Abstract
The study presents results on the composition and vertical distribution of the near-bottom plankton community at an abyssal site in the NE Atlantic. Plankton samples were collected at 1, 15, 50 and 100 m above bottom (mab). Whereas the composition within the upper three layers was very similar, a major shift occurred in the immediate vicinity of the seafloor. Between 100 and 15 mab, the plankton was dominated by Copepoda, making up more than 75% of the total abundance and biomass (without gelatinous organisms). At 1 mab, Copepoda were still abundant, but their share decreased to ca. 50%, while Polychaeta, Malacostraca and Chaetognatha became important groups. Within the Copepoda, the predominance of the genus Metridia (Calanoida) in the upper layers was replaced by the genus Benthomisophria (Misophrioida) at 1 mab. Despite enrichment in organic particles towards the bottom, the total abundance and biomass of plankton did not show marked differences between the four layers investigated. Several hypotheses are discussed which may explain why the presumably higher food concentrations near the deep-sea floor do not lead to increased standing stocks of the plankton community.
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
Introduction
Boundary layers are locations of increased biological and biogeochemical activity. For example, the contribution of the air–water interface, of frontal areas, thermoclines and haloclines to the biological production has widely been recognized. However, at abyssal depths of the deep sea, the transition zone between the pelagic zone and the sediment has rarely been studied biologically. One important feature of the near-bottom water layer in the deep sea is the enhanced particle concentration. Below a clear water minimum, particle concentrations typically increase towards the bottom forming the Bottom Nepheloid Layer (BNL; Nyffeler & Godet Citation1986). Tens of metres above the bottom there is a further sharp increase in particle concentrations, which then remain rather constant towards the sediment, coinciding with a homogenous temperature profile. This so-called Bottom Mixed Layer (BML) is particularly important for the transfer of energy and organic matter and acts as a filter for particle fluxes between the water column and the sediment (Turnewitsch & Springer Citation2001). The benthopelagic fauna may actually play a key role in this layer, because of their increased numbers as compared to the layers above (Wishner Citation1980a ,Citationb) and the predominantly detritivorous feeding behaviour of the Copepoda (Gowing & Wishner Citation1986), indicating their important contribution to the deep carbon cycling. A study by Hernes et al. (Citation2001) in the Pacific showed that the loss of particulate organic carbon (POC) in the sediment–water interface due to to remineralization is about two orders of magnitude.
Assuming that an enhanced organic particle concentration close to the sea floor of the abyssal plains can be utilized as food source by detritivores, we can expect increasing standing stocks of zooplankton towards the bottom. Additionally, the potential interaction of near-bottom zooplankton with epibenthic fauna and with the sea floor is likely to result in a different composition of the zooplankton community close to the bottom. In order to test these hypotheses, we studied the composition and distribution of the near-bottom zooplankton at an abyssal site in the NE Atlantic.
Material and methods
Sampling site
Sampling was performed at the BENGAL study site (), located in the centre of the Porcupine Abyssal Plain at 48°50' N 016°30' W (Billett & Rice Citation2001). Water depth is about 4850 m. Plankton tows were conducted at the so-called ‘trawling ground’, which features a very even seafloor without greater elevations (Billett & Rice Citation2001). The Porcupine Abyssal Plain is subject to a pronounced annual variability in vertical particles fluxes due the development of the phytoplankton spring bloom (Honjo & Manganini Citation1993; Newton et al. Citation1994; Lampitt et al. Citation2001). The Bottom Nepheloid Layer (BNL) extends to ca. 1000 m above bottom (mab) (Turnewitsch & Springer Citation2001). Within the immediate vicinity of the sea floor, a Bottom Mixed Layer (BML) was identified which varied in thickness from 10 to 65 m over a sampling period of 3 weeks in July 1997 (Turnewitsch & Springer Citation2001), but was thicker in 1998 (Vangriesheim et al. Citation2001). Current velocities were generally low (<15 cm s–1, usually <10 cm s–1; Lampitt et al. Citation2001; Vangriesheim et al. Citation2001).
