Abstract
This review investigates if goatfishes qualify as habitat indicators and play a role as key species for use in coastal ecosystem monitoring and management, emphasizing major gaps of knowledge in goatfish ecology and systematics. Currently, 66 species of goatfishes are known, the family occurring widely in tropical, subtropical and temperate habitats from the upper littoral down to the upper slope. Studies of goatfish occurrence and abundance in natural habitats have documented general preferences for sand-associated bottoms after post-larval settlement that goes hand in hand with the development of the characteristic barbels. Species, populations and later life-history stages may, however, differ significantly from each other in habitat use. Some species are more restricted to hard bottoms, others separate mainly by depth. Goatfishes respond to human-induced factors such as fisheries and habitat modification, as reflected by abundance, size, or weight changes, or changes in their distributional ranges. Temperature increase may lead to increased reproductive or growth rates and longer warming periods may induce goatfishes to migrate to higher latitudes, as exemplified by striped red mullet (Mullus surmuletus) in the North Sea. Isolated occurrences of this species in the Norwegian Sea at 60°N have been documented. Goatfishes may act as allochthonous ecosystem engineers through their vigorous foraging behaviour with barbels and mouth, which leads to the stirring-up of sediments and associated detritus particles high into the water column. Goatfishes play a key role in the formation of multi-species foraging associations as nuclear species that are followed by many other species. The role of goatfishes in food webs has been rarely evaluated and the many interactions goatfishes may be involved in have not yet been sufficiently considered. There is also a considerable lack of basic systematic and taxonomic knowledge, new species still being described and intraspecific morphological variation and genetic differentiation requiring more detailed studies. Goatfishes clearly deserve more attention in future coastal habitat exploration, monitoring and management efforts.
Introduction
Coastal waters are highly structured, covering a large variety of different bottom types that are inhabited by a diverse assemblage of organisms. Many of these habitats are still insufficiently known and require continued effort to sample, describe and register all species. However, due to increasing signs of human-induced local and global impacts (e.g. Cohen et al. Citation1997; Gommes et al. Citation1998; Phillippart Citation2007), there is also a pressing need to study further coastal organisms to understand their ecological role and function and to evaluate their potential use as indicators and/or key species for coastal ecosystem monitoring and management.
Indicators are here defined as a subset of organisms that strongly and transparently respond to distinct natural or human-induced factors or changes. ‘Strongly and transparently’ shall signify that observed responses should be directly related to distinct factors, relatively easy to measure and, hence, cost- and time-effective. The measuring of such responses can be based on occurrence and distribution patterns, local abundance, weight, size, behaviour or physiology (Nicholls Citation2002). Indicators should be relatively abundant and widespread, easy to sample and tolerant to a wide variety of environmental conditions.
Key species interact tightly with an entire assemblage and are able to modify it directly or indirectly. Some key species act as ‘ecosystem engineers’, as they physically change the environment, either by themselves or by manipulating distinct habitat features. Due to their interactive role, key species provide important information on ecosystem processes and, hence, can also be used as indicators of ecosystem integrity and state.
Most parsimonious, time- and cost-effective ecosystem monitoring and management may be achieved by using groups of easily accessible and widely distributed species that to some extent combine the features of indicator and key species (Nicholls Citation2002). Because these species will allow essential information to be obtained about distinct habitat features as well as about an overall assemblage within a certain area, they would be ‘ecosystem indicators’ in a very integrative way.
This study highlights the goatfishes, family Mullidae, as a group of mainly coastal organisms that have a high value for ecosystem monitoring and management, but also require intensified systematic and ecological research. Goatfishes are characterized by a pair of typical chin barbels that are very efficient tools for food search and location (). This family comprises 66 species () that are distributed worldwide in tropical, subtropical and temperate habitats between the upper littoral and the upper slope.
Figure 1. Bicolour goatfish, Parupeneus barberinoides, searching for prey with barbels (top) and mouth (bottom). Note the different degrees of penetration and sediment disturbance. A full behavioural sequence starting with barbel search and ending with mouth search deep in the sediment is shown in a supplementary video clip available at: http://www.informaworld.com/mpp/uploads/goatfish_food_search_video.avi Both the photographs and the video clip were made in the Okinawa Churaumi Aquarium, Japan, February 2007 by the author.

Table I. The 66 species of the family Mullidae, as of June 2007 (Randall Citation2004; Randall & Kulbicki Citation2006; Froese & Pauly Citation2007).
Goatfishes are relatively common and of high economic importance in many coastal areas. This study investigates if goatfishes qualify as coastal habitat indicators and if they may also play a role as key species in coastal assemblages. Gaps in the knowledge in goatfish ecology and basic systematics are pointed out to stimulate further research.
Goatfishes as habitat indicators
In the last few years, considerable research on coastal fishes has been carried out to examine the effects of both naturally varying factors and human-induced modifications on habitat utilization at different scales (e.g. Horn et al. Citation1999; Hart & Reynolds Citation2002; Sale Citation2006). Goatfishes have been increasingly considered in such kinds of study, either jointly with other fish taxa or as the major study subjects. The following overview is based mainly on quantitative, comparative data from recent research on goatfishes. The first section deals with goatfishes as indicators of natural habitat followed by studies of the impact of fisheries and human-induced habitat modification. The final section deals with temperature and climate change. The overview tables (Tables ) follow the same structure, listing the species, area, major factors and parameters, specific observations and the results of the respective investigation(s), and the literature source(s).
Table II. Goatfish species as indicators of natural habitat.
Table III. Goatfish species as indicators of fisheries impact.
Table IV. Goatfish species as indicators of habitat modification.
Table V. Goatfish species as indicators of temperature change.
Natural habitat
Goatfishes occur in a broad range of habitats, mostly close to or near the bottom of the littoral. However, some species may be found down to depths of 500 m (e.g. Golani Citation2001) and surface-dwelling goatfish larvae have sometimes been found drifting in the outer shelf (Hernandez et al. Citation2003) or in oceanic waters (Deudero Citation2002). Most goatfish species shift to bottom life soon after metamorphosis, coinciding with barbel development (McCormick Citation1993) and changes in eye structure (Shand Citation1997). However, some species may remain in the open water as juveniles (McCormick & Milicich Citation1993) or feed on plankton even during later ontogenetic stages (Krajewski & Bonaldo Citation2006).
Studies on goatfish habitat use have considered depth as well as various bottom types, including hard and soft bottoms, open sandy areas and those overgrown with vegetation (). Clear preferences for distinct habitat types, but also differences among species and size/age classes, have been reported. Goatfishes are most frequently found on sandy bottoms adjacent to hard bottoms, including coral reefs. Apart from daily short-distance movements within and among foraging and resting sites (Holland et al. Citation1993; Meyer et al. Citation2000), they may also show seasonal migrations, in particular during the reproductive period, leading to the formation of spawning aggregations (Colin & Clavijo Citation1978; Lobel Citation1978; Thresher Citation1984; Colin Citation1996; Machias & Labropoulou Citation2002; Claydon Citation2004).
