Abstract
Information on reproduction in temperate scleractinian corals is notably scant. Astroides calycularis is an azooxanthellate coral that inhabits the South-Western Mediterranean Sea, in shaded habitats from 0 to 50 m depth. Recently, it has been observed along the coast of the Adriatic Sea. This study is the first in-depth investigation of A. calycularis reproductive biology. Observations from the nineteenth century described A. calycularis as hermaphroditic; in contrast, we demonstrated gonochorism (male and female colonies) and brooding (planula releasing) as the reproductive mode, consistent with other members of the family Dendrophylliidae. Undifferentiated germ cells arose in the gastrodermis and subsequently migrated to the mesoglea, where they completed gametogenesis. During spermatogenesis, spermary diameter increased from 20 to 940 µm. During oogenesis, a conspicuous presence of lipid vesicles of exogenous origin (phagocytes) was observed in the ooplasma. As oogenesis progressed, the synthesis of yolk gradually reduced the nucleus to cytoplasm ratio. In the final stages of oogenesis, the nucleus migrated to the extreme periphery of the oocyte adhering to the oolemma, and became indented. Nuclear migration and shape change may facilitate fertilization and determine the future embryonic axis. During oogenesis, the oocyte diameter increased from 25 to 1590 µm. Embryogenesis took place in the coelenteron. Formation of a blastocoel was not observed, and development proceeded via stereoblastulae with superficial cleavage. Gastrulation took place by delamination. Embryo diameter ranged from 550 to 1140 µm. Released larvae (length 1700 to 2000 µm) were observed in the field during summer, along the benthos.
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
Introduction
Studying sexual reproduction is essential to understand the genetic structure, and the resistance and resilience of populations following natural or anthropogenic impacts (Connell & Keough Citation1985). Reaching sexual maturity requires a balance between somatic growth and survival, which in turn depends on the age and size of the organism. The age/size at first reproduction and the sex ratio influences the growth rates of populations (Fujiwara & Caswell Citation2001). Variation in life-history strategy is important as it may lead to evolutionary divergences (Richmond & Hunter Citation1990). Studying the reproductive biology of corals, including sexuality (hermaphroditic or gonochoric), reproductive mode (broadcasting or brooding), embryonic development (coeloblastic or stereoblastic) and larval behaviour (bentonic or planctonic) is the first step to understand the population dynamics of marine organisms (Goffredo et al. Citation2005).
Most scleractinian corals are hermaphroditic (Kruzic et al. Citation2008). Gonochorism accounts for less than 25% of examined species (Kruzic et al. Citation2008). Within the Scleractinia, the sexual condition tends to remain constant at the family level (Harrison Citation1985). Generally, the annual cycle of gametogenesis culminates in a short period of gametes being released into the environment, where fertilization occurs (Wilson & Harrison Citation2003). The regulation of the reproductive cycle has been connected to various environmental factors including water temperature and photoperiod, which seem to be fundamental for seasonality of reproduction (Penland et al. Citation2004). Environmental factors can also influence reproduction by acting as selective pressure elements on the sexuality of populations (Wilson & Harrison Citation2003). The synchronization of gamete development and release is important to maximize fertilization and reproductive success, since the rapid dispersion of gametes into water decreases the probability of fertile encounters (Harrison & Wallace Citation1990). Recently, particular photoreceptors (cryptochromes) perceiving lunar radiation have been detected in Acropora millepora (Ehrenberg, 1834) and probably trigger gamete release along the Great Barrier Reef (Levy et al. Citation2007).
While tropical and subtropical scleractinians are extensively studied (Fadlallah Citation1983a; Heltzel & Babcock Citation2002; Neves & Pires Citation2002), information on sexual reproduction for temperate zones is scarce (Szmant-Froelich et al. Citation1980; Beauchamp Citation1993). In particular, the only data from the Mediterranean Sea come from the observations of Lacaze-Duthiers (1873) on solitary (Caryophyllia smithii Stokes & Broderip, 1828, Balanophyllia regia Gosse, 1860, Leptopsammia pruvoti Lacaze-Duthiers, 1897) and colonial (Astroides calycularis (Pallas, 1766), Cladopsammia rolandi Lacaze-Duthiers, 1897) species, and from recent in-depth studies on the species Balanophyllia europaea (Risso, 1826) and L. pruvoti (Goffredo et al. Citation2006). More recently, observations have been made on the reproduction of Cladocora caespitosa (Linneo, 1767) in the Adriatic Sea (Kruzic et al. Citation2008). Studies on the sexual reproduction of temperate-Mediterranean corals are needed in order to address this lack of knowledge, and to quantify population resilience, especially in the face of global change-related shocks, whose magnitude is expected to be greater in temperate areas than in tropical ones (Solomon et al. Citation2007).
