Abstract
Mesodinium rubrum (Lohman) (=Myrionecta rubra Jankowsky) swims backwards in jumps of short duration interspersed by longer periods of rest. Cells attain a velocity of up to 1.2 cm s–1 during jumps and this is probably a speed record for ciliates. The ciliate carries long cirri that serve as mechanoreceptors and for orientating the cell at the initiation of jumps, while the ciliary rows on the posterior part of the cell are responsible for propulsion. The cirri are sensitive to shear so that they can orientate themselves against the current in a siphon flow (such as generated by filter-feeding copepods). Mesodinium cells do not reorientate their body axis during sinking, but they reorientate their direction during the initiation of jumps so that they always tend to move upwards. Because the cells sink between jumps they can regulate their vertical position by modulation of the frequency of jumps. The cells’ tendency to drift vertically up or down is light dependent. The jumps are so rapid that these phototrophic organisms can enhance their uptake of dissolved mineral nutrients beyond the limitation of molecular diffusion.
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
Introduction
Mesodinium rubrum (Lohman) (=Myrionecta rubra Jankowsky) is a ubiquitous plankton ciliate that occurs in all seas, including brackish water, and in all climatic zones. It harbours an intracellular phototrophic symbiont. It has recently been shown that the organism has an absolute requirement for feeding on small protist prey, but even so, carbon and energy requirements are primarily met by the photosynthesis of the symbiont (Taylor et al. Citation1971; Lindholm Citation1985; Gustafson et al. Citation2000; Yih et al Citation2004; Hansen in preparation). Mesodinium rubrum frequently forms conspicuous red tides in coastal waters with densities exceeding 104 cells ml−1 (Taylor et al. Citation1971).
Mesodinium rubrum is a “jumping ciliate” in the sense of Tamar (Citation1979). The ciliates remain motionless for shorter or longer periods interspersed with quick “jumps”. Some jumping ciliates are filter feeders that swim for a short distance at more or less regular intervals after they have exploited suspended food particles within a certain parcel of water (e.g. Cyclidium). In other cases, such as M. rubrum, the adaptive significance is less clear. Darwin described the jumping behaviour of M. rubrum in 1839 (cf. Taylor et al. Citation1971). Several authors have noted that the velocity attained during jumps is very high and Lindholm (1985) estimated that the ciliate attains swimming velocities up to 8 mm s−1. In fact the ciliate is too rapid to observe the motile behaviour in any detail by eye or with the time resolution of ordinary video recordings. Jonsson & Tiselius (Citation1990) observed that M. rubrum shows an and Fenchel effective escape response in the vicinity of copepods and that it is less vulnerable to predation than some other tested plankton ciliates. It is also known that M. rubrum can control its vertical position in the water column, but the actual mechanism is unknown (Lindholm Citation1985).
Here we present a study on the motile behaviour of M. rubrum using high-speed video in an attempt to clarify the details of their motile behaviour.
Material and methods
Mesodinium cells were isolated from a bloom in the Isefjord (northern Zealand, Denmark) in May 2004. The organism was maintained in f/2 medium (Guillard Citation1983) with seawater (30 ppt) at 15°C and a light:dark cycle of 16:8 h with an irradiance of 100 µmol photons m−2 s−1. Cultures were initiated with about 500 ml−1 M. rubrum cells and with the cryptomonad Teleaulax sp. (2000 cells ml−1) as food.
The external morphology of the cells was studied on living and fixed cells. These observations were supplemented with transmission electron microscopy and confocal scanning microscopy (see Hansen and Fenohel, in preparation).
The motility of the ciliates was observed in glass dishes with seawater using an inverted microscope or in 1 cm spectrophotometer cells submerged in a thermostat-controlled water bath and observed with a horizontally orientated microscope. For light-dependent behaviour the container with the ciliates was illuminated at different light intensities by a halogen lamp fitted with a heat filter. The motility patterns were recorded with an ordinary CCD camera (25 Hz frame rate) and with a high-speed CCD camera (CPL-MS100 CCD; Canadian Photonic Lab, Minnedota, Canada) with adjustable frame rates up to 1000 Hz. Swimming paths and velocities were recorded by plotting the positions of cells frame by frame on a transparent overlay on the video monitor. Jump lengths and velocities were based on tracks that remained in focus, thus securing that they were parallel to the optical plane.