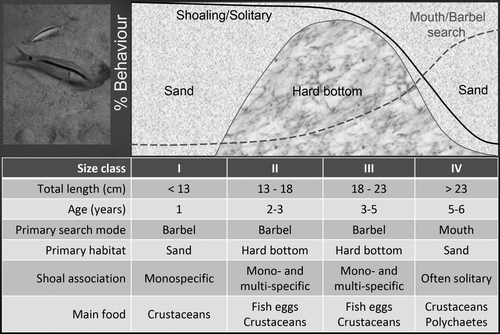
Juvenile goatfishes are often encountered on soft bottoms, in seagrass beds or mangroves, and at different depths than adults, reflecting both horizontal and vertical ontogenetic habitat shifts (). Serving as recruitment habitats, seagrass habitats may contribute positively to adult goatfish abundance in adjacent areas (Dorenbosch et al. Citation2005). Ontogenetic habitat shifts may also occur during later life history and coincide with changes in foraging mode, social behaviour and the formation of multi-species associations (Uiblein Citation1991; ).
Figure 2. Red Sea goatfish, Parupeneus forsskali, ontogenetic shifts in prey search, resource use, shoaling tendency and association with other species based on a field investigation of four size/age classes in the Gulf of Aqaba, Northern Red Sea (Uiblein Citation1991). Food selection information is based on Wahbeh & Ajiad (Citation1985).
There are marked differences among goatfish species with respect to preferred habitat type and depth (). For instance, the red mullet, Mullus barbatus, and the striped red mullet, M. surmuletus, show clear differences in distribution and abundance, with the latter occurring more on hard bottoms and shallower (Lombarte et al. Citation2000). Depth-related habitat segregation has also been observed in another species pair, the blue-lined goatfish, Upeneichthys lineatus, and U. stotti (Platell et al. Citation1998). There are also species differences in substrate preferences and in the flexibility of using alternative habitat types (McCormick Citation1995; Krajewski et al. Citation2006).
Fishing pressure
Goatfish species are relevant to fisheries in many areas worldwide and several species have high economic importance. For instance, in Hawaii, Central Pacific, at least six goatfish species are the target of fisheries (Williams et al. Citation2006). In the Mediterranean, the red mullet and the striped red mullet have been favourite food fishes at least since the Romans and have been heavily exploited in the last few years (e.g. Caddy Citation1993; European Commission Citation2005). Since the increase in abundance of striped red mullet in more northern areas (see also the section on temperature changes), a fisheries has been developing there (e.g. ICES Citation2006).
Goatfishes have been used as fisheries indicators (), often among other species, both to examine immediate pressure from ongoing fisheries or release from fishing impacts in marine protected areas (MPAs). Fisheries pressure leads to a reduction in goatfish abundance and landings, and a marked decrease in size and weight (). Opposite trends in these parameters are observed with release from fishing pressure, as particularly happens in MPAs with additional ‘spillover effects’ to surrounding areas (). Important variables that have to be considered when planning MPAs are site fidelity and home-range size, as goatfishes (e.g. the yellowstripe goatfish, Mulloidichthys flavolineatus, and the whitesaddle goatfish Parupeneus porphyreus) have distinct requirements for daily and seasonal movements (Holland et al. Citation1993; Meyer et al. Citation2000). Also, permanent closures to fisheries should be preferred above intermittent, rotational closures (Williams et al. Citation2006).
Habitat modification
Approximately 20% of the human population live within 30 km of the sea (Cohen et al. Citation1997; Gommes et al. Citation1998), exerting considerable direct or indirect influences on coastal habitats, which add to more globally acting impacts, such as climate change. To warrant sustainable use of coastal ecosystems in the future, negative influences have to be monitored and, if necessary, reduced or modified towards long-term ecological integrity.
Goatfishes may to some extent be very useful indicators of human-induced habitat changes other than fisheries, including introduced non-native flora and fauna, pollution, artificial habitat construction, and coastal degradation (). For instance, the introduction of the non-native common bluestripe snapper, Lutjanus kasmira (Forsskål, 1775), in Hawaii has resulted in vertical habitat shift in yellowfin goatfish, Mulloidichthys vanicolensis, towards staying more in open water with increased height above the bottom reflecting asymmetrical competition (Schumacher & Parrish Citation2005). The accidental introduction of the tropical alga Caulerpa taxifolia (Vahl 1802) in the Mediterranean resulted in decreased abundance and foraging actvitiy of striped red mullet, Mullus surmuletus, with increased algal cover (Longepierre et al. Citation2005).
Human-made constructions, such as artificial reefs, may lead to increased visits by goatfishes of the respective area and enhance abundance in the immediate surroundings (Golani & Diamant Citation1999; Angel et al. Citation2002). Since the Suez canal opened in the 19th century, three goatfish species have immigrated into the Mediterranean from the Red Sea, being so-called Lessepsian migrants (Ben-Tuvia Citation1966; ). This had consequences for the native red mullet and striped red mullet in the southwestern Mediterranean, which were replaced by two Lessepsian migrants, the goldband goatfish, Upeneus moluccensis, and Por's goatfish, U. pori, at shallower depths (Golani Citation1994).
Goatfishes may not always reliably indicate human-induced habitat changes, such as sewage pollution (Guidetti et al. Citation2003) or there may be no clearly traceable effects, as concluded in a study of fish faunal changes due to the construction of a nuclear power plant (Jan et al. Citation2001; ).
Temperature and climate change
Water temperature is affected by both climate variation and hydrographical features, including horizontal or vertical movement of water masses. Generally, fishes may respond sensitively to rather minimal changes in water temperatures in various ways, including changes in growth rate, reproductive activity, or development and this is also exemplified by goatfishes ().
Of particular interest is the immigration of goatfishes into previously less frequented or uninhabited areas with increasing temperatures, resulting in increased abundance, fisheries landings, or distributional extension (). Striped red mullet has recently increased in abundance in the English Channel (Vaz et al. Citation2004) and the North Sea (ICES Citation2006), including the Norwegian exclusive economic zone (Nedreaas et al. Citation2006). In the North Sea it was not collected by the international bottom trawl surveys before 1988 and a continuous northwards distributional shift has been demonstrated since, with steadily increasing abundance in the southwestern areas (Beare et al. Citation2004, http://www.ices.dk/marineworld/fishmap/ices/). This change in distribution and abundance has happened during a phase with demonstrated temperature increase due to global climate change (McCarty et al. Citation2001; Hulme et al. Citation2002). Similar findings have recently been documented for several other fish species (Perry et al. Citation2005).
The northernmost occurrence of striped red mullet, Mullus surmuletus, along the Norwegian Sea coast at 60°N has been documented by material caught by local fishermen and deposited in the scientific collections of the Bergen Museum and the Institute of Marine Research (). The examination of species identity was based on available keys (e.g. Hureau Citation1986; Quero et al. Citation2003), additional morphometric and meristic characters, and comparative material (Uiblein, unpublished data). The very first record derives from the island of Stolmen, Austevoll township, in 1943, close to the end of a relatively warm period that lasted from 1920 until 1950 (Southward Citation1963, Citation1974; Mason Citation1976; Cushing Citation1982). From 1992 onwards, four additional specimens have been collected on various islands southwest of Bergen () coinciding with the second, currently ongoing warming period and the increase in abundance of this species in the eastern English Channel and the North Sea (). However, other factors than temperature need to be considered, too, as it would also be required for the recently observed immigration of the West African goatfish, Pseudupeneus prayensis, from the Atlantic into the Mediterranean (Mercader Citation2002).
