Abstract
To provide data on the diets of hooded seals (Cystophora cristata) in the Greenland Sea, seals were collected for scientific purposes on expeditions conducted in the pack ice belt east of Greenland in September/October 1999, 2002 and 2003 (autumn), July/August in 2000 (summer), and February/March in 2001 and 2002 (winter). The results from analyses of stomach and intestinal contents from captured seals revealed that their diet was comprised of relatively few prey taxa. The squid Gonatus fabricii and polar cod (Boreogadus saida) were particularly important, whereas capelin (Mallotus villosus) and sand eels (Ammodytes spp.) occasionally contributed more. These four prey items constituted 60–97% of the diet biomass. Gonatus fabricii was the most important food item in autumn and winter, whereas polar cod dominated the summer diet, with important contributions from G. fabricii and sand eels. The latter was only observed on the hooded seal menu during the summer period, whereas polar cod, which was an important component during the autumn survey, was almost absent from the winter samples. During the latter survey, capelin also contributed to the hooded seal diet. Samples obtained from hooded seals in more coastal waters indicated a more varied diet based on fish such as polar cod, redfish (Sebastes sp.) and Greenland halibut (Reinhardtius hippoglossoides).
Keywords:
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
Introduction
Hooded seals, Cystophora cristata, occur in the drift ice waters of the Greenland Sea along the east coast of Greenland (also called the West Ice) during breeding and moult (March/April; see Rasmussen Citation1957, Citation1960; Folkow & Blix Citation1995; Folkow et al. Citation1996; Haug et al. Citation2000; Potelov et al. Citation2000). Recent information about the migratory patterns of the species obtained in satellite tagging programmes has indicated that hooded seals may also occur in the Greenland Sea pack ice in considerable periods outside the breeding and moulting period (Folkow et al. Citation1996). In total, they appeared to be based in the ice-covered waters off the east coast of Greenland for approximately 40% of the year and they made long excursions from these areas to distant waters (e.g. off the Faroe Islands, the Irminger Sea, north/northeast of Iceland, areas in the Norwegian Sea, and along the continental shelf edge from Norway to Bear Island), presumably to feed, before returning to the ice edge again (Folkow & Blix Citation1995, Citation1999; Folkow et al. Citation1996). During these excursions, which could last for more than 3 months, the seals apparently never hauled out even if they sometimes stayed very close to coastal areas. Migrations to distant waters were not synchronized in time, in that different seals made excursions at different times of the year. In that sense, hooded seals do not seem to display any general seasonal migration pattern, and part of the population will always be present in the Greenland Sea pack ice (the West Ice) areas (Folkow et al. Citation1996).
Previous studies of hooded seals in the Greenland Sea have concentrated mainly on stock size estimation, reproduction and migrational patterns (Rasmussen Citation1957, Citation1960; Øritsland Citation1959, Citation1964; Rasmussen & Øritsland Citation1964; Jacobsen Citation1984; Øien & Øritsland Citation1995; Øritsland & Øien Citation1995; Folkow & Blix Citation1995, Citation1999; Folkow et al. Citation1996; ICES Citation1998; Salberg et al. Citation2007). Little attention has been paid to the feeding habits of the seals, and our knowledge about the ecological significance of this stock is therefore very poor. Except for some observations made in the West Ice during breeding and moult (i.e. spring; see Haug et al. Citation2000; Potelov et al. Citation2000), which are known to be periods with low feeding intensity (Rasmussen Citation1960; Kovacs & Lavigne Citation1986), only occasional information, mostly from coastal areas of eastern Greenland and northern Iceland, was available (Hauksson & Bogason Citation1997; Kapel Citation2000). Although hooded seal migrational patterns outside the West Ice breeding and moulting periods are fairly well known from the recent satellite tagging programme (Folkow & Blix Citation1995, Citation1999; Folkow et al. Citation1996), the diet of these animals in the same period has remained poorly documented.
To enable an assessment of the ecological role of hooded seals during the considerable period they occur in drift ice waters along the east coast of Greenland, a project aimed at studying their feeding habits in the area was initiated in 1999. The project intended to pay special attention to the period July–February (i.e. between moulting and breeding), which is known to be a period of intensive feeding for the seals (Rasmussen Citation1960). Dedicated expeditions along the east Greenland pack ice edge were conducted during summer (July–August 2000), autumn (September/October 1999, 2002, 2003) and winter (February–March 2001, 2002). Additionally, some historical samples were obtained from coastal hunters who operated in the Ammassalik area on the southeastern coast of Greenland in May 1987.
In the research surveys performed during summer (July/August) in 2000 and winter (February/March) in 2001, it was observed that hooded seals co-occurred with harp seals, Phoca groenlandica, in the sampling areas. This enabled a comparison of the feeding habits of the two seal species, and Haug et al. (2004) observed that their diets varied remarkably. The present analyses included the hooded seal diet data used in Haug et al. (Citation2004). In addition, the data from 1987, 1999, 2002 and 2003 were included to give a more thorough description of the diet of hooded seals during all periods they occur in coastal and more offshore drift ice waters along the east coast of Greenland.
