Abstract
The three Arctic Calanus species, C. finmarchicus (Gunnerus, 1765), C. glacialis (Jaschov, 1955), and C. hyperboreus, are the most important herbivores in Arctic seas in terms of species biomass. They play a key role in the lipid-based energy flux in the Arctic, converting low-energy carbohydrates and proteins in ice algae and phytoplankton into high-energy wax esters. In this paper we review the over-wintering strategy, seasonal migration, stage development, life span, feeding strategy, body size, lipid biochemistry and the geographic distribution of the three dominant Calanus species in Arctic waters. We then relate these parameters to other biotic and abiotic factors, such as the timing of the Arctic phytoplankton and ice algae bloom, sea ice cover and climate variability. We also present new data on fatty acid and fatty alcohol content in the three Calanus species in addition to reviewing the available literature on these topics. These data are analysed for species homogeneity and geographic grouping. The dominance of diatom fatty acid trophic markers in the lipids of Calanus underpins the importance of diatoms as Arctic primary producers, even if dinoflagellates and Phaeocystis pouchetii can also be important food sources for the calanoid copepods. We conclude that the Arctic Calanus species are herbivores, engineered to feed on the Arctic bloom, and that the timing of the bloom is the most important factor in determining the life strategies of the individual species.
Introduction
The Calanus species, C. finmarchicus (Gunnerus, 1765), C. glacialis Jaschov, 1955, and C. hyperboreus, Krøyer, 1838, constitute a key role in the pelagic food web of the Arctic and northern seas. They are the most important biomass species and the prime herbivores in these waters (Conover & Huntley Citation1991; Mauchline 1998; Ringuette et al. Citation2002; Nielsen et al. Citation2007; Søreide et al. Citation2008). As light intensity increases in spring in high-latitude marine systems, ice melts and stratification of nutrient-rich water masses facilitate a short and intense Arctic bloom of phytoplankton and ice algae that propagate through Arctic waters producing a luxury of high-quality food available for zooplankton grazers. The carbon fixed through photosynthesis during the Arctic bloom is rapidly converted into large, specialized lipid (oil) stores by the Calanus herbivores (Lee Citation1975; Sargent & Henderson Citation1986; Falk-Petersen et al. Citation1987, Citation2000a; Lee et al. Citation2006), and the high-energy lipid compounds are transferred through the food chain in large amounts, being the major source of energy for the large stocks of fish, birds and marine mammals in the Arctic (Falk-Petersen et al. Citation1990, Citation2004; Dahl et al. Citation2003). These two factors (the timing of the Arctic bloom of ice algae and phytoplankton, and the lipid-driven energy flow through Arctic food webs) are the most prominent features of Arctic ecosystems that determine the structure of communities and populations in high-latitude ecosystems.
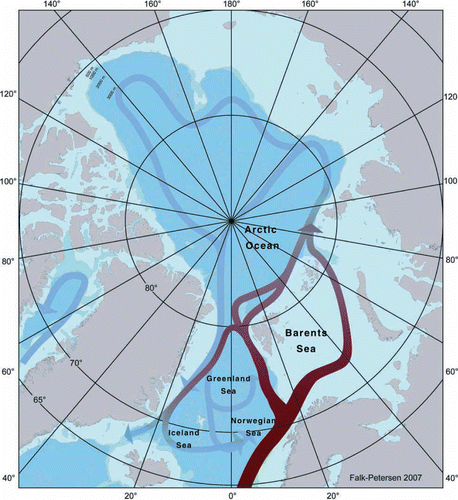
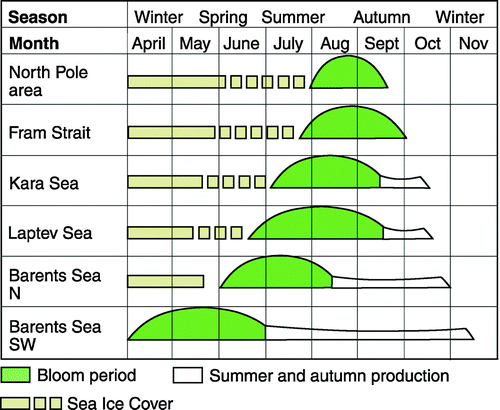
Towards higher latitudes, the amplitude of the primary production cycle becomes shorter due to the seasonality of light and ice conditions (Falk-Petersen et al. Citation2000b; Madsen et al. Citation2001; Ringuette et al. Citation2002; Hansen et al. Citation2003; Nilelsen et al. 2007). In the European Arctic the yearly ice melt starts in south west and blooms of phytoplankton propagate through the area (), starting in the southern Barents Sea in April–May, continuing in the seasonal ice covered seas as the northern Barents Sea and the Laptev Sea in July–August, culminating at the North Pole in August–September and eastern Fram Strait in September–October (Zenkevich 1963; Falk-Petersen et al. Citation2008). A similar situation exists in the American Arctic where the polynya opens up in early spring and the bloom propagate through the polynya as the ice is melting and the polynya increases in size. In the Canadian archipelago as well as in fjords on northeast Greenland the bloom starts as late as July–August (Welch et al. Citation1992; Rysgaard et al. Citation1999; Mei et al. Citation2002; Mundy et al. Citation2005). We suggest that the timing of the phytoplankton bloom, related to the dynamics of the sea ice cover and the sea climate, is a key in structuring the Arctic Calanus complex and the biodiversity of Arctic ecosystems (Ringuette et al. Citation2002; Blachowiak-Samolyk et al. Citation2008a; Madsen et al. Citation2008).
Figure 1. Time-related plankton blooms in the Arctic Oceans (modified after Zenkevitch Citation1963).
The three Calanus species are all widely distributed in the Arctic Ocean and the northern seas (Mauchline 1998). They also have some other common characteristics in that they are all herbivores, over-winter at depths in diapauses with reduced metabolic activity (Hirche 1997), have high total lipid contents of partially more than 50%, are rich in high energy wax esters (70–90%) and long-chain fatty alcohols and fatty acids (Lee Citation1975; Sargent & Henderson Citation1986; Falk-Petersen et al. Citation1987; Kattner et al. Citation1989; Scott et al. Citation2000, Citation2002; Lee et al. Citation2006).
The question still is: how and why do the three Calanus species co-exist in the Arctic? In this paper we discuss this question by relating the physical forcing of the Arctic marine system to distribution, over-wintering strategy, seasonal migration, stage development, life span, feeding strategy, size, lipid dynamics and lipid composition of the three Calanus species.
Distribution and seasonal migration
The distribution of the three Calanus species is strongly influenced by two large, interconnected gyres or current systems in the Nordic Seas and the Arctic Ocean (Rudels et al. Citation1999; Hansen & Østerhus Citation2000; ) and the cyclonic circulation in Baffin Bay (Melling et al. Citation2001).
Calanus finmarchicus, C. glacialis and C. hyperboreus are all found distributed in Arctic waters, including the Norwegian Sea, the Barents Sea, the White Sea, the Arctic Ocean, the Greenland Sea and the coastal waters bordering Siberia, East Canada and Alaska. However, the three species have different and distinct centres for over-wintering (Jaschnov Citation1970; Runge et al. Citation1986; Conover Citation1988; Hirche & Mumm Citation1992; Hirche Citation1997; Hirche & Kwasniewski Citation1997).
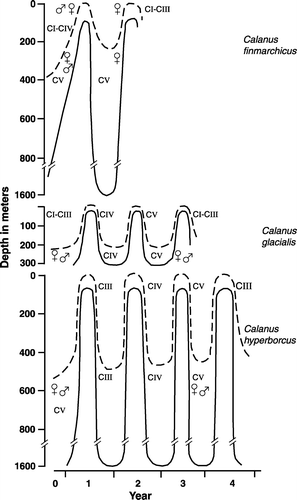
Calanus finmarchicus has its centre of distribution in the Norwegian Sea and the Labrador Sea, but it is also the dominant biomass zooplankton species south of Newfoundland, in the Barents Sea south of the Polar Front as well as along the Norwegian coast (Aksnes & Blindheim Citation1996; Planque et al. Citation1997). These are areas where a predictable annual spring bloom occurs between March and May. Calanus finmarchicus have also been recorded in low numbers all around the Arctic Ocean and East Greenland in connection with submerged Atlantic water that flow along the shelf margin (; Ringuette et al. Citation2002; Hirche & Kosobokova Citation2007; Nielsen et al. Citation2007; Madsen et al. Citation2008). Due to the Correolis Force the Atlantic flow is deflected into the Barents Sea north of Svalbard, the Kara Sea, the Siberian Shelf, the Canadian Arctic shelf as well as into the fjords on east Greenland (Rudels et al. Citation1999), transporting C. finmarchicus in low numbers into these areas. During winter the larger part of the population migrates down to deep water (). In the Norwegian Sea the over-wintering stages start migrating to depths of 500 m to more than 2000 m at the end of the vernal bloom (Østvedt Citation1955; Kaartvedt Citation1996; Visser & Jonasdottir Citation1999; Gislason & Astthorson 2000) while, in fjords and shelf seas, the over-wintering populations are found in deep trenches and basins (Kaartvedt Citation1996). During late winter (February–April) the population, now ready to spawn, migrates to surface waters, and the animals are transported by surface currents from their centre of distribution in the Norwegian Sea to all bordering seas (Sundby Citation2000).