Table VI. Striped red mullet, Mullus surmuletus, collected at 60°N, Norwegian Sea coast, Norway.
Goatfishes as key species
The term key species has been used in ecology to rule out those taxa that significantly contribute to the formation and sustaining of community structure and interaction among co-occurring species. The absence of key species would lead to a considerable decline in ecosystem coherence and integrity. Typical key species are those that control communities top-down as predators or bottom-up as important food or prey. Others are so-called ecosystem engineers that may either exert control directly by their simple presence (‘autochthonous ecosystem engineers’) or indirectly via other abiotic or biotic factors (‘allochthonous ecosystem engineers’). Classical examples for the first type are coral reefs and forests and for the second beavers and earthworms.
Among fishes, several groups have been considered to be allochthonous ecosystem engineers, such as the parrotfishes (Scaridae) that contribute significantly to the sedimentation of coral reefs (Rotjan & Lewis Citation2005) or the characins (Characidae) that process detritus in streams (Flecker Citation1996). Goatfishes have hitherto not been sufficiently considered. Due to their very active foraging behaviour with vigorous stirring up of sediments by their barbels and mouths (Randall Citation1967; Uiblein Citation1991; McCormick Citation1995; Krajewski et al. Citation2006; ), goatfishes may provide important ecosystem services, including resuspension and the formation of mixed-species foraging associations. These and additional characteristics of their resource use may render goatfishes essential components of food webs in sand-associated coastal ecosystems.
Resuspension
Many littoral hard bottoms undergo a continuous erosion process due to wave action and diverse mining or scraping organisms that contribute to sedimentation and the formation of sandy areas in the immediate surroundings. This is particularly evident on coral reefs, which are usually surrounded by sand habitats in the back- and fore-reef areas, as well as in reef canals, crevices and between reef patches. Corals feed themselves on microscopic food organisms that may, to a large extent, derive from currents transporting them towards the reefs, but there may also be a trophic link between sand bottoms and reef-forming corals, one possible mechanism being the looping back of nutrients from bottom sediments into the open water and surrounding areas by resuspension.
The resuspension of bottom sediments can be enhanced by currents or wave action, but also by distinct organisms. Recent evidence suggests that goatfishes are involved in resuspension (Yahel et al. Citation2002). For instance, each square metre of a reef site off Eilat, northern Red Sea, has been found to be subjected to, on average, 10 s h−1 resuspension activity by the Red Sea goatfish, Parupeneus forsskali, with plumes being formed up to 1 m above the bottom and being visible 1–2 min afterwards (Yahel et al. Citation2002). Apart from dislocation of a large amount of sediment during foraging, this should also contribute to nutrient cycling and transport, thus enriching the plankton. This may, however, also have the rather contrasting effect of damage and the clogging of filter feeders due to the increased abundance of relatively large, suspended detritus (Yahel et al. Citation2002). In both cases, very different but drastic effects on the overall filter feeding assemblage can be expected that would justify regarding goatfishes as allochthonous ecosystem engineers. There may also be important indirect effects on the sediment-dwelling fauna itself (Choat & Kingett Citation1982) and on other fish species that often follow goatfishes, thus forming mixed-species foraging associations.
Multi-species foraging associations
The formation of multi-species foraging associations (also called mixed-species, heterospecific or interspecific associations or shoals) may arise if food sources occur that can be shared with advantage. The stirring-up of sediments by goatfishes leads to the uplifting of formerly hidden detritus and other organic material into the water column. This activity attracts other species that follow goatfishes and feed on the newly available particles. Goatfishes themselves may profit, because foraging in larger groups reduces the predation risk. Heterogeneous shoaling may also facilitate access to defended resources by swamping the territories of egg-caring reef-dwellers, such as damselfishes (Fishelson et al. Citation1974).
Quite a number of studies have reported goatfishes being the primary agent of mixed-species formation, i.e. the nuclear species (see Sazima et al. Citation2006a; Lukoschek & McCormick Citation2002a and citations therein). One recent study in the tropical West Atlantic found that spotted goatfish, Pseudupeneus maculatus, was the nuclear fish that attracted the largest number of follower species among 27 observed reef fish species (Sazima et al. Citation2006b). Seventeen (68%) of the total of 25 follower species observed in this study were associated with spotted goatfish.
Multi-species feeding associations are not stable and may change significantly among different habitats, but also during life history. In a study of ontogenetic shifts in resource use in Red Sea goatfish, Uiblein (Citation1991) reported a size-/age-related change in foraging behaviour, habitat use, shoaling tendency and multi-species association. Intermediate size classes were more often found on hard substrates and were also more often associated with other species. One advantage for the goatfishes to form mixed-species flocks on a hard substrate would be to gain access to damselfish territories where they may dislodge fish eggs, a favourite food source during this life-history period (Wahbeh & Ajiad Citation1985; ).
Role in coastal food webs
Assemblage structure and interaction within an ecosystem can best be characterized and predicted by food web models that consider all possible trophic pathways (Polis & Winemiller Citation1995; Belgrano et al. Citation2005; de Ruiter et al. Citation2005). Food webs also allow the estimation of the number of indirect interactions between organisms of the same or different trophic levels and evaluation of the overall trophic ‘connectedness’ of a single species within an ecosystem. Although food web models may become rather complex constructions and may lead to uncertainty about causal relationships between distinct species, they are very valuable tools to obtain a measure on a species’ importance in an ecosystem. Species at intermediate trophic levels are often involved in a large number of interactions and may – depending on their own behavioural activity – also exert many important direct and indirect influences on other organisms in the same habitat.
Goatfishes have been relatively rarely considered in food web models, especially at the level of single species. This may derive from insufficient information on their feeding biology in the respective habitat, but may also reflect an underestimation of the overall importance of single goatfish species, populations, or even age classes in coastal ecosystems. One recent study, for instance, included the family Mullidae in a diagram on trophic relationships of estuarine fishes off south Portugal, although the investigation was based only on the diet selection of one species, the red mullet Mullus barbatus (Sá et al. Citation2006).
Because of the hitherto known species-specific differences in goatfish foraging behaviour and diet selection [McCormick Citation1995; Platell et al. Citation1998; Nakamura et al. Citation2003; Krajewski et al. Citation2006; see also Labropoulou & Eleftheriou (Citation1997) and Aguirre & Sánchez (Citation2005) for the often co-occurring red mullet and striped red mullet], it may be preferable to include only those species that have been thoroughly studied in food web models. Apart from species-specific differences, whether goatfishes also undergo ontogenetic shifts in foraging behaviour, diet, and habitat selection during early (McCormick & Molony Citation1992; McCormick Citation1995) as well as later life history (Wahbeh & Ajiad Citation1985; Uiblein Citation1991; Labropoulou et al. Citation1997; Lukoschek & McCormick Citation2002b; Nakamura & Sano Citation2003) should also be considered in food web models.