In order to study escape responses, a siphon flow was established by drawing water through a glass capillary with an internal diameter of 0.15 mm connected to a peristaltic pump. The flow was adjusted so that the water flow immediately outside the tip of the capillary was 1 cm s−1. Suspended 2 µm latex beads were used to calibrate and describe the flow field.
Results
External morphology
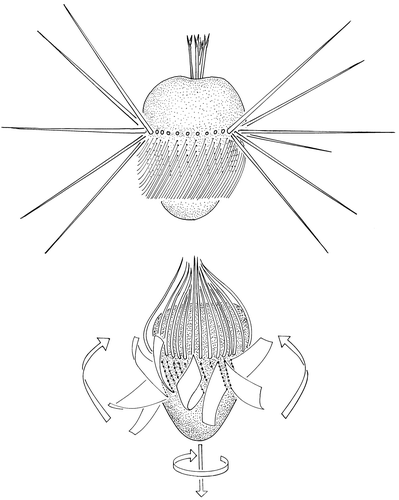
The external morphology of M. rubrum has previously been described in detail (e.g. Lindholm Citation1985) and will only be briefly re-described here as a background for the following. The cell consists of two parts, a hemispherical anterior part and a posterior cone-shaped part (). Here and in the following we define the position of the cytostome as the anterior end of the organism, although with this definition the ciliate always swims backwards. The size of the cells varies among populations; the mean length of our strain was 23 (17–28) µm, which is in the lower range of lengths reported by Lindholm (Citation1985). The oral end carries about six bifurcated tentacles that serve for catching prey (Yih et al. Citation2004). The ciliate carries an equatorial ring of 35 cirri (length about 25 µm), each consisting of eight cilia. Beneath them there are 35 parallel double rows of cilia that reach to about the middle of the posterior part; these form longitudinal membranelles. When the cells are at rest, the cirri stretch out at angles ranging from 45 to 135° relative to the longitudinal axis, while the membranelles are folded to the right close to the cell surface.
Jumping behaviour
The mean time interval between jumps ranges between approximately 1 and 10 s. The recorded extremes were 1.13 s (standard error = 0.15, n = 42) and 10.38 s (standard error = 1.60, n = 42). There is, however, a large variation in jumping intervals. Sometimes the interval between two jumps exceeds 20 s and occasionally the cells may make two to three jumps in immediate succession. A high jumping frequency leads to a net upwards drift of cells, whereas less frequent jumps lead to a net sinking of cells (see below).
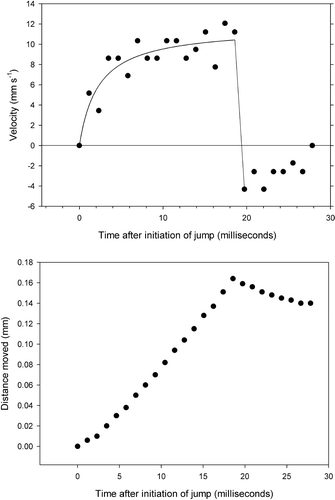
Jumps are initiated by the cirri, which then fold up around the anterior pole. This may take place symmetrically ( and ) or it may be initiated on one side of the cell. In the latter case the cell is rotated so that the direction of the jump is changed relative to the previous jump. The folding of the cirri is completed within 6 ms. Propulsion is primarily due to the membranelles, whose effective stroke is in an anterior-left direction. The cells are therefore propelled in a posterior direction and simultaneously rotate in a clockwise direction as observed from the oral pole (). During a jump the cell rotates about 360° (the rotation rate is about 54 Hz) around its longitudinal axis. The membranelles have only one beat cycle per jump, and the effective strokes occur pairwise on opposite sides of the cells, so that the jump paths are almost linear.
