Abstract
Demersal zooplankton were captured with traps from a set of tropical coastal habitats (seagrass bed, coral reef, gravel, and sand bottoms) to allow comparisons among communities. Sampling was carried out during dry and rainy seasons in 2000 and 2001. Traps with and without light were placed at 18:00 and removed at 06:00 the next day for three consecutive days. Eighty-eight zooplankton taxa were identified. Copepoda was the most abundant group, outranking in relative abundance in seagrass and in sandy bottoms. Copepoda was mainly represented by Oithona oculata, Pseudodiaptomus acutus, and Acartia lilljeborgi. No significant differences were found among substrates (P=0.1464); however, differences were significant between light and dark traps communities (P=0.0410). The average density was 7113 (±3966) ind m−2 in the light and 4759 (±4825) ind m−2 in the dark. In the light traps, Amphipoda and O. oculata were more representative. Without light, the main group was Foraminifera (>40%). Cluster analysis presented two main groups, Itamaracá Island and Tamandaré Bay; light and dark traps formed separate groups within these location groups. The results allow us to assess the efficiency of the used traps in a set of habitats of the tropical coastal area and gives information on the preference of specific organism groups in one of the tested substrates.
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
Introduction
The seagrass, saltmarsh, and coral reef patches provide a structure with ecological functions that support high species diversity of many invertebrates (Sheridan 1997; Beck et al. Citation2001; Heck et al. Citation2003; Touchette Citation2007). This high biological diversity is due to important ecological linkages in the heterogeneous complex mosaic of ecosystems in tropical coastal areas (Ogden Citation1997; Heidelberg et al. Citation2004).
Among the invertebrates, zooplankton is an important intermediate component in food webs, acting as a trophic link between small particles (detritus and microalgae) and planktivorous fishes (Morgan Citation1990; Boltovskoy Citation1999). It also includes larvae of diverse organisms; some are commercially important.
Demersal zooplankton are small, active organisms that reside or hide in or near the substrata, migrating up into the water column from the bottom at night and then returning to the substratum before daylight; therefore, these zooplankton cannot be captured by daytime tows (Sorokin Citation1990; Gross & Gross Citation1996). Studies have reported high abundances of demersal zooplankton emerging nightly from coral reefs, kelp beds, and soft-bottom habitats (e.g. Hammer Citation1981; Alldredge & King Citation1985; Jacoby & Greenwood Citation1988, Citation1989; Cahoon & Tronzo Citation1992; Carleton et al. Citation2001), suggesting that demersal zooplankton may play an important role in the ecology and trophic pathways of many benthic communities.
Also, numerous experiments in the field and in the laboratory have shown that vertical migration by demersal species is an adaptation, in part, to predation and behaviours that can be induced by endogenous (reproductive events and biological rhythms) and exogenous factors (light, gravity, dissolved oxygen, temperature, predator, and prey abundance). However, light is generally accepted to be the most significant external factor (Putzeys & Hernández-León Citation2005), as well as a limiting factor for the optical efficiency of visual predators (Ohlhorst Citation1982; Gliwicz Citation1986; Ringelberg Citation1995).
Conventional sampling methods used by zooplankton ecologists are inadequate in most cases for answering questions regarding demersal plankton drift ecology (Greene Citation1990). This difficulty is explained by the sporadic or cyclic emergence, or low densities, of demersal drifting biota (Dahms & Qian Citation2004). In studies of coral reefs, Emery (Citation1968) and Sale et al. (Citation1976) found different qualitative compositions of zooplankton in samples collected by airlift and light traps as compared with net tows or even with samples collected by the usual traps at night or traps without light. The difference in these areas might be due to the zooplankton being composed of demersal and meroplanktonic forms, which hide at the bottom during the day and appear in the water column mostly at night (Renon Citation1977). Similar traps were used by McWilliam et al. (Citation1981) to compare two lagoonal patch reefs, including an open sand environment that is 1–3 m away from the reef. They distinguished two types of fauna, ‘coral’ and ‘sand’ fauna.
Emery (Citation1968) reported that different species of reef zooplankton, including some holoplanktonic species, prefer different types of shelter at the bottom. According to Sorokin (Citation1990), these places include seagrasses, macrophytes, and soft-bottom substrates (sand or gravel).
Among the marine habitats, the tropical South Western Atlantic (SWA) is poorly known to many planktonic dermersal groups and many coastal habitats are under threat by human activities (e.g. mariculture, fishing, dumping of waste and pollution), and until we have a firmer idea of their biodiversity and what controls the emergence behaviour, we have little hope of conserving its ecological functions and biodiversity.
In our study, due to the successful use of a trap-sampler in coral reef areas, we used this sampling tool for the first time on seagrass beds (Halodule wrightii Aschers) to assess the composition and existence of vertical migration patterns of demersal zooplankton.
Thus, the aim of the present study was to test the same trap-sampler in a set of habitats (seagrass bed, coral reef, gravel, and sand bottoms) that normally occur together in the same tropical coastal area in a narrow connectivity to allow for comparisons among communities sampled with the same method; and to assess the preference of specific organism groups in one of the tested substrates.