The family Dendrophylliidae is cosmopolitan; it includes both solitary and colonial corals and has 148 living species divided into 19 genera (Cairns Citation1999). Seven species live in the Mediterranean Sea grouped into five genera; three of these (Astroides, Cladopsammia and Dendrophyllia) are colonial (Minelli et al. Citation1995). The genus Astroides contains a single species, A. calycularis (Cairns et al. Citation1999).
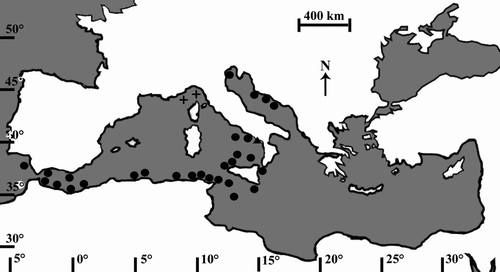
In the Pleistocene, A. calycularis was present throughout the Western Mediterranean Sea, as some fossils testify (Zibrowius Citation1995; ). Following a Pleistocene glaciation that lowered seawater temperature in this area, the species disappeared from the Northern Mediterranean Sea (Peres Citation1967). Currently, A. calycularis is spread in the south-central part of the Western Mediterranean Sea (Zibrowius Citation1995; Ocaña et al. Citation2000; Alvarez-Pérez et al. Citation2005; ), with some recent records in the north-eastern part of the Adriatic Sea, along the coasts of Croatia (Grubelic et al. Citation2004; Kruzic et al. Citation2005; Bianchi Citation2007; ) up to the Gulf of Venice (Casellato et al. Citation2007; ). The range expansion into the Adriatic Sea is thought to be due to seawater warming and to the Ionian cyclonic stream (Bianchi Citation2007), with the ascending circulation that seems to have favoured the flow of larvae along the Croatian coasts (Grubelic et al. Citation2004). Although A. calycularis is a Mediterranean endemic species, it has been found outside the Strait of Gibraltar, along the Atlantic coasts of Morocco and Spain (Bianchi Citation2007; ), probably due to the currents dispersing larvae out of the Strait (Ocaña et al. Citation2000). A. calycularis is found at depths of 0–50 m (Rossi Citation1971), but is typically found in the shallow infralittoral (0–15 m depth), on vertical walls or inside caves (Kruzic et al. Citation2002). It is an azooxanthellate species (Cairns Citation1999), living in both light and dark and seems to prefer elevated hydrodynamism (Kruzic et al. Citation2002). The population density can be high, with colonies covering up to 90% of the bottom (personal observations). The colonies generally have an ellipsoid shape with polyps densely crowded or separated, depending on flow (Kruzic et al. Citation2002). Near the surface (high hydrodynamism), massive-shaped colonies have polyps with a polygonal calyx (Kruzic et al. Citation2002). In these colonies the new polyps bud both in the outskirts of the colonies and between existing polyps. In deeper water (low hydrodynamism), the bush-shaped colonies have polyps with a circular calyx (Kruzic et al. Citation2002). In these colonies, the same polyp can produce buds at different heights of the calyx.
Figure 1. Astroides calycularis. Variations of the range of the scleractinian coral within the Mediterranean Sea. (+ Pleistocene fossil records; • current confirmed distribution; ▴ site where corals were collected during this study, Palinuro).
This study on the sexual reproduction of A. calycularis was conducted in the Southern Tyrrhenian Sea, at Palinuro (Salerno, Italy; ). This paper reports the morphologic aspects of spermatogenesis, ovogenesis, embryogenesis and larval development. Quantitative data on the annual sexual reproduction cycle (fecundity, size at sexual maturity, gonadal development in relation to environmental parameters, sexual allocation, planulation timing) will be presented in a separate paper.