Figure 2. The position of cirri and membranelles during a jump traced from a high-speed video camera recording. The numbers indicate milliseconds after initiation of the jump.
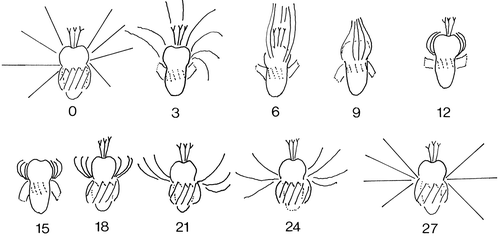
The initial acceleration is about 4 mm ms−2 (4 m s−2) and after 10 ms the velocity reaches about 1 cm s−1. After 20 ms the membranelles fold up against the cell and the cirri stretch out to their initial position within another 5–6 ms. Stretching of the cirri results in a slight reversal of the cell. The net length of a single jump is about 0.16 mm ( and ).
Mesodinium rubrum is also capable of sustained swimming for a longer period; this is attained solely by the membranelles, while the cirri are held motionless around the anterior pole of the cell. This behaviour was rarely observed, except during escape responses.
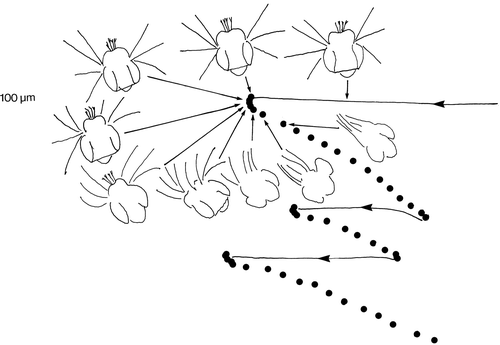
Escape response in a siphon flow
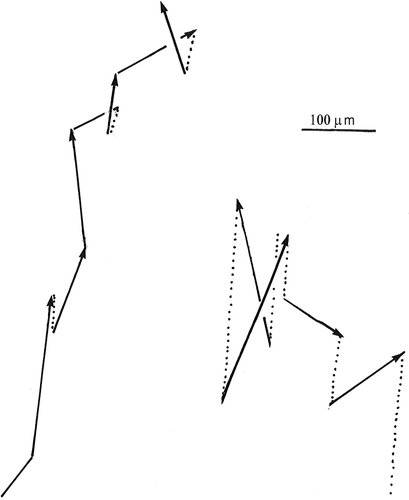
In a siphon flow, the ciliates are drawn passively along flow lines without any rotation of the cell. At some distance, the cells jump (). At the initiation of the jump, the cells adjust their orientation so that they tend to swim opposite to the direction of the flow. After the jump the ciliates will again be drawn towards the siphon and this will induce another jump. The cell may jump up to five times and each time the direction of the jump is more precisely orientated away from the siphon. Eventually, the ciliate swims continuously for a longer period and thus escapes from the flow (). In the experiments, practically all ciliates managed to stay away from the zone around the tip of the capillary where flow velocity exceeds that of the jump velocity and they thus avoided being drawn into the capillary.
Figure 4. The behaviour of Mesodinium cells in a siphon flow. The numbers refer to fluid velocity in mm s−1. Left: tracks of two ciliates in the flow. The dotted lines are the passive movement along flow lines; the solid lines are jumps. Right: initiation of jumps (solid circles) and of sustained swimming against the flow (triangles). The internal opening of the capillary is 0.15 mm.
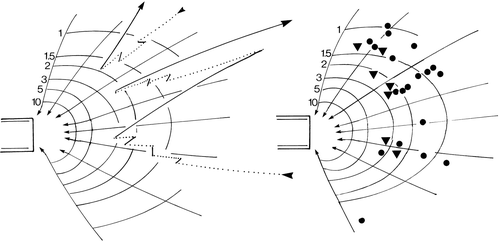
Figure 5. Angles of successive jumps of five Mesodinium cells relative to flow lines in a siphon flow. A value of 0° means swimming directly along the flow and a value of 180° that the cells swim directly against the current.
