova T, Chernozemsky IN, Castegnaro M. 1988. Ochratoxin A in human blood in relation to Balkan endemic nephropathy and urinary system tumours in Bulgaria. Food Additives & Contaminants. 5(3):299–301. doi:10.1080/02652038809373707.
- Pitt JI. A laboratory guide to common Penicillium species. 2000. Food Science Australia a Joint Venture of CSIRO and AFISC, Australia.
- Pitt JI, Hocking AD. 2009. Fungi and food spoilage. New York (NY): Springer.
- Purchase IFH, Van der Watt JJ. 1973. Carcinogenicity of sterigmatocystin to rat skin. Toxicology and Applied Pharmacology. 26:274–281. doi:10.1016/0041-008X(73)90262-7.
- Raper KB, Fennell DI. 1965. The genus Aspergillus. Baltimore (MD): Williams & Wilkins.
- Resgalla C Jr, Weber LI, Conceição MB. 2008. O Mexilhão Perna perna (L.): biologia, ecologia e aplicações. Rio de Janeiro: Interciência.
- Ricci I, Damiani C, Scuppa P, Mosca M, Crotti E, Rossi P, Rizzi A, Capone A, Gonella E, Ballarini P, et al. 2011. The yeast Wickerhamomyces anomalus (Pichia anomala) inhabits the midgut and reproductive system of the Asian malaria vector Anopheles stephensi. Environmental Microbiology. 13(4):911–921. doi:10.1111/j.1462-2920.2010.02395.x.
- Rivalier E, Seydel S. 1932. Nouveau procedé de culture sur lames gélosées appliqué a l’étude microscopique des champignos deteignes. Annals of Parasitology. 10:444–452. doi:10.1051/parasite/1932105444.
- Salazar-Vallejo SI, González NE. 1986. A preliminary study of the relation between Nemertopsis gracilis (Nemertea), Chthamalaus fisus (Cirripedia) and Collisella spp (Gastropoda). Ciencias Marinas. 12(1):51–71. doi:10.7773/cm.v12i1.485.
- Salehi M, Zibafar E, Mahmoudi S, Hashemi SJ, Gatmiri SM, Shoar MG, Manshadi SAD, Jahanbin B, Alizadeh R, Hosseinpour L, et al. 2019. First report of invasive pulmonary infection by Didymella microchlamydospora and successful treatment with voriconazole. Clinical Microbiology and Infection. 25:392–393. doi:10.1016/j.cmi.2018.10.018.
- Sallenave C, Pouchus YF, Bardouil M, Lassus P, Roquebertc M, Verbist J. 1999. Bioaccumulation of mycotoxins by shellfish: contamination of mussels by metabolites of a Trichoderma koningii strain isolated in the marine environment. Toxicon. 37:77–83. doi:10.1016/S0041-0101(98)00135-4.
- Sallenave-Namont C, Pouchus YF, Pont TR, Lassus P, Verbist JF. 2000. Toxigenic saprophytic fungi in marine shellfish farming areas. Mycopathologia. 149:21–25. doi:10.1023/A:1007259810190.
- Samson RA, Visagie CM, Houbraken J, Hong S-B, Hubka V, Klaassen CHW, Perrone G, Seifert KA, Susca A, Tanney JB, et al. 2014. Phylogeny, identification and nomenclature of the genus Aspergillus. Studies in Mycology. 78:141–173. doi:10.1016/j.simyco.2014.07.004.
- Santos A, Hauser-Davis RA, Santos MJS, Simone SG. 2017a. Potentially toxic filamentous fungi associated to the economically important Nodipecten nodosus (Linnaeus, 1758) scallop farmed in southeastern Rio de Janeiro, Brasil. Marine Pollution Bulletin. 115:75–79. doi:10.1016/j.marpolbul.2016.11.058.
- Santos AL, Medeiros JVF, Grault CE, Santos MJS, Souza ALA, Carvalho RW. 2020. The fungus Pestalotiopsis sp., isolated from Perna perna (Bivalvia: Mytilidae) cultured on marine farms in Southeastern Brazil and destined for human consumption. Marine Pollution Bulletin. 153:110976. doi:10.1016/j.marpolbul.2020.110976.
- Santos GR, Ferreira MASV, Pessoa-Filho MACP, Ferreira ME, Café-Filho AC. 2009. Host specificity and genetic diversity of Didymella bryoniae from Cucurbitaceae in Brazil. Journal of Phytopathology. 157:265–273. doi:10.1111/j.1439-0434.2008.01475.x.
- Santos LS, Cândido WS, Rabelo HO, Marin MV, Gaion LA, Gomes RF, Camargo M, Braz LT. 2017b. Reaction of melon genotypes to Didymella bryoniae (Fuckel) Rehm. Chilean Journal of Agricultural Research. 77(1):71–77. doi:10.4067/S0718-58392017000100009.