There are also more interactions than just those between goatfishes and their prey that deserve consideration in food web models, such as interactions with predators (McCormick & Kerrigan Citation1996; Cruz-Escalona et al. Citation2005), cleaners (Sazima et al. Citation1999), competitors for food or space (Schumacher & Parrish Citation2005), territory holders (Alwany et al. Citation2005), or followers (see previous section). Prey may also profit indirectly from foraging goatfishes due to sediment manipulation (Choat & Kingett Citation1982) and resuspension (see previous section). Moreover, as some goatfish species are more active at night than during the day (Hobson Citation1974), nocturnal interactions should also be considered.
Diet selection may vary significantly among goatfish populations leading to variation in their trophic levels among habitats (Stergiou & Karpouzi Citation2002). Hence, it will be preferable to include diet studies on goatfishes in all habitats where food web models will be established, and from different seasons (Caragitsou & Tsimenides Citation1982). Of particular interest in this respect would be to also consider the effect of goatfishes on ecosystems they invade as non-native species (Golani Citation1994) and fishing pressure (Badalamenti et al. Citation2002; Pinnegar et al. Citation2003). This would also contribute to the integration of human-induced ecosystem changes in food web models.
There are obviously many possibilities still left open in goatfish ecology to better understand their role in ecosystems. At the same time, there is also a pressing need to further advance with systematic and taxonomic studies of this family.
Goatfish systematics and taxonomy
Detailed morphological studies of an organism group are the prerequisite for understanding systematics, ecology and diversity. Still today, most species are described based on morphological characters, although genetics is becoming increasingly important. Knowledge of the shape, structure, and relative size of external and internal body characters facilitates the interpretation of a species’ capability to adapt to distinct environmental conditions. Behavioural studies build firmly on morphological characters that allow an animal to sense the environment, move, feed, rest or interact. Species differences in morphology have clear consequences for niche partitioning as have differences among different life-history stages of the same species. In addition, populations or even co-occurring individuals of the same size may differ morphologically from each other. In recent years, genetics has been employed to study the evolutionary background of morphological differentiation and species formation. This also applies to the goatfishes.
The goatfishes are a family characterized by their conspicuous barbels that differ clearly from similar organs of other fish groups (Kim et al. Citation2001). Barbels have been found to vary considerably in structure, size, and sensory equipment (e.g. Gosline Citation1984; Uiblein et al. Citation1998; Lombarte & Aguirre Citation1997). However, many other morphological traits of goatfishes, such as body size, coloration, head form, otolith form, or the number of countable characters, such as gillrakers, fin rays, or vertebrae, may vary interspecifically (e.g. Lachner Citation1954; Thomas Citation1969; Labropoulou & Eleftheriou Citation1997; Platell et al. Citation1998; Uiblein et al. Citation1998; Aguirre & Lombarte Citation1999; Kim Citation2002; Randall Citation2004) or intraspecifically (e.g. Fage Citation1909; Rosenblatt & Hoese Citation1968; Aguirre Citation1997; McCormick Citation1993, Citation1995; Mamuris et al. Citation1998; Uiblein et al. Citation1998; Mahé et al. Citation2005; Pothin et al. Citation2006; Sabatini Citation2007).
Currently, 66 species of goatfishes are known and in the last 7 years, seven new goatfish species have been described (). Some genera have been proven to be particularly specious, the most diverse being Parupeneus, which consists of 27 species (Randall Citation2004) followed by Upeneus with 23 species. Some species of both genera have a rather restricted occurrence, such as Parupeneus posteli and Upeneus mascareinsis, which are endemic to Reunion Island (Letourneur et al. Citation2004). No recent revisions of Upeneus and the other less specious genera exist. The status of an additional species from the Eastern Pacific, Mulloidichthys xanthogrammus (Gilbert, 1892), is unclear (Byung-Jik Kim, pers. comm.) and, hence, it was not included in the present list (). From future revisions, more detailed systematic information can be obtained and from further exploration of remote areas, like isolated islands, new discoveries of goatfish species can be expected.
All descriptions of goatfish species so far have been based exclusively on morphological data. Genetic studies using various methods have largely confirmed the conclusions from ‘classical’ systematics (e.g. Shaklee et al. Citation1982; Stepien et al. Citation1994; Golani & Ritte Citation1999; Mamuris et al. Citation1999; Apostolidis et al. Citation2001). In some cases, morphological variation may be higher than differentiation found at the genetic level (Stepien et al. Citation1994). Even among populations from neighbouring or close-by habitats considerable morphological variation exists (Mamuris et al. Citation1998; Uiblein et al. Citation1998), which may, to some extent, reflect phenotypic plasticity.
Much more information may still be hidden behind morphological differentiation, as recently discussed by Nielsen (Citation2000) based on a specimen of Mullus from the Skagerrak that shows a head shape intermediate between red mullet, M. barbatus, and striped red mullet, M. surmuletus. A similar observation was previously reported by Fage (Citation1909), who distinguished a southern and a northern form of striped red mullet based mainly on head shape. There have also been problems correctly identifying Mullus species during regular bottom trawls in the North Sea (e.g. ICES Citation2007). Additional confusion may arise, too, from the partly ongoing use of the common name ‘red mullet’ for both species. Recently, a detailed comparison of Mullus specimens from the North Sea with material of M. barbatus and M. surmuletus from other areas including the two subspecies of red mullet, M. barbatus barbatus Linneus, 1758 and M. b. ponticus Essipov, 1927, has been started as part of an intended revision of the genus (Byung-Jik Kim & Franz Uiblein).
Conclusions
Many knowledge gaps still exist in goatfish ecology and systematics. However, the currently available data suggest that goatfishes may indeed be suitable habitat indicators and may also qualify as key species in coastal sand-associated ecosystems. Because of considerable inter- and intraspecific variations in habitat preferences, food selection, behaviour, and body structure, special attention should be paid to treat species, populations, and size classes separately from each other. Because not all goatfish species are equally well known and even some new ones may be encountered, exploration, monitoring, and management focusing on this group should be co-ordinated worldwide, thus enhancing information exchange and initiating joint research efforts in goatfish ecology and systematics. At the same time, this study may also serve as a model for screening other organism groups for their potential as ecosystem indicators. Editorial responsibility: Tom Fenchel
smar_a_268578_sup_20840369.avi
Download Microsoft Video (AVI) (3.4 MB)goatfish_food_search_video.avi
Download Microsoft Video (AVI) (3.4 MB)Acknowledgements
The author wishes to thank Keiichi Sato, Okinawa Churaumi Aquarium, Japan, for making a research stay in Japan and experimental work on goatfish foraging behaviour possible. Thanks to Kent Carpenter, Old Dominion University, Norfolk, USA, Byung-Jik Kim, Cheju National University, South Korea, Jørgen G. Nielsen, Zoological Museum, Copenhagen, Denmark, and ‘Jack’ E. Randall, Bishop Museum, Honolulu, Hawaii, USA, for fruitful discussions and advice on goatfish taxonomy, systematics, and related topics. Thanks are also due to Ingvar Byrkjedal and Gunnar Langhelle at Bergen Museum for the loan of material. Two anonymous referees are gratefully acknowledged.
