Abstract
Small-scale grazing events where sea urchins have grazed kelp forests to barren grounds have been reported all along the NE Atlantic coast. One large-scale event has been reported where kelp forests along the Norwegian and Russian coast were grazed by sea urchins during the early 1970s. The barren ground area has persisted since. Different theories to explain the grazing event have been presented. This paper seeks to sort and summarize earlier published papers and national reports and to critically examine the most important theories presented to explain the grazing event. The conclusion is that the reason for the event is unknown and it is too late to find causes 40 years after it took place. However, new data and new geographical analysis tools provide insight into the extent and consequences of this dramatic event, and show re-vegetation of kelp forests in the southernmost area. Emphasis in future studies should be given to understand the reasons for shifts between the two ecosystem states and take advantage of the ongoing shift. Such basic ecological knowledge could provide an important basis for better understanding the system and further, to understand the extent to which other observed large-scale changes (e.g. climatic changes, fish stock reductions) affect kelp forest–sea urchin interaction.
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
Introduction
While destructive grazing by sea urchins has been reported from both hemispheres, from different latitudes, and has been reported to affect algal communities, seagrass beds and coral reefs (e.g. Lawrence Citation1975; Harrold & Pearse Citation1987; Birkeland Citation1989), large-scale shifts from highly productive, pristine kelp forests to desert-like barren grounds are mainly reported from temperate areas. According to Steneck et al. (Citation2002, Citation2004), large brown seaweeds (mainly kelps) have been predominant at northern temperate (boreal) rocky coasts throughout history, while mass occurrences of sea urchins and over-grazing of kelp forests to barren grounds are historically new events. These events are thought by some authors to have been caused by human activities that over-exploit predators of sea urchins, producing cascading perturbations down the food web (e.g. Estes et al. Citation1998; Tegner & Dayton Citation2000; Steneck et al. Citation2002, Citation2004). Kelp–sea urchin interactions are well studied in the Pacific and NW Atlantic, while this paper is focused on the situation in the NE Atlantic.
Naturally, various species of sea urchin inhabit kelp forests in low densities in the North Atlantic (Skadsheim et al. Citation1995; Steneck et al. Citation2004; Sjøtun et al. Citation2006), while densities during blooms may exceed 100 individuals m2 (Lang & Mann Citation1976; Hjörleifsson et al. Citation1995; Sivertsen Citation1997a). These aggregations of sea urchins form fronts that can remove macroalgae. Catastrophic shifts can switch ecosystems into a new stable state (Scheffer et al. Citation2001) and the resulting barren grounds may be dominated by sea urchins for decades (Elner & Vadas Citation1990; Keats Citation1991; Sivertsen Citation1997b; Steneck et al. Citation2004). The ability of sea urchin populations to persist on barren grounds is due to great phenotypic plasticity in response to low food availability (Russell Citation1998). A large primary and secondary production is lost and biodiversity is lowered when these rich underwater forests are denuded. Hence, there is a need for improving our understanding of the processes that cause changes from one ecological state to the other, and to understand the processes responsible for the persistence of the two states. Blooms of sea urchins and subsequent loss of kelp forests usually attract much attention and are important issues for nature and resource management, and have been intensely studied in the NW Atlantic and in the NE Pacific (Pringle Citation1986; Elner & Vadas Citation1990; Steneck et al. Citation2004). Although understanding of the importance of the kelp forest of the NE Atlantic has increased (Mann Citation2000; Christie et al. Citation2003; Norderhaug et al. Citation2005; Bartsch et al. Citation2008), the largest affected area of the Atlantic has largely been ignored by the authorities: approximately 2000 km2 of kelp forest along the Norwegian and Russian coast was grazed in the early 1970s and the sea urchin-dominated barren grounds have persisted since.
Ecological processes responsible for maintenance of the two states or shifts from one state to the other have been debated by, for example, Elner & Vadas (Citation1990), but there are still many questions that need to be answered (see Anon. Citation2002; Sivertsen Citation2006). Particularly, data that can contribute to identify the causes of sea urchin outbreaks and overgrazing of kelp in the NE Atlantic are generally lacking (Anon. Citation2002; Sivertsen Citation2006). The hypothesis that predators regulate sea urchin densities is in some areas well-documented (key stone predators like sea otters in the east Pacific, see Peterson & Estes Citation2001; predator functional groups among fish and larger invertebrates, see Steneck et al. Citation2004; Hereu et al. Citation2005), and there are strong indications that over-fishing of cod and other fish stocks has indirectly caused outbreaks of sea urchins in the NW Atlantic (Tegner & Dayton Citation2000; Steneck et al. Citation2004).
The kelp forest and barren ground state are fundamentally different in ecosystem structure and function: kelp forests are structurally complex, diverse systems (e.g. Marstein Citation1997; Christie et al. Citation2003), which have been ranked among the most highly productive systems on the planet (Mann Citation2000), while barren grounds are structurally simple with low productivity (Chapman Citation1981) and low biological diversity. Thus, over-grazing and formation of barren grounds cause cascading effects up the food web (Branch & Griffiths Citation1988; Field et al. Citation1980), and probably lead to implications for fish stocks and other organisms dependent on kelp production and shelter. On the other hand, grazing of kelp may be induced when sea urchins are free from predation control because of over-exploitation of sea urchin predators at higher food web levels (Elner & Vadas Citation1990; Estes et al. Citation1998; Steneck et al. Citation2004). Thus, it may be questioned whether sea urchin outbreaks are caused by over-fishing (Jackson et al. Citation2001), or if declining fish populations in coastal waters are due to the loss of kelp forests. Also, over-fishing may start a self-reinforcing process where the grazing of kelp forests further reduces coastal fish stocks by removing nursery areas, food sources and habitats.
This paper summarizes earlier published papers and more unavailable information (national reports) to update the status of knowledge and understanding of sea urchin grazing in the NE Atlantic. Emphasis is put on presenting recent and as yet unpublished data on changes in the distribution of barren ground and kelp forest along the Norwegian coast. For understanding the ecological implications of shifts between kelp forests and barren grounds, new and recent published data regarding the importance of kelp as habitat are discussed. We also want to identify the most important gaps of knowledge that need to be filled in order to make hypotheses for future studies focused on the understanding of causes and consequences of this ecological catastrophe. For the understanding of the NE Atlantic situation, it is important to take advantage of the more intensive research that has been done for many years in the NW Atlantic, which is a system with many similarities to the NE Atlantic. The NE and NW Atlantic share important ecosystem and dominant species similarities. In both regions, Strongylocentrotus droebachiensis (O.F. Müller, 1776) is the dominant grazer, Laminaria kelp the dominant primary producer, and predators include amphipods, crabs within the genus Cancer and Hyas and cod (Gadus morhua, Linnaeus, 1758) (McNaught Citation1999; Anon. Citation2002; Leland Citation2002; Leland et al. Citation2002; Steneck et al. Citation2002). However, there are also important differences between the NW and the NE Atlantic, e.g. the dominant kelp in the NW is Laminaria longicruris (de la Pylaie, 1824) while L. hyperborea ((Gunnerus) Foslie, 1884) dominate in the NE. Thus, results and explanations concerning kelp–sea urchin interactions in the NW Atlantic cannot be used in the NE Atlantic without caution.
Small-scale grazing events reported from the NE Atlantic
Small-scale local events of overgrazing of macrophytes by different species of sea urchins have been reported from the entire NE Atlantic. The southernmost incident is described by Clemente et al. (Citation2007), who found local areas overgrazed by Diadema antillarum (Philippi, 1845) in the Canary Islands. In France, Boudouresque et al. (Citation1980) described Paracentrotus lividus (Lamarck, 1816) as being responsible for grazing macrophytes, and they also observed mass mortality in local sea urchin populations. Paracentrotus lividus is also described as an important grazer at different sites in the Mediterranean (Hereu et al. Citation2005) and is reported to have caused local grazing of macroalgae in Lough Ine in Ireland (Norton Citation1978). In the UK, an episode of grazing of the kelp Laminaria hyperborea by high densities of the red sea urchin Echinus esculentus (Linnaeus, 1758) was reported by Jones & Kain (Citation1967). The green sea urchin Strongylocentrotus droebachiensis (also responsible for over-grazing of marine vegetation in both the NW Atlantic and in the N Pacific) is found all along the Norwegian coast (see below) and as far south as Swedish (Vasseur Citation1952) and Danish waters (Kattegat). Both Psammechinus miliaris (Gmelin, 1778) and S. droebachiensis have recently been reported to graze destructively, and to an increasing degree caused destructive grazing on vegetation related to boulder reefs in Denmark (Dahl et al. Citation2005; Ærtebjerg Citation2007; Dahl & Carstensen Citation2008).