The near-bottom fauna was sampled on two cruises: Discovery 226 (D226) in March/April 1997 and Meteor 42/2 (M42/2) in August 1998. The sampling gear on the Discovery cruise was a 1 m2 MOCNESS (Wiebe et al. Citation1985) with nine black nets of 333 µm mesh size. On the Meteor cruise, a 1 m2 Double-MOCNESS with 2×10 nets was employed. The nets can be opened and closed sequentially allowing for successively taken horizontal and/or oblique samples at discrete depth intervals.
A total of five near-bottom hauls were conducted during the Discovery and Meteor cruises each. Three depth layers were sampled horizontally at each haul: 15, 50 and 100 mab, each varying by ca. ±5 m. The distance from the bottom was measured by means of a Simrad Mesotech altimeter. Sampling speed was approximately 2 knots. Tow duration was ca. 30 min per net, resulting in a volume filtered of ca. 1800 m3 per net, as calculated from the flowmeter readings and the net angle. The intermediate tows between sampling layers were not analysed. A sampling scheme is given in .
Table I. MOCNESS sampling scheme (MOC-1: 1 m2 MOCNESS; MOC-D1: 1 m2 double-MOCNESS).
During cruise M42/2, an epibenthic sledge (IHF Fototrawl) was used to sample the layer between 0.5 and 1.5 mab. It carried a MOCNESS-type opening/closing net system with 4 nets (mesh 333 µm). Bottom contact was monitored using an altimeter and an inclinometer.
The zooplankton samples were preserved in 4% formaldehyde–seawater solution buffered with sodium tetraborate. In the laboratory, the wet weight of the samples was determined following the method of Tranter (Citation1962). Organisms >5mm were weighed separately. Specimens were identified to different taxonomic levels (see ) and counted under a dissecting microscope. The data were standardised to a volume of 1000 m3. Epipelagic organisms, which sporadically occurred in the samples as contaminants, were excluded from the counts. Exoskeletons and carcasses according to Wheeler (Citation1967) and Weikert (Citation1977) were counted separately.
Table II. Taxa identified in near-bottom zooplankton samples at the BENGAL site. *Most probably contaminants.
Differences in abundance between depth layers (15–100 mab) and cruises were tested statistically by a two-way-ANOVA with post-hoc tests (Wilkinson et al. Citation1992). Abundances were log-transformed, and Bonferroni adjustments were performed on significance levels.
Results
Taxonomic composition
gives an overview of the zooplankton taxa found during cruise Meteor 42/2 in the near-bottom zooplankton samples from the Bengal site. Because in the Discovery 226 samples higher taxa only were identified, data from this cruise have not been included in the list. The taxonomic analysis focused on the Crustacea, particularly on Copepoda. At least two Copepoda species new to science were found, belonging to the families Tharybidae and Diaixidae, respectively. A relatively high percentage of Copepoda could not be identified to lower taxonomic levels. Most of them were very small Calanoida, partly juveniles, which are extremely difficult to identify. The Acartiidae and Oithonidae found in the samples were most probably contaminants from surface water layers.
Malacostraca included mainly Isopoda and Amphipoda; only a few Decapoda, Euphausiacea larvae and Mysidacea were found. Of the remaining taxa, only Polychaeta and Holothuroida were identified to lower taxonomic levels. The Polychaeta comprised mainly Vanadis sp., besides a few Tomopteridae and unidentified larvae. Only one benthopelagic Holothuroida species was found, Peniagone diaphana, with lengths up to 120 mm.
Differences in relative abundances between the layers 15 mab, 50 mab and 100 mab were small (), although there was an indication for increasing shares of Polychaeta and Malacostraca towards the 15 mab layer, at least in 1998. The main group were the Copepoda, making up 75% of all individuals, followed by Chaetognatha and Ostracoda with less than 10% each. The taxonomic composition was similar in both sampling periods, except that the percentage of Ostracoda was higher in 1997 (9–13%) than in 1998 (5–8%), whereas the percentage of Chaetognatha was higher in 1998 (7–8%) than in 1997 (2–3%).