Materials and methods
Sampling areas
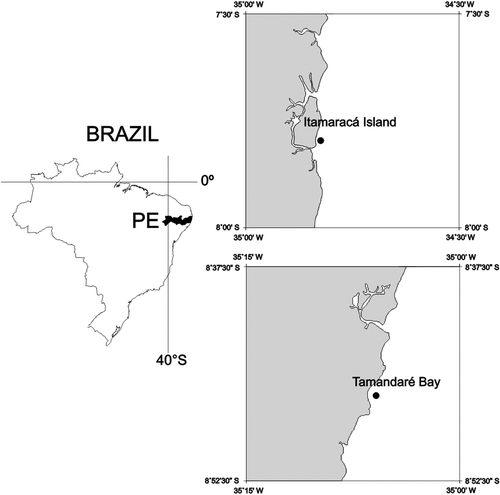
Itamaracá Island and Tamandaré Bay are located on the north and south coasts of Pernambuco, Brazil, respectively (). The area has a humid, tropical climate with two seasons: dry (September–February) and rainy (March–August). Itamaracá Island presents high ecosystem diversity and a strong connectivity among mangrove forests, small rivers estuaries, beach rocks, and seagrass beds (Neumann-Leitão & Schwamborn Citation2000). These seagrass beds are mainly composed of Halodule wrightii and occasionally Halophila decipiens Ostenfeld, which occur all along the east coast of the island (Cocentino et al. Citation2004), being commonly found at a depth of between 2 and 3 m. Tamandaré Bay has approximately 9 km of coastline, enclosed by coastal reefs. These coral reef formations are parallel to the coastline and resemble fringing reefs, with tops exposed during low tide (Maida & Ferreira Citation1997). Of the 18 species of hard corals described for the Brazilian coast, nine were observed in this area (Ferreira & Maida Citation2006). In this region, the reefs associated with the mangroves support intense artisanal fishing (Ferreira et al. Citation2000).
Figure 1. Demersal zooplankton sampling area at Itamaracá Island (sandy bottom and seagrass) and Tamandaré Bay (coral reef and gravel bottom), Pernambuco, Brazil, with the experimental localization.
At Itamaracá and Tamandaré, water temperatures vary from 24°C (rainy) to 31°C (dry season), and salinity from 27 psu (rainy) to 34 psu (dry season) (Moura & Passavante Citation1995; Medeiros et al. Citation2001).
Demersal zooplankton sampling and procedures
The demersal zooplankton samples were captured from two fixed stations at Itamaracá Island, one on a sandy bottom (A) and another on a seagrass bed (B), and two fixed stations at Tamandaré Bay, one on a coral reef (C) and another on a gravel bottom (D) located within the areas shown in . Sampling was carried out at neap tide during the dry (January and February 2001) and rainy (July 2000 and August 2001) seasons in both areas.
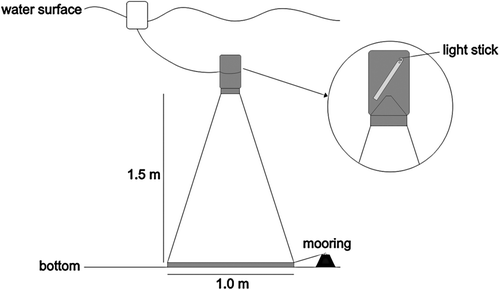
The samples were taken with emergence net traps (inverted cones placed over the bottom – according to Alldredge & King Citation1980 and Alldredge Citation1985) with 300 µm mesh size, 1 m mouth diameter and 1.5 m high (). Traps were placed at 18:00 and removed at 06:00 of the next day during three consecutive days.
For each type of bottom (A, B, C and D), two net traps were used: one was left in the light (L) and one in the dark (D). Triplicates were used for all treatments, totalling 24 traps. The treatment codes consisted of type of bottom + trap (e.g. BL). The samples were fixed in 4% formaldehyde buffered with borax (5 g l−1), according to Newell & Newell (Citation1963).
Zooplankton species were identified to the lowest possible taxonomic unit, as described in specific literature (Björnberg Citation1981; Boltovskoy Citation1981, Citation1999), and taxon abundance (per m2) was counted under a stereomicroscope, based on 5.0 ml subsamples. Three subsamples were taken with a Stempel pipette after sample dilution to 250 ml and homogenization. The samples with low density were completely analysed.
Data treatment
Density (ind m−2), relative abundance (%) and frequency of occurrence (%) were calculated and for the relative abundance the following scale were used: dominant (>70%), abundant (7040%), present (4010%), and rare (≤10%). The results of the frequency of occurrence were expressed in percentages as very frequent (>70%); frequent (7040%); infrequent (4010%), or sporadic (≤10%). The classification ‘others’ used in the figures comprised all the taxa occurring with low abundance or frequency.
The Shannon diversity index (H’) was applied for the estimation of community diversity (Shannon Citation1948), and the evenness was calculated according to Pielou (Citation1977).
Statistical analyses were based on density data (ind m−2) of the zooplanktonic community, with PRIMER 5 for similarity tests among samples. One-way Kruskall–Wallis ANOVA (Zar Citation1996) was used to test for significant (P<0.05) effects of the factors ‘bottom types (A, B, C and D)’ and ‘traps (L and D)’. The Spearman correlation was applied to determine the association between the traps.