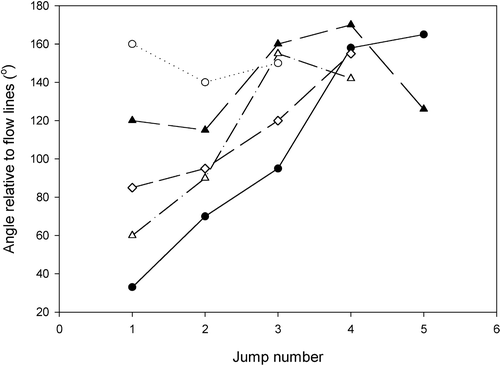
The mechanism of reorientation is shown in . When cells jump, the cirri on the leading side of the cell bend first. In this way the cell becomes orientated against the flow and the subsequent jump will tend to be opposite to the flow.
Figure 6. The behaviour of a cell in a siphon flow traced from a high-speed video recording. The cell does not reorientate while flowing passively along flow lines (solid lines), but reorientates against the flow at the initiation of jumps by activating the cirri in the leading edge of the cell. The time interval between dots is 1.5 ms.
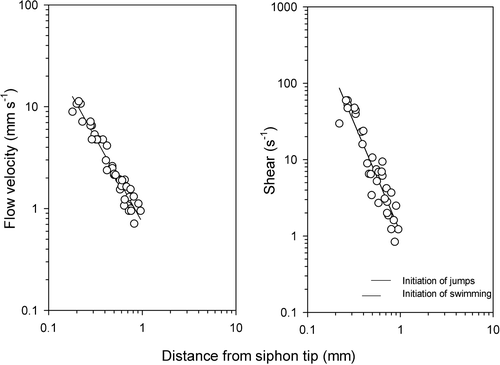
The flow field in a siphon flow is almost cone shaped. Consequently, flow velocity decreases with the square of distance, and linear shear (change in velocity as a function of distance) decreases with the third power of distance (). Because the flow velocity at a distance x from the pipette tip v(x) is proportional to x−2, then shear, dv/dx = const.×x−3. It is seen that jumping is induced at a shear of 1–3 s−1.
Figure 7. Flow velocity and shear in the siphon flow shown in as a function of distance from the tip of the capillary. The horizontal bars in the right frame show the range of shear that induces the first jumps, and the range of shear that induces sustained swimming against the water flow, respectively.
Vertical orientation
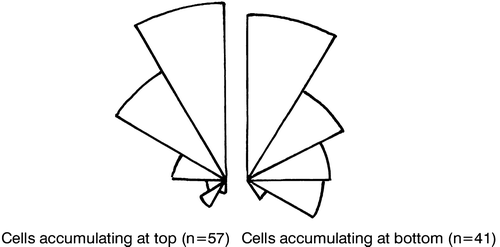
Cells often tend to migrate to the top of containers. They may also retain a rather even vertical distribution or tend to migrate towards the bottom. Motionless cells sink without any change in their orientation in the water. The sinking rate of motionless cells was measured to be 8.4 µm s−1 (standard error = 0.98 µm s−1, n = 34). In the water column, cells tend to point their posterior end upwards irrespective of whether they tend to migrate upwards or downwards (). The reason for this is that when cells jump, the cirri on the side of the cell pointing downwards tend to beat first. In this way, the cells become orientated so that the oral pole points downwards (). If the cells jump rarely (at intervals exceeding about 5 s), the sinking velocity exceeds the tendency to swim upwards so that the cells tend to be evenly dispersed or drift downwards. Conversely, if the cells jump more frequently, the upwards jumping exceeds the sinking velocity. In one case, where the cells tended to accumulate at the top, the jumping frequency was measured to be about 1 s−1, and as the displacement per jump is about 0.16 mm, the effective velocity was 0.16 mm s−1. The mean cosine of the angle (ϕ) between the cell axis and the vertical was 0.539 (n = 59). The vertical upward velocity component is velocity×cos ϕ or 86.2 µm s−1 and deducting the sinking velocity (8.4 µm s−1), the net upward drift was 77.8 µm s−1 (= 0.28 m h−1). When the jumping frequency was about 0.1 s−1, the upward velocity component, calculated in a similar manner, was only about 4.7 µm s−1 (n = 33 jumps) and so there was a slow net downward migration.