Materials and methods
Sampling of seals
Data from 20 hooded seals taken by local hunters in the Ammassalik area on the southeast coast of Greenland () in May 1987 were included. The stomachs were frozen by the hunters and later transferred to a laboratory for analysis.
Figure 1. Catch positions for hooded seals taken for scientific purposes east of Greenland in 1999–2003.
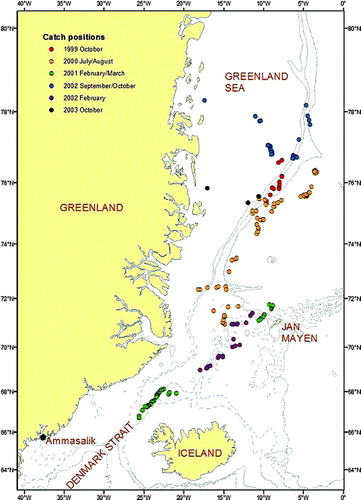
The remaining material originated from five dedicated research expeditions to open drift ice areas along the east coast of Greenland from the Denmark Strait (between Iceland and Greenland) to approximately 78°N () carried out in 1999–2003 using the ice-going research vessel Jan Mayen. The sampling was allocated to three periods of the year: summer (July/August), autumn (September/October) and winter (February/March). The cruise track generally followed the ice edge as an a priori planned “transect” line – attempts were made to shoot and sample all seals spotted along this edge. The seals were shot in the water or on ice floes and immediately brought on board the research vessel for dissection. Samples of digestive tracts (stomachs and large intestines) were frozen, and the lower jaw (with teeth) was collected from each seal for age determination (see Born Citation1982; Haug et al. Citation2004).
During the period 2–6 October 1999, 15 hooded seals were sampled in the Greenland Sea along a north–south cruise track, starting at position 76°40′N 7°07′W and ending at position 75°21′N 9°07′W. Most sampling was carried out over deep waters (1500–2800 m). Two of the seals were taken in pack ice located over more shallow waters (c. 330 m) on the Greenland continental shelf (approximate position 76°45′N 8°00′W).
In 2000, 65 hooded seals were sampled in the Greenland Sea during the period 22 July to 1 August along a south–north cruise track, starting to the west of the island Jan Mayen, between positions 70°58′N 14°30′W and 76°54′N 3°55′W. Most sampling was carried out in areas with sea depths ranging between 1000 and 3000 m. Two hooded seals were taken in pack ice located over more shallow waters (c. 250 m) on the Greenland continental shelf (approximate position 72°20′N 18°10′W).
The 2001 survey, carried out 18 February to 1 March, covered areas of the Greenland Sea north of Jan Mayen (as far north as 72°24'N 8°50′W) and of the Denmark Strait northwest and west of Iceland, as far to the southwest as 66°41′N 22°29′W. In total, 13 hooded seals were sampled in the Greenland Sea area pack ice (depths ranging between 600 and 800 m). In the Denmark Strait, 57 hooded seals were taken in ice-filled waters with depths ranging between 800 and 1200 m.
In 2002, samples were obtained from two different surveys. During the first cruise, 25 hooded seals were obtained along the ice edge in the Greenland Sea between 71°25′N 11°24′W and 69°00′N 17°59′W in the period 17–28 February. Later in the same year, during the period 30 September to 4 October, 38 hooded seals were caught in the Greenland Sea along a cruise track that first went from north to south between positions 78°11′N 4°39′W and 76°43′N 6°20′W, and then turned in a northwestward direction up to 78°18′N 17°24′W. The seals were taken in areas overlaying the Greenland continental shelf (250–1000 m depths).
The 2003 samples were obtained during 5–6 October when 11 hooded seals were caught in the Greenland Sea along a cruise track that started in the outer areas of the continental shelf (at 78°34′N 10°38′W), proceeded towards the coast and ended at position 75°50′N 17°02′W. All seals were taken in areas overlaying the Greenland continental shelf (250–1000 m depths).
Digestive tract contents analyses
In the laboratory, the stomachs and intestines were cut open after thawing. Stomach contents were weighed and, after flushing the intestine with fresh water, the contents sorted. Most of the stomach and intestinal contents were partly or completely digested, and the prey organisms were identified to the lowest possible taxonomic level, preferably species, using intact fresh specimens or hard structures such as pieces of exoskeletons and otoliths with references to Enckell (Citation1980), Pethon (Citation1985), Breiby (Citation1985), Clarke (Citation1986) and Härkönen (Citation1986).
Estimates of the number of crustaceans present in the digestive tract (stomach and large intestines) were obtained by counting the carapaces and/or tails of each species. In cases with large contents, subsamples were taken. Approximate average weights of crustaceans were obtained from fresh prey specimens found in the stomachs or from previously published values (see Haug et al. Citation1996, Citation2004; Potelov et al. Citation2000), and these were used to reconstruct the original biomass of crustaceans. The number of upper and lower squid beaks were recorded – the most numerous category was used to estimate the total number of squid in the digestive tract. Only one squid species (Gonatus fabricii) was recorded. Due to the importance of this particular prey item in the hooded seal diets, a subsample of beaks was sent to Dr Thomas K. Kristensen (present head of the Mandal-Barth Research Center for Biodiversity and Health, DBL – Institute for Health Research and Development, Charlottenlund, Denmark) who is an expert on squid of the genus Gonatus (e.g. Kristensen Citation1981, Citation1983), to confirm the species identifications. Back-calculation of squid biomass and mantle lengths from lower rostral lengths were carried out using regression equations given by Clarke (Citation1986).