Figure 2. Relative abundance of major zooplankton groups at the sampling layers. Left: March 1997; right: August 1998. Cop: Copepoda; Chae: Chaetognatha; Pol: Polychaeta; Ost: Ostracoda; Mal: Malacostraca.
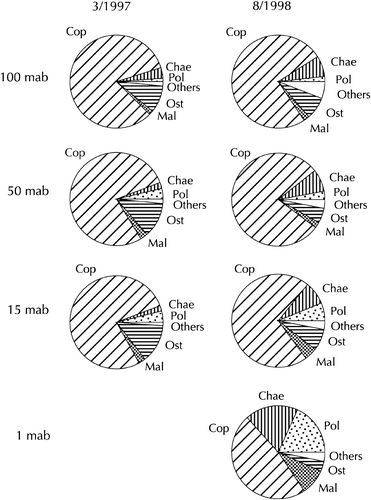
Proceeding to the 1 mab layer, there was a drastic change. Although Copepoda were still the predominating group, their share dropped to below 50%. On the other hand, the percentages of Polychaeta, Chaetognatha and Malacostraca (both Amphipoda and Isopoda) increased more than two- to threefold.
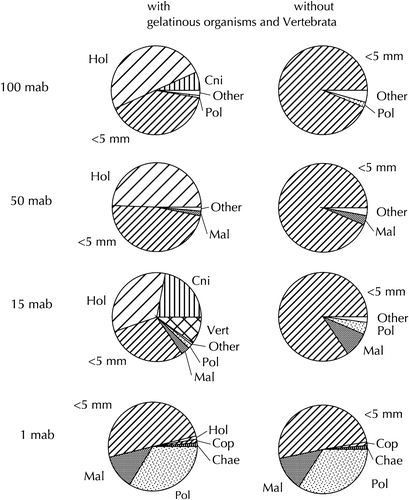
The change in composition towards the bottom is also evident based on biomass (wet weight) (): large gelatinous organisms like Holothuroida and Cnidaria dominated the biomass in the upper layers. Without these two groups, 80–95% of the biomass between 15 and 100 mab was made up by small organisms <5 mm (mainly Copepoda). Close to the bottom, however, also Polychaeta and Malacostraca contributed significantly to the bulk of the biomass.
Figure 3. Relative biomass of major zooplankton groups at the sampling layers in August 1998. Left: with gelatinous organisms and Vertebrata; right: without gelatinous organisms and Vertebrata. See caption of for abbreviations.
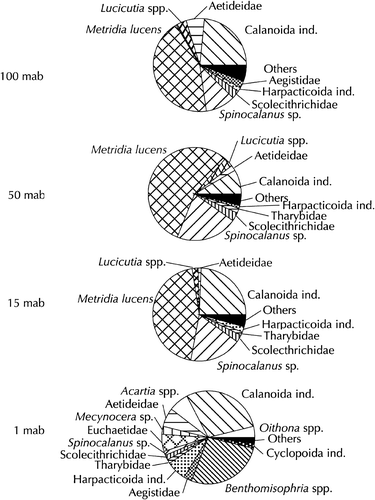
The Copepoda collected in 1998 were analysed further to family level and, if possible, to genus and species level (). The predominating species at the three upper layers were Metridia lucens, making up ca. 43–55% of all Copepoda. Apart from unidentified Calanoida (10–25%), Spinocalanus spp. (Pseudocalanidae) ranked next with 10–20%. Other abundant Copepoda were Aetideidae, Lucicutia spp., Tharybidae and Scolecithrichidae.
The layer closest to the bottom showed a clearly different composition: M. lucens was extremely rare, and Spinocalanus spp. were also found in low numbers only. The most abundant group, apart from unidentified Calanoida, were the typically benthopelagic Misophrioida, comprising several species of the genus Benthomisophria. Their share was 27%. Aetideidae, Euchaetidae and Harpacticoida followed with ca. 6% each.