Results
Eighty-eight zooplankton taxa were identified ( and ), with some occurring in different life-cycle stages. The frequency of occurrence showed that 5 in 88 taxa were very frequent (Cumacea, Amphipoda, Ostracoda, Mysidacea and Foraminifera) ( and ). Seven taxa were frequent and 10 were infrequent. The remaining taxa were sporadic, representing 75%, of which 29 occurred in only one sample (2.94%). As no significant differences were obtained between replicas, in our results we used the mean of each treatment.
Table I. Composition, relative abundance and frequency of occurrence (for sample) of demersal zooplankton (without Copepoda) captured with traps at Itamaracá Island and Tamandaré Bay, PE, Brazil. Values used in calculation are means.
Table II. Composition, relative abundance and frequency of occurrence (for sample) of demersal Copepoda captured with traps at Itamaracá Island and Tamandaré Bay, PE, Brazil. Values used in calculation are means.
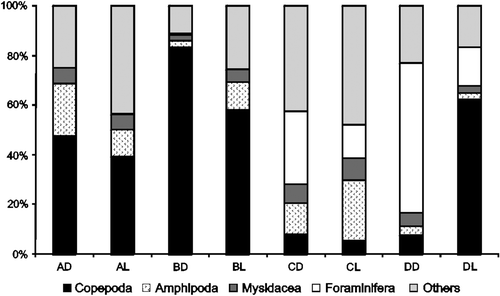
Copepoda was the most abundant group in nearly all the treatments, outranking all other groups in sandy and seagrass substrate, as well as in the gravel with light treatment; a total of 83.2% were attained in the seagrass substrate with dark treatment (). This group was represented mainly by Oithona oculata Farran, 1913, Pseudodiaptomus acutus (F. Dahl, 1894), and Acartia lilljeborgi Giesbrecht, 1889. Besides Copepoda, in coral reef with both light and dark treatment, as well as in gravel substrate during dark treatment, Foraminifera and others groups (mainly Radiolaria and Ostracoda) had larger contributions besides Amphipoda for CL. In sandy substrate, both in dark and light, the largest contribution was from Amphioxus larvae, besides Ostracoda, for AD and Gastropoda for AL. Among the Copepoda, O. oculata was dominant in gravel with light and occurring in less than 10% in the three other substrates. This species did not occur in sandy and seagrass bottoms. A. lilljeborgi was present in sandy and seagrass substrates and rare in CL and DD. P. acutus was present in A and B and rare in D and CL. Another abundant Copepoda was Calanopia americana F. Dahl, 1894, which was rare, but comprised almost 10% of the community in seagrass during dark treatment.
Figure 3. Relative abundance of demersal zooplankton captured with traps at Itamaracá Island and Tamandaré Bay, Pernambuco, Brazil. (A) Sandy bottom, (B) seagrass, (C) coral reef and (D) gravel bottom. (L) light and (D) dark.
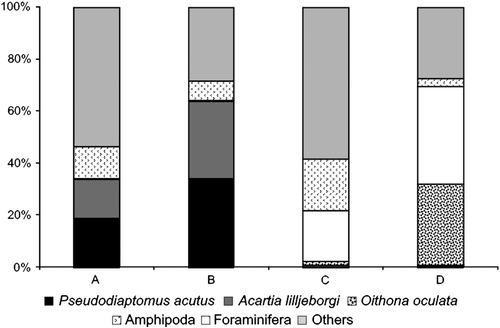
Considering the different substrates (), similarity was observed between the main taxa (A. lilljeborgi, P. acutus, Mysidacea, Cumacea, and Amphipoda) in the composition of sandy and seagrass communities. Calanopia americana, Anomura (Glaucothoea), Teleostei (egg), and Ostracoda presented greater affinity for seagrass bed, while Amphioxus (larvae) and Gastropoda (veliger) demonstrated a preference on the sandy substrate. The coral reef and gravel substrates also showed similarity between the main groups.
Figure 4. Composition of demersal zooplankton captured with traps for the substrates at Itamaracá Island and Tamandaré Bay, Pernambuco, Brazil. (A) Sandy bottom, (B) seagrass, (C) coral reef and (D) gravel bottom.
When comparing sandy and seagrass with coral reef and gravel substrates, a divergence, caused mainly by an important decrease on A. lilljeborgi and P. acutus, was observed. Also, an increase in the Foraminifera and the appearance of O. oculata in C and D could be noted.
Analysing the type of trap (light or dark), the average density was 7113±3966 ind m−2 in light and 4759±4825 ind m−2 in dark. The relative abundance of some groups had similar importance, but a difference could be noted (). In light-traps Amphipoda, O. oculata, and other groups were more representative. Without light, the main group was Foraminifera at more than 40%. In addition, there were considerable decreases in the abundances of Gastropoda (veliger) and O. oculata, from 6.5% to 1.1% and 27% to 1%, respectively ().
Figure 5. Composition of demersal zooplankton captured with light and dark traps at Itamaracá Island and Tamandaré Bay, Pernambuco, Brazil.
