Abstract
The Pacific red alga Heterosiphonia japonica has dispersed rapidly along European shores. Due to the species’ high abundance in many habitats, an impact on species richness and composition of local macroalgal communities might be expected. Higher sea temperatures may also influence local macroalgal composition, by providing more favourable conditions for species requiring higher temperatures. Macroalgal composition at 22 sublittoral sites along the south-western coast of Norway investigated prior to the establishment of H. japonica (1994–1995) were reinvestigated in 2003–2004, using similar methods. The total number of species collected in the area was approximately the same in the present investigation as in the previous study. With regard to number and composition of species at each site, there were no significant differences between sites with high abundance of H. japonica and sites with low or no abundance. Similarity percentage analysis (SIMPER) showed that there were temporal changes in composition of the macroalgal communities, mainly caused by higher frequency of ‘southern species’ (species with a northern limit on our coast). There was a significant increase in the percentage share of such species in the reinvestigations. The temporal differences observed are most likely caused by several warm summers/autumns and mild winters since the first investigation, which may favour a higher abundance of ‘southern species’. Heterosiphonia japonica was the most important species contributing to temporal dissimilarity in the area. This paper concludes that H. japonica has caused no negative impact on regional algal species richness in the relatively short time span since its introduction.
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark.
Introduction
Introduced seaweed species with a high potential for recruitment, rapid growth and general high competitive abilities may become very abundant within their new habitat. They may supersede indigenous species, and reduce local biodiversity. A negative impact on native algal communities has been demonstrated for the introduced Sargassum muticum (Yendo) Fensholt (Phaeophyceae, Fucales) in Europe and at the northeast Pacific coast of the USA (Viejo Citation1997; Stæhr et al. Citation2000; Britton-Simmons Citation2004; Sánchez et al. Citation2005). The same is reported for Undaria pinnatifida (Harvey) Suringar (Phaeophyceae, Laminariales) in Argentina (Casas et al. Citation2004) and for the two chlorophytes Caulerpa taxifolia (Vahl) C. Agardh and Caulerpa racemosa (Forsskål) J. Agardh (Caulerpales) in the Mediterranean Sea (Verlaque & Fritayre Citation1994; Piazzi et al. Citation2001; Balata et al. Citation2004).
Recent climatic trends (Bindoff et al. Citation2007) show a warming period in the world oceans since 1960, and in particular in the last decade. Increased seawater temperatures are expected to influence species geographical distribution ranges and also richness and composition of coastal assemblages (Harley et al. Citation2006).
A pronounced increase of seawater temperature of the North Sea basin has taken place during the last 15 years (data from the Institute of Marine Research, Norway). During 1997, 2002 and 2006 the average sea surface temperatures in August were exceptionally high along the coasts of south-western Norway, between 3 and 4°C higher than normal (Husa et al. Citation2007). Periods with warmer surface water will most likely influence the distribution pattern and abundance of macroalgae that have their main area of distribution south of Norway. Sagarin et al. (Citation1999) investigated the intertidal communities during a long-term warming period and showed that the abundance of southern species increased dramatically when sea temperature rose. Schiel et al. (Citation2004) showed that habitat-building seaweeds decreased substantially in an area impacted by thermal wastewater from a plume. A recent study shows that several southern macroalgal species have extended their northern distribution limits along the Portuguese coast (Lima et al. Citation2007). Thermal disturbances of macroalgal communities may also facilitate the establishment of exotic species in the marine environment (Harley et al. 2007).
A new red alga in the family Dasyaceae was first reported from European shores in 1994 (Bárbara & Cremades Citation1996; Stegenga Citation1997; Bárbara Citation2003), but has been present in Bretagne since 1984 (Verlaque & Cabioch, personal communication). The first observation of the new species in Norway was on the west coast in 1996 (Lein Citation1999), and at present the species is distributed along the main part of the coast of southern Norway (Bjærke & Rueness Citation2004; Husa et al. Citation2004). The new species was tentatively called Dasysiphonia sp. when first observed in Europe (Stegenga Citation1997). Genetic studies have shown that the new species is conspecific with specimens from Korea identified as Heterosiphonia japonica (sensu Choi Citation2001) (Bjærke Citation2004). However, this species most likely does not belong to the genus Heterosiphonia and a taxonomic revision within the Dasyaceae is needed. While waiting for a clear taxonomic status of the species it will be referred to as ‘Heterosiphonia japonica’ in this paper.
The rapid dispersal of Heterosiphonia japonica to new countries along European coasts (e.g. Bárbara & Cremades Citation1996; Stegenga Citation1997; Lein Citation1999; Maggs & Stegenga Citation1999; Verlaque Citation2001; Bárbara Citation2003; Wallentinus Citation2003; Axelius & Karlsson Citation2004; Husa et al. Citation2004; Peña & Bárbara Citation2006) suggests exceptional spreading abilities of the species. Heterosiphonia japonica has been observed to be rather abundant at many sites in Spain and Norway (Bárbara Citation2003; Husa et al. Citation2004; Peña & Bárbara Citation2006) and in a short period of time it has become one of the most common species in semi-exposed to sheltered communities at the Norwegian west coast (Husa et al. Citation2004). This raises the question whether this species has the ability to out-compete other macroalgae and cause a decrease in local macroalgal diversity. In addition, it has shown wide tolerance ranges with regard to temperature and salinity (Bjærke & Rueness Citation2004), and has been observed to a depth of 42 m (Husa Citation2003), suggesting low light requirements for growth. Heterosiphonia japonica thus possesses many traits that are advantageous for establishing in a wide range of habitats.