Figure 3. Generalized seasonal migration and stage development of Calanus finmarchicus, Calanus glacialis and Calanus hyperboreus. Upper and lower line delineates the general depth of the population.
Calanus glacialis is a typical shelf species distributed along the Arctic shelf seas including the Barents Sea shelf north of the Polar Front, along the east Greenland shelf, through the Canadian islands, in Baffin Bay, along the north west coast of North America, in the White Sea as well as on the Siberian shelf and as far east as the northern part of the Sea of Okhotsk. It spawns on the shelf, along the slope and in fjords (; Conover Citation1988). These areas are often ice-covered until summer or autumn and the Arctic blooms occur late depending on the local ice conditions.
Calanus hyperboreus has its centre of distribution associated with deep-water areas such as the Greenland Sea, the Fram Strait, the Labrador Sea, the Baffin Sea and the Arctic Ocean Basin. However, it is transported with the major currents and can be found in low numbers over most of the shelf seas, the Nordic Seas, along the Norwegian coast and even in the North Sea. The over-wintering stages of C. hyperboreus migrate down to deep waters (500–2000 m) during winter (; Vinogradov Citation1997). The centres of distribution are associated with areas of high ice cover, where the light available for phytoplankton and ice algae blooms are dependent on both seasonal and large scale climate forces.
Life cycle strategies
Calanus finmarchicus has a one-year life cycle in Baffin Bay, the Barents and the Norwegian Seas, and it spawns in coincidence with the period of maximum phytoplankton bloom ( and and and ). Calanus. finmarchicus mainly spawn in April–May during or just after the bloom peak (Tande Citation1982; Tande et al. Citation1985; Niehoff et al. Citation2002; Madsen et al. 2008). The stage I copepodites (1–2.2 µg dry mass (DM)) develop to the lipid-rich over-wintering stage IV (40–70 µg DM) and stage V copepodites (130–240 µg DM) by June–July (; Mauchline 1988; Kattner et al. Citation1989). The lipid-rich over-wintering stages then descend to deep water in June-July to undergo diapause (Kaartvedt Citation1996). The over-wintering stage, mainly stage V copepodites, develops into males and females in January, and the subsequent energy-intensive development of ovaries in females between January and March is reflected in large decreases in dry mass and in the energy-rich wax ester deposits (Tande Citation1982; Falk-Petersen et al. Citation1987).
Figure 4. Life span of Calanus finmarchicus, Calanus glacialis and Calanus hyperboreus in Arctic waters. The numeration generalizes a gradient of geographic regions from the southern Barents Sea to the North Pole as indicated in .
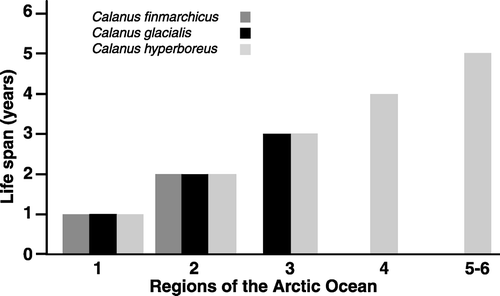
Table I. Life span of Calanus finmarchicus, Calanus glacialis and Calanus hyperboreus in Arctic waters.
Table II. Over-wintering stages and spawning periods of Calanus finmarchicus, Calanus glacialis and Calanus hyperboreus in Arctic waters.
Table III. Prosome length (mm), wet mass (WW), dry mass (DW) and lipid mass (LW) as mg ind–1, and % total lipid of DW in different developmental stages of Calanus finmarchicus, C. glacialis and C. hyperboreus. Length from Hirche et al. (Citation1994), WW, DW and % total lipid of DW from Scott et al. (Citation2000). DM of stages I–II are calculated after Mauchlene (1989).
Calanus glacialis often has a two-year life cycle (Tande et al. Citation1985; Eilertsen et al. Citation1989; Michel et al. Citation2006) and spawns during the spring of its third year ( and and and ; Smith Citation1990; Hirche & Kwasniewski Citation1997). MacLellan (Citation1967) observed a one-year cycle for C. glacialis in West Greenland, while Kosobokova (Citation1999) found that part of the White Sea population can be up to 3 years old, and that the females can spawn twice. Calanus glacialis develops its gonads to an advanced stage on internal lipid reserves well before the phytoplankton bloom, while spawning takes place just before or during the Arctic algae bloom, and may be partly fuelled by ice algae (Smith Citation1990; Hirche & Kattner Citation1993; Hirche & Kwasniewski Citation1997; Niehoff et al. Citation2002; Hirche & Kosobokova Citation2003). Successful spawning probably requires an energy input either from under-ice algae or from the phytoplankton bloom during egg production (Niehoff et al. Citation2002). Depending on the availability of food, i.e. on the timing of the bloom, C. glacialis can be found spawning from April until the end of August (Diel 1989; Hirche Citation1989a). After months of starvation, gonads developed in only 2 weeks (Hirche Citation1989a). In open shelf waters, C. glacialis spawns at the end of April, and it then develops rapidly into lipid-rich stage III or IV copepodites (Tande & Henderson Citation1988). Eggs of C. glacialis have been found floating in large numbers under the ice, consistent with a high lipid content and pre-bloom spawning (Werner & Hirche Citation2001). The lipid content of the younger stages of C. glacialis is unknown, but the lipids increase from stage IV to stage V copepodites and then to females from approx. 55, 60 to 70% DM, respectively ().
Calanus hyperboreus spawns during winter (Hirche & Niehoff Citation1996) between October and March ( and ), the spawning being fuelled entirely by pre-existing, internal lipid reserves. After the onset of the subsequent bloom, the eggs develop rapidly via nauplii into stage II and III copepodites, which are concentrated in the surface layers during the entire summer (May–October), probably feeding actively during this period. Large numbers of lipid-rich CIV, CV and females C. hyperboreus has been recorded active feeding on blooms of phytoplankton late autumn in the southern Arctic Ocean (Falk-Petersen et al. Citation2008). Daase et al. (Citation2008), however, found that young stages of C. hyperboreus were feeding in the chlorophyll maximum in the surface waters along the on the Spitsbergen shelf in mid September, while adult stages had migrated down to deep waters. In general, C. hyperboreus over-winter at depths of 800–1500 m, mainly as stages III, IV and V copepodites, in the Greenland Sea Gyre, Baffin Sea and the central Arctic Ocean ( and ; Vinogradov Citation1997). The first over-wintering stage is stage III, and they subsequently then develop into stage IV copepodites during their second summer, with a body mass of approximately 2 mg and a lipid content of more than 50% of their dry mass (). They then descend again to deep water (800–1500 m depth) and do not ascend to surface waters until late in the following summer. During this third summer they grow rapidly, increasing their dry mass 7 times and their lipid content probably exceeds 65% of their dry mass, as they develop into stage V copepodites. The stage V copepodites finally develop to adults during winter and then reproduce in the following spring, i.e. in the fourth year of their life. Stage V copepodites and females are segregated in two depth strata, with stage V copepodites over-wintering at 500–1000 m and females being concentrated at depths of 200–500 m, where they shed eggs (Vinogradov Citation1997). Life spans from 1–2 years and up to 4–6 years have been suggested for C. hyperboreus, depending on the geographical region and the food availability (, ). This probably reflects the large plasticity developed by the species to cope with the variability in available food. Interestingly, C. hyperboreus over-winters in deep water at relatively high temperatures of 0–2oC, whereas it actively feeds and grows in the surface layers at temperatures of –1 to –1.8oC.
Plasticity
The impressive plasticity of the three Calanus species is shown in and . In the North Sea, C. finmarchicus can have 1–3 generations per year (Wiborg Citation1954; Marshall & Orr Citation1955), while in the Norwegian Sea, along the coasts of north Norway, Greenland and east Canada and the Nordic Seas, the life span is mainly one year. A two-year life span is described for C. finmarchicus only from the Canadian archipelago (Longhurst et al. Citation1984). Calanus glacialis has a life span of 1–3 years, but for most areas a life span of 2 years is reported. Calanus hyperboreus shows the most impressive plasticity with a life span from 1 to 5 years or even longer (Hirche pers. comm.). Such plasticity in the different species reflects differences in plasticity towards their environments. The central Arctic Ocean has the highest variability in sea climate and, therefore, the lowest predictability of food available for herbivorous zooplankton, both between years and seasons.
The abilities of the different developmental stages of calanoids to over-winter depend on their capacity to store energy as lipids, principally wax esters (Lee Citation1974; Sargent & Falk-Petersen Citation1988). Stage III copepodites of C. hyperboreus have dry masses 3 times higher than C. glacialis and 11 times higher than C. finmarchicus (). The same is also evident for stage IV copepodites, and even more for stage V copepodites. Thus, the dry masses (and probably also the lipid contents) of the first over-wintering stages, stage III for C. hyperboreus, stage IV for C. glacialis, and stage V for C. finmarchicus, are approximately the same (0.09–0.15 mg dry mass per individual).