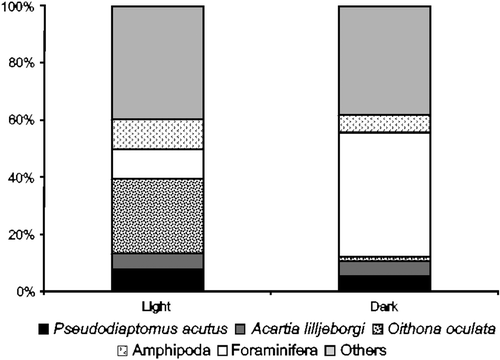
The affinity observed by different groups for the type of substrate and trap was very specific for Amphioxus (larvae) and Gastropoda (veliger) in AL, Labidocera fluviatilis F. Dahl, 1894 in AD, C. americana in BD, Anomura in BL, Harpacticoida Copepoda in CD, Brachyura (zoea and megalopa) and Oikopleura dioica Fol, 1872 in CL, Foraminifera in DD, and O. oculata in DL.
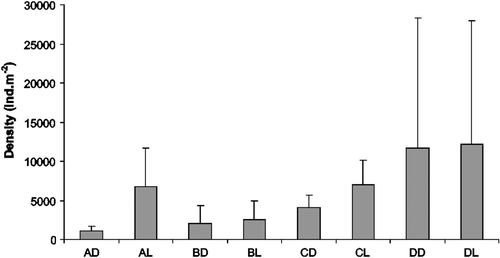
The average density observed for the treatments varied from 1093±592 ind m-2 (AD) to 12,209±15,765 ind m−2 (DL) (). Light-traps had higher density than dark for all substrates, but a significant difference was registered only for the sandy substrate (t-test, p=0.0319).
Figure 6. Density average (+standard deviation) for the treatments of demersal zooplankton captured with traps at Itamaracá Island and Tamandaré Bay, Pernambuco, Brazil.
The species diversity ranged from 1.653 bits ind−1 (DL) to 2.642 bits ind−1 (CD), and was classified between low and medium diversity (). Comparing the treatments, the substrates A, C, and D had higher diversity in the dark rather than light-traps. Evenness values ranged from 0.369 (DL) and 0.59 (CD). Generally, the coral reef substrate showed higher diversity and evenness than other substrates, followed by the sandy bottom. The evenness index showed better uniformity for sandy substrate samples, whereas in the seagrass samples, only one or two species were dominant. In general, the α diversity was 2.348 bits ind−1 to Itamaracá Island and 2.472 bits ind−1 to Tamandaré Bay while the β diversity was 2.725 bits ind−1.
Figure 7. Diversity index (bits ind−1) and evenness of demersal zooplankton captured with traps at Itamaracá Island and Tamandaré Bay, Pernambuco, Brazil.
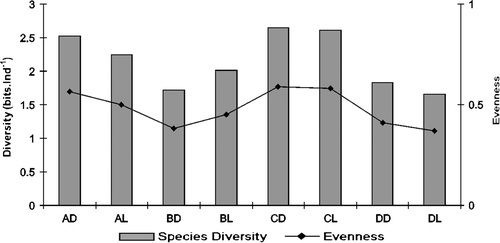
No significant difference was found among substrates (A, B, C, and D with ANOVA, p=0.1464) and between traps communities (L and D) (t-test, p=0.4510).
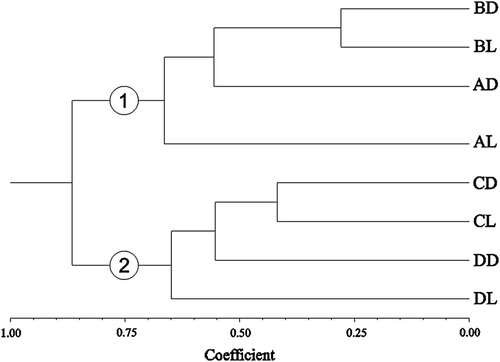
The Cluster analysis grouped the treatments into two main groups, one containing the samples from Itamaracá Island and another from Tamandaré Bay. For Itamaracá Island, BD and BL formed a subgroup (0.25 dissimilarity). In Tamandaré Bay, this subgroup was between CD and CL (0.43 dissimilarity) ().
Figure 8. Cluster analysis of demersal zooplankton captured with traps at Itamaracá Island and Tamandaré Bay, Pernambuco, Brazil. Group 1: Itamaracá Island; group 2: Tamandaré Bay.
The Spearman correlation between the set of treatments presented a significant positive correlation (p<0.05) for type of trap (0.4740), indicating that when the number of individuals in one type of trap increases, the same happens to the other treatment.
Discussion
The constant presence of organisms in early life stages highlights the importance of seagrass beds and reefs as nurseries for fish and invertebrates (Heck et al. Citation2003). The occurrence of these stages, including some species of socio-economic interest in the region, is an essential argument for the conservation of these habitats (Dorenbosch et al. Citation2006).
Other studies in the area presented higher richness than our research, but we observed the importance of certain groups that normally are not observed in samples collected by net tows, such as Cumacea and Amphioxus (larvae), demonstrating the usefulness of this trap-sampler for studies of zooplankton diversity and temporal variations. Adults and larvae of Amphioxus normally live in sandy bottoms, presenting a diurnal vertical migration immediately after sunset (Wickstead & Bone Citation1959), which could explain its absence in daylight net zooplankton tows.