References
- Aguirre , H . 1997 . Presence of dentition in the premaxilla of juvenile Mullus barbatus and M. surmuletus . Journal of Fish Biology , 51 : 1186 – 91 .
- Aguirre , H and Lombarte , A . 1999 . Ecomorphological comparisons of sagittae in Mullus barbatus and M. surmuletus . Journal of Fish Biology , 55 : 105 – 14 .
- Aguirre , H and Sánchez , P . 2005 . Feeding resource partitioning between Mullus barbatus and M. surmuletus in the Catlan Sea (northwestern Mediterranean) . Ciencias Marinas , 31 : 429 – 39 .
- Al-Rousan , SA , Rasheed , MY , Khalaf , MA and Badran , MI . 2005 . Ecological and geochemical characteristics of bottom habitats at the northern Jordanian coast of the Gulf of Aqaba . Chemistry and Ecology , 21 : 227 – 39 .
- Alwany , M , Thaler , E and Stachowitsch , M . 2005 . Territorial behaviour of Acanthurus sohal and Plectroglyphidodon laucozona on the fringing Egyptian Red Sea reefs . Environmental Biology of Fishes , 72 : 321 – 34 .
- Angel , DL , Eden , N , Breitstein , S , Yurman , A , Katz , T and Spanier , E . 2002 . In situ biofiltration: a means to limit the dispersal of effluents from marine finfish cage aquaculture . Hydrobiologia , 469 : 1 – 10 .
- Apostolidis , AP , Mamuris , Z and Triantaphyllidis , C . 2001 . Phylogenetic relationships among four species of Mullidae (Perciformes) inferred from DNA sequences of mitochondrial cytochrome b and 16S rRNA genes . Biochemical Systematics and Ecology , 29 : 901 – 9 .
- Badalamenti , F , Anna , GD , Pinnegar , JK and Polunin , NVC . 2002 . Size-related trophodynamic changes in three target fish species recovering from intensive trawling . Marine Biology , 141 : 561 – 70 .
- Beare , DJ , Burns , F , Peach , K and Reid , DG . 2004 . Red mullet migration into the northern North Sea during late winter . Journal of Sea Research , 53 : 205 – 12 .
- Belgrano A , Scharler U , Dunne J , Ulanowicz B 2005 . Aquatic Food Webs: an Ecosystem Approach . Oxford : Oxford University Press .
- Ben-Tuvia , A . 1966 . Red Sea fishes recently found in the Mediterranean . Copeia , 1966 : 254 – 75 .
- Caddy , JF . 1993 . Some future perspectives for assessment and management of Mediterranean fisheries . Scientia Marina , 57 : 121 – 30 .
- Caragitsou , E and Tsimenidis , N . 1982 . Seasonal changes and comparative analysis of the food of the red mullet (Mullus barbatus) in the Gulf of Saronikos and Thermaikos . Thalassographica , 5 : 41 – 61 .
- Choat , JH and Kingett , PD . 1982 . The influence of fish predation on the abundance cycles of an algal turf invertebrate fauna . Oecologia , 54 : 88 – 95 .
- Çinar , ME , Bilecenoglu , M , Öztürk , B and Can , A . 2006 . New records of alien species on the Levantine coast of Turkey . Aquatic Invasions , 1 : 84 – 90 .
- Claudet , J , Pelletier , D , Jouvenel , J-Y , Bachet , F and Galzin , R . 2006 . Assessing the effects of marine protected areas (MPA) on a reef fish assemblage in a northwestern Mediterranean marine reserve: identifying community-based indicators . Biological Conservation , 130 : 349 – 69 .
- Claydon , J . 2004 . Spawning aggregations of coral reef fishes: characteristics hypotheses, threats and management . Oceanography and Marine Biology: An Annual Review , 42 : 265 – 302 .
- Cohen , JF , Small , C , Mellinger , A , Gallup , J and Sachs , J . 1997 . Estimates of coastal populations . Science , 278 : 1211 – 12 .
- Colin , PL . 1996 . Longevity of some coral reef fish spawning aggregations . Copeia , 1996 : 189 – 92 .
- Colin , PL and Clavijo , IE . 1978 . Mass spawning by the spotted goatfish, Pseudopeneus maculatus (Bloch) (Pisces: Mullidae) . Bulletin of Marine Sciences , 28 : 780 – 2 .
- Cruz-Escalona , VH , Peterson , MS , Campos-Dávila , L and Zetina-Rejón , M . 2005 . Feeding habits and trophic morphology of inshore lizardfish (Synodus foetens) on the central continental shelf off Veracruz, Gulf of Mexico . Journal of Applied Ichthyology , 21 : 525 – 30 .
- Cushing , DH . 1982 . Climate and Fisheries , London : Academic Press .
- De Ruiter PC , Wolters V , Moore JC 2005 . Dynamic Food Webs . San Diego : Academic Press .
- Deudero , S . 2002 . Unexpected large numbers of Mullus surmuletus juveniles in open waters of the Mediterranean sampled with light attraction devices . Journal of Fish Biology , 61 : 1639 – 42 .
- Dorenbosch , M , Grol , MGG , Christianen , MJA , Nagelkerken , I and van der Velde , G . 2005 . Indo-Pacific seagrass beds and mangroves contribute to fish density and diversity on adjacent coral reefs . Marine Ecology Progress Series , 302 : 63 – 7 .
- Dorenbosch , M , Grol , MGG , Nagelkerken , I and van der Velde , G . 2006 . Different surrounding landscapes may result in different fish assemblages in East African seagrass beds . Hydrobiologia , 563 : 45 – 60 .
- Dufour , V , Jouvenel , J-V and Galzin , R . 1995 . Study of a Mediterranean reef fish assemblage. Comparisons of population distributions between depths in protected and unprotected areas over one decade . Aquatic Living Resources , 8 : 17 – 25 .
- European Commission . 2005 . Dissemination of the Results of Biological Studies 1997–2000 . Studies and Support Services Related to the Common Fisheries Policy . Luxembourg : Office for Official Publications of the European Communities, XXXI .
- Fage , L . 1909 . Étude de la variation chez le rouget (Mullus barbatus L.M. surmuletus L.) . Archives de Zoologie Expérimentale et Générale , 5 : 389 – 445 .
- Fishelson , L , Popper , D and Avidor , A. 1974 . Biosociology and ecology of pomacentrid fishes around the Sinai Peninsula (northern Red Sea) . Journal of Fish Biology , 6 : 119 – 33 .
- Flecker , AS . 1996 . Ecosystem engineering by a dominant detritivore in a diverse tropical stream . Ecology , 77 : 1845 – 54 .
- Friedlander , AM and DeMartini , EE. 2002 . Contrasts in density, size, and biomass of reef fishes between the northwestern and the main Hawaiian islands: the effects of fishing down apex predators . Marine Ecology Progress Series , 230 : 253 – 64 .
- Froese R , Pauly D 2007 . FishBase . www.fishbase.org , July 2007 .
- Garcia-Rubies , A and Macpherson , E . 1995 . Substrate use and temporal pattern of recruitment in juvenile fishes of the Mediterranean littoral . Marine Biology , 124 : 35 – 42 .