The total number of each fish species in the digestive tract was estimated by adding the number of whole specimens, the number of intact skulls and half the number of “free” otoliths. Otoliths were measured, and published otolith length to fish length and fish wet weight correlations were used to reconstruct the initial weight of the most numerous fish species: polar cod (Boreogadus saida), capelin (Mallotus villosus), sand eels (Ammodytes sp.), redfish (Sebastes sp.), and flatfish; see Härkönen (Citation1986) and Lindstrøm et al. (Citation1998). Unidentifiable gadoid otoliths, probably from polar cod, were treated similarly. No corrections were made for otolith erosions, but only otoliths that were little or moderately digested were used in the biomass back-calculations. All digested otoliths were, however, counted and used to estimate total prey biomass in that their numbers were multiplied by the average biomass of undigested fish. Due to a general lack of published otolith guides for Arctic fishes, the biomass of other minor components such as sculpins (Cottidae), blennies (Lumpenidae) and snailfishes (Liparidae) were calculated using correlations based on our own unpublished material (see also Haug et al. Citation2004).
Feeding indices
The following two feeding indices were used to analyse the dietary data (see also Hyslop Citation1980; Pierce & Boyle Citation1991):
(1) The frequency of occurrence of each prey item, FOi:
(2) The bulk biomass index, Bi:
(3) The combined index, Ci:
To construct 95% confidence intervals for the relative importance, as the combined index, of each prey group, the data from each sampling trip were bootstrapped 1000 times using each stomach/intestine as the sampling unit. All confidence intervals were corrected for possible acceleration and bias (see Efron & Tibishirani Citation1993). Pairwise bootstrapped-based hypothesis testing was used to test for possible differences in diet composition between years and age groups (Efron & Tibishirani Citation1993). Because multiple testing tends to lead to exaggerated P-values, all P-values were Bonferroni corrected. A Friedman test, a non-parametric counterpart of the two-way analysis of variance, was run to test for possible temporal differences in length (mantle) composition of squid.
Results
Prey occurrence
Most seals (95%) taken by local hunters in east Greenland coastal waters of the Ammassalik area had food in their stomachs, whereas the majority of stomachs (60–80%) from animals taken in the offshore drift ice areas were empty (). Intestine material was not available from the local hunter sample, but intestines from the drift ice samples generally contained food remains (only 6–24% were empty).
Table I. Frequency of occurrence (%) of empty stomachs and intestines, and identified species in stomachs and intestines of hooded seals caught in drift ice areas east of Greenland in May (1987), July/August (2000), September/October (1999, 2002 and 2003), and in February/March (2001 and 2002).
At least 22 different prey items were identified – of these there were four crustaceans, one mollusc and at least 17 fish species (). Not all items appeared to be of equal importance – of particular importance in all periods in the offshore samples were the cephalopod G. fabricii (occurred in 10–82% of most samples, fewest were observed in October 2003 when samples were obtained nearest the coast). Polar cod also occurred quite frequently in intestines in all periods (6–68%), whereas other fish species occurred more sporadically in particular periods (e.g. sand eels in summer, cottids in autumn, and capelin in winter, see ). The frequency of occurrence of amphipods of the genus Parathemisto was considerable in most periods. Seals sampled in the coastal areas in May 1987 were not observed to have eaten cephalopods, whereas fish species such as polar cod and redfish, to some extent also Greenland halibut (Reinhardtius hippoglossoides), appeared to be important.
Relative prey biomass
Polar cod and redfish contributed 66 and 29%, respectively, to the prey biomass found in the stomachs of seals taken by local hunters in east Greenland coastal waters of the Ammassalik in May 1987. The combined contribution from other fish, particularly Greenland halibut, Arctic cod (Arctocephalus glacialis), and sculpins was 5%, whereas the contribution from invertebrates was negligible.