In order to examine whether the combination of higher temperatures along the coast of Norway and the introduction of ‘H. japonica’ may have influenced local macroalgal communities, re-examinations of the algal composition at a number of sites along the south-western coast of Norway, which were formerly investigated during 1994–1995, has been carried out during 2003–2004. The first study was in the beginning of the period with higher temperatures of the coastal waters in Norway, and one to two years before the first recording of H. japonica in Norway. The investigation during 1994–1995 may therefore be regarded as a good baseline study for this purpose.
Material and methods
During August 2003 (Florø–Stadt area) and August 2004 (Solund area), macroalgae were collected at 22 sites previously investigated by Brattenborg (Citation1997) and Lein (unpublished data) in August 1994 (one site in 1995).
The study area
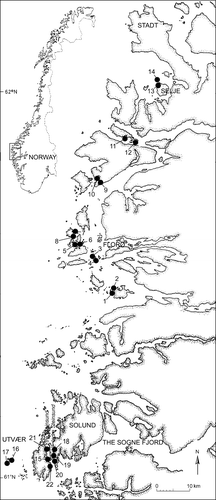
The sites are located on the southwest coast of Norway in the county of Sogn and Fjordane in two areas, the area from south of Florø to Stadt and the area west of Solund (). The distance between the areas is approximately 30 km. Fourteen sites were examined in the Florø–Stadt area and eight sites in Solund just north of the Sognefjord. The sites investigated in the Florø–Stadt area cover a distance of approximately 70 km along the coast, while the sites in the Solund area are more concentrated. The sites are situated in an open coastal area where archipelagos create protection from ocean waves. Mean sea surface winter temperature (1936–1989) measured at 1 m at the outlet of the Sognefjord and at Stadt (February) is 4.6 and 4.7°C, respectively, while corresponding summer temperatures (August) are 13.7 and 14.4°C. The subsequent salinities in the area range from 29.0 to 33.1 psu (Aure & Strand Citation2001).
Sampling
The sites were localized from coordinates, map descriptions and depth registrations from the previous studies. Benthic algal vegetation was collected through dredging with a triangular dredge (70×70×70 cm). In the Florø–Stadt area sample depths at sites 1, 3, 5, 7, 9, 11, 13 were 5–15 m (shallow interval group), while depths at sites 2, 4, 6, 8, 10, 12, 14 were 16–28 m (deep interval group). Sampling was done in the same way as during the first investigation of these sites, with three parallel dredgings in each depth interval (Brattenborg Citation1997). One deep and one shallow site in the Florø–Stadt area were paired (sites one and two, three and four, etc.) (). Only sites one and two were situated close to each other. Here, the dredging transects started at the same point and ended up approximately 40–50 m apart. The distance between the other of the paired sites varied between 250 m and 2 km. In the Solund area the two sites at Utvær were situated quite close to each other (40–50 m apart) (), while the distance between the other sites was always 1 km or more. The sites in the Solund area were not separated in depth groups. At three sites (15, 16 and 20) sample depths were 4–14 m, while sample depths at the rest of the sites were 8–30 m. During the first investigation in Solund (1994–1995), the samples were collected through one to three parallel dredgings at each site, while in the reinvestigations sampling was done through three parallel dredgings per site. The substrate at the sites was determined from the content of mud, sand, gravel or calcareous sand in the dredge. When the dredge contained little inorganic material and much kelp material, it was deduced that the substratum was rock.
The abundance of Heterosiphonia japonica at each site was recorded on a semi-quantitative scale where 0 = not found, 1 = rare (one or a few specimens), 2 = common (many specimens, estimated to <10% of the total algal biomass), 3 = plenty (estimated to > 10% of the total algal biomass). The abundance of larger species in Laminariales and Fucales was recorded in situ, and selected parts of their thalli with epiphytic algae were fixed in formalin (4% in seawater). The rest of the algal material was carefully sorted, and samples of different species and unidentified algae were fixed in formalin. The algal material collected at each site was examined systematically in the laboratory to identify taxa down to the lowest possible level. As crustose forms, maerl species and microscopic species <5–10 mm had not been systematically identified in the first investigations; these species were not identified in the late study and are not included in the analysis. The Porphyra and Cladophora species, except for easily recognizable Cladophora rupestris, were only identified to genus level and appears as ‘spp. groups’ in the analysis. The same applies to the species in the genus Aglaothamnion, as many specimens were not possible to identify to species level. Moreover, both Rhodomela and Ectocarpus species were analysed at genus level in the reinvestigation, as many specimens were atypical and difficult to identify to species level. The non-spiny, corticated Ceramium species were pooled in a Ceramium ‘rubrum’ group in the data analysis, while the rest of the Ceramium species were identified to species level, as in the previous studies. The kelp species, most likely present, were not noted from the Solund area in the first study and are not included in the analysis from Solund. Occasionally observations of species obviously belonging to the littoral zone in the samples are not included in the analysis. The species identified at each site were recorded as present, and no quantitative data for species abundance, except H. japonica, were obtained in this study. Nomenclature follows Hardy & Guiry (Citation2003) and newer taxonomic changes are given in additional references. A voucher specimen of each species found at each site is kept at The University of Bergen.