Local grazing by S. droebachiensis has also been reported from the northernmost coasts of the Atlantic, Jan Mayen Island (Gulliksen et al. Citation1980) and the Svalbard archipelago (Gulliksen & Sandnes Citation1980), and into the Barents Sea (Novaja Semlja: Nordenskiöld Citation1880). Also, before the large over-grazing event along the Norwegian coast that took place 30–40 years ago (see below), there is evidence for high local densities of green sea urchins and destructive grazing in several fjords along the Norwegian coast (Mortensen Citation1943; Vasseur Citation1952; Anon. Citation2002). These events have later been confirmed by divers, own observations, and student theses (Frid & Thomassen Citation1995; Fredriksen Citation1999).
On a regional scale, Einarsson (Citation1994) and Hjörleifsson et al. (Citation1995) have described the distribution of the green sea urchin S. droebachiensis around Iceland, and found high densities and over-grazing in a few areas, particularly in fjords. Hjörleifsson et al. (Citation1995) described a sea urchin front during grazing moving through a kelp forest from deeper water towards the shore.
Large-scale grazing along the Norwegian and Russian coast
The most extensive and also long-lasting event of over-grazing in the NE Atlantic occurred along the Norwegian and Russian coast. Vast areas of Laminaria hyperborea kelp forests were reported to disappear due to grazing by the green sea urchin Strongylocentrotus droebachiensis at the start of the 1970s. This phenomenon was first reported by fishermen on the Norwegian coast, but later documented by a number of studies (Propp Citation1977; Sivertsen & Bjørge Citation1980; Sivertsen Citation1982, Citation1997a, Citationb, Citation2002a, Citation2006; Hagen Citation1983, Citation1987; Sivertsen & Wentzel-Larsen Citation1989; Skadsheim et al. Citation1993, Citation1995; Christie & Rinde Citation1995). Historical data suggest that kelp forests dominated rocky substrates in shallow waters along the Norwegian coast before 1970 (Grenager Citation1955, Citation1956, Citation1958; fishermen and kelp trawling industry reported observations), and only a few local grazing events by Strongylocentrotus and Echinus were reported before 1970 (in 1847, 1900, and 1935; see Anon. Citation2002). The start of this large-scale grazing event probably occurred more or less simultaneously and was reported from mid-Norway (from 63°30′ N) and northwards to north of 71° N and continued into the Russian waters (Murman and Kola area; Propp Citation1977). According to Levin et al. (Citation1998), S. droebachiensis is mainly confined to shallow waters in the south western Barents Sea (Murman coast and southern parts of Spitsbergen), but is rare or absent around Franz Josefs Land and the Laptev and Kara Sea. On the Norwegian coast, the outermost wave-exposed areas were still covered by kelp, while the less-exposed areas were dominated by sea urchins (Sivertsen Citation1982, Citation1997a; Skadsheim et al. Citation1995). On the barren grounds, densities of 20–120 sea urchins m−2 have been found. Based on data on kelp distributions from the 1960s, Sivertsen (Citation1997b) roughly estimated the total affected area on the Norwegian coast to be 2000 km2, but accurate estimates of kelp distribution and affected areas have not been made.
The distribution of kelp and barren grounds along the Norwegian coast has not been monitored systematically, but the distribution of kelp forests and barren grounds have been observed in a number of studies. The sea urchin dominated barren ground phase has now, with a few small-scale exceptions, dominated the affected area in Norwegian and Russian waters for almost 40 years (e.g. Christie Citation1998; Levin et al. Citation1998; Sivertsen Citation2002a, Citationb; Norderhaug & Christie Citation2007). Both Hagen (Citation1987, Citation1995) and Christie et al. (Citation1995) reported local sea urchin mortality and kelp re-vegetation, later followed by new grazing events.
Recent development
On a regional scale, a shift from barren ground to kelp forest is presently occurring in mid-Norway. A northward movement of the southern border between kelp forest dominated areas and sea urchin dominated barren grounds has occurred since the late 1980s (). Røv et al. (Citation1990) found kelp forest re-vegetation on earlier barren grounds around the island Frøya (at 63°40′ N), and reduced densities of sea urchins on other sites near the southern border (when compared to the findings of Sivertsen Citation1982). A further reduction of sea urchin densities followed by kelp re-vegetation was later documented by Skadsheim et al. (Citation1993, Citation1995), and Christie & Rinde (Citation1995) found a northwards movement of the border between barren grounds and kelp forests from 1990 to 1995. In 2000 the border had at least reached 64°10′ N (Sjøtun et al. Citation2001).
Figure 1. Retreating sea urchins. Movement of the border between kelp-dominated areas and barren ground from 1980 until 2007. The barren ground area had its largest extent in 1980 where the border extended south to 63°30′ N (Sivertsen Citation1982). Røv et al. (Citation1990) showed that the border had moved to 63o40′ N by 1990 and in 2000 the border had at least moved to 64o10′ N (Sjøtun et al. Citation2001). In 2007 the border was situated at 65°30′ N (Norderhaug & Christie Citation2007).
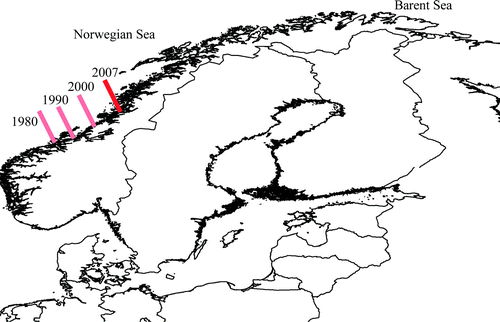
New, efficient sampling techniques combined with new software for spatial modelling and mapping (Lehman et al. Citation2002) provide powerful tools for describing the distribution of barren grounds and hence quantify the grazed areas. Recently, comprehensive data sets describing the distribution of kelp and barren grounds in the area around the southern border for the barren grounds were sampled by use of drop camera (including 833 stations; see Norderhaug & Christie Citation2007). These data confirm that the southern border between vast areas with barren ground and dominance of kelp forests has moved northward and was in 2007 situated at 65°30′ N.
Binomial data of kelp forest presence/absence (between south of Vikna 64°43′ N and north of Vega 65°30′ N) was used as response in a GAM (generalised additive model, GRASP software: Lehman et al. Citation2002) to analyse the distribution of kelp and barren grounds in the border area. The response data were analysed with respect to the available physical and topological data from the area (predictor variables). The predictors were depth, wave exposure, slope and latitude. Depth is important for light conditions and the growth of kelp (Sjøtun et al. Citation1995) down to 25–30 m, and depth also has an influence on the distribution of sea urchins (highest abundances on shallow subtidal hard bottom; Sivertsen & Hopkins Citation1995). Wave exposure is both important for the occurrence and growth of kelp (L. hyperborea is dependant on strong or moderate exposure; Sjøtun et al. Citation1998) and the survival and growth of sea urchins (sea urchins avoid strong exposure; Himmelmann Citation1986). Modelled wave exposure (according to Isæus Citation2004) was used in the analysis. Topological conditions may be expected to affect where kelps grow and where sea urchins can graze effectively (e.g. steep walls contra gentle slopes). Along the Norwegian coast, the kelp forests are grazed in the north but not in the south (which could, for example, be related to temperature or climatic-driven processes as well as biological processes: see later). Therefore, latitude was included as a factor in the model. All the predictors were included in a full model and significant predictors were selected according to the Aikake Selection Criterion (AIC; see Burnham & Anderson Citation2001). The selected statistical model was validated cross- (see Lehman et al. Citation2002).
Laminaria hyperborea was found in shallow waters from the surface and down to approximately 30 m (). It was found at many levels of exposure (inner to outer coastal areas), but was most abundant in outer highly exposed areas.
Figure 2. Occurrence of kelp (Laminaria hyperborea) in relation to different variables. Columns show total number of registrations for different values of the variables depth, exposure (SWM), slope (SLOPE) and latitude (LAT, WGS84 UTM 33). Dark shading shows the registration of kelp and light shading no occurrence of kelp. Solid lines show the proportion of kelp registrations out of the total number of registrations.
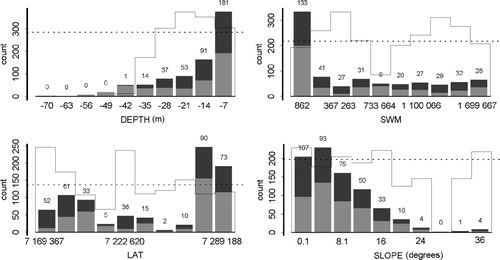
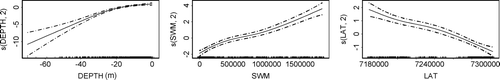
Of the tested predictors depth, exposure and latitude had a significant impact on the distribution of kelp within the examined area (). Depth had a negative effect, exposure a positive effect, and latitude a negative effect northward. The explanatory power of the statistical model was high according to cross-validation (cvROC = 0.80).