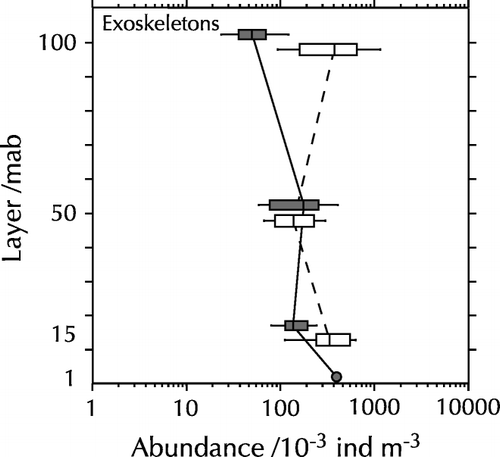
The percentage of copepod exoskeletons (including carcasses) in relation to the total Copepoda in the layers 15 mab, 50 mab, and 100 mab ranged from 40 to 49% in March 1997, being higher than in August 1998 with 16–26% in the layers 15 mab, 50 mab, and 100 mab. Vertical gradients in these layers were weak. However, in the 1 mab layer the share of exoskeletons amounted to 44%, thus being higher than in the upper layers in the same cruise, but comparable to the March 1997 data.
Distribution of biomass and abundance
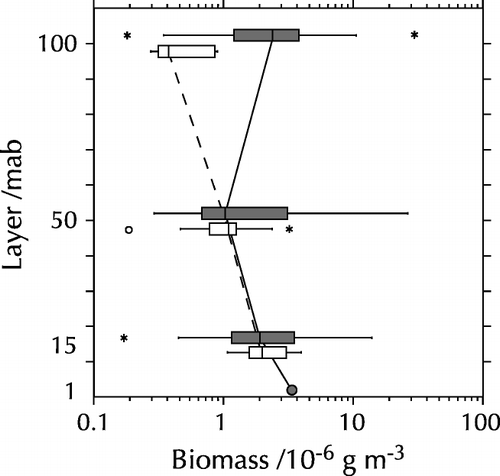
The vertical distribution of the total zooplankton biomass (without Holothuroida and Cnidaria), which was largely dominated by the Copepoda, is shown in for both cruises. Vertical gradients were rather small, particularly in the light of the high variability within the sampling layers, although the data from 1997 indicate a slight increase towards the bottom. Similarly, differences between the two cruises were small; only in the 100 mab layer the biomass appeared to be higher in 1998 than in 1997.
Figure 5. Vertical distribution of zooplankton biomass. White bars: March 1997 data; black bars: August 1998 data. The vertical line in the box denotes the median; the boundaries of the box correspond to the 25% and 75% percentiles, respectively, the whiskers extend to the 10% and 90% percentiles. Circles and asterisks denote outliers.
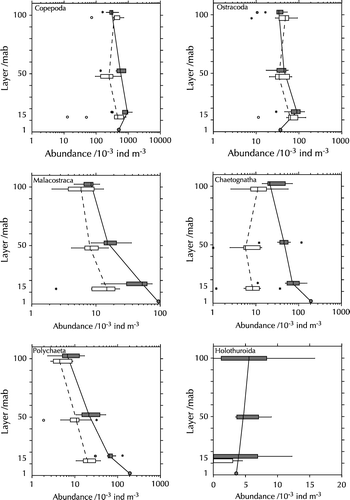
presents the abundance of the major taxonomic groups in the sampling layers. The distribution of the groups differed clearly: Copepoda were distributed fairly even and had only slightly higher abundances in August 1998. Similarly, the abundance of Chaetognatha showed only small differences between depth layers, except for higher values at 1 mab, but they were much more abundant in August 1998 than in March 1997. Ostracoda, on the other hand, had highest abundances at 15 mab, but no differences between cruises. The strongest trends appeared in Malacostraca and Polychaeta: both were more abundant in August 1998 than in March 1997, and both showed a strong increase towards the bottom on both cruises.