Data analyses
Multidimensional scaling ordination (MDS) (Shepard Citation1962; Kruskal Citation1964) was used to examine patterns in community structure in the two different studies (years). Similarity matrices based on the Bray–Curtis index (Bray & Curtis Citation1957) were used to construct the plots. A similarity percentage (SIMPER) analysis, with a ‘cut off’ for low contribution at 90%, was used to identify which species primarily contributed to the difference (between years) and similarity (within years) in macroalgal composition. Similarity analyses were performed using Primer software (Plymouth Routines In Multivariate Ecological Research 5.2.9, Plymouth, UK). The multidimensional scaling ordination and the SIMPER analyses were performed separately on the data from the two study areas. In the Florø–Stadt area, where two depth intervals were investigated (paired sites) in each sub area, the two depth groups were analysed separately.
Temporal differences in species number were tested on data from pooled sites in each area by non-parametric analysis (Mann–Whitney U test, Statistica 7.0, STAT SOFT) Temporal differences in the share of southern species were tested on Arcsin transformed data from pooled sites (Mann–Whitney U test, Statistica 7.0, STAT SOFT). The same analysis was performed on species number at sites with high or low abundance of H. japonica in 2003/2004 (all sites pooled).
Results
Macroalgal species richness
The sites were divided into two groups according to abundance of Heterosiphonia japonica, one with high (3 – plenty, 2 – common) and one with low (1 – rare, 0 – absent) abundance. There was no significant difference in species number between the two groups (Mann–Whitney U test, P=0.3).
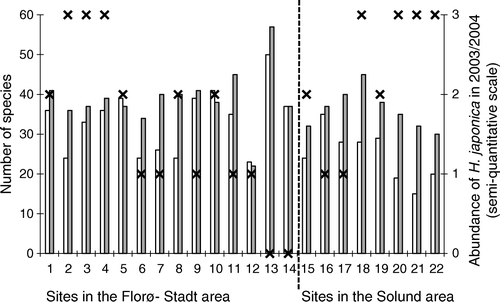
A total of 81 taxa were found in the shallow interval in the Florø–Stadt area in 2003 while 74 taxa were recorded there in 1994 (Appendix A). In the deep interval, the number of taxa recorded was almost unchanged: 65 taxa in 1994 and 68 taxa in 2003. Similarly, the number of taxa recorded in the reinvestigation in the Solund area was higher in 2004 (77 taxa) than in 1994 (65 taxa) (Appendix B). The species number recorded at each site was nearly significantly different in the Florø–Stadt area between years (Mann–Whitney U test, P=0.05) (), while a significantly higher number of species per site was recorded in the Solund area in 2004 than during the first investigation (Mann–Whitney U test, P<0.05) (). The lowest number of species in the reinvestigation was found at site 12 (Gangsøy) where loose-lying Phyllophora crispa (Hudson) Dixon dominated the algal community both in 1994 and in 2003. The highest number of species was found at the shallow exposed site 13 at the Selje Island south of Stadt. A new red alga for Norway was recorded in 2003 at Selje Island. Several fertile female and some non-fertile specimens of Haraldiophyllum bonnemaisonii (Kylin) Zinova were found in both the deep and shallow site (sites 13 and 14). This species is widely distributed at the west coast of the British Isles to Shetland, and must be considered to be a natural immigrant.
Figure 2. Macroalgal species richness represented by the presence of species at subtidal sites in Sogn and Fjordane investigated in 1994 and in 2003–2004. Sites 1–14 are situated in the Florø–Stadt area and sites 15–22 are situated in the Solund area. Open squares = 1994–1995; filled squares = 2003–2004. X = the abundance of Heterosiphonia japonica on sites in 2003–2004 recorded on a semi-quantitative scale where 0 = not found, 1 = rare (one or a few specimens), 2 = common (many specimens, estimated to <10% of the total algal biomass), 3 = plenty (estimated to > 10% of the total algal biomass).
Composition of macroalgal communities and temporal stability
Heterosiphonia japonica was found at the majority of sites in both areas in 2003/2004; it was only absent from the two exposed sites at the Selje Island (sites 13 and 14). The abundance of H. japonica was recorded as high (plenty) at seven sites, common at six sites and rare at seven sites (). The sites where H. japonica was common or plentiful are shown as large black circles in .
Figure 3. Two-dimensional MDS (non-parametric multi-dimensional scaling) ordination showing Bray–Curtis similarities for: A. Seven sites in the shallow interval (5–15 m) in the Florø–Stadt area investigated in 1994 and 2003. B. Seven sites in the deep interval (16–28 m) in the Florø–Stadt area investigated in 1994 and 2003. C. Eight sites in the Solund area investigated in 1994–1995 and 2004. Depth ranges at these sites were between 4 and 30 m. The last two numerals in the labels represent the year of sampling. The abundance of H. japonica is illustrated with a large black circle where the species was common or plentiful.