Figure 3. Relative effects within the GAM model from the significantly important predictors (depth, wave exposure (SWM) and latitude (LAT, WGS84 UTM 33), Df) for the distribution of kelp (Laminaria hyperborea, solid line) with 95% C.I. (dotted lines).
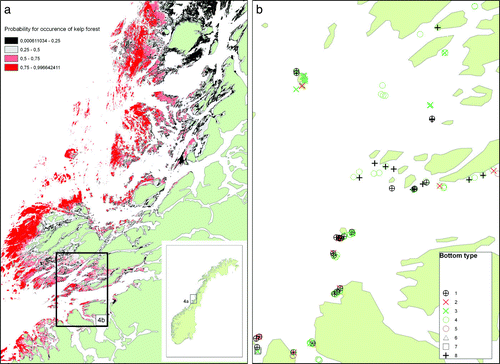
The probability of occurrence of kelp forest in the border area was modelled in GIS (a). The prediction shows the border clearly. The area just south of the border seems to be in an unstable ecological phase between barren ground and kelp forest (b). This area is a mosaic of patches with young, homogenous kelp forest and ‘pockets’ with green sea urchins Strongylocentrotus droebachiensis on barren grounds. Patches with scattered, young kelps being grazed by red sea urchins Echinus esculentus were also observed, and increasing densities of the red sea urchin E. esculentus on recently re-vegetated areas has also been observed further south (Sjøtun et al. Citation2006). In the outer wave-exposed areas, old heterogeneous kelp forest (characterized by kelps of different age and sizes, including large kelps over-grown with epiphytic organisms on the stipes) dominated. South of 64°43′ N, heterogeneous and well-developed kelp forest dominates. Here, few and mainly red sea urchins are observed. North of 65°30′ N, barren grounds with green sea urchins Strongylocentrotus droebachiensis dominate and kelp forests are only found in highly exposed outer areas.
Figure 4. Probability model for the distribution of kelp in the border area between barren grounds and areas dominated by kelp in 2007. In red areas there is a high probability for occurrence of kelp (Laminaria hyperborea), and in grey and black areas there is a low probability. White areas are outside the fundamental niche for kelp or outside the area of which the model can predict probabilities (e.g. in fjords). The spatial GIS model is predicted from statistical GAM models from the GRASP software (Lehman et al. Citation2002), with depth, wave exposure (according to the SWM fetch model: Isæus 2004) and latitude as predictors and kelp occurrence as the response variable. (b) Stations in a smaller area illustrating the mosaic of different bottom types just south of the kelp forest-barren ground border (marked with a rectangle in 2a). 1: Stations with scattered young Laminaria kelp being grazed by red sea urchins (Echinus esculentus). 2: Barren ground dominated by red sea urchins. 3: Barren ground dominated by green sea urchins (Strongylocentrotus droebachiensis). 4: Young, homogenous kelp (L. hyperborea) forest and few or no sea urchins. 5: Well-developed (old) kelp (L. hyperborea) forest and few sea urchins. 6: Laminaria digitata forest. 7: Laminaria saccharina forest. 8: Soft bottom.
System stability and shifts between kelp forests and sea urchin-dominated barren grounds
The kelp forest-dominated state
Kelp forests are stable ecosystems with a high capability to withstand mechanical stress and recover after disturbances (Elner & Vadas Citation1990; Christie et al. Citation1998). The high persistence and resilience is caused by high densities of (understorey) recruits that quickly replace adults as they die or are thrown off by waves or other disturbances (Christie et al. Citation1998; Rinde & Sjøtun Citation2005; Sjøtun et al. Citation2006; Rinde Citation2007). Associated communities do also recover shortly after disturbances (Christie et al. Citation1998; Norderhaug et al. Citation2002; Waage-Nielsen et al. Citation2003). Besides kelp (L. hyperborea), key components (functional groups) of the kelp forest are: epiphytic algae and fauna attached to the kelp stipes, mobile invertebrates, and fish. Mature kelp stipes are heavily over-grown by epiphytes, mainly red algae (Whittick Citation1983; Marstein Citation1997) and sessile animals (Schultze et al. Citation1990; Christie et al. Citation1998). Large densities of mobile invertebrates, dominated by crustaceans and gastropods, are associated with the kelp (Norderhaug et al. Citation2002; Christie et al. Citation2003). Fish aggregate over the kelp forest, in between the kelps and on the sea floor (Fosså Citation1995; Norderhaug et al. Citation2005). Fresh kelp is not the preferred food source of most grazers (see Christie & Rueness Citation1998; Norderhaug et al. Citation2003), but many species obtain their food from kelp-produced carbon (Fredriksen Citation2003), and the majority of the fauna feed on particulate organic matter from kelp enriched by microorganisms rather than grazing directly on kelp and epiphytic algae (Norderhaug et al. Citation2003; Norderhaug Citation2004). Fish predation, migration and space limitations contribute to limit the amount of small grazers (Jørgensen & Christie Citation2003; Norderhaug et al. Citation2005, Citation2007). All these factors and a possible high functional redundancy (see Duffy et al. Citation2001) among the kelp-associated flora and fauna increase the persistence and stability of the kelp ecosystem.
Sea urchins are scarce in this pristine kelp state. Settlement of sea urchin larvae may be high both in kelp forests and on barren grounds (Rowley Citation1989; Schroeter et al. Citation1996; Christie & Rueness Citation1998), but survival of post-settlement juveniles is typically low in macrophyte systems (Christie & Rueness Citation1998; Hereu et al. Citation2005). Kelp holdfasts are used as a habitat by juvenile sea urchins (Christie & Rueness Citation1998), but holdfasts are also inhabited by possible micro-predators including juvenile crabs and smaller invertebrates (Christie et al. Citation2003; Woll et al. Citation2004) that are found to prey on sea urchin recruits in the NW Atlantic (McNaught Citation1999; Vavrinec et al. Citation2001; Clemente et al. Citation2007; Scheibling & Robinson Citation2008). Micro-predation may decimate entire cohorts of juvenile sea urchins (McNaught Citation1999) and the negative effect for juvenile urchins by kelp as a predator habitat may be larger than the positive effect as a refuge (Vavrinec Citation2003). Adult green sea urchins are seldom found in dense kelp forest (Hjörleifsson et al. Citation1995; Skadsheim et al. Citation1995), but may accumulate along the edge of kelp forests or in clearings within the kelp forest (Norderhaug & Christie Citation2007). Why adult green sea urchins are hardly observed in dense kelp forests is not known.
Sea urchin aggregation and aggressive grazing
A behavioural change occurs when sea urchins aggregate in fronts and start grazing down the kelp forest. This perturbation is too large for the system to resist, since the main primary producer (kelp), the structural habitat, and thus, all species at higher ecosystem levels (except sea urchins) vanish. The reason why green sea urchins change their behaviour to form fronts and initiate aggressive grazing has been debated for decades, but none of the suggested hypotheses have been supported by scientific data (Vadas & Steneck Citation1995; Anon. Citation2002). The behavioural change of sea urchins is most likely caused by abrupt increases in population density, driven by changes in either pre- or post-settlement processes or both.
Sivertsen (Citation1997b, Citation2006) claims that successful recruitment of sea urchins is rare, and suggests that pre-settlement processes are important in controlling adult population size. Sivertsen (Citation2006) found a correlation between the abundance of pelagic echinoderm larvae and low water temperature, and used this observation to conclude that extensive settlement and subsequent recruitment of sea urchins leading to strong cohorts would be a rare and unpredictable event driven by climatic conditions. However, whenever it happens, it may increase the abundance of sea urchins to a level where it triggers a behavioural change leading to aggressive grazing, a situation that may then prevail for decades until the strong cohort dies.
Although appealing, we lack strong data to support this theory since the monitoring data used by Sivertsen (Citation2006) considered all echinoderm larvae and were obtained from deep water samples collected far from the coastline. On the other hand, some observations may provide support for this theory. For example, some observations from Norway (Sivertsen Citation2006) and Iceland (Hjörleifsson et al. Citation1995; Hjörleifsson and colleagues, personal communication) show that entire sea urchin populations can be comprised of one or a few cohorts, thus providing indirect evidence that pre-settlement processes (i.e. enhanced larval survival and subsequent settlement) may be important. We suggest that future studies should focus on sea urchin on-site settlement and recruitment combined with cohort studies over years as a basis to be able to understand the importance of these processes.