Figure 6. Vertical distribution of major zooplankton groups. Note different scales. White bars: March 1997 data; black bars: August 1998 data. The vertical line in the box denotes the median; the boundaries of the box correspond to the 25% and 75% percentiles, respectively, the whiskers extend to the 10% and 90% percentiles. Circles and asterisks denote outliers.
The two-way-ANOVA with post-hoc tests supports these findings. The results are shown in and summarized in . Although not testable, the trends for the 1 mab layer have been included in .
Figure 7. Zooplankton abundance: differences between depth layers and cruises. Results of the two-way-ANOVA and post-hoc tests with Bonferroni adjustments.
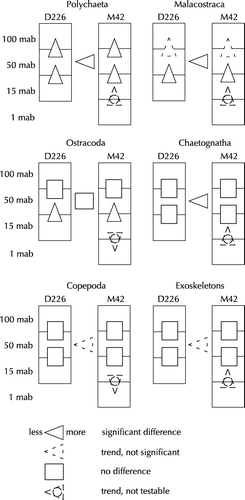
Table III. ANOVA table for major taxonomic groups. Significance levels (after Bonferroni adjustment, except for exoskeletons): n.s. not significant, *p<0.05, **p<0.01.
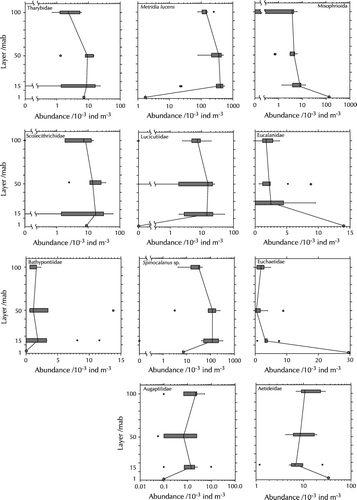
Although the vertical gradient in Copepoda abundance appears to be very small, there were marked differences in the vertical distribution between the families, as the results on the taxonomic composition of Copepoda at the sampling layers already suggest. All families have in common that gradients between 15 and 100 mab were only small (). However, three groups can be distinguished, representing different trends between 15 mab and the layer closest to the bottom. The first group, comprising Tharybidae, Scolecitrichidae and Bathypontiidae, did not show marked differences between 1 and 15 mab. The second group was made up of Metridinidae, Lucicutiidae, Spinocalanidae and Augaptilidae and showed a strong decline in abundance towards the bottom. The third group, including Misophrioidae, Eucalanidae, Euchaetidae and Aetideidae, had highest abundances directly above the bottom.
Figure 8. Vertical distribution of Copepoda families in August 1998. Note different scales. Left-hand graphs: bottom-neutral groups; centre graphs: presumable bottom avoiders; right-hand graphs: presumable bottom associates. The vertical line in the box denotes the median; the boundaries of the box correspond to the 25% and 75% percentiles, respectively, the whiskers extend to the 10% and 90% percentiles. Circles and asterisks denote outliers.
Vertical differences in exoskeleton abundance were small, although there appears to be a trend for an increase towards the bottom ()., Exoskeleton abundance was significantly higher in March 1997 than in August 1998 in the 15 mab to 100 mab layers.
Figure 9. Vertical distribution of exoskeletons. White bars: March 1997 data; black bars: August 1998 data. The vertical line in the box denotes the median; the boundaries of the box correspond to the 25% and 75% percentiles, respectively, the whiskers extend to the 10% and 90% percentiles. Circles and asterisks denote outliers.
Discussion
This study presents results from the near-bottom water layer in the deep sea, an oceanic realm that has rarely been investigated. Although the existence of a benthopelagic fauna in the deep sea has been recognized for a number of years (Marshall & Merrett Citation1977), the methodological difficulties encountered with sampling this habitat have essentially limited the possibilities for systematic studies of the fauna inhabiting the near-bottom water layers.