Copepoda, Mysidacea, Ostracoda, and Amphipoda, all with larger contributions, demonstrated their importance in the demersal plankton, in spite of being holoplanktonic organisms that live permanently in the water column. During the day, these organisms are found near the substrate searching for shelter (Emery Citation1968). A diverse array of small invertebrates exhibit this demersal behaviour, which is particularly prevalent among crustaceans, such as Copepoda, Ostracoda, and Peracarida (Amphipoda, Isopoda, Mysidacea, Cumacea, and Tanaidacea) (Hobson & Chess Citation1976; Hammer & Zimmerman Citation1979), as observed in our study.
The dominance of Copepoda, Mysidacea, Ostracoda and Amphipoda in our study may be due to sampling procedures, as compared to demersal zooplankton collected by Youngbluth (Citation1982) in three structurally different emergence traps, indicating that density and diversity estimates were affected by design features and sampling procedures. The smallest mesh netting (63 µm) contained larger catches than traps with 202- or 333-µm mesh. Samples from Porter–Porter traps tethered 1 and 10 cm above the bottom had statistically more zooplankton than all other traps set on the substratum. A consistently greater total number of demersal zooplankton was captured in the Alldredge–King traps when all three trap types were sampled at the same time and area. The abundance and rank order of all but the most numerous animals (harpacticoid copepods) differed between the traps. Alldredge–King traps caught more of the larger organisms (polychaetes, cumaceans, and gammaridean amphipods), whereas the Porter–Porter and Hobson–Chess traps contained larger densities of smaller zooplankton (copepod nauplii and gastropod veligers). However, we used the same mesh size and differences were mainly caused by the different substrates.
The dominance of Copepoda in Brazilian tropical areas is common (Björnberg Citation1981; Neumann-Leitão Citation1995), but this was not observed for substrates C and D, the last only in the dark treatment. This taxa did not exceed 20% in traps completely sealed at the bottom (Hobson & Chess Citation1979), indicating the importance of new methodologies in plankton studies.
Decapoda, Cumacea, and Polychaeta, considered as true demersal plankton (Sorokin Citation1990), also occurred in the samples, although with lesser representation. The Mysidacea showed greater fidelity to seagrass beds, and suffered the strong influence of light (Cebrián et al. Citation2001). The light intensity variation is the main cue controlling vertical migrations of many Mysidacea (Gal et al. Citation1999), and they are well known as nocturnally active, demersal zooplankton (Hobson & Chess Citation1976; Hammer & Zimmerman Citation1979).
Pseudodiaptomus acutus had a high abundance in light traps in A and B, in agreement with others studies which identified Pseudodiaptomus migration. Rios-Jara & González (Citation2000) verified this migration in seagrass bed, mud and sand in other tropical Bay.
The high abundance of Calanopia americana, which occurs in the water column at night and is buried in the mud bottom during the day (Boltovskoy Citation1981), in samples of seagrass bed without light can be justified by the efficient retention of fine sediments by seagrass bed environments (Alves Citation2000). Pseudodiaptomus trihamatus Wright, 1937 was another species that should be highlighted. This exotic species, native from Indo-Pacific coastal waters, was accidentally introduced in Northeastern Brazil in 1977 (Medeiros et al. Citation1991) and was not registered to Pernambuco coastal area until the present study.
Amphioxus larvae and other larvae, such as Gastropoda (veliger) and Crustacea (zoea), are included in the groups that spend the initial stages of ontogenetic development in the plankton, providing a link with the substrate.
The large variation in the average density observed can be related to preference, not only for the substrate, but also treatment. According to Hobson & Chess (Citation1979), the results obtained by this kind of trap in reef areas are difficult to interpret quantitatively, being more suitable for studies of taxonomic composition. This problem is related to the fact that the substrate is not completely flat and may allow organisms access through openings between the bottom and the base of the trap. The same authors mention that during net tows, Copepoda and Mysidacea may be overestimated, while Gammaridea (Amphipoda) can be underestimated, since this group swims near the bottom at night.
The high densities found in light-trap samples were in opposition to that observed by Alldredge & King (Citation1980). They demonstrated that in shallow waters, large organisms migrated less frequently into the water column during moonlit periods than small forms, suggesting that this behaviour is for avoidance of visually oriented predators.
The high densities of Oithona oculata in some samples, especially in gravel bottom and in one coral reef, achieved densities estimated for swarms (Ambler Citation2002). According to Ambler et al. (Citation1991), swarms occur near seagrass beds, algal beds, and coral reefs in both tropical and temperate areas. This species forms swarms in light shafts during the day and disperses to the water column at dusk (Ueda et al. Citation1983; Ambler et al. Citation1991; Buskey et al. Citation1996).
Alldredge & King (Citation1977) and Porter & Porter (Citation1977) suggest that most zooplankton live over structurally complex coral substrata, but differences among substrates communities were not found in this study, as observed by Birkeland & Smalley (Citation1981) and Ohlhorst (Citation1982). This indicates that the zooplankton moving along the coral reefs and seagrass become available in less complex adjacent habitats (sandy and gravel bottom). Fenchel & Uiblein (Citation2008) say that most people consider soft sediments to be rather homogeneous material notwithstanding the presence of burrowing invertebrates; however, their study in sediment indicate that sandy bottom is a spatially complex habitat and on a subcentimetre scale this marine sediment appear as spatially complex and exciting as do coral reefs. Bühring et al. (Citation2006) showed that sandy sediments are highly active and have fast turnover rates.