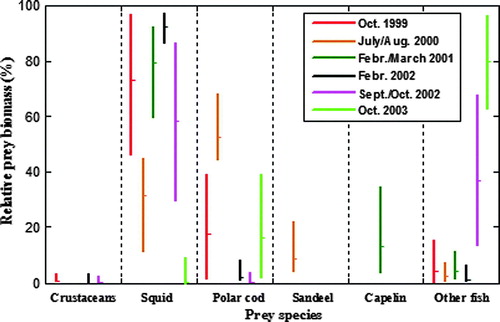
In terms of relative biomass, G. fabricii dominated the digestive tract contents in two of the autumn (1999 and 2002) samples and all winter (2001 and 2002) samples obtained from the open drift ice areas (58–94%, see ). Parts of the 2001 and the entire 2002 winter samples came from the Greenland Sea west and southwest of the island Jan Mayen () and these were particularly dominated by this squid – the 2001 winter samples from the Denmark Strait were, however, more a mixture of squid and fish, particularly capelin. The contribution from squid was negligible in the more near-coast samples obtained during autumn in 2003. In the summer 2000 samples, the contribution from G. fabricii (32%) was significantly lower than in autumn 1999 and in both the winter samples (74–94%, pairwise bootstrap-based testing, all P < 0.05). The summer sample in 2000 was particularly characterized by a large contribution from polar cod (54%) – this value was significantly higher for this species than in all other periods/areas (pairwise bootstrap-based testing, all P < 0.05), although polar cod also contributed considerably in the autumn samples in 1999 (19%) and 2003 (18%). Sand eels and capelin contributed with 11 and 14% in the summer (2000) and winter (2001) samples, respectively, but were otherwise scarce. The contribution from other fish, such as bottom-living sculpins and flatfishes, was significantly higher in the autumn 2002 (39%) and 2003 (81%) samples (which contained the highest proportion of seals taken at or inside the edge of the continental shelf in any sampling event, see ) than in any other samples (pairwise bootstrap-based testing, all P < 0.05). The contributions from crustaceans were low in all periods.
Figure 2. Relative prey importance, as percentage combined index (see text for explanation), in hooded seal diets east of Greenland in the period 1999–2003. The mean relative biomass estimates are plotted with 95% confidence intervals, determined from 1000 bootstrap replicates, and corrected for possible acceleration and bias.
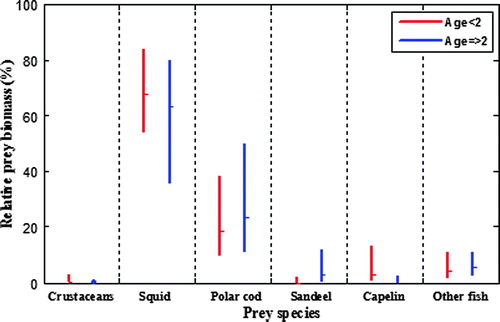
The entire material included digestive tract contents from 181 hooded seals, of which 111 were in their first or second year of life, whereas the remaining 70 were 2 years old or older. Apparently, there were no significant differences (paired bootstrap-based testing, all P > 0.05) in the diets observed for the youngest (<2 years old) seals as compared with those 2 years old or older ().
Figure 3. Relative prey importance, as percentage combined index (see text for explanation), in hooded seal diets east of Greenland in the period 1999–2003. The mean relative biomass estimates are plotted with 95% confidence intervals, determined from 1000 bootstrap replicates, and corrected for possible acceleration and bias. Results are given for young (<2 years) and older seals.
Size of prey
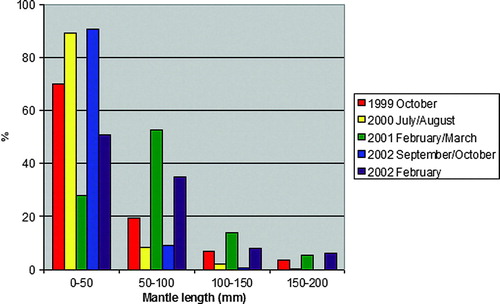
Estimated mantle lengths for G. fabricii, the most common prey found in the hooded seal digestive tracts, ranged from less than 5 mm to 200 mm (). Small squid (length groups 0–50 and 50–100 mm) were, however, significantly more numerous than larger squid (Friedman test; ). The smallest size groups (0–50 mm) appeared to dominate the samples from summer (July–August) and autumn (September–October), whereas in the winter samples (February–March) there seemed to be an increased contribution from larger squids, particularly the size groups 50–100 mm mantle lengths. This apparent change was, however, not statistically significant (Friedman test;
).
Discussion
The observed diet of hooded seals in the Greenland Sea pack ice appeared to be comprised of relatively few prey taxa, and the dominant role of the squid G. fabricii was conspicuous. Although the relative contribution from polar cod, capelin and sand eel varied both with species and sampling period/area, these four prey items (one squid, three fishes) constituted 60–97% of the observed diet biomass, irrespective of the sampling period. Gonatus fabricii appeared to contribute importantly during all sampling periods, whereas polar cod was confined mainly to the summer 2000 (July/August) and autumn 1999 and 2003 (September/October) periods, sand eels to the summer period, and capelin to the winter 2001 (January/February) period (, ). When interpreting the results from the presented digestive tract contents analyses, one must of course take into consideration that the sample sizes were small. The material contained a considerably larger number of younger (less than 2 years old) than older seals. However, no significant changes in the material were observed with increasing age of the seals ().
Satellite tracking data have revealed that hooded seals from the West Ice stock appeared to be based in the ice-covered waters off the east coast of Greenland, from where they made long excursions to distant waters (such as the waters off the Faroe Islands, the Irminger Sea, north/northeast of Iceland, areas in the Norwegian Sea, and along the continental shelf edge from Norway to Bear Island), presumably to feed, before returning to the ice edge again (Folkow & Blix Citation1995, Citation1999; Folkow et al. Citation1996). During excursions, which could last for more than 3 months, the seals apparently never hauled out, even if they sometimes stayed very close to coastal areas. The present observations indicate that hooded seals may both feed and haul out when they occur along the ice edge in the northern Greenland Sea.