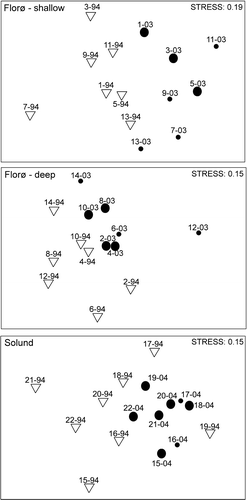
The MDS plot () of similarity between individual sites in the Florø–Stadt area shows the temporal differences in community structure (1994→2003). When comparing the similarity indexes (Bray–Curtis) from the shallow sites of 1994 and 2003 pairwise, six sites revealed relatively high temporal similarity (>60%) (), while site 7 shows the lowest temporal similarity. This site was relatively low in species number in 1994 (). A pairwise comparison of similarity indexes (Bray–Curtis) from the deep sites in the Florø–Stadt area shows that a very low temporal similarity occurred at site 12, which had a low species number in both investigations and represents a distinguished outlier in the plot. Separate SIMPER analyses of the two depth intervals in the Florø–Stadt area showed that the average temporal similarity was higher in the shallow interval (66.2%) than in the deep interval (60.1%). The macroalgal composition in the Solund area (MDS plot in B) shows higher temporal differences (1994→2004) between individual sites than in the Florø–Stadt area. A pairwise comparison of similarity indexes (Bray–Curtis) from individual sites shows that the temporal similarity between them barely exceeds 60% at two sites. Site 21 had the lowest temporal similarity (37.5%). In 2004, this site was dominated by high abundance of H. japonica and Chorda filum (L.) Stackhouse, which were not recorded here in 1994. The average temporal similarity in this area, determined by SIMPER analysis, was 51.5%, which is far lower than in the Florø–Stadt area.
Species contributing to similarity within areas
From 1994 to 2003–04 there was an increase in the number of species that accounted for 60% of the similarity between sites within the studied areas (). Some species have a temporal stability in their abundance; the most stable species being the exotic Bonnemaisonia hamifera Hariot. The highest number of temporal stable common species was found in the shallow interval in Florø–Stadt (). Some species were no longer among the most common in the reinvestigations, but all of them were still present at several sites in the later study. Polysiphonia fibrillosa (Dillwyn) Sprengel, which was one of the most common species in the shallow interval in the Florø–Stadt area in 1994, was only found at one of these sites in 2003. Heterosiphonia japonica was an important contributor in 2003–2004 to similarity between sites in all areas/depths (Florø–Stadt shallow interval: 2.73%, deep interval: 3.33%; Solund: 4.29%), and has in a short time become one of the most common species in both areas.
Table I. Species contributing to 60% of the similarity between sites in 1994/1995 and 2003/2004 determined by SIMPER analysis. Shallow interval = 5–15 m deep, deep interval = 16–28 m deep, all depths = 4–30 m deep.
Species contributing to dissimilarity between years
Heterosiphonia japonica was the most important species contributing to temporal differences in both areas, accounting for 2.94–3.14% of the observed dissimilarity ().
Table II. Average abundance of species contributing to approximately 30% of the net dissimilarity in macroalgal assemblages in the Florø-Stadt and Solund area between years (1994 and 2003/ 2004) determined by SIMPER analysis. Species marked: (S) = species with a southern distribution in Norway; (X) = species that occurs along the entire Norwegian coastline. Shallow interval = 5–15 m deep, deep interval = 16–28 m deep, all depths = 4–30 m deep. (AA= average abundance ranging on a scale from 0 to 1, where 1.00 means that the species is present at all sites.)
In the shallow interval of the Florø–Stadt area 15 species accounted for 30% of the observed dissimilarity between years (). Polysiphonia fibrillosa and Ectocarpus spp. were less frequently recorded here in the reinvestigation, while the remaining 13 species occurred more frequently. Five species which had a considerable increase in their average abundance in the shallow interval in 2003 were Rhodophyllis divaricata (Stackhouse) Papenfuss, Chylocladia verticillata (Lightfoot) Bliding, Acrothrix gracilis (Kylin), Neosiphonia harveyi Kim, Choi, Guiry & Saunders and Dictyota dichotoma (Hudson) Lamouroux.
In the deepest interval in this area, 14 species accounted for 30% of the dissimilarity between years (). Three of these species had a decline in abundance in 2003; the crustose form of Cutleria multifida (Smith) Greville (2n), Pterothamnion plumula (Ellis) Nägeli and Callithamnion corymbosum (Smith) Lyngbye. Three species had the same abundance in both studies but occurred on different sites, and the remaining species had an increased abundance in 2003. In the deepest interval, Chylocladia verticillata, Rhodophyllis divaricata, Brongniartella byssoides (Goodenough & Woodward) F. Schmitz and Sphacelaria caespitula Lyngbye were found much more often than in the former study. In the Solund area, 15 species were responsible for 30% of the dissimilarity between years (). Pterosiphonia parasitica (Hudson) Falkenberg was observed less frequently in this area, and the rest had a higher average abundance in the last investigation. Species with a remarkable higher average abundance in Solund in 2004 were: Bonnemaisonia asparagoides (Woodward) C. Agardh, Cystoclonium purpureum (Hudson) Batters, Rhodophyllis divaricata, Asperococcus bullosus Lamouroux, Phyllophora crispa and Spermatochnus paradoxus (Roth) Kützing.