The diversity ranged from low to medium, being smaller in BD, which can be attributed to the predominance of Acartia lilljeborgi and Pseudodiaptomus acutus, and DL, which was dominated by O. oculata and Foraminifera. Studies comparing the epifauna of seagrasses and adjacent areas without vegetation have shown higher faunal diversity and abundance in seagrass areas (Arrivillaga & Baltz Citation1999; Jackson et al. Citation2002). However, this was not observed in our study, mainly due to the intense degradation of this area caused by intense human seagrass removal. In gravel bottoms, the low diversity is probably due to the lack of shelter. The diversity on coral reef in our study was higher than found by Suárez-Morales & Gasca (Citation2000) (1.84 bits ind−1) by horizontal surface hauls, showing the need for other strategies to study these environments.
The differences ob served among the samples, both in terms of composition, densities, and proportions of the species, can be explained by the different abiotic characteristics of these environments (e.g. suspended materials, light, salinity, temperature, oxygen concentration, and pH), and with the fauna, thus presenting different patterns of diel migration. Therefore, grouping of samples by treatment () appears to be the most consistent with this approach. Divergence in some of these samples can be related to low density and diversity.
The positive correlation between the set of samples both with and without light indicated that some other factor might regulate diel migration. The other factor that may support this migration could be the tide, which causes a change in the community composition through the input of oceanic species or the export of local species. Another factor may be the season, but some authors (Heidelberg et al. Citation2004) found seasonal differences among only a few of the zooplankton taxa. In our study significant differences between dry and rainy seasons were observed only to the gravel bottom only. This may be caused by the lower atypical rainfall in the studied period.
Our research proved the effectiveness of trap-samplers for assessment of demersal zooplankton diversity in seagrass beds as well as coral reefs. The differences observed between treatments showed the importance of this type of trap in studies of communities with diel migration patterns, highlighting the need for further studies to clarify points about the other abiotic factors that affect these communities’ structure, composition, and behaviour.
Editorial responsibility: Torkel Gissel Nielsen
Acknowledgements
We would like to thank M. F. Costa for comments and suggestions on the manuscript and to American Journals Experts for editing the English. We declare that the experiments comply with the current laws of Brazil.
Notes
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
References
- Alldredge , AL. 1985 . Abundance of demersal zooplankton in tropical seagrass meadows . National Geographic Research Reports , 19 : 95 – 101 .
- Alldredge , AL and King , JM. 1977 . Distribution, abundance, and substrate preferences of demersal reef zooplankton at Lizard Island Lagoon, Great Barrier Reef . Marine Biology , 41 : 317 – 33 .
- Alldredge , AL and King , JM. 1980 . Effects of moonlight on the vertical migration patterns of demersal zooplankton . Journal of Experimental Marine Biology and Ecology , 44 : 133 – 56 .
- Alldredge , AL and King , JM. 1985 . The distance demersal zooplankton migrate above the benthos: Implications for predation . Marine Biology , 84 : 253 – 60 .
- Alves , MS. 2000 . “ Fauna associada aos prados de Halodule wrightii Aschers ” . In Gerenciamento Participativo de estuários e manguezais , Edited by: Barros , HM , Eskinazi-Leça , E , Macedo , SJ and Lima , T . 75 – 87 . Recife : Editora Universitária da UFPE .
- Ambler , JW. 2002 . Zooplankton swarms: Characteristics, proximal cues and proposed advantages . Hydrobiologia , 480 : 155 – 64 .
- Ambler , JW , Ferrari , FD and Fornshell , J. 1991 . Population structure and swarm formation of the cyclopoid copepod Dioithona oculata near mangrove shores . Journal of Plankton Research , 13 : 1257 – 72 .
- Arrivillaga , A and Baltz , DM. 1999 . Comparison of fishes and macroinvertebrates on seagrass and bare sand sites on Guatemala's Atlantic Coast . Bulletin of Marine Science , 65 : 301 – 19 .
- Beck , MW , Heck , KL Jr , Able , KW , Childers , DL , Eggleston , DB Gillanders , BM . 2001 . The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates . BioScience , 51 : 633 – 41 .
- Birkeland C , Smalley TL. 1981 . Comparison of demersal plankton from comparable substrata from a high Island and an Atoll . Proceedings of the 4th International Coral Reef Symposium 1 : 437 42 .
- Björnberg , TKS. 1981 . “ Copepoda ” . In Atlas del zooplancton del Atlántico Sudoccidental y métodos de trabajo con el zooplancton marino , Edited by: Boltovskoy , D . 587 – 680 . Mar del Plata : INIDEP .
- Boltovskoy , D. 1981 . Atlas del zooplancton del Atlántico Sudoccidental y métodos de trabajo con el zooplancton marino , 587 – 679 . Mar del Plata : INIDEP .
- Boltovskoy D. 1999 . South Atlantic Zooplankton . Leiden : Backhuys Publishers . 936 pages
- Bühring , SI , Ehrenhauss , S , Kamp , A , Moodley , L and Witte , U. 2006 . Enhanced benthic activity in sandy sublittoral sediments: Evidence from 13C tracer experiments . Marine Biology Research , 2 : 120 – 29 .
- Buskey , EJ , Peterson , JO and Ambler , JW. 1996 . The swarming behaviour of the copepod Dioithona oculata: in situ and laboratory studies . Limnology and Oceanography , 41 : 513 – 21 .