- SAP/MAPA. 2020. Boletim de resultados do acompanhamento dos contratos de cessão de uso da maricultura em águas da União. Boletim da Maricultura em Águas da União 2017–2018 – 2019.
- Sarquis MIM, Borba CM. 1997. Fusarium species in sandy soil Ipanema Beach, Rio de Janeiro, Brazil. Journal of Basic Microbiology. 37(6):425–429. doi:10.1002/jobm.3620370608.
- Sarquis MIM, Oliveira PC. 1996. Diversity of microfungi in the sandy soil of Ipanema Beach, Rio de Janeiro, Brazil. Journal of Basic Microbiology. 36:51–58. doi:10.1002/jobm.3620360111.
- Seifert K, Morgan-Jones G, Gams W, Kendrick B. 2011. The genera of hyphomycetes. Utrecht: CBS-KNAW Fungal Biodiversity Centre.
- Smaoui S, D’Amore T, Agriopoulou S, Khaneghah AM. 2023. Mycotoxins in seafood: occurrence, recent development of analytical techniques and future challengs. Separations. 10. doi:10.3390/separations10030217.
- Smit E, Leeflang P, Glandorf B, Elsas JD, Wernars K. 1999. Analysis of fungal diversity in the wheat rhizosphere by sequencing of cloned PCR-amplified genes encoding 18S rRNA and temperature gradient gel electrophoresis. Applied and Environmental Microbiology. 65(6):2614–2621. doi:10.1128/aem.65.6.2614-2621.1999.
- Sumi Y, Hamasaki T, Miyakawa M. 1987. Tumors and other lesions induced in germ-free rats exposed to aspergillus versicolor alone. Japanese Journal of Cancer Research. 78:480–486.
- Tongxin X, Fengrong W, Junling W, Zhendong Z. 1991. Sterigmatocystin induced adenocarcinoma of the lung and atypical hyperplasia of glandular stomach in mice. Chinese Journal of Cancer Research. 3(1):31–34. doi:10.1007/BF02672086.
- Visagie CM, Houbraken J, Frisvad JC, Hong S-B, Klaassen CHW, Perrone G, Seifert KA, Varga J, Yaguchi T, Samson RA. 2014. Identification and nomenclature of the genus Penicillium. Studies in Mycology. 78:343–371. doi:10.1016/j.simyco.2014.09.001.
- Wafa EW, Yahya RS, Sobh MA, Eraky I, El-Baz M, El-Gayar HAM, Betbeder AM, Creppy EE. 1998. Human ochratoxicosis and nephropathy in Egypt: a preliminary study. Human & Experimental Toxicology. 17:124–129. doi:10.1177/096032719801700207.
- Watkinson SC, Boddy L, Money NP. 2015. The Fungi. Amsterdam: Elsevier.
- Webster J, Weber RWS. 2007. Introduction to Fungi. New York (NY): Cambridge University Perss.
- Wellbaum C, Schoenlein-Crusius IH, Malasso E, Tauk-Tornisielo SM. 2007. Fungos filamentosos isolados de folhas em decomposição na Represa de Guarapiranga, São Paulo, SP. HOLOS Environment. 7(2):171–190. doi:10.14295/holos.v7i2.1378.
- White TJ, Bruns T, Lee SJWT, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editor. PCR protocols: a guide to methods and applications. New York (NY): Academic press; p. 315–322.
- WoRMS. 2021. World Register of Marine Species. LifeWatch Belgium; [accessed 2021 Nov 3]. http://www.marinespecies.org.
- Xião B, Li E, Du Z, Jiang R, Chen L, Yu N. 2014. Effects of temperature and salinity on metabolic rate of the Asiatic clam Corbicula fluminea (Mülle, 1774). SpringerPlus. 3:455. doi:10.1186/2193-1801-3-455.
- Zhou G, Whong WZ, Ong T, Chen B. 2000. Development of a fungus-specific PCR assay for detecting low-level fungi in an indoor environment. Molecular and Cellular Probes. 14:339–348. doi:10.1006/mcpr.2000.0324.
- Zouhair S, Laaziz A, Qjidaa S, Bouseta A. 2017. Growth and ochratoxin a production by Aspergillus carbonarius and Aspergillus niger in relation to culture medium, water activity and temperature. Glo Adv Res J Agric Sci. 6(10):314–322.
- Zuluaga-Montero A, Toledo-Hernández C, Rodríguez JA, Sabat AM, Bayman P. 2010. Spatial variation in fungal communities isolated from healthy and diseased sea fans Gorgonia ventalina and seawater. Aquatic Biology. 8:151–160. doi:10.3354/ab00218.
- Zvereva LV, Vysotskaya MA. 2005. Filamentous fungi associated with bivalve mollusks from polluted biotopes of Ussuriiskii Bay, Sea of Japan. Russian Journal of Marine Biology. 31:382–385. doi:10.1007/s11179-006-0007-3.