Temporal temperature variations and abundance of ‘southern species’
A considerable number of the species contributing to temporal dissimilarity in algal communities were ‘southern species’ (species with a northern boundary at the Norwegian coast; Brattegaard & Holte Citation1997) which occurred more frequently in the reinvestigations (). Rhodophyllis divaricata was recorded for the first time at in the Florø–Stadt area in 1994, and was then present at three sites. In the later investigations the species was present at 12 sites. In Solund the species was present at one site in 1994 and at seven sites in 2004. The species was quite common at many sites in the reinvestigations. Chylocladia verticillata was only recorded at two sites in the shallow interval in Florø–Stadt in 1994, but was present at 10 sites in both depth intervals in 2003. Acrothrix gracilis: this species is considered rare in Norway and has only been found occasionally north to Troms. acrothrix gracilis was not recorded in either area in the previous investigations but was present at 10 sites, and quite common in many of them (personal observation), in the later investigations. Neosiphonia harveyi: an introduced red alga on the Norwegian coast (1983), common in the Oslofjord but with few records outside the Skagerrak area (Bjærke Citation2004). This species was not recorded in either of the areas in 1994, but was sparsely present at five sites in the Florø–Stadt area in 2003, mainly in the shallow subtidal interval. The exotic species Sargassum muticum (Yendo) Fensholt was only recorded at one site in the Solund area. Dictyota dichotoma was not recorded in the Florø area in 1994 but was present at four sites here in 2003. Brongniartella byssoides was recorded at three sites in the Florø–Stadt area in 1994, but was present in nine sites in 2003, and had also increased its frequency in the Solund area in 2004. The gamethophytic erect stage of Cutleria multifida was not recorded in either of the areas in 1994, but was present at 10 sites altogether in the reinvestigations and was quite common in many of them (persoanl observation). Nitophyllum punctatum (Stackhouse) Greville was not recorded in the Solund area in the previous investigation, but was observed for the first time at four sites in the Florø area in 1994. In the later investigations the species was present at 12 localities in total in both areas, and was quite abundant at many of those.
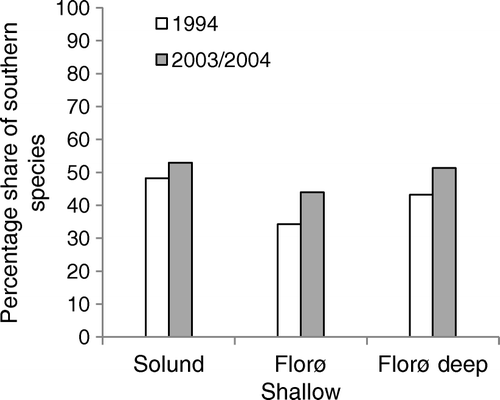
The increase in occurrence of ‘southern species’ was most pronounced in the Florø–Stadt area (). When pooling all sites the percentage shares of ‘southern species’ as a total of all recognized species, was significantly higher in the reinvestigations (Mann–Whitney U test, P=0.02).
Figure 4. The percentage share of species with a southern distribution on the Norwegian coast as a total of all species collected in the two studies (1994–2003/2004).
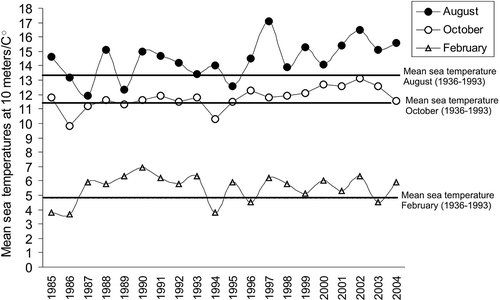
shows mean seawater temperatures during 1985–2004 at 10 m at the outlet of the Sognefjord (Temperature series from Institute of Marine Research, Norway). After 1995 all August measurements are above normal (mean temperature during 1936–1993). Also, the high sea temperatures persist during the autumn (October). The winter temperatures (February) in the year of the first investigations were below normal. Since 1994 only two measurements of mean temperature in February showed mean temperatures slightly below 5°C, and the rest were above normal ().
Figure 5. The mean seawater temperatures measured at 10 m at the outlet of the Sognefjord in August (warmest month), October and February (coldest month). Normal seawater temperatures during August, October and February (shown as vertical lines) are mean seawater temperature in the period 1936–1993 (Aure & Strand Citation2001).
Discussion
Almost 10 years after the first observation of Heterosiphonia japonica in Norway, the species was present at 20 of 22 of the reinvestigated sites. There was no evidence of a negative impact on macroalgal species richness of the introduced H. japonica in the present study. This result is in agreement with a number of other studies where the effects of introduced algae on local biodiversity have been investigated (e.g. Stæhr et al. Citation2000; Balata et al. Citation2004; Britton-Simmons Citation2004; Sánchez & Fernández Citation2005). However, several of these studies have shown an impact on indigenous algal species of an introduced alga, in terms of decreased abundances. For example, several studies have shown that Sargassum muticum can have a negative impact on abundance of other macroalgae (Stæhr et al. Citation2000; Britton-Simmons Citation2004; Sánchez et al. Citation2005). On the other hand, an experiment lasting two years in the intertidal of the same area and habitat showed only negligible effects of S. muticum on the native algae (Sánchez & Fernández Citation2005). It is possible that the time factor may be crucial in studying the impact of introduced species on native communities. Effects of introduced species on native communities should therefore be investigated on a long-term study basis.