On the other hand, Hereu et al. (Citation2004, Citation2005) and Clemente et al. (Citation2007) argue that post-settlement processes are more important for the regulation of sea urchin population size. Sivertsen (Citation2006) precluded the importance of predation control of sea urchins along the Norwegian coast based on estimates of predation rates combined with abundance estimates of some of the most obvious predators on sea urchins. However, reliable estimates of the impact of predation are difficult to make based on such numbers, and the importance of predation control on sea urchin populations may be different during the outbreak phase and in the barren ground phase. Studies from the NW Atlantic suggest, however, that predation from fish and invertebrate predators on juvenile and adult sea urchins is the most important factor controlling sea urchins abundance (Steneck & Carlton Citation2001; Steneck et al. Citation2002; Leland et al. Citation2002; Vavrinec et al. Citation2001). If the controlling predator(s) is(are) removed for some reason, cascading effects may be expected, and sea urchin numbers should increase (Steneck et al. Citation2004). However, we lack proper population analyses and studies that can identify the causes of mortality and potential predator impacts on different life-stages of sea urchins. This is needed to assess if predation is important in regulating urchin abundance in NE Atlantic.
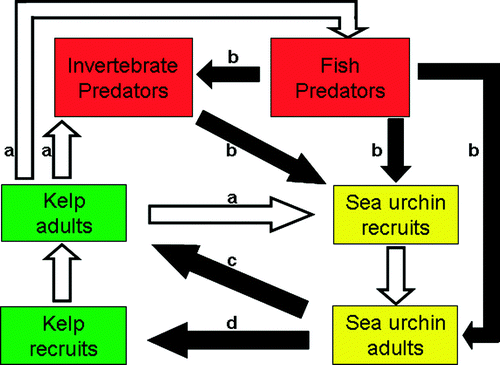
So far, the reasons behind sea urchin blooms in the NE Atlantic are not fully understood, but potentially important interactions between kelp, sea urchins and possible predators are summarized in . Finally, sea temperature, other environmental factors, and predation pressure on pelagic larvae will influence sea urchin recruitment success.
Figure 5. Conceptual figure describing possibly important interactions (arrows) between kelp (Laminaria hyperborea), sea urchins (Strongylocentrotus droebachiensis) and potentially important predators. Black arrows indicate a negative effect and white arrows a positive effect. (a) Adult kelp provides a habitat for sea urchin recruits, invertebrate predators and a nursery area and habitat for fish. (b) Invertebrate and fish predators prey on sea urchin recruitment. (c) If sea urchins become abundant they aggregate and graze down the kelp forest. Then the kelp and associated predators are lost and only sea urchins survive. (d) When the kelp forest is lost, sea urchin populations prevent kelp from re-establishing by grazing.
The barren ground state
Having removed the kelp forest, sea urchins prevail on the barren ground in a new stable state (Elner & Vadas Citation1990; Sivertsen Citation1997a, Citationb). The kelp habitat and, thus, most of the sea urchin predators that live associated with kelp have disappeared, leaving the sea urchin populations more or less undisturbed. Sea urchins may live for many years (Russell Citation1998; Vadas et al. Citation2002; Ebert & Southon Citation2003), but the fact that the barren ground has lasted for almost 40 years in Norwegian waters indicates that recruitment to the adult population is needed in order to sustain the high population density. However, the survival of post-settlement juveniles is probably sufficient for maintaining high population density on the barren grounds (Christie & Rueness Citation1998; Hereu et al. Citation2004). The juvenile urchins recruit to the adult population after about two years (Himmelmann Citation1986; Sivertsen Citation1997a). Sea urchins on barren grounds feed on drift algae and newly settled algae (Chapman Citation1981), but food supplies on barren grounds are typically low. Consequently, the condition of adult urchins is sub-optimal and mortality tends to be high (Sivertsen Citation1997a; Christie & Rueness Citation1998). Christie & Rueness (Citation1998) suggested, therefore, that barren ground populations depend on regular recruitment to prevent a population crash, but this idea is being debated by other authors, who suggest that urchin populations on barrens grounds only need occasional recruitments due to a long life span (Sivertsen Citation1997b; Vadas et al. Citation2002). Sivertsen (Citation2006) suggested that recruitment strategies may differ with latitudes, but this has not been studied by repeated sampling of recruitment and mortality between years.
The distribution of local sea urchin populations inside fjords seems to be controlled by salinity and fjord circulation. Fredriksen (Citation1999) found Strongylocentrotus droebachiensis occurring below the halocline in the Oslo fjord, South Norway, and similar occurrences have been observed in fjords further north (personal observations). A possible explanation for the maintenance of isolated fjord populations is that S. droebachiensis is trapped in cold and saline waters below the halocline (according to Lange Citation1964), and by spawning in deeper waters larvae are kept inside the fjords and avoid being flushed out by warm and less saline surface water typical for these fjord systems (estuarine circulation). This seems to be valid at least for several fjords with shallow sills, where cold and saline waters dominate in the layers below the halocline and support S. droebachiensis further south than in coastal water.
Reduction of sea urchins and re-establishment of kelp forests
Since the barren ground state seems persistent, some sort of perturbation may be necessary to induce a shift from barren ground to kelp forest state. Shifts from barren grounds to kelp forests are often initiated by reduced sea urchin density caused by local mass mortality (Hagen Citation1987; Christie et al. Citation1995; Leinaas & Christie Citation1996; see also Scheibling & Stephenson Citation1984 from the NW Atlantic). Events of mass mortality among sea urchins may be caused by an increased presence and/or activity by predators, increased frequency of diseases and/or parasites or by changes in environmental factors that may affect survival.
Changes in the physical environment may cause the observed disappearance of sea urchins along the coast of mid-Norway () if green sea urchins are sensitive to temperature increases. Since the mid-1980s there has been an increasing trend in Northern Hemisphere temperature and also the North Atlantic Oscillation index (NAO) has influenced temperature (see Beaugrand et al. Citation2003; Hurrell & Dickson Citation2004). The temperature increase is expected to have an opposite effect on the growth conditions for kelp and sea urchins, which may affect the interaction between the two species. Strongylocentrotus droebachiensis has a northern distribution and is associated with cold water (Stephens Citation1972), and the temperature increase may have affected the latitudinal distribution of this species. Strongylocentrotus droebachiensis may retreat further north if the temperature increase continues. Laminaria hyperborea is a more temperate distributed species, with highest recruitment and faster growth rates further south (mid-Norway: Rinde & Sjøtun Citation2005), and it has been shown that the distribution of kelp is affected by temperature fluctuations (Walker Citation1956; Gray & Christie Citation1983). To our knowledge, similar episodes of retreating sea urchins have not been reported from other areas in the NE Atlantic, but the distributions of pelagic echinoderm larvae and larvae from many other species have been found to change along latitudinal gradients (Lindley & Batten Citation2002).
Direct effects from physical factors could be enforced by indirect effects: predators on sea urchins are moving northwards with increasing temperatures. It has been reported that several fish stocks have moved their distribution northwards, and fisheries statistics document that the crab Cancer pagurus (Linnaeus, 1758) has been more common northwards over the last 10 years (see later). Also, other predators that are not monitored may move northwards and contribute to the observed termination of the sea urchin dominated barren grounds.
In the NW Atlantic, sea urchin fishery during the 1990s reduced adult populations to levels low enough to allow kelp forests to re-establish. Increased populations of crustaceans (amphipods, Cancer and Hyas crabs) inhabiting the re-established kelp forest seemed to play a crucial role in increasing the regulation of the population density of S. droebachiensis (McNaught Citation1999; Leland Citation2002; Leland et al. Citation2002; Steneck et al. Citation2002), since crabs were able to decimate sea urchin recruits shortly after settlement (Vavrinec et al. Citation2001). Thus, reduced adult sea urchin density allowed re-establishment of the kelp forest. This affected sea urchin populations further by increasing the predation pressure and reducing sea urchin recruitment.
Other perturbations that have been suggested to cause population collapse of green sea urchin populations in the NE Atlantic include outbreaks of disease and parasite control, but these theories have later been rejected. Amoeba-induced mass mortality among urchins has been observed along the Canadian coast (Jones & Scheibling Citation1985; Scheibling & Hennigar Citation1997), but microbial screenings of sea urchins from the Norwegian coast did not provide any evidence for infections which could have been responsible for local mass mortality of sea urchins observed at 66°44′ N (Christie et al. Citation1995). It has also been suggested that parasites may cause local urchin mass mortality (Hagen Citation1995), but the observed infection frequency by the nematode Echinomermella matsi (Jones and Hagen, 1987) did not regulate sea urchin populations (Stien et al. Citation1995, Citation1998), and Skadsheim et al. (Citation1995) observed a local sea urchin population crash in the same area where E. matsi did not occur at all. However, heavy parasite infections reduced the fitness of the sea urchins (Sivertsen Citation1996; Stien et al. Citation1998), and may thus contribute to affect the urchin population stability on barren grounds by reducing the ecosystem robustness from outside disturbances (see Begon et al. Citation1990).