- Garpe , KC and Öhman , MC . 2003 . Coral and fish distribution patterns in Mafia Island Marine Park, Tanzania: fish–habitat interactions . Hydrobiologia , 498 : 191 – 211 .
- Golani , D . 1994 . Niche separation between colonizing and indigenous goatfish (Mullidae) along the Mediterranean coast of Israel . Journal of Fish Biology , 45 : 503 – 13 .
- Golani , D . 2001 . Upeneus davidaromi, a new deepwater goatfish (Osteichthyes, Mullidae) from the Red Sea . Israel Journal of Zoology , 47 : 111 – 21 .
- Golani , D and Diamant , A . 1999 . Fish colonization of an artificial reef in the Gulf of Elat, northern Red Sea . Environmental Biology of Fishes , 54 : 275 – 82 .
- Golani , D and Ritte , U . 1999 . Genetic relationships in goatfishes (Mullidae: Perciformes) of the Red Sea and the Mediterranean, with remarks on Suez Canal migrants . Scientia Marina , 63 : 129 – 35 .
- Gommes R , du Guerny J , Nachtergaele F , Brinkman R. 1998 . Potential Impacts of Sea-level Rise on Populations and Agriculture . Rome : FAO, Sustainable Development Department ( http://www.fao.org/sd/eidirect/EIre0045.htm ).
- Goren , M and Galil , BS . 2005 . A review of changes in the fish assemblages of Levantine inland and marine ecosystems following the introduction of non-native fishes . Journal of Applied Ichthyology , 21 : 364 – 70 .
- Gosline , WA . 1984 . Structure, function, and ecology in the goatfishes (family Mullidae) . Pacific Science , 38 : 312 – 23 .
- Gullström M , Dahlberg M. 2004 . Fish community structure of seagrass meadows around Inhaca Island, southern Mozambique . Minor Field Study 106. Uppsala University.
- Guidetti , P , Terlizzi , A , Fraschetti , S and Boero , F . 2003 . Changes in Mediterranean rocky-reef assemblages exposed to sewage pollution . Marine Ecology Progress Series , 253 : 269 – 78 .
- Hart PJB , Reynolds JD 2002 . Handbook of Fish Biology and Fisheries , Vols 1 and 2 . Maiden, MA : Blackwell Publishing .
- Hernandez , FJ , Shaw , RF , Cope , JS , Ditty , JG , Farooqi , T and Benfield , MC . 2003 . The across-shelf larval, postlarval, and juvenile fish assemblages collected at offshore oil and gas platforms of the Mississipi River Delta . American Fisheries Society Symposium , 36 : 39 – 72 .
- Hobson , ES . 1974 . Feeding relationships of teleostean fishes on coral reefs in Kona, Hawaii . Fishery Bulletin , 72 : 915 – 1031 .
- Holland , KN , Peterson , JD , Lowe , CG and Wetherbee , BM . 1993 . Movements, distribution and growth rates of the white goatfish Mulloides flavolineatus in a fisheries conservations zone . Bulletin of Marine Science , 52 : 982 – 92 .
- Horn , MH , Martin , KLM and Chotkowski , MA . 1999 . Intertidal Fishes: Life in Two Worlds , San Diego : Academic Press .
- Hulme M , Jenkins GJ , Lu X , Turnpenny JR , Mitchell TD , Jones RG , et al. . 2002 . Climate Change Scenarios for the United Kingdom: The UKCIP02 Scientific Report . Tyndall Centre for Climate Change Research, School of Environmental Sciences, University of East Anglia.
- Hureau J-C. 1986 . Mullidae. 1986 . In: Whitehead PJP , Bauchot M-L , Hureau J-C , Nielsen J , Tortonese E Fishes of the North-eastern Atlantic and the Mediterranean , Vol. 2 . Paris : UNESCO . p 877 – 82 .
- ICES . 2006 . Report of the ICES Advisory Committee on Fishery Management, Advisory Committee on the Marine Environment and Advisory Committee on Ecosystems, 2006. ICES Advice.
- ICES . 2007 . Report of the International Bottom Trawl Survey Working Group (IBTSWG) , 27–30 March 2007, Sète, France. ICES Document CM 2007/RMC:05.
- Jan , RQ , Chen , JP , Lin , CY and Shao , KT. 2001 . Long-term monitoring of the coral reef fish communities around a nuclear power plant . Aquatic Ecology , 35 : 233 – 43 .
- Katselis , G , Koutsikopoulos , C , Dimitriou , E and Rogdakis , Y . 2003 . Spatial patterns and temporal trends in the fishery landings of the Messolonghi-Etoliko lagoon system (western Greek coast) . Scientia Marina , 67 : 501 – 11 .
- Kim , B-J . 2002 . Comparative anatomy and phylogeny of the family Mullidae (Teleostei: Perciformes) . Memoirs of the Graduate School of Fisheries Sciences, Hokkaido University , 49 : 1 – 74 .
- Kim , B-J , Yabe , M and Nakaya , K . 2001 . Barbels and related muscles in Mullidae (Perciformes) and Polymixiidae (Polymixiiformes) . Ichthyological Research , 48 : 409 – 13 .
- Krajewski , JP and Bonaldo , RM . 2006 . Plankton-picking by the goatfish Pseudupeneus maculatus (Mullidae), a specialized bottom forager . Journal of Fish Biology , 68 : 925 – 30 .
- Krajewski , JP , Bonaldo , RM , Sazima , C and Sazima , I . 2006 . Foraging activity and behaviour of two goatfish species (Perciformes: Mullidae) at Fernando de Noronha Archipelago, tropical West Atlantic . Environmental Biology of Fishes , 77 : 1 – 8 .
- Labropoulou , M and Eleftheriou , A . 1997 . The foraging ecology of two pairs of congeneric demersal species: importance of morphological characteristics in prey selection . Journal of Fish Biology , 50 : 324 – 40 .
- Labropoulou , M , Machias , A , Tsimenides , N and Eleftheriou , A . 1997 . Feeding habits and ontogenetic diet shift of the striped red mullet, Mullus surmuletus Linnaeus, 1758 . Fisheries Research , 31 : 257 – 67 .
- Labropoulou , M and Papacontantinou , C . 2004 . Community structure and diversity of demersal fish assemblages: the role of fishery . Scientia Marina , 68 ( Suppl.1 ) : 215 – 26 .
- Lachner , EA . 1954 . A revision of the goatfish genus Upeneus with descriptions of two new species . Proceedings of the United States National Museum , 103 : 497 – 532 .
- Letourneur , Y , Chabanet , P , Durville , P , Taquet , M , Teisseir , E Parmentier , M . 2004 . An updated checklist of the marine fish fauna of reunion Island, south-western Indian Ocean . Cybium , 28 : 199 – 216 .
- Levi , D , Andreoli , MG , Bonanno , A , Fiorentino , F , Garofalo , G Mazzola , S . 2003 . Embedding sea surface temperature anomalies into the stock recruitment relationship of red mullet (Mullus barbatus L. 1758) . Scientia Marina , 67 ( Supp.1 ) : 259 – 68 .
- Lipej , L , Orlando Bonaca , M and Šiško , M . 2003 . Coastal fish diversity in three marine protected areas and one unprotected area in the Gulf of Trieste (Northern Adriatic) . Marine Ecology , 24 : 259 – 73 .