Most of the introduced species, which have been shown to have an impact on native communities, are large (Verlaque & Fritayre Citation1994; Stæhr 2000; Balata et al. Citation2004; Britton-Simmons Citation2004; Casas et al. Citation2004; Farrell & Fletcher Citation2006; Sánchez et al. Citation2005) or form dense carpets like Caulerpa racemosa (Balata et al. Citation2004). The negative effects of large introduced algae on biodiversity or abundance of native algae may be, for example, due to shading of indigenous species (Britton-Simmons Citation2004) or monopolizing space (Levin et al. Citation2002). A positive effect of introduced macroalgae on biodiversity can be to provide substratum for epiphytes (Sánchez et al. Citation2005). Heterosiphonia japonica is a relatively small species, which also shows strong seasonality in size (Husa et al. Citation2004). It is very common as an epiphyte. As an epiphyte it may have a negative impact on host species, but with its small size it is unlikely to be able to compete with larger dominant species.
In the Solund area (stations 15–22) the reinvestigation showed a significant increase in species number at the sites. This may be partly due to a higher sampling effort in the reinvestigation, during which three parallel dredgings at each site were consequently carried out. The dredge samples the algal community haphazardly, and dredging repeatedly at the same site will increase the chances of collecting as many of the species present as possible. However, also in the Florø–Stadt area (stations 1–14), where the sampling effort was similar during the two investigations, several of the stations had a higher species number in the second investigation than in the first one. The increase in species numbers may therefore partly reflect a genuine increase in species richness. Macroalgal communities may vary temporarily and the abundance and presence of species may be strongly influenced by temporal variation in environmental factors such as temperature. For example, Forrest & Taylor (Citation2002) observed that the richness of macroalgal taxa showed a high year-to-year variation when studying the composition of communities in the Lyttelton Harbour (New Zealand) during three years.
Besides the two introduced species since 1994–1995 (H. japonica, Neosiphonia harveyi (Bailey) Kim, Choi, Guiry & Saunders), the main cause of the differences in the algal composition between the two investigations was a pronounced increase in abundance of ‘southern species’. Together with the temperature recordings this indicates that temporal variations in seawater temperatures have influenced the composition and diversity in the macroalgal communities. Several years of high summer and autumn temperatures, when the majority of temperate red algae have their fertile period (Kain Citation1989), might be beneficial for species in the northern part of their distribution area. The winter of 1994, when the first investigations took place, had lower temperatures than normal, and might have restricted populations of warm–temperate species. There are currently only a few studies showing changes in macroalgal composition due to higher seawater temperatures. Sagarin et al. (Citation1999) noted a remarkable decline in the cover of the dominant fucoid algae during a warming period. Hiscock et al. (Citation2004) predict that the northern distribution limits of species generally and gradually will shift northwards as temperature increases around Ireland and Britain. A long-term study performed in an area where the seawater temperatures were elevated by 3.5°C due to wastewater from a power plant showed no evidence of an increase in warm-water species (Schiel et al. Citation2004). Instead there was a decline in habitat-building key species, such as kelps, causing indirect effects on the algal community structure. Lima et al. (Citation2007) showed that the distribution range of several southern species had expanded significantly northwards in the warming period since 1970 at the Portuguese coast. The increased abundance of many southern species in this study is consistent with the predictions in Harley et al. (Citation2006); marine organisms living near their range boundaries are exhibited to thermal stress and their distribution and abundance may shift when the environmental conditions is more favourable.
The impact of introduced algae on native communities can be studied through experiments where the introduced alga is removed (e.g. Britton-Simmons Citation2004; Sánchez & Fernández Citation2005), by monitoring studies (e.g. Stæhr et al. Citation2000; Sánchez et al. Citation2005) or by comparing sites where the introduced species is established to sites where it is not present (e.g. Forrest & Taylor Citation2002). The removal studies normally only show changes on a small scale, while the ‘comparing sites’ method may fail to account for the differences in environmental conditions at the impact and control sites. The need for long-term ‘before–after’ studies in invasion ecology is emphasized by many authors (Forrest & Taylor Citation2002; Steinbeck et al. Citation2005; Strayer et al. Citation2006). However, the lack of pre-invasion baseline studies has unfortunately restricted this practice in the marine environment. The ‘before–after’ approach has been tried in New Zealand on the impact of the introduced Undaria pinnatifida on biodiversity (Forrest & Taylor Citation2002), but their study showed no significant influence of the invasive species. As suggested by the authors, this method also has its limitations, as it probably will miss the worst-case scenario; U. pinnatifida grew sparsely in the study sites, while dense populations were observed just outside the study area (Forrest & Taylor Citation2002). In the search for the most reliable method of accounting for the impact from introduced seaweeds, the present ‘before–after’ study shows that the temporal variations in environmental factors, especially summer/winter temperatures, may confound possible effects of an introduced species on biodiversity. Previous studies have shown that an introduced species might have a significant impact on the biomass or cover of native species, even though the diversity is not affected (Balata et al. Citation2004; Britton-Simmons Citation2004). Here, on a regional scale and at present, it is concluded that H. japonica has not yet had an impact on overall macroalgal species richness. However, it cannot be ruled out that it may cause a local decline in abundance of indigenous species. Due to the high optimal growth temperature of H. japonica (20–25°C), high vegetative fragmentation and survival of fragments at > 20°C (Bjærke & Rueness Citation2004; Husa & Sjøtun Citation2006), and expected higher temperatures of coastal waters (Meehl et al. Citation2007), the question remains whether one may expect a stronger impact from H. japonica in the future.