Consequences of deforestation, the importance of the kelp forest to the larger coastal ecosystem
The barren ground state found along the Norwegian coast has lasted for almost 40 years and has affected production and diversity along a coastline of about 1300 km. Preliminary estimates indicate that roughly 2000 km2 kelp forests have been lost due to over-grazing by sea urchins (Sivertsen Citation1997b; Anon. Citation2002). These estimates imply that a standing stock of 20 million tons of kelp (fresh weight, which could tie up 7–8 millions of tonnes of CO2) has been lost.
The lost kelp forest represents an annual primary production of 20 million tons (fresh weight estimated from Sjøtun et al. Citation1995; Abdullah & Fredriksen Citation2004) that is no longer available for the coastal secondary producers. Kelp forests contain a rich diversity of marine plants and animals (Whittick Citation1983; Schulze et al. Citation1990; Marstein Citation1997; Christie et al. Citation1998, Citation2003) and the invertebrate fauna (often exceeding 105 individuals m−2) makes up an important food source for most fish found in or near the kelp forest (Fredriksen Citation2003; Norderhaug et al. Citation2005). Many coastal fish stocks use the kelp forests as nursery areas (Keats et al. Citation1987; Godø et al. Citation1989). Due to the great spatial and temporal extent of kelp forest losses, this event must be considered as one of the largest ecological catastrophes reported in Norway, regardless if the causes to the sea urchin bloom is man-made or not.
Large-scale deforestation has been suggested to be one of several causes for the observed decline of coastal cod north of 62°N (reported by Berg Citation2006). The reduction started in the early 1980s, and cod from that area has been put on the Norwegian ‘red list’ for vulnerable and endangered species (Kålås et al. Citation2006). The disappearance of kelp forests is a possible cause of the reduction of coastal fish stocks because kelp disappeared before the decline in, for example, cod stocks. On the other hand, over-fishing may well have started a self-reinforcing process where reduced predatory fish stocks have stimulated an outbreak of sea urchins followed by loss of the kelp forest. The loss of kelp forests may then have had a negative feedback effect on the fish stocks because important nursery grounds have been lost.
There are also some noticeable similarities between the events of possible cascade effects on the coast of Maine and the events on the coast of Norway. Crabs of the genus Cancer have been reported to occur in higher densities in areas where this species earlier was scarce during the last years (see Vavrinec et al. Citation2001 from the Maine coast). This is also documented from the NE Atlantic, because the catch reports from Norwegian Fishery Authorities indicates an extension of Cancer crab fisheries from about 65o N to 69o N during the last 10 years (see also Woll et al. Citation2004). According to Steneck et al. (Citation2004) crabs may take over the niche of predators if fish (cod) disappear, including the controlling impact on sea urchin populations.
The loss of kelp production will also affect food chains in the adjacent coastal ecosystems, because kelp forests are export systems that provide primary and secondary production for ecosystems on deep and shallow waters (Field et al. Citation1980; Branch & Griffiths Citation1988; Duggins et al. Citation1989; Vetter Citation1995; Jørgensen & Christie Citation2003). In Norwegian waters, kelp forests are also feeding areas for a number of seabirds (Bustnes et al. Citation1997; Fredriksen Citation2003). Therefore, local communities and regional and national authorities have called for actions to restore the kelp forests.
Concluding remarks
Many local events of destructive grazing by sea urchins in the NE Atlantic have been reported, but the large-scale event covering the mid- and north- Norwegian coast continuing into Russian waters is the largest and most long-lasting event, and must be considered as one of the largest ecological catastrophes reported in Norway. Different theories have been proposed to explain this large-scale grazing event, but the causes remain virtually unproven. The main reason why we do not fully understand the factors and processes that led to the massive blooms of sea urchins 30–40 years ago is simply that no investigations were undertaken at the time of the major outbreak. Investigations that have been conducted in the NE Atlantic instead represent more or less loose inferences performed many years after the events took place (Hagen Citation1983, Citation1987; Skadsheim et al. Citation1995; Sivertsen Citation1997a, Citationb, Citation2006). Now, it is obviously too late to perform field studies to test the causes for the event, and we must therefore try to identify the factors and processes that are responsible for: (1) the continuous dominance of sea urchins in barren grounds, and (2) the potential of kelp forests to recover when and if the sea urchin dominance comes to an end. The spatial distribution of kelp forests and sea urchins should also be monitored in order to correlate distributional changes to possible processes that may act as forcing agents in determining where kelp forest and sea urchins dominate.
In order to identify the factors that control sea urchin population size during an outbreak and under barren ground conditions, it seems important to test the hypothesis proposed by Sivertsen (Citation2006) about the importance of pre-settlement processes and regular versus episodic sea urchin recruitment. It seems equally important to investigate further the importance of predation control on different life-stages of sea urchins under different conditions (i.e. barren ground vs. kelp forest state), and particularly so for the younger stages because they may be very important for the future population size. Finally, long-term cohort studies of sea urchin populations under barren ground and kelp forest conditions, respectively, should provide valuable insight into the population dynamics under different conditions.
It may also be important to investigate whether or not kelp forests are able to re-establish if and when the urchin blooms comes to an end. Re-establishment of the kelp forest has been observed locally in some places and is quite likely in areas where intact patches of kelp forest are present in the neighbourhood and can serve as source populations. We have no idea, however, what will happen if urchins disappear from vast areas of barren ground – i.e. in over-grazed areas with a very large spatial extension and without nearby source populations of kelp.
Elner & Vadas (Citation1990) stated that the understanding of the kelp–sea urchin system should be built on properly designed research, instead of weak evidences or inferences based on field observations. They stressed that caution should be taken when drawing conclusions from weak evidences, and they referred to the paradigm of lobster as a proposed controlling predator of sea urchin populations. This paradigm has been used in textbooks without being tested properly. In a recent review, Bartsch et al. (Citation2008) refer to the endoparasite Echinomermella matsi (Hagen Citation1987) and the wolfish (Anarhichas lupus (Linnaeus, 1758), Sivertsen & Bjørge Citation1980) as controlling predators of sea urchin populations along the Norwegian coast, although evidence for their importance is lacking and has been questioned (see Stien et al. Citation1995, Citation1998; Anon. Citation2002). The statements of Elner & Vadas (Citation1990) still seem to be valid for the NE Atlantic kelp–sea urchin system.
This paper points out a number of uncertainties, particularly in the section discussing stability and shifts between the ecological states represented by kelp forests and barren grounds. Thus, further studies of sea urchins and their responses to environmental and interfering biological factors may improve our understanding. Relationships between sea urchin mortality and recruitment over following years are still possible to investigate. This paper has presented new observations on decreasing dominance of sea urchins in mid-Norway, and this ongoing large-scale shift at the southern border of where sea urchins used to dominate provides a unique opportunity to study the dynamics of the system to increase the understanding of controlling factors and reasons for shifts between the two stable states.
Editorial responsibility: Franz Uiblein
Acknowledgements
We thank the editors, Professor Morten Foldager Pedersen, Professor Stein Fredriksen and Dr Eli Rinde, for useful comments to earlier versions of the manuscript.
Notes
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
References
- Abdullah , MI and Fredriksen , S . 2004 . Production, respiration and exudation of dissolved organic matter by the kelp Laminaria hyperborea along the west coast of Norway . Journal of the Marine Biological Association of the United Kingdom , 84 : 887
- Anon 2002 . Nedbeiting av tareskog i Norge . Report to Ministry of Fishery and Coastal Affairs. 49 pages
- Bartsch , I , Wiencke , C , Bischof , K , Buchholz , CM , Buck , BH Eggert , A . 2008 . The genus Laminaria sensu lato: Recent insights and developments . European Journal of Phycology , 43 : 186
- Beaugrande , G , Brander , KM , Lindley , JA , Souissi , S and Reid , PC. 2003 . Plankton effect on cod recruitment in the North Sea . Nature , 426 : 661 – 64 .
- Begon M , Harper JL , Townsend CR . 1990 . Ecology. Individuals, Populations and Communities . Oxford : Blackwell Scientific Publication . 945 pages
- Berg E. 2006 . Norsk kysttorsk. Havets ressurser, Fisken og havet special issue 1 Bergen : IMR 121 23
- Birkeland C. 1989 . The Influence of Echinoderms on Coral-reef Communities . Echinoderm Studies 3 . Rotterdam : Balkema . 79 pages
- Boudouresque , CF , Nedelec , H and Shepherd , SA. 1980 . The decline of a population of the sea urchin Paracentrotus lividus in the Bay of Port-Cros (Var, France) . Travaux Scientifique du Parc national de Port-Cros , 6 : 243 – 51 .