The results of this study on the near-bottom abyssal plankton communities feature two major outcomes:
-
a major taxonomic shift occurred within a few metres off the bottom; and
-
vertical gradients in total zooplankton abundance and biomass within the water layer 100 mab were only small.
In the only detailed study of the community ecology of benthopelagic Copepoda living deeper than 3000 m, Wishner (Citation1980a) made an attempt to distinguish between bottom avoiders and bottom associates, depending on their presence in samples taken at 10 mab and 100 mab. We can only partially confirm her classification: She identified species of the families Euchaetidae, Scolecitrichidae, Aetideidae, Metridinidae, Bathypontiidae and Tharybidae as probable bottom associates, whereas Lucicutiidae and Aetideopsis were classified as probable bottom avoiders. In our samples, Metridia and Spinocalanus, like Lucicutia, appeared to avoid the bottom, and Tharybidae, Scolecitrichidae and Bathypontiidae showed no trend. The only bottom-associates we found were the non-Calanoida family Misophrioidae, together with the Calanoida Euchaetidae, Eucalanidae and, possibly, Aetideidae. Part of this discrepancy may be explained by different species making up the copepod families in the two areas, but bottom distance or water depth may also play a role: in our samples, the strongest trend occurred between 15 mab and 1 mab, and thus possibly below Wishner's (1980a) minimum bottom distance of 10 mab.
A major taxonomic shift within a few decimetres off the bottom has also been found in the upper bathyal zone by, e.g. Grice (Citation1972), who used plankton nets attached to a submersible and observed a distinct benthopelagic fauna 20–50 cm above bottom at 1000–1500 m. All 14 ‘planktobenthic’ Copepoda species in his samples belonged to the families Aetideidae, Phaennidae and Tharybidae.
Obviously only the layer closest to the bottom is affected by the taxonomic shift, although the quality and quantity of suspended organic particles as potential food changes already further up. What makes the bottom layer attractive for one group, but unattractive for the other? There was no obvious shift in Copepoda feeding types between the two guilds in our study (Bühring & Christiansen Citation2001), but, although many benthopelagic Copepoda appear to be generalist detritivores (Gowing & Wishner Citation1986), information on dietary preferences in deep-sea plankton is far from complete, and it is not known whether the ‘true’ benthopelagic Copepoda prefer to feed on the bottom or in the water column (Wishner Citation1980a). The higher abundance of carnivores like Chaetognatha, Polychaeta and Decapoda may probably shape the Copepoda community near the bottom, but little is known about e.g. escape responses of benthopelagic Copepoda, or dietary preferences of potential predators.
Although differences in total zooplankton biomass and abundance were rather small between the two cruises, the Polychaeta, Malacostraca and Chaetognatha had significantly higher abundances in 1998. We can only speculate whether this was a seasonal effect or due to variability on another temporal scale. Generally, only little is known about the temporal variability in deep ocean plankton.
The small vertical gradients in total biomass and abundance are in contrast to former hypotheses on increasing zooplankton biomass towards the bottom due to the presumed higher concentration of potential food particles in the immediate vicinity of the sea floor (Wishner Citation1980b). However, Christiansen et al. (Citation1999) have already presented only small vertical differences in zooplankton abundance and biomass between 20 and 100 mab in the nearby BIOTRANS area, but pointed out that probably the minimum distance from the bottom sampled (20 m) was too far from the seafloor to detect significant changes. The present study supports their findings and shows that there is no notable biomass increase even down to 1 mab. One problem linked to net samples is an under-representation of gelatinous organisms, which are often destroyed during sampling, resulting in a gross under-estimation of zooplankton standing stocks. For example, during dives at the Charlie-Gibbs Fracture Zone, Vinogradov (Citation2005) visually observed high densities of gelatinous Appendicularia houses close to the bottom, forming the most abundant group in this layer. Although the houses of Appendicularia are not caught in net samples, the animals are usually retained in the nets, but we cannot exclude that other gelatinous organisms have been missed.