Response to light
The vertical distribution of the ciliates in spectrophotometer cells was influenced by light. In darkness, cells appeared to be randomly distributed. Above a certain light intensity the ciliates tended to migrate upwards; when irradiance exceeded about 350 mol photons m−2 s−1 the cells dispersed and above about 500 mol photons m−2 s−1 the cells drifted downwards (). Due to the high cell concentration, the results were affected by bioconvection when cells moved upwards. Bioconvection means that parcels of water with a high concentration of cells assume a higher density than the surrounding water and sink. This creates some convection in the container and consequently some mixing. In addition, shading by cells in the upper mm may have reduced light exposure for cells further down, resulting in two groups of cells with differential light exposure and consequently differential behaviour.
Figure 10. Vertical distribution of Mesodinium in a 6 mm high water column at different irradiance levels (numbers above the graphs are µmol photons m−2 s−1 after about 15 min exposure). Each distribution is based on the position of about 100 cells.
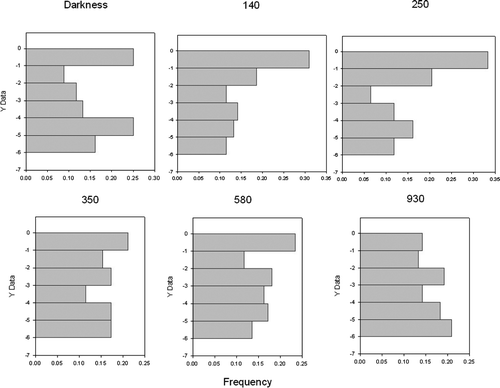
The described light response was also not quite consistent. It was strongest in the morning (after the cells had been exposed to darkness for 8 h), but became less evident during the day. It is therefore possible that there is some sort of circadian rhythm involved or that other factors influence their tendency to move up or down.
Discussion
The high velocity attained during jumping of Mesodinium could indicate a relatively high energy expenditure. An exact estimate is difficult to obtain. If we consider the ciliate as a sphere with a radius (r) of 0.0015 cm and a swimming velocity (v) of 1 cm s−1, then from Stokes’ law the required power is given by 6πηrv2, where η is viscocity (=0.01 Poise). This works out to be 2.8×10−4 erg s−1. Supposing that the efficiency of conversion to mechanical energy is 1%, and further that the cells jump 2% of the time (jump intervals of 1 s, duration of jumps 20 ms) then the necessary power would be 5.6×10−4 erg s−1; if they only jump 0.2% of the time, the power requirement would be only 5.6×10−5 erg s−1. At maximum metabolic rate, a ciliate with a size like Mesodinium will at 20°C consume about 2.8 pmol O2 h−1 (Fenchel & Finlay Citation1983). Assuming 50% efficiency in conversion to ATP this corresponds to a power generation of 1.8×10−3 erg s−1. With these assumptions, the cells may use about 30% of their available energy for motility when jumping is 1 s−1, and about 3% when the jumping frequency is 0.1 s−1. Such considerations are quite crude for several reasons, but they do suggest that motility may be energetically costly and so jumping can be assumed to be adaptive. The considerations also explain why frequent jumping takes place only during escape behaviour and in the light when more energy is available ( and ). Mesodinium may represent an exception to the generalization that micro-organisms spend a relatively small fraction of their power generation on motility (Fenchel & Finlay Citation1983).