- Branch , GM and Griffiths , CL. 1988 . The Benguela ecosystem. Part V. The coastal zone . Oceanography and Marine Biology Annual Review , 26 : 395 – 486 .
- Burnham , KP and Anderson , DR. 2001 . Kullback-Leibler information as a basis for strong inference in ecological studies . Wildlife Research , 28 : 111 – 19 .
- Bustnes JO , Christie H , Lorentsen SH . 1997 . Sjøfugl, tareskog og taretråling: en kunnskapsstatus . NINA Oppdragsmelding 472 . 43 pages .
- Chapman , ARO. 1981 . Stability of sea urchin dominated barren grounds following destructive grazing of kelps in St. Margaret's Bay, eastern Canada . Marine Biology , 62 : 307 – 11 .
- Christie H . 1998 . Økologiske effekter av varm sommer 1997 – kråkebolletetthet langs kysten av Midt-Norge . Rapport til Direktoratet for Naturforvaltning (DN) . 9 pages
- Christie , H , Fredriksen , S and Rinde , E. 1998 . Regrowth of kelp and colonisation of epiphyte and fauna community after kelp trawling at the coast of Norway . Hydrobiologia , 375/376 : 49 – 58 .
- Christie , H , Jørgensen , NM , Norderhaug , KM and Waage-Nielsen , E. 2003 . Species distribution and habitat exploitation of fauna associated with kelp (Laminaria hyperborea) along the Norwegian coast . Marine Biological Association of the United Kingdom , 83 : 687 – 99 .
- Christie , H , Leinaas , HP and Skadsheim , A. 1995 . “ Local patterns in mortality of the green sea urchin, Strongylocentrotus droebachiensis, at the Norwegian coast ” . In Ecology of Fjords and Coastal Waters , Edited by: Skjoldal , HR , Hopkins , C , Erikstad , KE and Leinaas , HP . 573 – 84 . Amsterdam : Elsevier .
- Christie H , Rinde E . 1995 . Endringer i kråkebolleforekomst, kråkebolleparasitt og bunnalgevegetasjon langs kysten av Midt-Norge . NINA Oppdragsmelding 359 . 39 pages .
- Christie , H and Rueness , J. 1998 . “ Tareskog ” . In Kystøkologi, den ressursrike norskekysten , Edited by: Rinde , E , Bjørge , A , Eggereide , A and Tufteland , G. 164 – 89 . Oslo : Universitetsforlaget .
- Clemente , S , Hernandez , JC , Toledo , K and Brito , A. 2007 . Predation upon Diadema aff. antillarum in barren grounds in the Canary Islands . Scientia Marina , 71 : 745 – 54 .
- Dahl K , Carstensen J . 2008 . Tools to assess conservation status on open water reefs in Nature-2000 areas . Aarhus University NERI Technical Report 663 . 32 pages .
- Dahl K , Lundsteeen S , Tendal OS . 2005 . Mejlgrund og Lillegrund. En undersøkelse af biologisk diversitet på et lavvandet område med stenrev i Samsø Bælt . Aarhus University DMU Report 529 . 87 pages .
- Duffy , JE , MacDonald , KS , Rhode , JM and Parker , JD. 2001 . Grazer diversity, functional redundancy, and productivity in seagrass beds: An experimental test . Ecology , 82 : 2417 – 34 .
- Duggins , DO , Simenstad , CA and Eestes , JA. 1989 . Magnification of secondary production by kelp detritus in coastal marine ecosystems . Science , 245 : 170 – 73 .
- Ebert , TA and Southon , JR. 2003 . Red sea urchin (Strongylocentrotus franciscanus) can live over 100 years: Confirmation with A-bomb (14) carbon . Fishery Bulletin , 101 : 91522
- Einarson , ST. 1994/k . The distribution and density of green sea urchin (Strongylocentrotus droebachiensis) in Icelandic waters. ICES . CM Documents , 38 : 1 – 20 .
- Elner , RW and Vadas , RL. 1990 . Inference in ecology: The sea urchin phenomenon in the Northwest Atlantic . American Naturalist , 136 : 108 – 25 .
- Estes , JA , Tinker , MT , Williams , TM and Doak , DF. 1998 . Killer whale predation on sea otters linking oceanic and nearshore ecosystems . Science , 282 : 473 – 76 .
- Field , JG , Grifiths , RJ , Jarman , N , Zoutendyk , P , Velimirov , B and Bowes , A. 1980 . Variation in structure and biomass of kelp communities along the South-West Cape coast . Transactions of the Royal Society of South Africa , 44 : 145 – 203 .
- Fosså JH . 1995 . Forvaltning av stortare: prioriterte forskningsoppgaver . Bergen : Institute of Marine Research . 102 .
- Fredriksen K. Vertikalfordeling og livshistorie hos kråkebollen Strongylocentrotus droebachiensis i Drøbaksundet Cand. Scient. Thesis University of Oslo 1999 81 pages
- Fredriksen , S. 2003 . Food web studies in a Norwegian kelp forest based on stable isotope (d13C and d15N) analysis . Marine Ecology Progress Series , 260 : 71 – 81 .
- Frid , LI and Thomassen , Ø. 1995 . Bunnslåing av kråkeboller (Echinoidea; Echinodermata) i Drøbaksundet , 84 pages Cand. scient. thesis : University of Oslo .
- Godø OR , Gjøsæter J , Sunnanå K , Dragesund O . 1989 . Spatial distribution of 0-group gadoids off mid-Norway . Report of the International Council for the Exploration of the Sea 191 : 273 Oslo 80 .
- Gray JS , Christie H . 1983 . Predicting long-term changes in marine benthic communities . Marine Ecology Progress Series 13 : 87 Oslo 94 .
- Grenager B . 1955 . Kvantitative undersøkelser av tareforekomster i sør-Helgeland 1952 og 1953. Norwegian Institute of Seaweed Research . Report 7 . 70 pages .
- Grenager B . 1956 . Kvantitative undersøkelser av tareforekomster i øst-Finmark 1953. Norwegian Institute of Seaweed Research . Report 13 . 37 pages .
- Grenager B . 1958 . Kvantitative undersøkelser av tang- og tareforekomster i Helgøy, Troms 1953 . Norwegian Institute of Seaweed Research . Report 21 . 31 pages
- Gulliksen , B , Haug , T and Sandnes , O. 1980 . Benthic macrofauna on new and old lava grounds at Jan Mayen . Sarsia , 65 : 137 – 48 .
- Gulliksen , B and Sandnes , O. 1980 . Marine bunndyrsamfunn, nøkkelarter og felteksperimenter på hardbunn . Fauna , 33 : 1 – 9 .
- Hagen , NT. 1983 . Destructive grazing of kelp beds by sea urchin in Vestfjorden, northern Norway . Sarsia , 68 : 177 – 90 .
- Hagen , NT. 1987 . Sea urchin outbreaks and nematode epizootics in Vestfjorden, northern Norway . Sarsia , 72 : 213 – 29 .
- Hagen , NT. 1995 . Recurrent outbreak of successionally immature kelp forest by green sea urchins in Vestfjorden, Northern Norway . Marine Ecology Progress Series , 123 : 95 – 106 .
- Harrold , C and Pearse , JS. 1987 . The ecological role of echinoderms in kelp forests . Echinoderm Studies , 2 : 137 – 233 .
- Hereu , B , Zabala , M , Linares , C and Sala , E. 2004 . Temporal and spatial variability in settlement of the sea urchin Paracentrotus lividus in the NW Mediterranean . Marine Biology , 144 : 1011 – 18 .
- Hereu , B , Zabala , M , Linares , C and Sala , E. 2005 . The effect of predator abundance and habitat structural complexity on survival of juvenile sea urchins . Marine Biology , 146 : 293 – 99 .
- Himmelmann , JH. 1986 . Population biology of green sea urchin on rocky barrens . Marine Ecology Progress Series , 33 : 295 – 306 .
- Hjörleifsson , E , Kaasa , Ö and Gunnarsson , K. 1995 . “ Grazing of kelp by green sea urchin in Eydafjordur, North Iceland ” . In Ecology of Fjords and Coastal Waters , Edited by: Skjoldal , HR , Hopkins , C , Erikstad , KE and Leinaas , HP . 525 – 36 . Amsterdam : Elsevier .
- Hurrel , JW and Dickson , RR. 2004 . “ Climate variability over the North Atlantic ” . In Marine Ecosystems and Climate Variability , Edited by: Stenseth , NC and Ottersen , G . 15 – 46 . Oxford : Oxford University Press .