Although particle concentration as measured by transmissometer was enhanced in the near-bottom water layer at the study site with a sharp increase well below our upper sampling depth of 100 mab (Vangriesheim et al. Citation2001), this potential surplus food source within the BML does obviously not allow for building up higher standing stocks of the planktic community. There are several possible reasons which could explain this result.
-
Most of the zooplankton production is utilized by higher trophic levels so that the standing stocks remain low. This would mean a strong top–down control. The relatively high abundances of carnivores like Chaetognatha and Polychaeta close to the bottom and the additional predation pressure by epibenthic planktivores may support this hypothesis.
-
The inferior nutritional value of the organic particles prevents their effective utilization by the zooplankton, meaning a bottom–up control of the near-bottom plankton community. However, although the food quality of sinking particles in terms of total organic carbon content, nitrogen, protein and lipid decreases with depth, the remaining compounds should generally be sufficient for utilization as food (Kiriakoulakis et al. Citation2001; Lampitt et al. Citation2001). The extremely small burrowing rate of organic matter in the deep-sea sediment, as assumed by Pfannkuche (Citation1992) for the nearby BIOTRANS station, suggests that most of the material is in fact utilized by deep-sea organisms, e.g. incorporated in the relatively high concentrations of phytoplankton-derived fatty acids found in deep-sea zooplankton by Bühring & Christiansen (Citation2001), and finally remineralized.
-
Close to the sea floor, zooplankton directly compete for food with epibenthic suspension feeders, which potentially rely on the same food sources as the near-bottom free-swimming organisms. This hypothesis is strongly supported by the high standing stocks of epibenthic suspension feeders in the area, like Cnidaria, Porifera and Xenophyophora (Christiansen unpublished).
We cannot decide yet on the validity of these hypotheses, as long as there is no more information available on trophic pathways, fluxes of energy and production in the deep sea.
Editorial responsibility: Tom Fenchel
Acknowledgements
We thank L. Neugebohrn, R. Gollembiewski and K. Schulz for their support in the identifications. The work was funded, in part, by EC contract MAS-3 950018 under the MAST III programme, and by the Deutsche Forschungsgemeinschaft.
Notes
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
References
- Berg , CJ and van Dover , CL. 1987 . Benthopelagic macrozooplankton communities at and near deep-sea hydrothermal vents in the eastern Pacific Ocean and the Gulf of California . Deep-Sea Research , 34 : 379 – 401 .
- Billett , DSM and Rice , AL. 2001 . The BENGAL programme: Introduction and overview . Progress in Oceanography , 50 ( 1–4 ) : 13 – 25 .
- Bühring , SI and Christiansen , B. 2001 . Lipids in selected abyssal benthopelagic animals: Links to the epipelagic zone? . Progress in Oceanography , 50 : 369 – 82 .
- Christiansen , B , Drüke , B , Koppelmann , R and Weikert , H. 1999 . The near-bottom zooplankton at the abyssal BIOTRANS-site, northeast Atlantic: Composition, abundance and variability . Journal of Plankton Research , 21 ( 10 ) : 1847 – 63 .
- Gowing , MM and Wishner , KF. 1986 . Trophic relationships of deep-sea calanoid copepods from the benthic boundary layer of the Santa Catalina Basin, California . Deep-Sea Research , 33 : 939 – 61 .
- Grice , GD. 1972 . The existence of a bottom-living calanoid copepod fauna in deep water with descriptions of five new species . Crustaceana , 23 : 219 – 42 .
- Hernes , PJ , Peterson , ML , Murray , JW , Wakeham , SG , Lee , C and Hedges , JI. 2001 . Particulate carbon and nitrogen fluxes and compositions in the central equatorial Pacific . Deep-Sea Research I , 48 ( 9 ) : 1999 – 2023 .