Diapause is a part of the life cycle of all Calanus species. The three species described here migrate down to deep water, where they over-winter as lipid-rich stages (Conover Citation1988). The similarity of the energy content of the first over-wintering copepodite stages, V, IV and III for C. finmarchicus, C. glacialis and C. hyperboreus, respectively, is consistent with the energy demand for the first over-wintering being the same for the three species. It has been shown by model studies where respiration, lipid stores and food availability were used as parameters, that C. finmarchicus has substantially higher probability of survival when it over-winters as stage V copepodites than as stage IV (Einane pers. comm.). By inference, therefore, stage IV C. glacialis and stage III C. hyperboreus both have more than sufficient energy stores to over-winter successfully in diapause.
Lipids and trophic markers
The role of wax esters
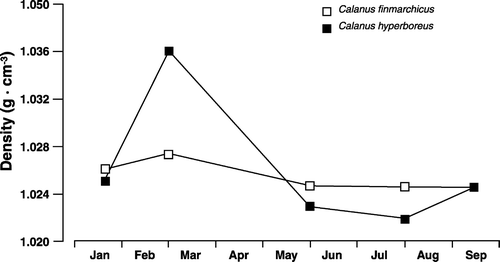
The three Calanus species build up their lipid reserves rapidly and have high total lipid and wax ester contents. All this is associated with a herbivorous strategy well fitted to the Arctic environment with short intense blooms of phytoplankton (Sargent & Henderson Citation1986; Sargent & Falk-Petersen Citation1988; Falk-Petersen et al. Citation2000b, Citation2004, Citation2007; Hagen & Auel Citation2001; Scott et al. Citation2002; Varpe et al. Citation2007). An interesting effect of the high lipid content is that lipids and especially wax esters, being much lighter than seawater, will create a positive up thrust in the lipid-rich stages of the three Calanus species (; Koegeler et al. Citation1987).
Figure 5. Seasonal variation in the specific gravity (buoyancy) of Calanus finmarchicus and Calanus hyperboreus (after Koegeler et al. Citation1987).
The biosynthesis of wax esters is a special adaptation of Calanus species and some other zooplankter to cope with the high seasonality of food availability. It is a very effective way to quickly produce high amounts of lipids during food plenty. The biosynthesis of fatty alcohols for the formation of wax esters is a mechanism for removing end-product inhibition in the fatty acid biosynthesis while producing a more reduced, i.e. more energy-rich, end product. Therefore, the formations of wax ester accelerate de novo biosynthesis of lipids utilizing the abundant diet (Sargent & Henderson Citation1986). Part of the fatty alcohol moieties of wax esters may originate from dietary fatty acids, but they are mainly produced from fatty acids, which are themselves biosynthesized de novo from protein and carbohydrate dietary precursors. The fatty acid moieties in the wax esters are derived from dietary fatty acids and are also to a smaller extent produced de novo (Sargent & Henderson Citation1986; Kattner & Hagen Citation1995). A longer chain fatty alcohol (or fatty acid) has higher energy content per unit mass than a shorter chain one. Therefore, the energy content of lipid is maximized by increasing the chain lengths of their constituent fatty alcohols and/or fatty acids. The very high amounts of de novo synthesized 22:1 and 20:1 fatty alcohols and acids is a store of exceptional high energy value lipids (Sargent & Falk-Petersen Citation1988). A large amount of energy, 182 moles ATP, is generated from the catabolism of one mole of 22:1 fatty acid.
The results presented here establish that Calanus finmarchicus, C. glacialis and C. hyperboreus have very high levels of 20:1 and 22:1 alcohols, ranging from 67 to 93%. Ratios of 22:1(n–11)/20:1(n–9) in females of C. hyperboreus, C. glacialis and C. finmarchicus of 1.74, 0.74 and 1.04, respectively, are reported from Kongsfjorden (Scott et al. Citation2002). The same trend can be seen in the ratios of Calanus from other regions () even if there is a great variation in the ratios between season and location. Furthermore, C. hyperboreus has the largest lipid stores with the highest percentages of 22:1(n–11) alcohol and fatty acid. This species is the most active of the three species in biosynthesizing lipid de novo and accumulates wax esters with the highest energy content (Albers et al. Citation1996). This is consistent with C. hyperboreus being the most highly adapted of the three species, maximizing formation of the longest chain wax esters (Graeve & Kattner Citation1992), reflecting its main location in the most extreme environment, the Greenland Sea and the Arctic basin.
Table IV. Fatty acid composition of polar lipids of Calanus finmarchicus, Calanus glacialis and Calanus hyperboreus from the Arctic.
Fatty acids of polar lipids
The polar lipids presented in represent three different seasons. Spring data were from the Northern Barents Sea and Kongsfjorden, summer data from the western Fram Strait and from all three season from the Arctic Ocean. The polar lipids were very rich in the typical diatom fatty acid marker 20:5(n–3) (eicosapentanoic acid; EPA), and especially the dinoflagellate marker 22:6(n–3) (docosahexaenoic acid; DHA). There are, however, some interesting differences between developmental stages and geographical region and season. In Calanus finmarchicus, the level of 20:5(n–3) increases from approximately 13 to 20% and 22:6(n–3) from approximately 10 to 30–40% between copepodite stage V and females. Another interesting feature is that the levels of the very important 22:6(n–3) fatty acid is much higher in all the three Calanus species in summer and autumn both in the Fram Strait and the Kongsfjorden (34–46%) than the Northern Barents Sea (Central Bank) (13–20%) in spring. 22:6(n–3) is a unique fatty acid, having a multifunctional role in cell membranes including functions as fluidity, elastic compressibility and permeability. It is produced by dinoflagellates and plays a role in locomotion of the flagella. For human health, for example, DHA is linked positively to the prevention of cancer and heart disease and is essential to neurological functions (Stillwell & Wassall Citation2003). The level of the dinoflagellate-derived DHA in phospholipids (34–46%) in Calanus females are much higher than in wax esters (0–10%). This shows that DHA is preferentially incorporated into copepod phospholipids and cell membranes. Although diatoms are the major food of Calanus copepods, and contribute much more to the animals’ total lipids than flagellates, the flagellates may have a more critical and fundamental role in the successful growth and reproduction of this species by contributing DHA to the formation of cell membranes (Scott et al. Citation2002).
Lipid trophic markers
The use of fatty acid trophic markers (FATM) (Dalsgaard et al. Citation2003) is based on the observation that marine primary producers lay down certain fatty acid patterns characteristic of different taxa that may be transferred conservatively through the food chain (Sargent & Whittle Citation1981; Dalsgaard et al. Citation2003). Of the important phytoplankton species in polar waters, diatoms are rich in the fatty acids 20:5(n–3), 16:1(n–7) and C16 PUFA (polyunsaturated fatty acids) but deficient in C18 PUFA. Dinoflagellates are rich in 18:4(n–3), 18:5(n–3) and especially 22:6(n–3) and deficient in 16:1(n–7). The haptophyceae Phaeocystis pouchetii, which often dominates the blooms in sub-Arctic waters (Falk-Petersen & Hopkins Citation1981), is rich in C18 PUFA, especially 18:4(n–3) and 18:5(n–3), together with 20:5(n–3) and 22:6(n–3), while 16:1(n–7) is only found in very small amounts (Sargent et al. Citation1985; Hamm et al. 2000). The 20:1(n–9) and 22:1(n–11) units, present in very large amounts in Calanus copepods, are formed by de novo biosynthesis in these animals, which in our present knowledge are the major site of the formation of 20:1(n–9) and 22:1(n–11) units to the marine food web (Sargent & Whittle Citation1981). The FATM produced by microalgae together with the specific markers produced by Calanus species de novo can be followed through the ecosystem to predators such as fish, mammals and seabirds (Falk-Petersen et al. Citation1986a, Citationb, Citation1990, Citation2004; Dahl et al. Citation2003).
Fatty acids and alcohols of wax esters
The composition of wax ester fatty acids and fatty alcohols are presented in and . Calanus from the northern Barents Sea and Kongsfjorden were collected in spring and autumn. The dominance FATM of diatoms, 20:5(n–3) and 16:1(n–7), up to 70%, in the wax ester underpins the importance of diatoms among the Arctic marine primary producers and its importance as the base of the Arctic food chain. Also striking are low levels of the dinoflagellate marker 22:6(n–3) (0 and 10%), which is in contrast to findings from the Norwegian fjords and the southern Barents Sea, where dinoflagellates and especially Phaeocystis pouchetii can be a dominating food source (Sargent & Falk-Petersen Citation1981; Falk-Petersen et al. Citation1987).
Table V. Fatty alcohol composition of wax esters of Calanus finmarchicus, Calanus glacialis and Calanus hyperboreus from the Arctic.