Materials and methods
Samples of Astroides calycularis (a) were collected at Palinuro (10 km south of Salerno, Italy, Southern Tyrrhenian Sea, 40°01.81′N; 15°16.74′E; ) during 16 monthly collections from April 2004 to September 2005. Scuba divers collected 10 colonies every month at 7–10 m depth along a random transect line, parallel to the coast line; the distance between two consecutive sampled colonies was 2 m. Water temperature was measured directly in the field at the depth and time of sampling using a mercury thermometer. Astronomical almanacs were used to calculate photoperiods. Colonies had approximately elliptical shape (b). Of each collected colony, colony length (L
C, major axis of the colony) and colony width (W
C, minor axis of the colony) were measured, and colony area (A
C) was calculated using the formula . Colonies were fixed in saturated Formalin solution (10% formaldehyde and 90% seawater; the solution was saturated with calcium carbonate) and transferred to the laboratories for histological analysis. A biometric analysis of each polyp in each colony was performed: polyp length (L
P, major axis of the oral disc), width (W
P, minor axis of the oral disc) and height (h, oral–aboral axis) were measured and body volume (V
P) was calculated using the formula
(Goffredo et al. Citation2009).
Figure 2. Astroides calycularis. (a) Specimens photographed at Palinuro (Salerno, 40°01.81′N; 15°16.74′E) at 10 m depth. (b) Colony photographed in the laboratory (L C major axis of the colony; W C minor axis of the colony).

Polyps were post-fixed in Bouin solution. After decalcification in EDTA and dehydration in a graded alcohol series from 80% to 100%, polyps were embedded in paraffin and serial transverse sections were cut at 7 µm intervals along the oral–aboral axis, from the oral to the aboral poles. Tissues were then stained with Mayer's haematoxylin and eosin. Histological observations were made under a light microscope. Cyto-histological readings were made with a LEICA Q5001 W image analyser. The maximum and minimum diameters of the spermaries and oocytes in nucleated sections were measured. The size of each reproductive element was determined as the mean of the two diameters and was classified into developmental stages in accordance with earlier studies on gametogenesis in scleractinians (Goffredo et al. Citation2005).
The following definitions were used: active polyp, a polyp which showed gametogenetic or embryogenetic activity; male polyp, a polyp which showed spermaries; female polyp, a polyp which showed oocytes or embryos; inactive polyp, a polyp which did not show gametogenetic or embryogenetic activity; active colony, a colony where at least one analysed polyp showed gametogenetic activity; male colony, a colony formed by male polyps; female colony, a colony formed by female polyps; undetermined colony, a colony where only inactive polyps were found.
Results
Sexual condition and reproductive mode
In this study 96 polyps from 46 colonies were analysed ( and ). We found Astroides calycularis to be gonochoric both at the polyp and the colony level. All mature polyps and colonies examined had either male or female germ cells, none had both ( and ). We did not observe sexual dimorphism at either the polyp or the colony level, nor were there significant differences in mean size between male and female at either level (Student's t-test for L P: t=0.563, p>0.05; Student's t-test for V P: t=0.417, p>0.050; Student's t-test for L C: t=0.613, p>0.050; Student's t-test for A C: t=0.013, p>0.05; ). Sex ratio was 1:1 for sexually active colonies examined (Chi-square test: χ2=3.667, p>0.05). Thirty polyps were inactive; six were from five female colonies (L P=4.49 mm, SE = 0.51, N=6; V P=78.27 mm3, SE = 20.68, N=6), and the remaining 24 inactive polyps (L P=5.18 mm, SE = 0.14, N=24; V P=94.43 mm3, SE = 9.42, N=24) were from 13 indeterminate colonies collected in the summer–autumn period, from July to October (). The mean size of the six inactive polyps was not significantly different from the mean size of the 39 analysed female polyps (Student's t-test for L P: t=1.848, p>0.05; Student's t-test for V P: t=1.349, p>0.05; ), and the mean size of the 24 inactive polyps was not significantly different from the mean size of the 66 sexually active polyps (Student's t-test for L P: t=0.266, p>0.05; Student's t-test for V P: t=1.048, p>0.05; ). In addition, the mean size of the 13 indeterminate colonies was not significantly different from the mean size of the 33 sexually active colonies (Student's t-test for L C: t=0.903, p>0.05; Student's t-test for A C: t=0.885, p>0.05; ). Four out of five (80%) analysed females of the May sample had embryos in the coelenteron, indicating a brooding reproductive mode ().