- Cahoon , LB and Tronzo , CR. 1992 . Quantitative estimates of demersal zooplankton abundance in Onslow Bay, North Carolina, USA . Marine Ecology Progress Series , 87 : 197 – 200 .
- Carleton , JH , Brinkman , R and Doherty , PJ. 2001 . Zooplankton community structure and water flow in the lee of Helix Reef (Great Barrier Reef, Australia) . Marine Biology , 139 : 705 – 17 .
- Cebrián , CB , Cunha , MR , Jerez , PS and Esplá , AAR. 2001 . Misidáceos asociados a fanerógamas marinas en el sudeste ibérico . Bolletin Instituto Español de Oceanografia , 17 : 97 – 106 .
- Cocentino , AM , Magalhães , KM and Pereira , SMB. 2004 . “ Estrutura do macrofitobentos marinho ” . In Oceanografia: um cenário tropical , Edited by: Eskinazi-Leça , E , Neumann-Leitão , S and Costa , MF . Recife : Bagaço .
- Dahms , H and Qian , PY. 2004 . Drift-pump and drift-net – two devices for the collection of bottom-near drifting biota . Journal of Experimental Marine Biology and Ecology , 301 : 29 – 37 .
- Dorenbosch , M , Grol , MGG , Nagelkerken , I and Van der Velde , G. 2006 . Seagrass beds and mangroves as potential nurseries for the threatened Indo-Pacific humphead wrasse, Cheilinus undulatus and Caribbean rainbow parrotfish, Scarus guacamaia . Biological Conservation , 129 : 277 – 82 .
- Emery , AR. 1968 . Preliminary observations on coral reef plankton . Limnology and Oceanography , 13 : 293 – 303 .
- Fenchel , T and Uiblein , F. 2008 . Heterogeneity on a small scale . Marine Biology Research , 4 : 241 – 42 .
- Ferreira BP , Maida M. 2006 . Monitoramento dos recifes de coral do Brasil . MMA , Brasília . 120 pages
- Ferreira BP , Maida M , Cava F. 2000 . Características e perspectivas para o manejo da pesca na APA marinha Costa dos Corais . Anais do Congresso Brasileiro de Unidade de Conservação. p 50 58 .
- Gal , G , Loew , ER , Rudstam , LG and Mohammadian , AM. 1999 . Light and diel vertical migration: spectral sensitivity and light avoidance by Mysis relicta . Canadian Journal of Fisheries and Aquatic Science , 56 : 311 – 22 .
- Gliwicz , ZM. 1986 . A lunar cycle in zooplankton . Ecology , 67 : 883 – 97 .
- Greene , CH. 1990 . A brief review and critique of zooplankton sampling methods: copepodology for the larval ecologist . Ophelia , 32 : 109 – 13 .
- Gross MG , Gross E. 1996 . Oceanography, a View of Earth . Englewood Cliffs, NJ : Prentice Hall . 472 pages
- Hammer , RM. 1981 . Day–night differences in the emergence of demersal zooplankton from a sand substrate in a kelp forest . Marine Biology , 62 : 275 – 80 .
- Hammer , RM and Zimmerman , RC. 1979 . Species of demersal zooplankton inhabiting a kelp forest ecosystem off Santa Catalina Island, California . Bulletin of the South California Academy of Science , 78 : 199 – 206 .
- Heck , KL , Hays , G and Orth , RJ. 2003 . Critical evaluation of the nursery role hypothesis for seagrass beds . Marine Ecology Progress Series , 253 : 123 – 36 .
- Heidelberg , KB , Sebens , KP and Purcell , JE. 2004 . Composition and sources of near reef zooplankton on a Jamaican forereef along with implications for coral feeding . Coral Reefs , 23 : 263 – 76 .
- Hobson , ES and Chess , JR. 1976 . Trophic interactions among fishes and zooplankters near shore at Santa Catalina Island, California . Fisheries Bulletin , 74 : 567 – 98 .
- Hobson , ES and Chess , JR. 1979 . Zooplankters that emerge from the lagoon floor at night at Kure and Midway Atolls, Hawaii . Fisheries Bulletin , 77 : 275 – 80 .
- Jackson , EL , Rowden , AA , Attrill , MJ , Bossy , SF and Jones , MB. 2002 . Comparison of fish and mobile macroinvertebrates associated with seagrass and adjacent sand at St. Catherine Bay, Jersey (English Channel): Emphasis on commercial species . Bulletin of Marine Science , 71 : 1333 – 41 .
- Jacoby , A and Greenwood , JG. 1988 . Spatial, temporal, and behavioural patterns in emergence of zooplankton in the lagoon of Heron Reef, Great Barrier Reef, Australia . Marine Biology , 97 : 309 – 28 .
- Jacoby , A and Greenwood , JG. 1989 . Emergent zooplankton in Moreton Bay, Queensland, Australia: Seasonal, lunar, and diel patterns in emergence and distribution with respect to substrata . Marine Ecology Progress Series , 51 : 131 – 54 .
- Maida M , Ferreira BP. 1997 . Coral reefs of Brazil: An overview . Proceedings of the 8th International Coral Reef Symposium 1 : 263 74 .