Table VI. Fatty acid composition of wax esters of Calanus finmarchicus, Calanus glacialis and Calanus hyperboreus from the Arctic.
The diatom FATM 16:1(n–7), 16PUFAs and 20:5(n–3) were very low (10–15%) in C. finmarchicus and C. glacialis during early spring at the northern Barents Sea (Central Bank) in 1986, while it increased in late spring at the same location (39%) and during summer and especially autumn, up to about 30% for both C. finmarchicus and C. glacialis. This indicates a clear seasonality of food available. The animals from early spring in the northern Barents Sea were collected before or at the beginning of the spring bloom (Melle & Skjoldal Citation1998) and had low levels of diatom FATM compared to animals collected later in spring at the same location, and from summer and autumn in Kongsfjorden and the Fram Strait East and West.
Calanus hyperboreus had moderate level of diatom FATM (14–28%) in the Fram Strait and northern Barents Sea during summer, while the summer data from the Arctic Ocean and autumn data from the western Fram Strait and the Arctic Ocean (North Water polynya) had very high levels of diatom FATM (up to 65%). This can be associated with late diatom blooms as the pack ice brakes up (; Falk-Petersen et al. Citation2004). High values of 18:4(n–3) were recorded in summer in the eastern Fram Strait, indicating that the animals feed on dinoflagellates and possibly also on Phaeosystis pouchetii (Kattner et al. Citation1989). The exchange of fatty acids due to changes in phytoplankton composition is provided by feeding experiments. The assimilation of dietary fatty acids and the de novo biosynthesis are rapid processes (Graeve et al. Citation1994, Citation2005).
The very high level of 20:1(n–9) and 22:1(n–11) fatty acids and alcohols, recorded in females of C. glacialis and C. hyperboreus from the northern Barents Sea (Central Bank) in early spring, indicate active de novo biosyntheses and feeding in this area at the start of the spring bloom. It is well known that Calanus has a flexible feeding strategy and is able to switch to alternative prey when fresh phytoplankton are scarce, including nauplii (Bonnet et al. Citation2004; Basedow & Tande Citation2006) and protozooplankton (Mauchline Citation1998; Levinsen et al. Citation2000). However, based on the fatty acid composition and life strategy, we conclude that the three Arctic Calanus species are herbivores heavily utilizing the Arctic phytoplankton bloom (Søreide et al. Citation2008).
Discrimination of Calanus species as preys based on fatty acids and fatty alcohols
The central position of the three major species of Calanus in the Arctic and sub-Arctic pelagic food web makes it important to define those fatty acid trophic markers and alcohols which could be used to discriminate between the three species. The wax ester composition is used because it integrates relatively long time scales (days to weeks) and represents the main lipid class. It also yields a dual definition based on both fatty acids and fatty alcohols. The approach used here is based on the one hand on a hierarchical cluster analysis to ascertain the degree of homogeneity or heterogeneity between wax esters and species at all locations, and on the other hand on a PLS discriminant analysis (PLS-DA; Wold et al. Citation1984), which is a principal component analysis of the averages of the three species, weighted by their respective sample sizes. All data were normalized to constant sum, and observations were standardized by dividing each variable by the square root of its standard deviation.
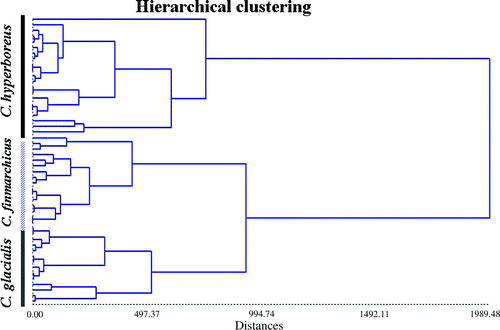
The initial cluster analysis, based on the Ward's hierarchical clustering model (reciprocal neighbour; Ward Citation1963), was performed on both fatty acid and fatty alcohol data sets. As expected from the influence of dietary fatty acids, a large variability was recorded for the analysis based on the wax ester fatty acid fraction (cluster not shown) associated to a large part to sampling location. On the contrary, when hierarchical clustering was based on fatty alcohols, species homogeneity was the dominant feature () as anticipated from components resulting from de novo synthesis. A PLS-DA (not shown) suggested that Calanus hyperboreus can be best discriminated by higher percentages of 22:1(n–11) alcohols, whereas C. glacialis and C. finmarchicus can be best discriminated by higher values of 22:1 + 20:1 and 16:1(n–7). This further strengthens the understanding that fatty alcohols are mainly biosynthesized de novo from protein and carbohydrate dietary precursors, and that the biosynthesis of fatty alcohol moieties is a process that is species-specific.
Figure 6. Hierarchical cluster analysis of fatty alcohol composition for C. hyperboreus, C. glacialis and Calanus finmarchicus collected in different sectors of the European Arctic Ocean. Symbols: Ch = C. hyperboreus, Cg = C. glacialis, Cf = C. finmarchicus
The regional heterogeneity of the wax ester fatty acid composition does not exclude that, at a given location, the three species could be correctly discriminated. To test this possibility, we used PLS-DA for three groups of locations: the northern Barents Sea and Kongsfjorden, the eastern Fram Strait and Arctic Ocean, and the western Fram Strait. Three indices characteristics of each species are computed based on the results of the analysis: sum of 14:0 + 16:0 for C. finmarchicus, 16:1(n–7) +20:1(n–9) for C. glacialis and 22:1 + 16:0 for C. hyperboreus. Results show that, regionally, the three species can be discriminated with 100% correct assignment, including the indices (Figures ). Interestingly, the fatty acid descriptors of each species differ with the location, but the indices remain characteristic of the related species.
Figure 7. PLS-DA for the three main species of Calanus sampled in the Northern Barents Sea and Kongsfjorden based on the fatty acid composition of wax esters. Projection on the plane defines by the first two discriminant functions (DS1 = 45.9% and DS2 = 33.2% total inertia). Factor loading for the fatty acid descriptors. Italic: centroid of the factorial group. Ind = indices for each Calanus species (see text).
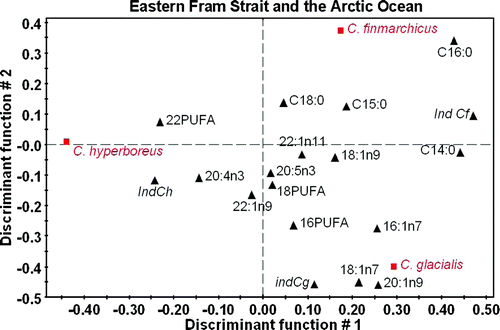
Figure 8. PLS-DA for the three main species of Calanus sampled in the eastern Fram Strait and Arctic Ocean based on the fatty acid composition of wax esters. Projection on the plane defines by the first two discriminant functions (DS1 = 37.7% and DS2 = 32.3% total inertia). Factor loading for the fatty acid descriptors. Italic: centroid of the factorial group. Ind = indices for each Calanus species (see text).
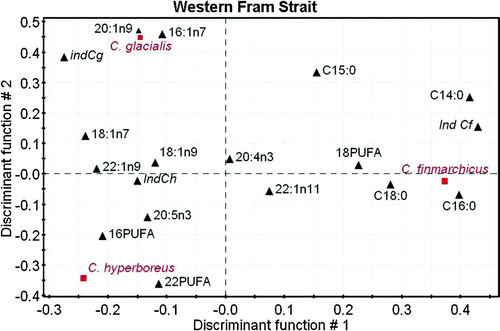
Figure 9. PLS-DA for the three main species of Calanus sampled in the western Fram Strait based on the fatty acid composition of wax esters. Projection on the plane defines by the first two discriminant functions (DS1 = 36% and DS2 = 32% total inertia). Factor loading for the fatty acid descriptors. Italic: centroid of the factorial group. Ind = indices for each Calanus species (see text).
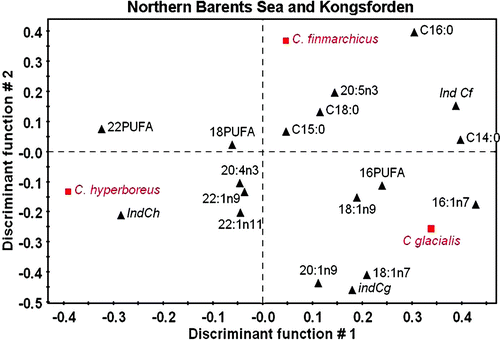
In two of the three sectors considered, the first discriminant function opposes Calanus hyperboreus to the other two species, while the second function separates C. glacialis from C. finmarchicus. In the northern Barents Sea and Kongsfjorden (), C. hyperboreus is associated to the profile of C22 PUFA, while 20:1(n–9), 18:1(n–9) profiles are related to C. glacialis and 16:0, 20:5(n–3) with C. finmarchicus. The fatty acid 16:1(n–7) and C16 PUFA are descriptors for the latter two species, suggesting that at these locations C. glacialis and C. finmarchicus fed more heavily on phytoplankton dominated by diatom. PLS-DA for East Fram Strait and Arctic Ocean waters () shows slightly different descriptors with C22 PUFA, 20:4(n–3) for C. hyperboreus, 20:1(n–9), 18:1(n–7), 16:1(n–7) for C. glacialis and 16:0 for C. finmarchicus. At these locations C. glacialis seems to be the most herbivorous species. The results for East Fram Strait are quite different since the first discriminant function opposes C. finmarchicus to the other two species (). The fatty acid descriptors comprise most saturated acids and C18 PUFA, while C. hyperboreus was associated to changes in C22 PUFA, C16 PUFA and 20:5(n–3), and C. glacialis to 16:1(n–7) and 20:1(n–9). These last two species are clearly feeding on phytoplankton in this area.