- Lobel , PS . 1978 . Diel, lunar, and seasonal periodicity in the reproductive behavior of the pomacanthid fish, Centropyge potteri, and some other reef fishes in Hawaii . Pacific Science , 32 : 193 – 207 .
- Lombarte , A and Aguirre , H . 1997 . Quantitative differences in the chemoreceptor systems in the barbels of two species of Mullidae (Mullus surmuletus and M. barbatus) . Marine Ecology Progress Series , 150 : 57 – 64 .
- Lombarte , A , Recasens , L , González , M and de Sola , LG . 2000 . Spatial segregation of two species of Mullidae (Mullus surmuletus and M. barbatus) in relation to habitat . Marine Ecology Progress Series , 206 : 239 – 49 .
- Longepierre , S , Robert , A , Levi , F and Francour , P . 2005 . How an invasive alga species (Caulerpa taxifolia) induces changes in foraging strategies of the benthivorous fish Mullus surmuletus in coastal Mediterranean ecosystems . Biodiversity and Conservation , 14 : 365 – 76 .
- Lukoschek , V and McCormick , MI . 2002a . A review of multi-species foraging associations in fishes and their ecological significance . Proceedings of the 9th International Coral Reef Symposium , 1 : 467 – 74 .
- Lukoschek , V and McCormick , MI . 2002b . Ontogeny of diet changes in a tropical benthic carnivorous fish, Parupeneus barberinus (Mullidae): relationship between foraging behaviour, habitat use, and prey selection . Marine Biology , 138 : 1099 – 113 .
- Machias , A and Labropoulou , M . 2002 . Intra-specific variation in resource use by red mullet, Mullus barbatus . Estuarine, Coastal and Shelf Science , 55 : 565 – 78 .
- Mahé K , Destombes A , Coppin F , Koubbi P , Vaz S , Le Roy D , Carpentier A. 2005 . Le rouget barbet de roche Mullus surmuletus (L. 1758) en Manche orientale et mer du Nord . Rapport de Contrat IFREMER/CRPMEM Nord-Pas-de-Calais.
- Mamuris , Z , Apostolidis , AP , Panagiotaki , P , Theodorou , AJ and Triantaphyllidis , C . 1998 . Morphological variation between red mullet populations in Greece . Journal of Fish Biology , 52 : 107 – 17 .
- Mamuris , Z , Stamatis , C , Bani , M and Triantaphyllidis , C . 1999 . Taxonomic relationships between four species of the Mullidae family revealed by three genetic methods: allozymes, random amplified polymorphic DNA and mitochondria DNA . Journal of Fish Biology , 55 : 572 – 87 .
- Maravelias , CD and Papaconstantinou , C . 2006 . Geographic, seasonal and bathymetric distribution of demersal fish species in the eastern Mediterranean . Journal of Applied Ichthyology , 22 : 35 – 42 .
- Mason , BJ . 1976 . Towards the understanding and prediction of climate variations . Quarterly Journal of the Royal Meteorological Society , 102 : 473 – 98 .
- McCarty , JJ , Canziani , OF , Leary , NA , Dokken , DJ and White , KS . 2001 . Climate Change 2001: Impacts, Adaptation, and Vulnerability , Cambridge : Cambridge University Press .
- McCormick , MI . 1993 . Development and changes at settlement in the barbel structure of the reef fish, Upeneus tragula (Mullidae) . Environmental Biology of Fishes , 37 : 269 – 82 .
- McCormick , MI . 1995 . Fish feeding on mobile benthic invertebrates: influence of spatial variability in habitat associations . Marine Biology , 121 : 627 – 37 .
- McCormick , MI and Kerrigan , BA . 1996 . Predation and its influence on the condition of a newly settled tropical demersal fish . Marine and Freshwater Research , 47 : 557 – 62 .
- McCormick , MI and Milicich , MJ . 1993 . Late pelagic-stage goatfishes: distribution patterns and inferences on schooling behaviour . Journal of Experimental Marine Biology and Ecology , 174 : 15 – 42 .
- McCormick , MI and Molony , BW . 1992 . Effects of feeding history on the growth characteristics of a reef fish at settlement . Marine Biology , 114 : 165 – 73 .
- McCormick , MI and Molony , BW . 1995 . Influence of water temperature during the larval stage on size, age and body condition of a tropical reef fish at settlement . Marine Ecology Progress Series , 118 : 59 – 68 .
- Mercader , L . 2002 . Premiére capture de Pseudupeneus prayensis (Mullidae) en Mer Catalane . Cybium , 26 : 235 – 6 .
- Meyer , CG , Holland , KN , Wetherbee , BM and Lowe , CG . 2000 . Movement patterns, habitat utilization, home range size and site fidelity of whitesaddle goatfish, Parupeneus porphyreus, in a marine reserve . Environmental Biology of Fishes , 59 : 235 – 42 .
- Muhando CA , Mndeme YE , Kamukuru AT. 1998 . Mnazi Bay-Ruvuma estuary proposed marine park: Environmental Assessment Report . Commissioned and sponsored by IUCN and World Bank.
- Nakamura , Y , Horinouchi , M , Nakai , T and Sano , M . 2003 . Food habits of fishes in a seagrass bed on a fringing coral reef at Iriomote Island, southern Japan . Ichthyological Research , 50 : 15 – 22 .
- Nakamura , Y and Sano , M . 2003 . Comparison between community structures of fishes in Enhalus acoroides- and Thalassia hemprichii-dominated seagrass beds on fringing coral reefs in the Ryukyu Islands, Japan . Ichthyological Research , 51 : 38 – 45 .
- Nakamura , Y and Sano , M . 2004 . Overlaps in habitat use of fishes between a seagrass bed and adjacent coral and sand areas at Amitori Bay, Iriomote Island, Japan: importance of the seagrass bed as juvenile habitat . Fisheries Science , 70 : 788 – 803 .
- Nedreaas , K , Hesthagen , T , Borgstrøm , R , Brabrand , Å , Byrkjedal , I Christiansen , JS . 2006 . “ Fisker – ‘Pisces’ ” . In Norsk Rødliste 2006 – 2006 Norwegian Red List. Artsdatabanken Edited by: Kålås , JA , Viken , Å and Bakken , T . 341 – 50 .
- Nicholls , P . 2002 . Determining impacts on marine ecosystems: the concept of key species . Water and Atmosphere , 10 : 22 – 3 .
- Nielsen , JG . 2000 . Mystisk mulle fra Skagen . Dyr i Natur of Museum , 2000 : 27
- Ordines , F , Moranta , J , Palmer , M , Lerycke , A , Suau , A Morales-Nin , B . 2005 . Variations in a shallow rocky reef fish community at different spatial scales in the western Mediterranean Sea . Marine Ecology Progress Series , 304 : 221 – 33 .
- Perry , AL , Low , PJ , Ellis , JR and Reynolds , JD . 2005 . Climate change and distribution shifts in marine fishes . Science , 308 : 1912 – 5 .
- Phillippart CJM 2007 . Impacts of Climate Change on the European Marine and Coastal Environment: Ecosystems Approach . Strasbourg : European Science Foundation, Marine Board .