Figure 3. Astroides calycularis. Spermary developmental stages. (a) Localization of the spermaries in the mesentery. (b) Spermary early stage (I, II). Male germ cells, arising in the mesenterial gastrodermal layer, are clustering. (c) Stage III: the spermary, containing spermatocytes undergoing meiosis, is delineated by a wall that has arisen from the mesoglea (arrow). (d) Stage III: particular of the spermary periphery, showing the mesoglea wall (arrows). (e) Stage IV: the spermary presents an external layer of spermatocytes and an internal mass of spermatids. (f) Stage V: the spermary is made up of a mass of spermatozoa. (g) Stage V: shortly before leaving the spermary, mature spermatozoa form ‘bouquets’, with their tails all facing the same direction (t, spermatozoa tails; cc, coelentric cavity; g, gastrodermis; mf, mesenterial filament; sdi, spermatids; sni, spermatogonia; sp, spermary; sti, spermatocytes; szoi, spermatozoa; I, II, III, IV, V, spermary developmental stages).
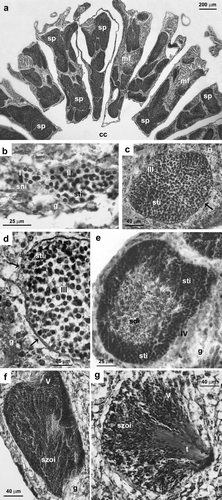
Table I. Astroides calycularis. Size, sex and brooding condition of the analysed polyps (L P, major axis of the oral disc; W P, minor axis of the oral disc; h, oral–aboral diameter; V P, body volume; F, female; M, male; I, inactive).
Table II. Astroides calycularis. Size, sex and brooding condition of the analyzed colonies (L C, major axis of the colony; W C, minor axis of the colony; A C, colony area; F, female; M, male; I, indeterminate).
Table III. Astroides calycularis. Mean size and standard error of sexually active (males and females) and inactive/indetermined polyps or colonies (L P, major axis of the oral disc of the polyp; V P, polyp volume; L C, major axis of the colony; A C, colony area; N, number of polyps or colonies examined).
Spermaries and oocytes
The gastrodermal tissue lining the mesenteries with clearly visible gametocytes was swollen and had a granular appearance ( and ). A total of 30,801 spermaries and 1326 oocytes were observed and measured.
Spermaries () were made up of groups of germ cells and were located in the mesentery (a). Spermaries were delineated by the mesogleal envelope. We identified five spermary developmental stages.
Stage I: undifferentiated germ cells were lined up in the mesenterial gastroderm layers. Spermaries were formed by the migration of undifferentiated germ cells moving from the gastrodermis and clustering in the mesoglea. Stage I spermary was made up of a group of spermatogonia and had a diameter of 48.1 µm (SE = 2.2, N=73; b).
Stage II: the spermary was made up of a group of spermatocytes undergoing meiosis (b). The mesogleal layer enveloping the spermary had not yet formed a wall. Stage II spermary diameter was 70.9 µm (SE = 0.9, N=929).
Stage III: the spermary, still made up of a group of spermatocytes undergoing meiosis, was delineated by a clearly differentiated wall formed by the mesoglea (c, d). Stage III spermary diameter was 144.5 µm (SE = 0.5, N=11,452).
Stage IV: the spermary showed a centripetal maturation gradient: less mature and larger germ cells (spermatocytes) were located at the periphery of the spermary, while more mature and smaller ones (spermatids) were located in the center (e). Stage IV spermary diameter was 186.6 µm (SE = 0.5, N=15,517).
Stage V: the spermary was made up of a mass of spermatozoa with their tails prejecting in the same direction (an arrangement known as a ‘bouquet’; Fadlallah & Pearse Citation1982; f, g). At the time of fertilization, the spermatozoa were released into the coelenteron. Stage V spermary diameter was 173.6 µm (SE = 1.4, N=2830).