Biodiversity and ocean climate variability
Polar systems are characterized by pronounced seasonal oscillations in incident solar radiation. The light regime changes dramatically during the year, from a period of winter darkness to a period of midnight sun during summer north of the Arctic Circle. The ice cover also changes dramatically on very short time scales from hours to days and on long time scales from years to decades (Proshutinsky et al. Citation1999; Falk-Petersen et al. Citation2000b, Citation2008; Vinje Citation2001). These changes strongly influence the light available for primary production with little or no light penetrating to waters underlying dense ice (Hansen et al. Citation2003). The extent to which ice cover can vary is emphasized by the fact that the North Pole can be largely ice-free during summer in some years, while it is totally ice-covered in other years. In the North Pole region, on average more than 15% of the area is open water in late summer (Polyakov et al. Citation1999), and phytoplankton blooms are regularly registered in North Pole waters (; Zenkevich 1963). Large variations in ice cover can also be seen in the marginal ice-covered seas. Large parts of the Barents Sea and the Norwegian Sea were totally ice-covered in spring of 1966, while in 1995 most of the same areas were ice free (Vinje Citation2001). Oscillations in ice conditions on an interannual and decadal scale are related to environmental phenomena such as the changes of the cyclonic and anticyclonic regimes over the Arctic Ocean, which occurs in periods of 10-years (Proshutinsky et al. Citation1999), and the North Atlantic Oscillation Index, which occurs in periods of 7 years (Dickson et al. Citation2000).
Plankton communities at high latitudes have developed as a result of the history of climate changes, not only the recent history, but changes that have occurred over millions of years. Diatoms and copepods have evolved during a period of global cooling, largely since the K-T extinction event 65 million years ago , when polar ice caps were formed, and marine production in high latitudes became seasonally pulsed (Rigby & Milsom Citation2000). Kattner et al. (Citation2007) hypothesize that the present composition of the polar herbivore community in the Arctic, mainly consisting of the three Calanus species, as well as the development of highly efficient lipid synthesis of wax ester in these animals, is a consequence of the variations in climate observed over the past centuries and millennia. According to our present understanding, the Arctic Calanus species are engineered to feed on the Arctic bloom and convert the low-energy carbohydrates and proteins produced by phytoplankton and ice algae to high-energy wax ester lipids (Sargent & Henderson Citation1986; Hagen & Auel Citation2001; Falk-Petersen et al. Citation2007; Kattner et al. Citation2007; Varpe et al. Citation2007). The pelagic Calanus species, being one of the major members of the Arctic marine ecosystem, has adapted to an environment changing markedly on different time scales. This readily accounts for the biodiversity of the Calanus complex in terms of the species’ different life strategies, different ecological niches and different centres of distribution.
Thus, the most polar species, Calanus hyperboreus, has its centre of distribution in deep-sea areas such as the central Arctic Ocean, the Fram Strait and the Greenland Sea. These are areas with marked annual and interannual variations in ice conditions. In favourable seasons, when the ice-cover opens up for longer periods, food is plenty, while in years with permanent ice cover food is effectively absent. Calanus hyperboreus has adapted to this condition by developing a large plasticity in its life strategy. Under favourable conditions, where primary production is high, the animal has a life span of only 1–2 years (), while under less favourable conditions when ice cover had been extensive it has a life span from 3 to5 years or even longer. When C. hyperboreus has accumulated sufficient oil reserves as high-energy wax esters, it migrates down to deep waters (500–2000 m) and enters diapause, probably neutrally buoyant. Under these conditions it has sufficient high-energy reserves to meet its minimal metabolic requirements for long periods, exceeding a single season if need be. It is logical that the greater the variations in ice cover and especially the greater the likelihood of prolonged ice cover, the greater the advantage for a herbivorous copepod to arrest development at any stage. This is more likely to occur in large copepods with large reserves of high-energy lipids in early as well as late developmental stages.
Calanus glacialis is a typical shelf species which spawns in waters all around the shelf and in the White Sea. Although the shelf areas experience large variations in ice cover, the ice mostly opens up for shorter or longer period during the summer or autumn, allowing an annual algal bloom. Calanus glacialis has a life span of 1–3 years. Scott et al. (Citation2000) suggested that this species is well capable of developing from eggs to wax ester-rich stages III and IV copepodites within a single year. However, the large increment in body mass and lipid reserves as it develops further from stages IV to V copepodites is unlikely to be achieved in a single year, except under very favourable conditions. Thus, development from stage IV to stage V probably occurs in two-year-old C. glacialis. The resulting large, wax ester-rich stage V copepodites then over-winter and spawn immediately prior to or during the spring bloom in their subsequent third year of life.
The boreal–arctic Calanus finmarchicus is also a deep-water species, but its centre of distribution is further south in the Norwegian Sea and the Labrador Sea. Breeding populations are also found along the Norwegian coast, the west coast of Greenland and in the southern Barents Sea. These are areas where a predictable annual spring bloom occurs between March and May. Calanus finmarchicus is the smallest of the three species and develops from eggs to wax ester-rich, over-wintering stage IV and stage V copepodites, within 6–10 weeks in favourable years. Stage IV copepodites complete their development to stage V and then to females in the spring of the following year, prior to spawning. This final development depends on both internal wax ester reserves and the availability of algal food. A successful spawning depends strongly on the spring bloom.
Based on the lipid composition and the biology of the Arctic Calanus species we generally conclude that:
| • | all Calanus species are herbivores engineered to feed on the Arctic bloom; | ||||
| • | the timing of the bloom is the important factor in determining the life strategy of the individual species and biodiversity of the Calanus complex; | ||||
| • | Calanus finmarchicus is a deep-water species adapted to an environment with a regular yearly spring bloom, as occurs in the Norwegian Sea; | ||||
| • | Calanus glacialis is a shelf species adapted to an environment subjected to large variations in timing and length of the annual bloom, as found in the northern Barents Sea and on the Siberian and American shelves; and | ||||
| • | Calanus hyperboreus is a deepwater species adapted to an environment with large interannual variations in ice cover and algal blooms, as found in the central Arctic Ocean and the Fram Strait. | ||||
Editorial responsibility: Tom Fenchel
References
- Aksnes , DL and Blindheim , J . 1996 . Circulation patterns in the North Atlantic and possible impact on population dynamics of Calanus finmarchicus . Ophelia , 44 : 7 – 28 .
- Albers , CS , Kattner , G and Hagen , W . 1996 . The compositions of wax esters, triacylglycerols and phospholipids in Arctic and Antarctic copepods: Evidence of energetic adaptations . Marine Chemistry , 55 : 347 – 58 .
- Basedow , SL and Tande , KS . 2006 . Cannibalism by female Calanus finmarchicus on naupliar stages . Marine Ecology Progress Series , 327 : 247 – 55 .
- Blachowiak-Samolyk K , Søreide JE , Kwasniewski S , Sundfjord A , Hop H , Falk-Petersen S , et al . 2008a . Hydrodynamic control of mesozooplankton abundance and biomass in northern Svalbard waters (79–81 °N) . Deep-Sea Research II. doi:10.1016/jdrs2.2008.05.024
- Blachowiak-Samolyk , K , Kwasniewski , S , Hop , H and Falk-Petersen , S . 2008b . Magnitude of mesozooplankton variability: A case study from the Marginal Ice Zone of the Barents Sea in spring . Journal of Plankton Research , 30 : 311 – 23 .
- Bonnet , D , Titelman , J and Harris , R . 2004 . Calanus the cannibal . Journal of Plankton Research , 26 : 937 – 48 .
- Conover , RJ . 1962 . Metabolism and growth in Calanus hyperboreus in relation to its life cycle. Rapports et procès-verbaux des reunions (Conseil international pour l . exploration de la mer) , 153 : 190 – 97 .
- Conover , RJ . 1988 . Comparative life histories in the genera Calanus and Neocalanus in high latitudes of the Northern Hemisphere . Hydrobiologia , 167/168 : 127 – 42 .
- Conover , RJ and Huntley , M . 1991 . Copepods in ice-covered seas – Distribution, adaptations to seasonally limited food, metabolism, growth patterns and life cycle strategies in polar seas . Journal of Marine Systems , 2 : 1 – 41 .