- McWilliam , PS , Sale , PF and Anderson , DT. 1981 . Seasonal changes in resident zooplankton sampled by emergence traps in one tree lagoon, Great Barrier Reef . Journal of Experimental Marine Biology and Ecology , 52 : 185 – 203 .
- Medeiros , C , Kjerfve , B , Araújo , M. and Neumann-Leitão , S. 2001 . “ The Itamaracá Estuarine Ecosystem, Brazil ” . In Ecological Studies: Coastal Marine Ecosystems of Latin America , Edited by: Seelinger , U and Kjerfve , B . 71 – 81 . Berlin : Springer .
- Medeiros GF , Rocha CEF , Silva ML. 1991 . A note on the occurrence of Pseudodiaptomus trihamatus Wright, 1937 (Crustacea Copepoda) in Natal, Brazil . Boletim do Departamento de Oceanografia e Limnologia da Universidade Federal do Rio Grande do Norte 8 : 113 .
- Morgan , SG. 1990 . Impact of planktivorous fishes on dispersal, hatching, and morphology of estuarine crab larvae . Ecology , 71 : 1639 – 52 .
- Moura , RT and Passavante , JZO. 1995 . Biomassa Fitoplanctônica da Baía de Tamandaré, Rio Formoso – Pernambuco, Brasil . Trabalhos Oceanograficos Universidade Federal de Pernambuco , 23 : 1 – 16 .
- Neumann-Leitão , S. 1995 . Resenha Literária Sobre Zooplâncton Estuarino no Brasil . Trabalhos Oceanograficos Universidade Federal de Pernambuco , 23 : 25 – 53 .
- Neumann-Leitão , S and Schwamborn , R. 2000 . “ Interações tróficas do Canal de Santa Cruz ” . In Gerenciamento Participativo de estuários e manguezais , Edited by: Barros , HM , Eskinazi-Leça , E , Macedo , SJ and Lima , T . 163 – 80 . Recife : Editora Universitária da UFPE .
- Newell GE , Newell RC. 1963 . Marine Plankton: A Practical Guide . London : Hutchison Educational . 221 pages
- Ogden , JC. 1997 . “ Ecosystem interactions in the tropical coastal seascape ” . In Life and Death of Coral Reefs , Edited by: Birkeland , C . 288 – 97 . New York : Chapman and Hall .
- Ohlhorst , SL. 1982 . Diel migration patterns of demersal reef zooplankton . Journal of Experimental Marine Biology and Ecology , 60 : 1 – 15 .
- Pielou EC. 1977 . Mathematical Ecology . New York : Wiley . 386 pages .
- Porter , JW and Porter , KG. 1977 . Quantitative sampling of demersal plankton migrating from different coral reef substrates . Limnology and Oceanography , 22 : 553 – 56 .
- Putzeys , S and Hernández-León , SA. 2005 . Model of zooplankton diel vertical migration off the Canary Islands: Implication for active carbon flux . Journal of Sea Research , 53 : 213 – 22 .
- Renon , JP. 1977 . Zooplancton du lagon de Takapoto (Polynésie française) . Annales l'Insitute Oceanographique , 53 : 217 – 36 .
- Ringelberg , J. 1995 . Changes in light intensity and diel vertical migration: A comparison of marine and freshwater environments . Journal of the Marine Biology Association of the United Kingdom , 75 : 15 – 25 .
- Rios-Jara , E and González , JG. 2000 . Effects of lunar periodicity on the emergence behaviour of the demersal copepod Pseudodiaptomus cokeri in Phosphorescent Bay, Puerto Rico . Bulletin of Marine Science , 67 : 887 – 901 .
- Sale , PF , McWilliam , PS and Anderson , DT. 1976 . Composition of the near reef zooplankton at Heron Reef, Great Barrier Reef . Marine Biology , 34 : 59 – 66 .
- Shannon , CE. 1948 . A mathematical theory of communication . Bell System Technical Journal , 27 : 379 – 423 .
- Sheridan , P. 1977 . Benthos of adjacent mangrove, seagrass and non-vegetated habitats in Rookery Bay, Florida, USA . Estuarine Coastal and Shelf Science , 44 : 455 – 69 .
- Sorokin , YI. 1990 . “ Plankton in the reef ecosystems ” . In Ecosystems of the World, Coral Reefs , Edited by: Dubinsky , Z . 291 – 327 . Amsterdam : Elsevier .
- Suárez-Morales , E and Gasca , R. 2000 . The planktonic copepod community at Mahahual reef, Western Caribbean . Bulletin of Marine Science , 66 : 255 – 67 .
- Touchette , BW. 2007 . The biology and ecology of seagrasses . Journal of Experimental Marine Biology and Ecology , 350 : 1 – 2 .
- Ueda , H , Kuwahara , A , Tanaka , M and Azeta , M. 1983 . Underwater observations on copepod swarms in temperate and subtropical waters . Marine Ecology Progress Series , 11 : 165 – 71 .
- Wickstead , JH and Bone , Q. 1959 . Ecology of acraniate larvae . Nature , 184 : 1849 – 51 .
- Youngbluth , MJ. 1982 . Sampling demersal zooplankton: A comparison of field collections using three different emergence traps . Journal of Experimental Marine Biology and Ecology , 61 : 111 – 24 .
- Zar JH. 1996 . Biostatistical Analysis , 3rd ed Englewood Cliffs, NJ : Prentice Hall . 662 pages