- Isæus M . 2004 . Factors structuring Fucus communities at open and complex coastlines in the Baltic Sea . Doctoral thesis, University of Stockholm . 40 pages .
- Jackson , JBC , Kirby , MX , Berger , WH , Bjorndal , KA , Botsford , LW Bourque , BJ . 2001 . Historical overfishing and the recent collapse of coastal ecosystems . Science , 293 : 629 – 38 .
- Jones , GM and Scheibling , R. 1985 . Paramoeba invadens n.sp. (Amoebida, Paramoebidae), a pathogenic amoeba from the sea urchin Strongylocentrotus droebachiensis in eastern Canada . Journal of Protozoa , 32 : 564 – 69 .
- Jones , NS. and Kain , JM. 1967 . Subtidal algal colonization following the removal of Echinus . Helgolander Wissen Meeresunters , 15 : 460 – 66 .
- Jørgensen , NM and Christie , H. 2003 . Diurnal, horizontal and vertical dispersal of kelp-associated fauna . Hydrobiologia , 50 : 69 – 76 .
- Kålås , JA , Viken , Å and Bakken , T. 2006 . Norsk rødliste. Norwegian red list , 416 pages Norway : Artsdatabanken .
- Keats , DW. 1991 . Refugial Laminaria abundance and reduction in urchin grazing in communities in the north-west Atlantic . Journal of the Marine Biological Association of the United Kingdom , 71 : 867 – 76 .
- Keats , DW , Steel , DH and South , GR. 1987 . The role of fleshy macroalgae in the ecology of of juvenile cod (Gadus morhua L.) in offshore waters of eastern Newfoundland . Canadian Journal of Zoology , 65 : 49 – 53 .
- Lang , C and Mann , KH. 1976 . Changes in sea urchin populations after the destruction of kelp beds . Marine Biology , 36 : 321 – 26 .
- Lange , R. 1964 . The osmotic adjustment in the echinoderm, Strongylocentrotus droebachiensis . Comparative Biochemistry and Physiology , 13 : 205 – 16 .
- Lawrence , JM. 1975 . On the relationship between marine plants and sea urchins . Oceanography and Marine Biology: An Annual Review , 13 : 213 – 86 .
- Lehman , A , Overton , J McC and Leathwick , JR. 2002 . GRASP: Generalized regression analysis and spatial prediction . Ecological Monograph , 157 : 189 – 207 .
- Leinaas , HP and Christie , H. 1996 . Effects of removing sea urchins (Strongylocentrotus droebachiensis): Stability of the barren state and succession of kelp forest recovery in the east Atlantic . Oecologia , 105 : 524 – 36 .
- Leland AV . 2002 . A new apex predator in the Gulf of Maine? Large, mobile crabs (Cancer borealis) control benthic community structure . Orono : Masters thesis, University of Maine . 82 pages .
- Leland AV , Vavrinec J , Steneck RS . 2002 . Reseeding the green sea urchin in depleted habitats . Final report to the Maine Department for Marine Research . 25 pages .
- Levin , VS , Muller , OF and Anisimova , NA. 1998 . “ Part II Bottom invertebrates ” . In Harvesting and Prospective Algae and Invertebrates for Uses of the Barents and White Seas , 394 – 440 . Murmansk : Kola Science Centre Apatity .
- Lindley , JA and Batten , SD. 2002 . Long-term variability in the diversity of North Sea zooplankton . Journal of the Marine Biology Association of the United Kingdom , 82 : 34 – 40 .
- Mann KH . 2000 . Ecology of Coastal Waters . With Implications for Management . Oxford : Blackwell Science . 406 .
- Marstein , AC. 1997 . Epiphytic algae on kelp stipes from Vega – an area with varying densities of sea urchins . Blyttia , 3 : 123 – 29 .
- McNaught , DC. 1999 . The indirect effects of macroalgae and micropredation on the post-settlement success of the green sea urchin in Maine. PhD Dissertation , 161 pages Orono : University of Maine .
- Mortensen , T. 1943 . A monograph of the Echinoidea. III. 3. Camarodonta. II. Echinidae, Strongylocentrotidae, Parasaleniidae, Echinometridae , 208 – 09 . Copenhagen : CA Reitzel .
- Nordenskiöld AE . 1880 . Vegas färd kring Asien och Europa jemte en historisk återblick på föregående resor längs Gamla Verldens nordkust . 1 . Stockholm : F & G Beijers Förlag . 510 .
- Norderhaug , KM. 2004 . Use of red algae as hosts by kelp-associated amphipods . Marine Biology , 144 : 225 – 30 .
- Norderhaug KM , Christie H . 2007 . Reetablering av tareskog i områder av midt-Norge som tidligere har vært beitet av kråkeboller . NIVA rapport 5516 . 20 pages .
- Norderhaug , KM , Christie , H , Fosså , JH and Fredriksen , S. 2005 . Fish–macrofauna interactions in a kelp (Laminaria hyperborea) forest . Journal of the Marine Biological Association of the United Kingdom , 85 : 1279 – 86 .
- Norderhaug , KM , Christie , H and Fredriksen , S. 2007 . Is habitat size an important factor for faunal abundances on kelp (Laminaria hyperborea)? . Journal of Sea Research , 58 : 120 – 24 .
- Norderhaug , KM , Christie , H and Rinde , E. 2002 . Colonisation of kelp imitations by epiphyte and holdfast fauna; A study of mobility patterns . Marine Biology , 141 : 965 – 73 .
- Norderhaug , KM , Fredriksen , S and Nygaard , K. 2003 . The trophic importance of Laminaria hyperborea to kelp forest consumers and the importance of bacterial degradation for food quality . Marine Ecology Progress Series , 255 : 135 – 44 .
- Norton , TA. 1978 . The factors influencing the distribution of Sacchoritza polyschides in the region of Lough Ine . Journal of the Marine Biological Association of the United Kingdom , 58 : 527 – 36 .
- Peterson , CH and Estes , JA. 2001 . “ Conservation and management of marine communities ” . In Marine Community Ecology , Edited by: Bertness , MD , Gaines , SD and Hay , ME . 469 – 508 . Sunderland, MA : Sinauer Associated Inc .
- Pringle , JD. 1986 . A review of urchin/macroalgal associations with a new synthesis for nearshore, eastern Canadian waters . Monographs in Biology , 4 : 191 – 218 .
- Propp , MV. 1977 . Ecology of the sea urchin Strongylocentrotus droebachiensis of the Barents Sea: Metabolism and regulation of abundance . Soviet Journal of Marine Biology , 3 : 27 – 37 .
- Rinde E . 2007 . Studies of processes in Laminaria hyperborea kelp forest ecosystem – Contribution to a scientifically based resource management . PhD thesis, University of Oslo . 33 pages .
- Rinde , E and Sjøtun , K. 2005 . Demographic variation in the kelp Laminaria hyperborea along a latitudal gradient . Marine Biology , 146 : 1051 – 62 .
- Røv N , Christie H , Fredriksen S , Leinaas HP , Lorentsen SH . 1990 . Biologiske forundersøkelser i forbindelse med planer om taretråling i Sør-Trøndelag . NINA Oppdragsmelding 052 . 20 pages .
- Rowley , RJ. 1989 . Settlement and recruitment of sea urchins (Strongylocentrotus spp.) in a sea-urchin barren ground and a kelp bed: are populations regulated by settlement or post-settlement processes? . Marine Biology , 100 : 485
- Russell , MP. 1998 . Resource allocation plasticity in sea urchins: Rapid, diet induced, phenotypic changes in the green sea urchin, Strongylocentrotus droebachiensis (Muller) . Journal of Experimental Marine Biology and Ecology , 220 : 1 – 14 .
- Scheffer , M , Carpenter , S , Foley , JA , Folke , C and Walker , B. 2001 . Catastrophic shifts in ecosystems . Nature , 413 : 591 – 96 .
- Scheibling , RE and Hennigar , AW. 1997 . Recurrent outbreaks of disease in sea urchins Strongylocentrotus droebachiensis in Nova Scotia: Evidence for a link with large-scale meteorological and oceanographic events . Marine Ecology Progress Series , 152 : 155 – 65 .
- Scheibling , RE and Robinson , MC. 2008 . Settlement behaviour and early post-settlement predation of the sea urchin Strongylocentrotus droebachiensis . Journal of Experimental Marine Biology and Ecology , 365 : 59 – 66 .
- Scheibling , RE and Stephenson , RL. 1984 . Mass mortality of Strongylocentrotus droebachiensis (Echinodermata: Echinoidea) off Nova Scotia, Canada . Marine Biology , 78 : 153 – 64 .