- Conover RJ , Harris LR , Bedo AW . 1991 . Copepods in cold oligotrophic waters – How do they cope? Bulletin of Plankton Society Japan 177 – 98 .
- Conover , RJ and Siferd , TD . 1993 . Dark-season survival strategies of coastal zone zooplankton in the Canadian Arctic . Arctic , 46 : 303 – 11 .
- Daase , M , Eiane , K , Aksnes , DL and Vogedes , D . 2008 . Vertical distribution of Calanus spp. and Metridia longa at four Arctic locations . Marine Biology Research , 4 : 193 – 207 .
- Dahl , TM , Falk-Petersen , S , Gabrielsen , GW , Sargent , JR , Hop , H and Millar , RM . 2003 . Lipids and stable isotopes in common eider, black-legged kittiwake and northern fulmar: A trophic study from an Arctic fjord . Marine Ecology Progress Series , 256 : 257 – 69 .
- Dalsgaard , J , St. John , M , Kattner , G , Müller-Navarra , D and Hagen , W . 2003 . Fatty acid trophic markers in the pelagic marine environment . Advances in Marine Biology , 46 : 227 – 318 .
- Dawson , JK . 1978 . Vertical distribution of Calanus hyperboreus in the central Arctic Ocean . Limnology and Oceanography , 23 : 950 – 57 .
- Dickson , RR , Osborn , TJ , Hurrel , JW , Meincke , J , Blindheim , J Ådlandsvik , B . 2000 . The Arctic Ocean response to the North Atlantic Oscillation . Journal of Climate , 13 : 2671 – 96 .
- Diel , S . 1991 . On the life history of dominant copepod species (Calanus finmarchicus, C. glacialis and C. hyperboreus, Metridia longa) in the Fram Strait . Reports on Polar Research , 88 : 1 – 113 .
- Eilertsen , HC , Taasen , JP and Weslawski , JM . 1989 . Phytoplankton studies in the fjords of west Spitzbergen – Physical environment and production in spring and summer . Journal of Plankton Research , 11 : 1245 – 60 .
- Falk-Petersen , S , Falk-Petersen , I-B and Sargent , JR . 1986a . Structure and function of an unusual lipid storage organ in the Arctic fish Lumpenus maculatus (Fries) . Sarsia , 71 : 1 – 6 .
- Falk-Petersen , S , Hagen , W , Kattner , G , Clarke , A and Sargent , JR . 2000a . Lipids, trophic relationships and biodiversity in Arctic and Antarctic krill . Canadian Journal of Fishery and Aquatic Sciences , 57 : 178 – 91 .
- Falk-Petersen , S , Haug , T , Nilsen , KT , Wold , A and Dahl , TM . 2004 . Lipids and trophic linkages in harp seal (Phoca groenlandica) from the eastern Barents Sea . Polar Research , 23 : 21 – 50 .
- Falk-Petersen , S , Hop , H , Budgell , WP , Hegseth , EN , Korsnes , R Løyning , TB . 2000b . Physical and ecological processes in the Marginal Ice Zone of the northern Barents Sea during the summer melt periods . Journal of Marine System , 27 : 131 – 59 .
- Falk-Petersen , S and Hopkins , CCE . 1981 . Zooplankton sound scattering layers in North Norwegian fjords: Interactions between fish and krill shoals in a winter situation in Ullsfjorden and Øksfjorden. Kieler Meeresforschungen . Sonderheft , 5 : 191 – 201 .
- Falk-Petersen , S , Hopkins , CCE and Sargent , JR . 1990 . “ Trophic relationships in the pelagic food web ” . In Trophic Relationships in the Marine Environment , Edited by: Barnes , M and Gibson , RN . 315 – 33 . Aberdeen : Aberdeen University Press .
- Falk-Petersen S , Leu E , Berge J , Nygaard H , Røstad A , Kwasniewski S , et al . 2008 . Vertical migration in high Arctic waters during autumn 2004 . Deep Sea Research II. doi:10.1016/j.dsr2.2008.05.010
- Falk-Petersen , S , Pedersen , G , Kwasniewski , S , Hegseth , EN and Hop , H . 1999 . Spatial distribution and life cycle timing of zooplankton in the marginal ice zone of the Barents Sea during the summer melt season in 1995 . Journal of Plankton Research , 21 : 1249 – 64 .
- Falk-Petersen , S , Sargent , JR and Middleton , C . 1986b . Level and composition of triacylglycerols and wax esters in commercial capelin oils from the Barents Sea Fishery, 1983 . Sarsia , 71 : 49 – 54 .
- Falk-Petersen , S , Sargent , JR and Tande , KS . 1987 . Lipid composition of zooplankton in relation to the Subarctic food web . Polar Biology , 8 : 115 – 20 .
- Falk-Petersen S , Timofeev S , Pavlov V , Sargent JR . 2007 . Climate variability and the effect on arctic food chains. The role of Calanus. Monograph Springer Verlag. Environmental Challenges in Arctic-Alpine Regions . Arctic-Alpine Ecosystems and People in a Changing Environment .
- Gislason , A and Astthorsson , OS . 1998 . Seasonal variations in biomass, abundance and composition of zooplankton in the sub waters north of Iceland . Polar Biology , 20 : 85 – 94 .
- Graeve , M , Albers , C and Kattner , G . 2005 . Assimilation and biosynthesis of lipids in Arctic Calanus species based on 13C feeding experiments with a diatom . Journal of Experimental Marine Biology and Ecology , 317 : 109 – 25 .
- Graeve , M and Kattner , G . 1992 . Species-specific differences in intact wax esters of Calanus hyperboreus and C. finmarchicus from Fram Strait–Greenland Sea . Marine Chemistry , 39 : 269 – 81 .
- Graeve , M , Kattner , G and Hagen , W . 1994 . Diet-induced changes in the fatty acid composition of Arctic herbivorous copepods: Experimental evidence of trophic markers . Journal of Experimental Marine Biology and Ecology , 182 : 97 – 110 .
- Grainger , EH . 1959 . The annual oceanographic cycle at Igloolik in the Canadian Arctic. I. The zooplankton and physical and chemical observations . Journal of the Fishery Research Board of Canada , 16 : 453 – 501 .
- Grainger EH . 1963 Copepods of the genus Calanus as indicators of eastern Canadian waters . In: Dunbar MJ Marine Distributions. Research Society Canadian Special Publications 5 : 68 – 94
- Grainger , EH . 1965 . Zooplankton from the Ocean and adjacent Canadian waters . Journal of the Fishery Research Board of Canada , 22 : 543 – 64 .
- Hagen , W and Auel , H . 2001 . Seasonal adaptation and the role of lipids in oceanic zooplankton . Zoology , 104 : 313 – 16 .
- Hamm , C , Reigstad , M , Wexels-Riser , C , Mühlebach , A and Wassmann , P . 2001 . On the trophic fate of Phaeocystis pouchetii: VII. Sterols and fatty acids reveal sedimentation of Phaeocystis-derived organic matter via krill fecal strings . Marine Ecology Progress Series , 209 : 55 – 69 .
- Hansen , AS , Nielsen , TG , Levinsen , H , Madsen , SD , Thingstad , F and Hansen , BW . 2003 . Impact of changing ice cover on pelagic productivity and food web structure in Disko Bay, West Greenland: a dynamic model approach . Deep Sea Research Part I , 50 : 171 – 87 .
- Hansen , B and Østerhus , S . 2000 . North Atlantic–Nordic Seas exchanges . Progress In Oceanography , 45 : 109 – 208 .
- Harding GC . 1966 . Zooplankton distribution in the Ocean with notes of life cycles . M.S. thesis, McGill University, Montreal .
- Hirche , H-J . 1989a . Egg production of the copepod Calanus glacialis: Laboratory experiments . Marine Biology , 103 : 311 – 18 .
- Hirche , H-J . 1989b . Spatial distribution of digestive enzyme activities of Calanus finmarchicus and C. hyperboreus in Fram Strait/Greenland Sea . Journal of Plankton Research , 11 : 431 – 43 .
- Hirche , H-J . 1991 . Distribution of dominant calanoid copepod species in the Greenland Sea during late fall . Polar Biology , 11 : 351 – 62 .
- Hirche , H-J. 1997 . Life cycle of the copepod Calanus hyperboreus in the Greenland Sea . Marine Biology , 128 : 607 – 18 .
- Hirche , H-J , Hagen , W , Mumm , N and Richter , C . 1994 . The Northeast Water Polynya, Greenland Sea. III. Meso- and macroplankton distribution and production of dominant herbivorous copepods during spring . Polar Biology , 14 : 491 – 503 .
- Hirche , H-J and Kattner , G . 1993 . Egg production and lipid content of Calanus glacialis in spring: Indication of a food-dependent and food-independent reproductive mode . Marine Biology , 117 : 615 – 22 .
- Hirche , H-J and Kosobokova , K . 2003 . Early reproduction and development of dominant calanoid copepods in the sea ice zone of the Barents Sea – Need for a change of paradigms? . Marine Biology , 143 : 769 – 81 .