The adaptive significance of the escape jumps in siphon flow is obvious. Such escape jumps of plankton ciliates have previously been demonstrated by Jonsson & Tiselius (Citation1990) and by Jakobsen (Citation2001) in the vicinity of filter-feeding copepods and in artificial siphon flows. Jakobsen (Citation2001) found that the escape reactions were induced at a shear of 1–3 s−1 and this is consistent with the present findings. These also explain the mechanism by which the ciliates manage to jump away against the flow. The cirri serve both as mechanoreceptors and as effectors: the cirri in the leading edge experience a higher shear and respond first, thus turning the ciliates in a direction opposite to the flow.
Jumping also serves as a mechanism by which the cells can orientate themselves vertically in the water column. Jonsson (Citation1989) showed for several species of plankton ciliates that they tend to reorientate their axis while sinking through the water column so that their anterior end tends to point upwards. This reorientation is due to an asymmetry in cell shape or in the distribution of mass within the cells. Mesodinium rubrum presents another mechanism. It does not reorientate the direction of its length axis as it sinks through the water column. Instead the cells reorientate at the initiation of jumps and this is accomplished by an asymmetric response of the cirri: those on the leading side of the sinking cells initiate the jump by a power stroke so that the cells turns in a more upright position. As in the escape response, the cirri must function as a mechanoreceptor. The only obvious explanation is that as the cells sink the cirri on the leading side experience diverging flow lines, while those on the opposite side of the cell experience converging flow lines. The exact mechanism is, however, unclear and the involved forces would seem slight given that the sinking velocity of the resting ciliates is only about 8 µm s−1.
The response to light appears adaptive. In darkness, or at very low light intensities, the cells jump infrequently and the cells tend to disperse. At somewhat higher light intensities, the cells migrate upwards, but at an irradiance exceeding about 300 µmol photons m−2 s−1, jumping frequency falls again and the cells disperse or drift slowly downwards. This accords with Stoecker et al. (Citation1991), who found that the light saturation of M. rubrum photosynthesis is around 350 µmol photons m−2 s−1.
Previous studies on the in situ vertical distribution of M. rubrum in the water column have not given consistent results. Crawford & Lindholm (Citation1997) found limited diurnal vertical migration and also that many cells were found close to halocline at depths where light intensity was lower than the compensation point for photosynthesis. Dale (Citation1987) found an indication for diel vertical migrations: in the dark the cells dispersed and during the day the maximum density of the cells was closer to the surface. This basic pattern accords with the present results, but the estimated migration velocity (about 1 m h−1) is higher than the present results can account for: that is, a maximum upwards drift of 28 cm h−1, while downwards drift that depends on sinking can only be a few cm h−1. If the ciliates can jump more frequently than observed in the present study, this could account for a higher upwards drift. A higher downwards drift would require that the cells tend to reorientate downwards during the initiation of jumps, but this was not observed in the present study. Although Dale's (Citation1987) data do show an increased concentration of cells at particular depths and also indications of diel migrations, ciliates always occurred over a wide range of depths. It appears that the actual vertical distribution of Mesodinium was also influenced by convective water movements or by turbulent mixing.
It is generally assumed that microbes cannot enhance the uptake of solutes by swimming: at the small scale and relatively low absolute velocities, translation of the cells is slower than molecular diffusion.Being a phototroph, M. rubrum depends on the uptake of dissolved mineral nutrients that may be limiting for photosynthesis. Light-dependent uptake of inorganic nitrogen (nitrate and ammonium) has previously been demonstrated for this species by Wilkerson & Grunseich (Citation1990). As Mesodinium can swim at a velocity that is an order of magnitude higher than that of most other ciliates and at about two orders of magnitude higher than most other phototrophic protists, the jumps may actually play a role in nutrient uptake.