- Schroeter , SC , Dixon , JD , Ebert , TA and Rankin , JV. 1996 . Effects of kelp forests Macrocystis pyrifera on the larval distribution and settlement of red and purple sea urchins Strongylocentrotus fransiscanus and S. purpuratus . Marine Ecology Progress Series , 133 : 125 – 34 .
- Schultze , K , Janke , K , Krüß , A and Weidemann , W. 1990 . The macrofauna and macroflora associated with Laminaria digitata and L. hyperborea at the island of Helgoland (German Bight, North Sea) . Helgoländer Meeresunters , 44 : 39 – 51 .
- Sivertsen K . 1982 . Utbredelse og variasjon i kråkebollenes nedbeiting av tareskogen på vestkysten av Norge . Nordlandsforskning, Bodø, Rapport 7/82 . 31 pages .
- Sivertsen K. 1996 . The incidence, occurrence and distribution of the nematode parasite Echinomermella matsi in its echinoid host, Strongylocentrotus droebachiensis, in northern Norway . Marine Biology 126 : 703 – 14 .
- Sivertsen , K. 1997a . Geographical and environmental factors affecting the distribution of kelp beds and barren grounds and changes in biota associated with kelp reduction at sites along the Norwegian coast . Canadian Journal of Fisheries and Aquatic Sciences , 54 : 2872 – 87 .
- Sivertsen K . 1997b . Dynamics of sea urchins and kelp during overgrazing of kelp forests along the Norwegian coast . Dr. scient. Thesis, University of Tromsø . 127 pages .
- Sivertsen , K. 2002a . Kartlegging av kråkebolleforekomster i Indre Laksefjord i Finnmark . HiFi-Rapport , 2002 : 13
- Sivertsen , K. 2002b . Kartlegging av fangstbare forekomster av kråkeboller i Vest-Finnmark . HiFi Rapport , 2002 : 14
- Sivertsen , K. 2006 . Overgrazing of kelp beds along the coast of Norway . Journal of Applied Phycology , 18 : 599 – 610 .
- Sivertsen , K and Bjørge , A. 1980 . Reduksjon av tareskogen på Helgelandskysten . Fisken og Havet , 4 : 1 – 9 .
- Sivertsen , K and Hopkins , CCE. 1995 . “ Demography of the echinoid Strongylocentrotus droebachiensis related to biotope in northern Norway ” . In Ecology of Fjords and Coastal Waters , Edited by: Skjoldal , HR , Hopkins , C , Erikstad , KE and Leinaas , HP . 549 – 71 . Amsterdam : Elsevier .
- Sivertsen K , Wentzel-Larsen T . 1989 . Fangstbare forekomster av kråkeboller . Nordlandsforskning , Bodø, Rapport 3/89 . 59 pages .
- Sjøtun , K , Christie , H and Fosså , JH. 2001 . Overvaking av kråkebolleførekomstar og gjenvekst av stortare etter prøvetråling i Sør-Trøndelag . Fisken og Havet , 5 : 1 – 24 .
- Sjøtun , K , Christie , H and Fosså , JH. 2006 . Effects of kelp recruitment and sea urchin grazing on stability in kelp forest (Laminaria hyperborea) . Marine Biology Research , 2 : 24 – 32 .
- Sjøtun , K , Fredriksen , S and Rueness , J. 1998 . The effect of canopy biomass and wave exposure on growth in Laminaria hyperborea (Laminariaceae: Phaeophyta) . European Journal of Phycology , 33 : 337 – 43 .
- Sjøtun , K , Fredriksen , S , Rueness , J and Lein , TE. 1995 . “ Ecological studies of the kelp Laminaria hyperborea (Gunnerus) Foslie in Norway ” . In Ecology of Fjords and Coastal Waters , Edited by: Skjoldal , HR , Hopkins , C , Erikstad , KE and Leinaas , HP . 525 – 36 . Amsterdam : Elsevier .
- Skadsheim , A , Christie , H and Leinaas , HP. 1995 . Population reduction of Strongylocentrotus droebachiensis (Echinodermata) in Norway and the distribution of its endoparasite Echinomermella matsi (Nematoda) . Marine Ecology Progress Series , 119 : 199 – 209 .
- Skadsheim A , Rinde E , Christie H . 1993 . Forekomst og endringer i kråkebolletetthet, kråkebolleparasitt og gjenvekst av tareskog langs norskekysten fra Trøndelag til Troms . NINA Oppdragsmelding 258 . 39 pages .
- Steneck , RS and Carlton , JT. 2001 . “ Human alterations of marine communities: Students beware ” . In Marine Community Ecology , Edited by: Bertness , MD , Gaines , SD and Hay , ME . 445 – 68 . Sunderland, MA : Sinauer Associates Inc .
- Steneck , RS , Graham , MH , Bourque , BJ , Bruce , J , Corbett , D Erlandson , JM . 2002 . Kelp forest ecosystems: Biodiversity, stability, resilience and future . Environmental Conservation , 29 : 436 – 59 .
- Steneck , RS , Vavrinec , J and Leland , AV. 2004 . Accelerating trophic-level dysfunction in kelp forest ecosystems of the Western North Atlantic . Ecosystems , 7 : 323 – 32 .
- Stephens , RE. 1972 . Studies on the development of the sea urchin Strongylocentrotus droebachiensis. I. Ecology and normal development . Biological Bulletin , 142 : 132 – 44 .
- Stien , A , Halvorsen , O and Leinaas , HP. 1995 . “ No evidence of Echinomermella matsi (Nematoda) as a mortality factor in a local mass mortality of Strongylocentrotus droebachiensis (Echinoidea) ” . In Ecology of Fjords and Coastal Waters , Edited by: Skjoldal , HR , Hopkins , C , Erikstad , KE and Leinaas , HP . 585 – 92 . Amsterdam : Elsevier .
- Stien , A , Leinaas , HP , Halvorsen , O and Christie , H. 1998 . Population dynamics of the Echinomermella matsi (Nematoda)–Strongylocentrotus droebachiensis system: Effects on host fecundity . Marine Ecology Progress Series , 163 : 193 – 201 .
- Tegner , MJ and Dayton , PK. 2000 . Ecosystem effects of fishing in kelp forest communities . ICES Journal of Marine Science , 57 : 579 – 89 .
- Vadas , RL , Smith , BD , Beal , B and Dowling , T. 2002 . Sympatric growth morphs and size bimodality in the green sea urchin (Strongylocentrotus droebachiensis) . Ecological Monographs , 72 : 113 – 32 .
- Vadas , RL and Steneck , RS . 1995 . “ Overfishing and inferences in kelp–sea urchin interactions ” . In Ecology of Fjords and Coastal Waters , Edited by: Skjoldal , HR , Hopkins , C , Erikstad , KE and Leinaas , HP . 509 – 24 . Amsterdam : Elsevier .
- Vasseur , E. 1952 . Geographic variation in the Norwegian sea-urchins Strongylocentrotus droebachiensis and S. pallidus . Evolution , 6 : 87 – 100 .
- Vavrinec J . 2003 . Resilience of green sea urchin (Strongylocentrortus droebachiensis) populations following fishing mortality: Marine protected areas, alternate stable states, and larval ecology . Doctor thesis, the graduate school, The University of Maine . 127 pages .
- Vavrinec J , Meidel SK , Wahle RA , Steneck RS . 2001 . Sea urchin no-fish areas in Maine: Rates of recovery and resilience in urchin populations, gonad indices, and algal habitats in fished and unfished areas . Report to the Maine Department of Marine Resources . 26 pages .
- Vetter , EW. 1995 . Detritus-based patches of high secondary production in the nearshore benthos . Marine Ecology Progress Series , 120 : 251 – 262 .
- Waage-Nielsen , E , Christie , H and Rinde , E. 2003 . Short-term dispersal of kelp fauna to cleared (kelp harvested) areas . Hydrobiologia , 503 : 77 – 91 .
- Walker , FT. 1956 . Periodicity of the Laminariacea around Scotland . Nature , 177 : 1246
- Whittick , A. 1983 . Spatial and temporal distribution of dominant epiphytes on the stipes of Laminaria hyperborea (Gunn) Fosl (Phaeophyta: Laminariales) in S.E. Scotland . Journal of Experimental Marine Biology and Ecology , 73 : 1 – 10 .
- Woll AK , van der Meeren GI , Fossen I , Tveite I 2004 Ressursundersøkelse av taskekrabbe langs Norskekysten, Sluttrapport 2001–2003 . Møreforskning Report Å0406 . 25 pages
- Ærtebjerg G . 2007 . Marine områder 2005–2006 – Tilstanden og udvikling i miljø- og naturkvalitet. NOVANA. Danmarks Miljøundersøkelser. Aarhus University . Faglig rapport DMU 639 . 95 pages .