- Hirche , H-J and Kosobokova , KN . 2007 . Distribution of Calanus finmarchicus in the northern North Atlantic and Arctic Ocean – Expatriation and potential colonization . Deep Sea Research Part II , 54 : 2729 – 47 .
- Hirche , H-J and Kwasniewski , S . 1997 . Distribution, reproduction and development of Calanus species in the Northeast Water in relation to environmental conditions . Journal of Marine System , 10 : 299 – 317 .
- Hirche , H-J and Mumm , N . 1992 . Distribution of dominant copepods in the Nansen Basin, Arctic Ocean, in summer . Deep Sea Research , 39 : 485 – 505 .
- Hirche , H-J and Niehoff , B . 1996 . Reproduction of the copepod Calanus hyperboreus in the Greenland Sea – Field and laboratory observations . Polar Biology , 16 : 209 – 19 .
- Huntley , M , Strong , KW and Dengler , AT . 1983 . Dynamics and community structure of zooplankton in the Davis Strait and Northern Labrador Sea . Arctic , 36 : 143 – 61 .
- Jaschnov , WA . 1939 . Life cycle and seasonal variability in distribution of age stages of Calanus finmarchicus in the Barents Sea . Trudy VNIR , 4 : 225 – 44 .
- Jaschnov , WA . 1961 . Water masses and plankton. 1. Species of Calanus finmarchicus s.l. as indicators of definite water masses . Zoology Zürich , 40 : 1314 – 34 .
- Jaschnov , WA . 1970 . Distribution of Calanus species in the seas of the northern hemisphere . International Revue der Gesamten Hydrobiologie/International Review of hydrobiology , 55 : 197 – 212 .
- Johnson , MW . 1963 . Zooplankton collections from the high polar basin with special reference to the copepoda . Limnology and Oceanography , 8 : 89 – 102 .
- Kaartvedt , S . 1996 . Habitat preference during overwintering and timing of seasonal vertical migration of Calanus finmarchicus . Ophelia , 44 : 145 – 56 .
- Kamshilov , MM . 1955 . Materials on the biology of Calanus finmarchicus Gunner in the White and Barents Seas . Trudy – Murmanskja Biologiceskij Stancija –Moskva , 2 : 62 – 86 .
- Kattner , G , Hirche , H-J and Krause , M . 1989 . Spatial variability in lipid composition of calanoid copepods from Fram Strait, the Arctic . Marine Biology , 102 : 473 – 80 .
- Kattner , G and Hagen , W . 1995 . Polar herbivorous copepods – Different pathways in lipid biosynthesis . ICES Journal of Marine Science , 52 : 329 – 35 .
- Kattner , G , Hagen , W , Lee , RF , Campbell , R , Deibel , D Falk-Petersen , S . 2007 . Perspectives on marine zooplankton lipids . Canadian Journal of Fisheries and Aquatic Sciences , 64 : 1628 – 39 .
- Koegeler , JW , Falk-Petersen , S , Kristiansen , Å , Pettersen , F and Dahlen , J . 1987 . Soundspeed- and density contrasts in Sub-Arctic zooplankton . Polar Biology , 7 : 231 – 35 .
- Kosobokova , KN . 1986 . Estimation of production of common herbivorous copepods of the Central Basin . Oceanology (Moscow) , 26 : 749 – 52 .
- Kosobokova , KN . 1999 . The reproductive cycle and life history of the copepod Calanus glacialis in the White Sea . Polar Biology , 22 : 254 – 63 .
- Kosobokova , KN and Hirche , H-J . 2001 . Reproduction of Calanus glacialis in the Laptev Sea, Arctic Ocean . Polar Biology , 24 : 33 – 43 .
- Lee , RF . 1974 . Lipid composition of the copepod Calanus hyperboreus from the Arctic Ocean: Changes with depth and season . Marine Biology , 26 : 313 – 18 .
- Lee , RF . 1975 . Lipids of zooplankton . Comparative Biochemical and Physiology , 51 : 263 – 66 .
- Lee , RF , Hagen , W and Kattner , G . 2006 . Lipid storage in marine zooplankton . Marine Ecological Progress Series , 307 : 273 – 306 .
- Levinsen , H , Turner , JTY , Nielsen , TG and Hansen , BW . 2000 . On the trophic coupling between protests and copepods in Arctic marine ecosystems . Marine Ecology Progress Series , 204 : 65 – 77 .
- Lie , U . 1968 . Variations in the quantity of zooplankton and the propagation of Calanus finmarchicus at Station M in the Norwegian Sea . Fiskeri Direktoratets Skrifter: Havundersøkelser , 14 : 121 – 28 .
- Longhurst , A , Sameoto , D and Herman , A . 1984 . Vertical distribution of zooplankton in summer: Eastern Canadian archipelago . Journal of Plankton Research , 6 : 137 – 68 .
- MacLellan , DC . 1967 . The annual cycle of certain calanoid species in west Greenland . Canadian Journal of Zoology , 45 : 101 – 15 .
- Madsen , SD , Nielsen , TG and Hansen , BW . 2001 . Annual population development and production by Calanus finmarchicus, C. glacialis and C. hyperboreus in Disko Bay, western Greenland . Marine Biology , 139 : 75 – 93 .
- Madsen , SD , Nielsen , Tervo , OM and Söderkvist , J . 2008 . On the importance of feeding for egg reproduction of Calanus finmarchicus and C. glacialis during the Arctic spring . Marine Ecology Progress Series , 353 : 177 – 90 .
- Manteiphel , BP . 1941 . Plankton and herring in the Barents Sea . Trudy PINRO , 7 : 125 – 218 .
- Marshall , SM and Orr , AP. 1955 . The Biology of Marine Copepod Calanus finmarchicus (Gunnerus) , 188 London/Edinburg : Oliver and Boyd .
- Matthews , JBL , Hestad , L and Bakke , JLW . 1978 . Ecological studies in Korsfjorden, Western Norway. The generations and stocks of Calanus hyperboreus and C. finmarchicus in 1971–1974 . Oceanology Acta , 1 : 277 – 84 .
- Mauchline , J. 1998 . The biology of calanoid copepods . Advances in Marine Biology , 33 : 1 – 710 .
- Mei , ZP , Legendre , L , Gratton , Y , Tremblay , J-É , LeBlanc , B Mundy , CJ . 2002 . CHv. Physical control of spring–summer phytoplankton dynamics in the North Water, April–July 1998 . Deep Sea Research Part II , 49 : 4959 – 82 .
- Melle , W and Skjoldal , HR . 1998 . Reproduction and development of Calanus finmarchicus, C. glacialis and C. hyperboreus in the Barents Sea . Marine Ecology Progress Series , 169 : 211 – 28 .
- Melling , H , Gratton , Y and Ingram , G . 2001 . Ocean circulation within the North Water Polynya of Baffin Bay . Atmosphere–Ocean , 39 : 301 – 25 .
- Michel , C , Ingram , RG and Harris , LR . 2006 . Variability in oceanographic and ecological processes in the Canadian Arctic Archipelago . Progress in Oceanography , 71 : 379 – 401 .
- Mundy , CJ , Barber , DG and Michel , C . 2005 . Variability of snow and ice thermal, physical and optical properties pertinent to sea ice algae biomass during spring . Journal of Marine Systems , 58 : 107 – 20 .
- Niehoff , B , Madsen , SD , Hansen , BW and Nielsen , TG . 2002 . Reproductive cycles of three dominant Calanus species in Disko Bay, West Greenland . Marine Biology , 140 : 567 – 76 .
- Nielsen , TG , Ottosen , LD and Hansen , BW . 2007 . “ Structure and function of the pelagic ecosystem in an ice covered arctic fjord ” . In Carbon Cycling in Arctic Marine Ecosystems: Case Study Young Sound. Meddr , Edited by: Rysgaard , S and Glud , RN . 88 – 107 . Greenland : Bioscience Special Issue .
- Østvedt , OJ . 1955 . Zooplankton investigations from weathership ‘M’ in the Norwegian Sea, 1948–49 . Hvalrådets Skrifter , 40 : 1 – 93 .
- Pavshtiks , EA . 1977 . Seasonal variations in the age composition of copepod populations (Calanoida) in the Central Basin . Issleddovanija Fauny Morej/Exploration of the Fauna of the Seas , 19 : 56 – 73 .
- Pavshtiks , EA . 1983 . Some patterns in the life of the plankton of the Central Basin . Canadian Translation of Fishery and Aquatic Sciences , 4917 : 1 – 24 .
- Pedersen , G , Tande , K and Ottesen , GO . 1995 . Why does a component of Calanus finmarchicus stay in the surface waters during the overwintering period in high latitudes? . ICES Journal of Marine Science , 52 : 523 – 31 .
- Planque , B , Hays , GC , Ibanez , F and Gamble , JC . 1997 . Large scale spatial variations in the seasonal abundance of Calanus finmarchicus . Deep-Sea Research Part I – Oceanographic Research Papers , 44 : 315 – 26 .