Editorial responsibility: Tom Fenchel
Acknowledgements
We thank Professor Jan Rueness at the University of Oslo for identifying/verifying difficult species, Vera Rønningen for assistance in the laboratory, Amund Ulfsnes for statistical assistance and two anonymous referees for valuable comments for improvement of the manuscript. Additionally we thank the crew at ‘RV Hans Brattström’ and ‘RV Fangst’ for valuable help during the fieldwork. The Norwegian Research Council supported the study.
Notes
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark.
References
- Aure J , Strand Ø. 2001 . Hydrografiske normaler og langtidsvariasjoner i norske kystfarvann mellom 1936 og 2000 . Fisken og havet 13 : 1 – 10 (in Norwegian) .
- Axelius B , Karlson J. 2004 . Japanplym, ny rødalg før Sverige. (Heterosiphonia japonica new for Sweden) . Svensk botanisk tidskrift 98 : 268 – 73 ( in Swedish ).
- Balata , D , Piazzi , L and Cinelli , F . 2004 . A comparison along assemblages in areas invaded by Caulerpa taxifolia and C. racemosa in a subtidal Mediterranean rocky bottom . Marine Ecology , 25 : 1 – 13 .
- Bárbara , I . 2003 . Dasysiphonia sp. (Ceramiales, Dasyaceae), Nuevo ródofito alóctono para la peninsula Iberíca . Anales Jardín Botaníco de Madrid , 60 : 440 – 3 .
- Bárbara , I and Cremades , J . 1996 . Seaweeds of the Ria de A Coruña (NW Iberian Peninsula, Spain) . Botanica Marina , 39 : 371 – 88 .
- Bindoff NL , Willebrand J , Artale V , Casenave A , Gregory J , Gulev S , et al. 2007 . Observations: oceanic climate change and sea level . In Qin D. , Manning M , Chen Z , Marquis M , Averyt KB , Tignor M , et al. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change . Cambridge, , UK : Cambridge University Press .
- Bjærke MR. 2004 . The introduced red alga Heterosiphonia japonica (Ceramiales, Rhodophyta) in the eastern north Atlantic: an investigation of molecular variation with focus on AFLP fingerprinting . Molecular and ecological studies on marine macroalgae in Norwegian waters. Thesis for the degree of Doctor Scientarium, University of Oslo, Norway , p 40 – 63 .
- Bjærke , MR and Rueness , J . 2004 . Effects of temperature and salinity on growth, reproduction and survival in the introduced red alga Heterosiphonia japonica (Ceramiales, Rhodophyta) . Botanica Marina , 47 : 373 – 80 .
- Brattegaard T , Holte T. 1997 . Distribution of marine, benthic macro-organisms in Norway. Research Report for DN 1997-1. Directorate for Nature Management , 409 pages .
- Brattenborg N. 1997 . Den marine benthosalgevegetasjonen i sublittoralen på middels eksponerte lokaliteter i Sogn og Fjordane . Cand. Scient. Thesis. University of Bergen , 103 pages .
- Bray , JR and Curtis , JT . 1957 . An ordination of the upland forest communities of Southern Wisconsin . Ecological Monographs , 27 : 325 – 49 .
- Britton-Simmons , KH . 2004 . Direct and indirect effects of the introduced alga Sargassum muticum on benthic, subtidal communities of Washington State, USA . Marine Ecology Progress Series , 277 : 61 – 78 .
- Casas , G , Scrosati , R and Luz Piriz , M . 2004 . The invasive kelp Undaria pinnatifida (Phaeophyceae, Laminariales) reduces native seaweed diversity in Nuevo Gulf (Patagonia, Argentina) . Biological Invasions , 6 : 411 – 416 .
- Choi H-G. 2001 . Morphology and reproduction of Heterosiphonia pulchra and H. japonica (Ceramiales, Rhodophyta) . Algae 16 : 387 – 409 (in Korean with English abstract, descriptions and legends) .
- Farrell , P and Fletcher , RL . 2006 . An investigation of dispersal of the introduced brown algae Undaria pinnatifida (Harvey) Suringar and its competition with some species on the man-made structures of Torquay Marina (Devon, UK) . Journal of Experimental Marine Biology and Ecology , 334 : 236 – 43 .
- Forrest , BM and Taylor , MD . 2002 . Assessing invasion impact: survey design considerations and implications for management of an invasive marine plant . Biological Invasions , 4 : 375 – 86 .
- Hardy FG , Guiry MD. 2003 . A checklist and atlas of the seaweeds of britain and ireland.1. Marine Algae – Britain and Ireland . British Library Cataloguing in Publication Data. The British Phycological society .
- Harley , CDG , Hughes , AR , Hultgren , KM , Miner , BG , Sorte , CJB Thornber , CS . 2006 . The impacts of climate change in coastal marine systems . Ecology Letters , 9 : 228 – 41 .
- Hayden , HS , Blomster , J , Maggs , CA , Silva , PC , Stanhope , MJ and Waaland , JR . 2003 . Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera . European Journal of Phycology , 38 : 277 – 94 .
- Hiscock , K , Southward , A , Tittley , I and Hawkins , S . 2004 . Effects of changing temperature on benthic marine life in Britain and Ireland . Aquatic Conservation – Marine and Freshwater Ecosystems , 14 : 333 – 62 .
- Husa V. 2003 . ‘Dasysiphonia’ en introdusert rødalge I Norge; utbredelse langs kysten av Sørvest Norge, habitat og sesongvariasjoner . Cand. Scient. Thesis. University of Bergen, Norway , 55 pages .