- Pinnegar , JK , Polunin , NVC and Badalamenti , F . 2003 . Long-term changes in the trophic level of western Mediterranean fishery and aquaculture landings . Canadian Journal of Fish and Aquatic Sciences , 60 : 222 – 35 .
- Platell , ME , Potter , IC and Clarke , KR . 1998 . Do the habitats, mouth morphology and diets of the mullids Upeneichthys stotti and U. lineatus in coastal waters of south-western Australia differ? . Journal of Fish Biology , 52 : 398 – 418 .
- Polis GA , Winemiller KO 1995 . Food Webs . Integration of Patterns and Dynamics . London : Chapman and Hall .
- Pothin , K , Gonzales-Salas , C , Chabanet , P and Lecomte-Finiger , R . 2006 . Distinction between Mulloidichthys flavolineatus juveniles from Reunion Island and Muritius Island (south-west Indian Ocean) based on otolith morphometrics . Journal of Fish Biology , 69 : 38 – 53 .
- Quero , J-C , Porché , P and Vayne , J-J . 2003 . Guide des poissons de l'Atlantique européen , Paris : Delachaux et Niestlé .
- Randall , JE . 1967 . Food habits of reef fishes of the West Indies . Studies in Tropical Oceanography (Miami) , 5 : 665 – 847 .
- Randall , JE . 2004 . Revision of the goatfish genus Parupeneus (Perciformes: Mullidae), with descriptions of two new species . Indo-pacific Fishes , 36 : 1 – 64 .
- Randall , JE and Kulbicki , M . 2006 . A review of the goatfishes of the genus Upeneus (Perciformes: Mullidae) from New Caledonia and the Chesterfield Bank, with a new species, and four new records . Zoological Studies , 45 : 298 – 307 .
- Rosenblatt , RH and Hoese , DF . 1968 . Sexual dimorphism in the dentition of Pseudupeneus and its bearing on the generic classification of the Mullidae . Copeia , 1968 : 175 – 7 .
- Rotjan , RD and Lewis , SM . 2005 . Selective predation by parrotfishes on the reef coral Porites astreoides . Marine Ecology Progress Series , 305 : 193 – 201 .
- Sá , R , Bexiga , C , Veiga , P , Viera , L and Erzini , K . 2006 . Feeding ecology and trophic relationships of fish species in the lower Guadiana River Estuary and Castro Marim e Vill Real de Santo António salt marsh. Estuarine . Coastal and Shelf Science , 70 : 19 – 26 .
- Sabatini , A , Follesa , MC , Pendugui , AA , Pesci , P and Cau , A . 2007 . Morphological description and intraspecific variability of Mullus surmuletus (Teleostei, Mullidae) vertebral column . Italian Journal of Zoology , 74 : 1 – 5 .
- Sale , PF . 2006 . Coral Reef Fishes , London : Academic Press .
- Sazima , C , Krajewski , JP , Bonaldo , RM and Guimarães , PR Jr . 2006a . The goatfish Pseudupeneus maculatus and its follower fishes at an oceanic island in the tropical West Atlantic . Journal of Fish Biology , 69 : 883 – 91 .
- Sazima , C , Krajewski , JP , Bonaldo , RM and Sazima , I . 2006b . Nuclear-follower foraging associations of reef fishes and other animals at an oceanic island . Environmental Biology of Fishes , 78 : 1 – 11 .
- Sazima , I , Moura , RL and Sazima , C . 1999 . Cleaning activity of juvenile angelfish, Pomacanthus paru, on the reefs of the Abrolhos Archipelago, western South Atlantic . Environmental Biology of Fishes , 56 : 399 – 407 .
- Schumacher , BD and Parrish , JD . 2005 . Spatial relationships between an introduced snapper and native goatfishes on Hawaiian reefs . Biological Invasions , 7 : 925 – 33 .
- Shaklee , JB , Tamaru , CS and Waples , RS . 1982 . Speciation and evolution of marine fishes studied by the electrophoretic analysis of proteins . Pacific Science , 36 : 141 – 57 .
- Stepien , CA , Randall , JE and Rosenblatt , RH . 1994 . Genetic and morphological divergence of a circumtropical complex of goatfishes: Mulloidichthys vanicolensis, M. dentatus, and M. martinicus . Pacific Science , 48 : 44 – 56 .
- Stergiou , KI and Karpouzi , VS . 2002 . Feeding habits and trophic levels of Mediterranean fish . Reviews in Fish Biology and Fisheries , 11 : 217 – 54 .
- Shand , J . 1997 . Ontogenetic changes in retinal structure and visual acuity: a comparative study of coral-reef teleosts with differing post-settlement lifestyles . Environmental Biology of Fishes , 49 : 307 – 22 .
- Southward , AJ . 1963 . The distribution of some plankton animals in the English Channel and approaches. III. Theories about longterm biological changes including fish . Journal of the Marine Biological Association of the United Kingdom , 43 : 1 – 29 .
- Southward , AJ . 1974 . Changes in the plankton community in the western English channel . Nature , 259 : 180 – 1 .
- Thomas , PA . 1969 . Goatfishes (Mullidae) of the Indian seas . Marine Biological Association of India Memoirs , 3 : 1 – 174 .
- Thresher , RE . 1984 . Reproduction in Reef Fishes , Neptune City : TFH Publications .
- Tissot BN. 1998 . Changes in the marine habitat and biota of Pelekane Bay, Hawaii over a 20-year period . US Fish and Wildlife Service , Pacific Islands Office, , Honolulu, Hawai'i . Technical Report.
- Uiblein , F . 1991 . Ontogenetic shifts in resource use and shoaling tendency related to body size in Red Sea goatfish (Parupeneus forsskali, Mullidae) . Marine Ecology , 12 : 153 – 61 .
- Uiblein , F , Köhler , C and Tian , MC . 1998 . Quantitative examination of morphological variability among goatfishes of the genus Upeneus from the Malayan Province (Pisces: Perciformes: Mullidae) . Senckenbergiana Maritime , 28 : 123 – 32 .
- Vaz , S , Carpentier , A and Coppin , F . 2004 . Eastern English Channel fish community from 1988 to 2003 and its relation to the environment . ICES CM , 2004/K : 40
- Wahbeh , MJ and Ajiad , A . 1985 . The food and feeding habits of the goatfish Parupeneus barberinus (Lacepede) from Aqaba, Jordan . Journal of Fish Biology , 27 : 147 – 54 .
- Wantiez , L , Thollot , P and Kulbicki , M . 1997 . Effects of marine reserves on coral reef fish communities from five islands in New Caledonia . Coral Reefs , 16 : 215 – 24 .
- Williams , ID , Walsh , WJ , Miyasaka , A and Friedlander , AM . 2006 . Effects of rotational closure on coral reef fishes in Waikiki-Diamond Head Fishery Management Area, Oahu, Hawaii . Marine Ecology Progress Series , 310 : 139 – 49 .
- Yahel , R , Yahel , G and Genin , A . 2002 . Daily cycles of suspended sand at coral reefs: a biological control . Limnology and Oceanography , 47 : 1071 – 83 .