- Polyakov , IV , Proshutinsky , AY and Johnson , MA . 1999 . Seasonal cycles in two regimes of Arctic climate . Journal of Geophysical Research , 104 ( C11 ) : 761 – 88 .
- Proshutinsky , AY , Polyakov , IV and Johnson , MA . 1999 . Climate state and variability of Arctic ice and water dynamics during 1946–1997 . Polar Research , 18 : 135 – 42 .
- Prygunkova , RV . 1968 . On the cycle of development of Calanus (Calanus glacialis Jaschnov) in the White Sea . Doklady Akademii Nauk USSR , 182 : 1447 – 50 .
- Prygunkova , RV . 1974 . Certain peculiarities in the seasonal development of zooplankton in the Chupa Inlet of the White Sea . Issleddovanija Fauny Morej/Exploration of the Fauna of the Seas , XIII ( XXI ) : 4 – 55 .
- Rigby , S and Milsom , CV . 2000 . Origins, evolution, and diversification of zooplankton . Annual Review of Ecology and Systematic , 31 : 293 – 313 .
- Ringuette , M , Fortier , L , Fortier , M , Runge , JA , Bélanger , S Larouche , P . 2002 . Advanced recruitment and accelerated population development in Arctic calanoid copepods of the North Water . Deep-Sea Research II , 49 : 5081 – 99 .
- Rudels , B , Friedrich , HJ and Quadfasel , D . 1999 . The Arctic Circumpolar Boundary Current . Deep-Sea Research II , 46 : 1023 – 62 .
- Runge , JA , McLaren , IA , Corkett , CJ , Bohrer , RN and Koslow , JA . 1986 . Molting rates and cohort development of Calanus finmarchicus and C. glacialis in the sea off southwest Nova Scotia . Marine Biology , 86 : 241 – 46 .
- Rysgaard , S , Nielsen , TG and Hansen , BW . 1999 . Seasonal variation in nutrients, pelagic primary production and grazing in high-arctic coastal marine ecosystem, Young Sound; North Eastern Greenland . Marine Ecology Progress Series , 179 : 13 – 25 .
- Sargent , JR , Eilertsen , HC , Falk-Petersen , S and Taasen , JP . 1985 . Carbon assimilation and production in phytoplankton in northern Norwegian fjords . Marine Biology , 85 : 109 – 16 .
- Sargent , JR and Falk-Petersen , S . 1981 . Ecological investigation on the zooplankton community of Balsfjorden, Northern Norway: Lipids and fatty acids in Thysanoessa inermis (Krøyer), Thysanoessa raschii (M. Sars) and Meganytiphanes norvegica (M. Sars) during mid-winter . Marine Biology , 62 : 131 – 37 .
- Sargent , JR and Falk-Petersen , S . 1988 . The lipid biochemistry of calanoid copepods . Hydrobiologia , 167/168 : 101 – 14 .
- Sargent , JR and Henderson , RJ . 1986 . “ Lipids ” . In The Biological Chemistry of Marine Copepods , Edited by: Corner , ECS . 59 – 108 . Oxford : Clarendon Press .
- Sargent , JR and Whittle , K . 1981 . “ Lipids and hydrocarbons in the marine food web ” . In Analysing Marine Ecosystems , Edited by: Longhurst , A . 491 – 533 . London : Academic Press .
- Scott , CL , Kwasniewski , S , Falk-Petersen , S and Sargent , JR . 2000 . Lipids and life strategies of Calanus finmarchicus, Calanus glacialis and Calanus hyperboreus in late autumn, Kongsfjorden, Svalbard . Polar Biology , 23 : 510 – 16 .
- Scott , CL , Kwasniewski , S , Falk-Petersen , S and Sargent , JR . 2002 . Species differences, origins and functions of fatty alcohols and fatty acids in the wax esters and phospholipids of Calanus hyperboreus, Calanus glacialis and Calanus finmarchicus from Arctic Waters . Marine Ecological Progress Series , 235 : 127 – 34 .
- Sekerak , AD , Thomson , D , Bain , H and Acreman , J . 1976 . Summer surveys of the marine ecology of Creswell Bay, Somerset Island, and Assistance Bay, Cornwallis Island, NWT. Unpublished Report by LGL Ltd, for Polar Gas. Ltd: 1–251. (Ref. by Longhurst A, Sameoto D, Herman A (1984). Vertical distribution of zooplankton in summer: Eastern Canadian archipelago . Journal of Plankton Research , 6 : 137 – 68 .
- Slagstad , D and Tande , KS . 1990 . Growth and production dynamics of Calanus glacialis in a pelagic food web . Marine Ecological Progress Series , 63 : 189 – 99 .
- Smith , SL . 1990 . Egg production and feeding by copepods prior to the spring bloom of phytoplankton in the Fram Strait area of the Greenland Sea . Marine Biology , 106 : 59 – 69 .
- Søreide JE , Falk-Petersen S , Nøst Hegseth E , Hop H , Carroll ML , Hobson K , et al . 2008 . Seasonal feeding strategies of Calanus in the high-Arctic Svalbard region . Deep-Sea Research II. doi:10.1016/jdrs2.2008.05.024
- Stillwell , W and Wassall , SR . 2003 . Docosahexaenoic acid: Membrane properties of a unique fatty acid . Chemistry and Physics of Lipids , 126 : 1 – 27 .
- Sundby , S . 2000 . Recruitment of Atlantic cod stocks in relation to temperature and advection of copepod populations . Sarsia , 85 : 277 – 98 .
- Tande , KS . 1982 . Ecological investigations on the zooplankton community in Balsfjorden, northern Norway: Generation cycles, and variation in body weight and body content of carbon and nitrogen related to overwintering and reproduction in the copepod Calanus finmarchicus (Gunnerus) . Journal of Experimental Marine Biology and Ecology , 62 : 129 – 42 .
- Tande , KS . 1991 . Calanus in North Norwegian fjords and in the Barents Sea . Polar Research , 10 : 389 – 408 .
- Tande , KS , Hassel , A and Slagstad , D . 1985 . “ Gonad maturation and possible life cycle strategies in Calanus finmarchicus and Calanus glacialis in the northwest part of the Barents Sea ” . In Marine Biology of Polar Regions and Effects of Stress on Marine Organisms , Edited by: Gray , JS and Christiansen , ME . 141 – 55 . New York , Chichester : Wiley and Sons .
- Tande , KS and Henderson , RJ . 1988 . Lipid composition of copepodite stages and adult females of Calanus glacialis in waters of the Barents Sea . Polar Biology , 8 : 333 – 39 .
- Ussing , HH . 1938 . The biology of some important plankton animals in the fjords of East Greenland . Meddeleser Groenland , 100 : 1 – 108 .
- Varpe , Ø , Jorgensen , C , Tarling , GA and Fiksen , O . 2007 . Early is better: Seasonal egg fitness and timing of reproduction in a zooplankton life-history model . Oikos , 116 : 1331 – 42 .
- Vinje T. 1997 . Ice fluxes through Fram Strait . Proceedings of the WCRP Conference on Polar Processes and Global Climate, 3–6 November 1997. ACSYS Project Office, Norwegian Polar Institute, Oslo .
- Vinje , T . 2001 . Anomalies and trends of ice extent and atmospheric circulation in the Nordic Seas during the period 1864–1998 . Journal of Climate , 14 : 255 – 67 .
- Vinogradov , ME . 1997 . Some problems of vertical distribution of meso- macroplankton in the ocean . Advances in Marine Biology , 32 : 1 – 92 .
- Visser , AW and Jonasdottir , SH . 1999 . Lipids, buoyancy and the seasonal migration of Calanus finmarchicus . Fishery and Oceanography , 8 : 100 – 06 .
- Ward , JH . 1963 . Hierarchical grouping to optimize an objective function . Journal of the American Statistical Association , 58 : 236 – 44 .
- Welch , HE , Bergmann , MA , Siferd , TD , Martin , KA , Curtis , MF Crawford , RE . 1992 . Energy flow through the marine ecosystem of the Lancaster Sound region, Canada . Arctic , 45 : 343 – 57 .
- Werner , I and Hirche , H-J . 2001 . Observations on Calanus glacialis eggs under the spring sea ice in the Barents Sea . Polar Biology , 24 : 296 – 98 .
- Wiborg , KF . 1954 . Animal plankton of the Norwegian coast and offshore waters of west and north-western Norway . Report Norwegian Fishery Investigations , 11 : 1 – 246 .
- With , C . 1915 . Copepoda I, Calanoida Amphiscandria . Report Dan Ingolf Expedition , 3 ( 4 ) : 1 – 210 .
- Wold , S , Albano , C , Dunn , J , Edlund , UK , Geladi , P Hellberg , S . 1984 . “ Multivariate data analysis in chemistry ” . In Chemometrics, Mathematics and Statistics , Edited by: Kowalski , BR . 17 – 95 . Norwell , , USA : D. Reidel Publishing Company .
- Zenkevitch , L. 1963 . Biology of the Seas of the USSR , 955 London : George Allen and Unwin .