From their satellite tag-based observations of diving behaviour and available published information about potential prey species, Folkow & Blix (Citation1999) suggested that the diet of hooded seals along the ice edge in the northern Greenland Sea might be comprised of Greenland halibut, redfish, polar cod and the squid G. fabricii. The present investigations did not give any evidence for demersal species such as Greenland halibut or redfish as important hooded seal food in the offshore drift ice area, whereas the more pelagic polar cod and particularly G. fabricii did prove to be major food items for the seals. Thus, even though the co-occurrence of predators and prey in time and space may be indicative of predation, it is evident that confirmatory observations by other means are also required. The presented coastal samples from Greenland, on the other hand, confirmed the importance of redfish and Greenland halibut. It is also worth noticing that the autumn 2002 and 2003 samples contained larger proportions of demersal fishes (sculpins in particular) than any other drift ice samples. A large number of these autumn samples were taken over the Greenland shelf (see ) – this may have made demersal fish species more available to the seals and suggest that hooded seals may well feed on bottom fishes in areas where they can obtain them, e.g. when the seals occur closer to coastal waters.
Gonatus fabricii is the most abundant squid of the Arctic and sub-Arctic waters of the North Atlantic (Kristensen Citation1981, Citation1983). Their biomass production in the Nordic Seas represents a considerable food resource, and the consumption of them by other top predators such as sperm whales (Physeter macrocephalus), northern bottlenose whales (Hyperoodon ampullatus) and long-finned pilot whales (Globicephala melaena) is assumed to be substantial (Bjørke Citation2001). Based on information from stranded animals, sperm whales have been suggested to feed primarily on adult specimens (mantle lengths generally more than 200 mm; see Santos et al. Citation1999, Citation2002; Simon et al. Citation2003), in particular mature females, which after mating undergo comprehensive ontogenetic changes that imply that they lose their swimming ability and presumably become floatation devices for their negatively buoyant eggs (Kristensen Citation1981; Arkhipin & Bjørke Citation1999). This seems not to be the case with hooded seals, for which the majority of the squid eaten had mantle lengths less than 50 mm during summer and autumn, and less than 100 mm in winter (). In his study of the life history of G. fabricii from west Greenland waters, Kristensen (Citation1983, Citation1984) concluded that the juveniles (<c. 50 mm mantle length) lived in shoals in the uppermost 80 m of the water column. At increasing size they would live deeper, and as sub-adults and adults they would live above the bottom from 200 m and downwards. Apparently, the main source of squid as food for the hooded seals was juveniles (). There was an increased occurrence of larger specimens (in particular individuals with mantle lengths ranging between 50 and 100 mm) in the observed winter diets, as compared with summer and autumn. A general increase in size of squid specimens due to their natural individual growth from summer to winter may have contributed to this (Kristensen Citation1983, Citation1984). However, there are important differences in the habitats where the seals were sampled during the summer and autumn (north of Jan Mayen, primarily in areas with water depths exceeding 1000 m) as compared with the winter samples, which were obtained south of Jan Mayen in water depths ranging primarily between 250 and 1000 m (see ). The larger depths north of Jan Mayen may simply have made the older, near-bottom dwelling squid unavailable for the seals.
Although hooded seals are known to feed much less intensively during breeding and moult than in other periods of the year (Rasmussen Citation1960), they have been shown to perform some food intake on their breeding and moulting grounds in pack ice areas of the Greenland Sea in April–June. As in the present investigations, G. fabricii, and to some extent also polar cod, dominated their diet (Potelov et al. Citation2000). The diet of hooded seal pups during their first independent feeding excursions in April has been observed to be mainly Parathemisto sp. (Haug et al. Citation2000).
Hooded seal stomachs examined further to the south in the Nordic Seas (southern Greenland Sea, Iceland Sea) and in more coastal waters seem to indicate a more varied and fish-based diet. Animals taken by local hunters in southeastern Greenland in June–July suggested low feeding intensity in this period (i.e. the moulting period when they are known to feed very little; see Rasmussen Citation1960) when particularly redfish and Greenland halibut were consumed (Kapel Citation1995, Citation2000). Later in the year (September), however, the few samples seem to indicate that hooded seal feeding was more intensive in the area, the diet being comprised of squid, shrimp (Pandalus sp.), polar cod and redfish. The importance of polar cod and redfish and, to some extent, also Greenland halibut, was confirmed by the present Greenland coast samples (from May 1987). Hooded seals examined south of the breeding and moulting lairs (in coastal waters of northern Iceland) during the period April–October were observed to feed mainly on redfish, cod (Gadus morhua) and various other fishes, occasionally also shrimps and squid (Hauksson & Bogason Citation1997). Fish-based hooded seal diets were also observed in Atlantic Canadian waters, where Greenland halibut, cod and redfish were the most important items (Hammill & Stenson Citation2000).