- Honjo S , Manganini SJ. 1993 . Annual biogenic particle fluxes to the interior of the North Atlantic Ocean studied at 37°N 21°W and 48°N 21°W . Deep-Sea Research II 40 1–2 : 587 607 .
- Kiriakoulakis , K , Stutt , E , Rowland , SJ , Vangriesheim , A , Lampitt , RS and Wolff , GA. 2001 . Controls on the organic chemical composition of settling particles in the Northeast Atlantic Ocean . Progress in Oceanography , 50 ( 1–4 ) : 65 – 87 .
- Lampitt , RS , Bett , BJ , Kiriakoulakis , K , Popova , EE , Ragueneau , O Vangriesheim , A . 2001 . Material supply to the abyssal seafloor in the Northeast Atlantic . Progress in Oceanography , 50 ( 1–4 ) : 27 – 63 .
- Marshall , NB and Merrett , NR. 1977 . “ The existence of a benthopelagic fauna in the deep-sea ” . In A Voyage of Discovery: George Deacon 70th Anniversary Volume , Edited by: Angel , MV . 483 – 94 . Oxford : Pergamon Press .
- Newton PP , Lampitt RS , Jickells TD , King P , Boutle C. 1994 . Temporal and spatial variability of biogenic particle fluxes during the JGOFS northeast Atlantic process studies at 47°N, 20°W . Deep-sea Research 41 11/12 : 1617 42 .
- Nyffeler , F and Godet , C-H. 1986 . The structural parameters of the benthic nepheloid layer in the northeast Atlantic . Deep-Sea Research , 33 ( 2 ) : 195 – 207 .
- Pfannkuche , O. 1992 . “ Organic carbon flux through the benthic community in the temperate abyssal northeast Atlantic ” . In Deep-Sea Food Chains and the Global Carbon Cycle. NATO ASI Series , Edited by: Rowe , GT and Pariente , V . 183 – 98 . Dordrecht : Kluwer Academic Publishers .
- Tranter , DJ. 1962 . Zooplankton abundance in Australian waters . Australian Journal of Marine and Freshwater Research , 13 : 106 – 42 .
- Turnewitsch , R and Springer , B. 2001 . Do bottom mixed layers influence 234Th dynamics in the abyssal near-bottom water column? . Deep-Sea Research I , 48 ( 5 ) : 1279 – 307 .
- Vangriesheim , A , Springer , B and Crassous , P. 2001 . Temporal variability of near-bottom particle resuspension and dynamics at the Porcupine Abyssal Plain, Northeast Atlantic . Progress in Oceanography , 50 ( 1–4 ) : 123 – 45 .
- Vinogradov , GM. 2005 . Vertical distribution of macroplankton at the Charlie-Gibbs Fracture Zone (North Atlantic), as observed from the manned submersible Mir-1 . Marine Biology , 146 ( 2 ) : 325 – 31 .
- Weikert , H. 1977 . Copepod carcasses in the upwelling region south of Cap Blanc, NW Africa . Marine Biology , 42 : 351 – 57 .
- Wheeler , EH. 1967 . Copepod detritus in the deep sea . Limnology and Oceanography , 12 : 697 – 702 .
- Wiebe , PH , Morton , AW , Bradley , AM , Backus , RH , Craddock , JE Barber , V . 1985 . New developments in the MOCNESS, an apparatus for sampling zooplankton and micronekton . Marine Biology , 87 : 313 – 23 .
- Wilkinson L , Hill M , Vang E. 1992 . SYSTAT: Statistics, Version 5 , 2 Edition Evanston, IL : SYSTAT, Inc . 724 pages
- Wishner , K. 1980a . Aspects of the community ecology of deep-sea, benthopelagic plankton, with special attention to gymnopleid copepods . Marine Biology , 60 : 179 – 87 .
- Wishner , KF. 1980b . The biomass of the deep-sea benthopelagic plankton . Deep-Sea Research , 27 : 203 – 16 .