In a diffusion-limited case, the concentration gradient of a dissolved nutrient around a stationary spherical cell is given by C = Co(1 – r/a) where C is the concentration, Co is the bulk concentration (far away from the cell), a is the cell radius and r is the distance from the centre of the cell (Berg Citation1983). The concentration therefore already approaches bulk concentration at a distance of two to three cell diameters (60–90 µm) away from the cell surface. The length of jumps is 0.16 mm. The Sherwood number Lv/D is a measure of the role of convective transport (caused by the motility of the cells) relative to diffusive transport. Here L is the distance (0.016 cm), v is the swimming velocity (1 cm s−1) and D is the diffusion coefficient of the solute (assumed to be 10−5 cm2 s). If the Sherwood number exceeds unity, then convective transport is significant relative to diffusion. It works out to be 1600, suggesting that Mesodinium can enhance nutrient uptake by jumping.
Editorial responsibility: Diane Stoecker
Acknowledgments
We are grateful to Marianne Saietz for technical assistance. The study was supported by grants from the Danish Natural Science Research Council to TF and PJH and from the Carlsberg Foundation to TF.
Notes
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
References
- Berg , HC . 1983 . Random Walks in Biology , Princeton : Princeton University Press .
- Crawford , DW and Lindholm , T . 1997 . Some observations on vertical distribution and migration of the phototrophic ciliate Mesodinium rubrum (=Myrionecta rubra) in a stratified brackish inlet . Aquatic Microbial Ecology , 13 : 267 – 74 .
- Dale , T . 1987 . Diel vertical distribution of planktonic ciliates in Lindåspollene, Western Norway . Marine Microbial Food Webs , 2 : 15 – 28 .
- Fenchel , T and Finlay , BJ . 1983 . Respiration rates in heterotrophic, free-living Protozoa . Microbial Ecology , 9 : 99 – 122 .
- Guillard , RRL . 1983 . “ Culture of phytoplankton for feeding invertebrate animals ” . In Culture of Marine Invertebrates , Edited by: Berg , CJ . 123 – 8 . Stroudsberg, PA : Hutchinson Ross .
- Gustafson Jr , DE , Stoecker , DK , Johnson , MD , Van Heukelem , WF and Sneider , K . 2000 . Cryptophyte algae are robbed of their organelles by the marine ciliate Mesodinium rubrum . Nature , 405 : 1049 – 52 .
- Jakobsen , HH . 2001 . Escape response of planktonic protists to fluid mechanical signals . Marine Ecology Progress Series , 214 : 67 – 8 .
- Jonsson , PR . 1989 . Vertical distribution of planktonic ciliates – an experimental analysis of swimming behaviour . Marine Ecology Progress Series , 52 : 39 – 53 .
- Jonsson , PR and Tiselius , P . 1990 . Feeding behaviour, prey detection and capture efficiency of the copepod Acartia tonsa feeding on planktonic ciliates . Marine Ecology Progress Series , 60 : 35 – 44 .
- Lindholm , T . 1985 . Mesodinium rubrum – a unique photosynthetic ciliate . Advances in Aquatic Microbiology , 8 : 1 – 48 .
- Stoecker , DK , Putt , M , Davis , LH and Michaels , AE . 1991 . Photosynthesis in Mesodinium rubrum: species-specific measurements and comparison to community rates . Marine Ecology Progress Series , 73 : 245 – 52 .
- Tamar , H . 1979 . The movement of jumping ciliates . Archiv für Protostenkunde , 122 : 290 – 327 .
- Taylor , FJR , Blackbourn , DJ and Blackbourn , J . 1971 . The red-water ciliate Mesodinium rubrum and its “incomplete symbionts”: a review including new ultrastructural observations . Journal of the Fisheries Research Board Canada , 28 : 391 – 407 .
- Wilkerson , FP and Grunseich , G . 1990 . Formation of blooms by the symbiotic ciliate Mesodinium rubrum: the significance of nitrogen uptake . Journal of Plankton Research , 12 : 973 – 89 .
- Yih , W , Kim , HS , Jeong , HJ , Myung , G and Kim , YG . 2004 . Ingestion of cryptophyte cells by the marine photosynthetic ciliate Mesodinium rubrum . Aquatic Microbial Ecology , 36 : 165 – 70 .