Very little is known about fish stocks such as sand eels and polar cod in the study area. However, the biology of capelin in the area is fairly well known (Vilhjalmsson Citation1997, Citation2002). Capelin spawn in areas south of Iceland, and their feeding areas are in the northern parts of the Denmark Strait and shelf areas between Iceland and the island Jan Mayen (see ). This restricted distribution explains both their appearance in hooded seal diets in the Denmark Strait during winter 2001, and their absence in all diets of seals taken to the north of the distributional areas for capelin.
Hooded seals have long been known to co-occur with harp seals in the drift ice waters of the Greenland Sea along the east coast of Greenland during spring, when both species breed (March/April: see Wollebæk Citation1907; Iversen Citation1927; Rasmussen Citation1957; Folkow & Blix Citation1995; Folkow et al. Citation1996, Citation2004; Haug et al. Citation2000; Potelov et al. Citation2000). Recent information, obtained in satellite tagging programmes, about the migratory patterns of the two seal species indicate that they may also co-occur in the Greenland Sea pack ice outside the breeding period (Folkow et al. Citation1996, Citation2004). The latter was further confirmed in the research surveys performed during summer (July/August) in 2000 and winter (February/March) in 2001, when it was observed that, although they co-occurred, the diets varied remarkably between the two seal species (Haug et al. Citation2004). These observed differences were probably the result of different foraging depths. Studies of the diving behaviour of harp and hooded seals in the Greenland Sea have revealed that both species usually perform more shallow dives during summer than during winter, and that hooded seals dive to deeper waters than harp seals in both periods (Folkow & Blix Citation1999; Folkow et al. Citation2004).
Many of the examined stomachs were empty, a common feature in seals when they are sampled while hauled out on ice (see Nilssen et al. Citation1995a, Citationb; Lindstrøm et al. Citation1998; Haug et al. Citation2004), and it may reflect rapid digestion (Helm Citation1984; Markussen Citation1993; Berg et al. Citation2002) and/or some migratory distance between feeding grounds and the haul out sites on the ice. In passing through the gastrointestinal tract of the predator, otoliths of different species and sizes erode at different rates, and some are completely digested (e.g. Tollit et al. Citation1997, Citation2003; Berg et al. Citation2002; Christiansen et al. Citation2005). The present recalculations of fish biomass were based only on little or very moderately eroded otoliths. Furthermore, although cephalopod beaks appear to be less susceptible to digestion, they may accumulate in the stomach (Pitcher Citation1980; Bigg & Fawcett Citation1985; Tollit et al. Citation1997), causing additional bias. The effect of passage through the pinniped gastrointestinal tract of crustaceans, as compared with, for example, fish is unknown.
Editorial responsibility: Aril Slotte
Acknowledgements
Thanks are due to the crew and field assistants on board the research vessel Jan Mayen. The seal investigations in the Nordic Seas are supported economically by the Norwegian Council of Research, project no. 133646/120.
Notes
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
References
- Arkhipin , AI and Bjørke , H . 1999 . Ontogenetic changes in morphometric and reproductive indices of the squid Gonatus fabricii (Oegopsida, Gonatidae) in the Norwegian Sea . Polar Biology , 22 : 357 – 65 .
- Berg , I , Haug , T and Nilssen , KT . 2002 . Harbour seal (Phoca vitulina) diet in Vesterålen, north Norway . Sarsia , 87 : 451 – 61 .
- Bigg , MA and Fawcett , I . 1985 . “ Two biases in diet determination of northern fur seals (Callorhinus ursinus) ” . In Marine Mammals and Fisheries , Edited by: Beddington , JR , Beverton , RJH and Lavigne , DM . 284 – 91 . London : George Allen & Unwin .
- Bjørke , H . 2001 . Possible predators of Gonatus fabricii (Lichtenstein) in its deep-water habitat . Fisheries Research , 52 : 113 – 20 .
- Born , EW . 1982 . Reproduction in the female hooded seal, Cystophora cristata Erxleben, at south Greenland . Journal of Northwest Atlantic Fishery Science , 3 : 57 – 62 .
- Breiby , A . 1985 . Otolitter fra saltvannsfisker i Nord Norge . Tromura Naturvitenskap , 53 : 1 – 30 .
- Christiansen , JS , Moen , A-GM , Hansen , TH and Nilssen , KT . 2005 . Digestion of capelin, Mallotus villosus (Müller), herring, Clupea harengus, and polar cod, Boreogadus saida (Lepechin), otoliths in a simulated seal stomach . ICES Journal of Marine Science , 62 : 86 – 92 .
- Clarke , MR . 1986 . A Handbook for the Identification of Cephalopod Beaks , Oxford : Clarendon Press .
- Efron , B and Tibshirani , RJ . 1993 . An Introduction to Bootstrap , New York : Chapman and Hall .
- Enckell PH . 1980 . Kräftdjur . Bokförlaget Signum i Lund .
- Folkow , LP and Blix , AS . 1995 . “ Distribution and diving behaviour of hooded seals ” . In Whales, Seals, Fish, and Man , Edited by: Blix , AS , Walløe , L and Ulltang , Ø . 193 – 202 . Amsterdam : Elsevier .