- Husa , V , Sjøtun , K and Lein , TE . 2004 . The newly introduced species Heterosiphonia japonica Yendo (Dasyaceae, Rhodophyta): geographical distribution and abundance at the Norwegian southwest coast . Sarsia , 89 : 211 – 17 .
- Husa V , Sjøtun K. 2006 . Vegetative reproduction in ‘Heterosiphonia japonica’ (Dasyaceae, Ceramiales, Rhodophyta), an introduced red alga on European coasts . Botanica Marina 49 : 191 – 9 .
- Husa V , Steen H , Åsen PA. 2007 . Hvordan vil makroalgesamfunnene langs norskekysten påvirkes av økt sjøtemperatur? Kyst og Havbruksrapporten 2007 . Report, Institute of Marine Research (in Norwegian with English abstract and figure legends) .
- Kain , JM . 1989 . The seasons in the subtidal . British Phycological Journal , 24 : 203 – 15 .
- Kruskal , JB . 1964 . Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis . Psychometrika , 29 : 1 – 27 .
- Lein , TE . 1999 . A newly immigrated red alga (‘Dasysiphonia’, Dasyaceae, Rhodophyta) to the Norwegian coast . Sarsia , 84 : 85 – 8 .
- Levin , PS , Coyer , JA , Petrik , R and Good , TP . 2002 . Community-wide effects of nonindigenous species on temperate rocky reefs . Ecology , 83 : 3182 – 93 .
- Lima , FP , Ribeiro , PA , Queiroz , N , Hawkins , SJ and Santos , AM . 2007 . Do distributional shifts of northern and southern species of algae match the warming pattern? . Global Change Biology , 13 : 2592 – 604 .
- Maggs , CA and Stegenga , H . 1999 . Red algal exotics on the North Sea coasts . Helgoländer Meeresuntersuchungen , 52 : 243 – 58 .
- Meehl GA , Stocker TF , Collins WD , Friedlingstein P , Gaye AT , Gregory JM , et al. 2007 . Global climate projections . In: Qin , D . Manning M , Chen Z , Marquis M , Averyt KB , Tignor M , et al. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change . Cambridge, , UK : Cambridge University Press .
- Peña , V and Bárbara , I . 2006 . Revision of the genus Dasya (Ceramiales, Rhodophyta) in Galicia (NW Spain) and the addition of a new alien species Dasya sessilis Yamada for the European Atlantic coasts . Anales Jardín Botaníco de Madrid , 63 : 13 – 26 .
- Piazzi , L , Ceccherelli , G and Cinelli , F . 2001 . Threats to macroalgal diversity: effects of the introduced green algae Caulerpa racemosa in the Mediterranean . Marine Ecology Progress Series , 210 : 161 – 5 .
- Sagarin , RD , Barry , JP , Gilman , SE and Baxter , CH . 1999 . Climate-related change in an intertidal community over short and long time scales . Ecological Monographs , 69 : 465 – 90 .
- Sánchez , I and Fernández , C . 2005 . Impact of the invasive seaweed Sargassum muticum (Phaeophyta) on an intertidal macroalgal assemblage . Journal of Phycology , 41 : 923 – 30 .
- Sánchez , I , Fernández , C and Arrontes , J . 2005 . Long-term changes in the structure of intertidal assemblages after invasion by Sargassum muticum (Phaeophyta) . Journal of Phycology , 41 : 942 – 9 .
- Schiel , DR , Steinbeck , JR and Foster , MS . 2004 . Ten years of induced ocean warming causes comprehensive changes in marine benthic communities . Ecology , 85 : 1833 – 9 .
- Shepard , RN . 1962 . The analysis of proximities: multidimensional scaling with an unknown distance function . Psychometrika , 27 : 125 – 40 .
- Stegenga H. 1997 . Een nieuve Japanse invasie – vooral een systematisch probleem . Het Zeepard 57 : 109 – 33 (in Dutch) .
- Steinbeck , JR , Schiel , DR and Foster , MS . 2005 . Detecting long-term change in complex communities: a case study from the rocky intertidal zone . Ecological Applications , 15 : 1813 – 32 .
- Stæhr , PA , Pedersen , MF , Thomsen , MS , Wernberg , T and Krause-Jensen , D . 2000 . Invasion of Sargassum muticum in Limfjorden (Denmark) and its possible impact on the indigenous macroalgal community . Marine Ecology Progress Series , 207 : 79 – 88 .
- Strayer , DL , Eviner , VT , Jeschke , JM and Pace , ML . 2006 . Understanding the long-term effects of species invasions . Trends in Ecology and Evolution , 21 : 645 – 51 .
- Verlaque , M . 2001 . Checklist of the macroalgae of Thau Lagoon (Hérault, France), a hot spot of marine species introduction in Europe . Oceanologica Acta , 24 : 29 – 49 .
- Verlaque , M and Fritayre , P . 1994 . Mediterranean algal communities are changing in the face of the invasive alga Caulerpa taxifolia (Vahl) C. Agardh . Oceanologica Acta , 17 : 659 – 72 .
- Viejo , RM . 1997 . The effects of colonization by Sargassum muticum on tidepool macroalgal assemblages . Journal of the Marine Biological Association of the United Kingdom , 77 : 325 – 40 .
- Wallentinus I. 2003 . Ny rødalg på västkusten? Havsutsikt 3 : 11 ( in Swedish ).