- Folkow , LP and Blix , AS . 1999 . Diving behaviour of hooded seals (Cystophora cristata) in the Greenland and Norwegian Seas . Polar Biology , 22 : 61 – 74 .
- Folkow , LP , Mårtensson , PE and Blix , AS . 1996 . Annual distribution of hooded seals (Cystophora cristata) in the Greenland and Norwegian Seas . Polar Biology , 16 : 179 – 89 .
- Folkow , LP , Nordøy , ES and Blix , AS . 2004 . Distribution and diving behaviour of harp seals Pagophilus groenlandica from the Greenland Sea stock . Polar Biology , 27 : 281 – 98 .
- Hammill , MO and Stenson , GB . 2000 . Estimated prey consumption by harp seals (Phoca groenlandica), hooded seals (Cystophora cristata), grey seals (Halichoerus grypus) and harbour seals (Phoca vitulina) in Atlantic Canada . Journal of Northwest Atlantic Fishery Science , 26 : 1 – 23 .
- Härkönen , T . 1986 . Guide to the Otoliths of the Bony Fishes of the Northeast Atlantic , Hellerup : Danbiu .
- Haug , T , Lindstrøm , U , Nilssen , KT , Røttingen , I and Skaug , HJ . 1996 . Diet and food availability for Northeast Atlantic minke whales Balaenoptera acutorostrata . Report of the International Whaling Commission , 46 : 371 – 82 .
- Haug , T , Nilssen , KT and Lindblom , L . 2000 . First independent feeding of harp seal (Phoca groenlandica) and hooded seal (Cysophora cristata) pups in the Greenland Sea . North Atlantic Marine Mammal Commission Scientific Publications , 2 : 29 – 39 .
- Haug , T , Nilssen , KT and Lindblom , L . 2004 . Feeding habits of harp and hooded seals in drift ice waters along the east coast of Greenland in summer and winter . Polar Research , 23 : 35 – 42 .
- Hauksson , E and Bogason , V . 1997 . Comparative feeding of grey (Halichoerus grypus) and common seals (Phoca vitulina) in coastal waters of Iceland, with a note on the diet of hooded (Cystophora cristata) and harp seals (Phoca groenlandica) . Journal of Northwest Atlantic Fishery Science , 22 : 125 – 35 .
- Helm , RC . 1984 . Rate of digestion in three species of pinnipeds . Canadian Journal of Zoology , 62 : 1751 – 6 .
- Hyslop , EJ . 1980 . Stomach content analysis – a review of methods and their application . Journal of Fish Biology , 17 : 411 – 29 .
- International Council for the Exploration of the Sea (ICES) . 1998 . Report of the Joint ICES/NAFO Working Group on Harp and Hooded Seals , ICES Headquarters, 28 August–3 September 1997. ICES CM 1998/Assess: 3 .
- Iversen , T . 1927 . Drivis og selfangst . Årsberetning vedkommende Norges Fiskerier , 1927 ( 2 ) : 1 – 84 .
- Jacobsen , NO . 1984 . Estimates of pup production, age at first parturition and natural mortality for hooded seals in the West Ice . Fiskeridirektoratets Skrifter Serie Havundersøkelser , 17 : 483 – 98 .
- Kapel , FO . 1995 . “ Feeding ecology of harp and hooded seals in the Davis Strait–Baffin Bay region ” . In Whales, Seals, Fish, and Man , Edited by: Blix , AS , Walløe , L and Ulltang , Ø . 287 – 304 . Amsterdam : Elsevier Science .
- Kapel , FO . 2000 . Feeding habits of harp and hooded seals in Greenland waters . North Atlantic Marine Mammal Commission Scientific Publications , 2 : 50 – 64 .
- Kovacs , KM and Lavigne , DM . 1986 . Cystophora cristata . Mammalian Species , 258 : 1 – 9 .
- Kristensen , TK . 1981 . The genus Gonatus Gray, 1849 (Mollusca, Cephalopoda) in the North Atlantic. A revision of the North Atlantic species and description of Gonatus steenstrupi n. sp . Steenstrupia , 7 : 61 – 99 .
- Kristensen , TK . 1983 . “ Gonatus fabricii ” . In Cephalopod Life Cycles, Vol. 1: Species Accounts , Edited by: Boyle , PR . 159 – 73 . New York : Academic Press .
- Kristensen , TK . 1984 . Biology of the squid Gonatus fabricii (Lichtenstein, 1818) from West Greenland waters. Meddelelser om Grønland . Bioscience , 13 : 3 – 17 .
- Lindstrøm , U , Harbitz , A , Haug , T and Nilssen , KT . 1998 . Do harp seals Phoca groenlandica exhibit particular prey preferences? . ICES Journal of Marine Science , 55 : 941 – 53 .
- Markussen , NH . 1993 . Transit time of digesta in captive harbour seals (Phoca vitulina) . Canadian Journal of Zoology , 71 : 1071 – 3 .
- Nilssen , KT , Haug , T , Potelov , V , Stasenkov , VA and Timoshenko , YK . 1995a . Food habits of harp seals (Phoca groenlandica) during lactation and moult in March–May in the southern Barents Sea and White Sea . ICES Journal of Marine Science , 52 : 33 – 41 .
- Nilssen , KT , Haug , T , Potelov , V and Timoshenko , YK . 1995b . Food habits and food availability of harp seals (Phoca groenlandica) during early summer and autumn in the northern Barents Sea . Polar Biology , 15 : 485 – 93 .
- Øien , N and Øritsland , T . 1995 . “ Use of mark-recapture experiments to monitor seal populations subject to catching ” . In Whales, Seals, Fish, and Man , Edited by: Blix , AS , Walløe , L and Ulltang , Ø . 35 – 45 . Amsterdam : Elsevier Science .
- Øritsland , T . 1959 . Klappmyss . Fauna (Oslo) , 12 : 70 – 90 .
- Øritsland , T . 1964 . Klappmysshunnens forplantingsbiologi . Fisken og Havet , 1964 ( 1 ) : 1 – 15 .
- Øritsland , T and Øien , N . 1995 . “ Aerial surveys of harp and hooded seal pups in the Greenland Sea pack-ice ” . In Whales, Seals, Fish, and Man , Edited by: Blix , AS , Walløe , L and Ulltang , Ø . 77 – 87 . Amsterdam : Elsevier Science .
- Pethon , P . 1985 . Aschehougs store fiskebok , Copenhagen : H. Aschehoug and Company .
- Pierce , GJ and Boyle , PR . 1991 . A review of methods for diet analysis in piscivorous marine mammals . Oceanography and Marine Biology (Annual Review) , 29 : 409 – 86 .
- Pitcher , KW . 1980 . Stomach contents and faeces as indicators of harbour seal, Phoca vitulina, foods in the Gulf of Alaska . Fishery Bulletin , 78 : 797 – 8 .
- Potelov , V , Nilssen , KT , Svetochev , V and Haug , T . 2000 . Feeding habits of harp Phoca groenlandica and hooded seals Cystophora cristata during late winter, spring and early summer in the Greenland Sea . North Atlantic Marine Mammal Commission Scientific Publications , 2 : 40 – 9 .
- Rasmussen , B . 1957 . Exploitation and protection of the east Greenland seal herds . Norsk Hvalfangsttidende , 46 : 45 – 59 .
- Rasmussen , B . 1960 . Om klappmyssbestanden i det nordlige Atlanterhav . Fisken og Havet , 1960 ( 1 ) : 1 – 23 .
- Rasmussen , B and Øritsland , T . 1964 . Norwegian tagging of harp and hooded seals in North Atlantic waters . Fiskeridirektoratets Skrifter Serie Havundersøkelser , 13 : 43 – 55 .
- Salberg A-B , Haug T , Nilssen KT . 2007 . Estimation of hooded seal (Cystophora cristata) pup production in the Greenland Sea pack ice during the 2005 whelping season . Polar Biology (in submission) . In press .
- Santos , MB , Pierce , GJ , Boyle , PR , Reid , RJ , Ross , HM Patterson , IAP . 1999 . Stomach contents of sperm whales Physeter macrocephalus stranded in the North Sea 1990–1996 . Marine Ecology Progress Series , 183 : 281 – 94 .
- Santos , MB , Pierce , GJ , Garcia-Hartmann , M , Smeenk , C , Addink , MJ Kuiken , T . 2002 . Additional notes on stomach contents of sperm whales Physeter macrocephalus stranded in the north-east Atlantic . Journal of the Marine Biological Association of the UK , 82 : 501 – 7 .
- Simon , MJ , Kristensen , TK , Tendal , OS , Kinze , CC and Tougaard , S . 2003 . Gonatus fabricii (Mollusca, Theuthida) as an important food source for sperm whales (Physeter macrocephalus) in the Northeast Atlantic . Sarsia , 88 : 244 – 6 .
- Tollit , DJ , Steward , MJ , Thompson , PM , Pierce , GJ , Santos , MB and Hughes , S . 1997 . Species and size differences in the digestion of otoliths and beaks: implications for estimates of pinniped diet composition . Canadian Journal of Fisheries and Aquatic Sciences , 54 : 105 – 19 .
- Tollit , DJ , Wong , M , Winship , AJ , Rosen , DAS and Trites , AWE . 2003 . Quantifying errors associated with using prey skeletal structures from fecal samples to determine the diet of Stellar's sea lion (Eumetopias jubatus) . Marine Mammal Science , 19 : 724 – 44 .
- Vilhjalmsson , H . 1997 . Interactions between capelin (Mallotus villosus) and other species and the significance of such interactions for the management and harvesting of marine ecosystems in the northern North Atlantic . Rit Fiskideildar , 15 : 31 – 63 .
- Vilhjalmsson , H . 2002 . Capelin (Mallotus villosus) in the Iceland–East Greenland–Jan Mayen ecosystem . ICES Journal of Marine Science , 59 : 870 – 83 .
- Wollebæk , A . 1907 . Über die Biologie der Seehunde und die Seehundjagd im Europäischen Eismeer . Rapports et Procés-Verbaux des Réunions Conseil International pour l'Exploration de la Mer , 8 : 109 – 19 .