Oocytes () were oval and located in the mesenteries (a). Oocytes had a diameter ranging from 25 to 1590 µm.
Figure 4. Astroides calycularis. Oogenesis. (a) Localization of the oocytes in the mesenteries. (b) Early stage: the oocyte located in the mesoglea of a mesentery is characterized by a high nucleus to cytoplasm ratio. Lipid vesicles are visible in the cytoplasm. (c) Early stage: a conspicuous mesogleal central cord (arrows) envelops the oocyte. (d) Intermediate stage: the spherical nucleus is still located in the centre of the cell. There is a conspicuous presence of lipid vesicles in the oocyte cytoplasm. Note the phagocytosis of a lipid droplet (arrow). (e) Intermediate stage: the spherical nucleus with a single nucleolus starts to migrate towards the cell periphery. Note the mesogleal thickening surrounding the cytoplasmatic membrane (arrow). (f) Late stage: the oocyte is located in the central portion of one of the mesenteries; the ooplasm is full of yolk plates. The nucleus to cytoplasm ratio is decreased. (g) Late stage: detail of the oolemma in a mature oocyte. The oolemma is surrounded by a mesogleal thickening (arrow). (h) Late stage: detail of the nucleus in a mature oocyte. The nucleus becomes concave and is located on the cell's periphery where it adheres to an invagination of the oolemma (cc, celentric cavity; g, gastrodermis; lv, lipid vesicles; m, mesoglea; mf, mesenteric filament; N, nucleus; n, nucleolus; o, oocyte; yp, yolk plates).
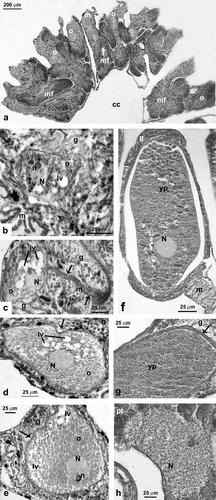
The early stages of oogenesis were visible in the mesentery's gastrodermal layers and showed a centrally located spherical nucleus and a high nucleus to cytoplasm ratio (b).
The intermediate stages of oogenesis developed in the mesoglea of the mesenteries (c, d). The nucleus was spherical, and a conspicuous yolk mass had begun to accumulate, causing a reduction in the ratio of nucleus to cytoplasm (c, d).
In the late stages of oogenesis, the oocytes were still located in the mesenteries and enveloped by a mesogleal layer, which formed an evident thickening surrounding the oolemma (e–g). During the late stages, yolk synthesis and differentiation were completed. The nucleus had migrated to the cell's periphery and changed its shape, adhering closely to the cell membrane and becoming indented and concave (dome-shaped; h). During oogenesis, the nucleolus was at the periphery of the nucleus (b, c, e).
In all stages of oogenesis the presence of lipid vesicles was observed in the ooplasma (b–e). The lipid material, of exogenous origin (phagocytes), was accumulated within oocytes through phagocytosis (d). For most examined oocytes, these lipid vesicles were concentrated at the periphery of the ooplasma or around the nuclear membrane (d, e).
Embryos and larvae
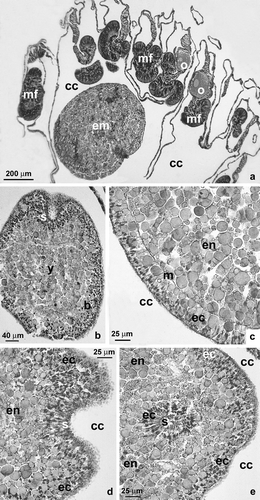
While the oocytes were found inside the mesenteries, embryos were located in the coelenteron of female polyps collected in May (a). Early stage embryos were stereoblastulae (solid and lacking a blastocoel). A visibly cleaved superficial layer surrounded the central yolk mass (b). Stereoblastulae had diameters ranging from 556 to 964 µm. During the intermediate developmental stage (called a stereogastrula since there was no archenteron), gastrulation took place by delamination (c). The ectodermal layer was clearly differentiated and appeared separated from the endodermal mass by a clearly defined mesogleal layer (c). Stereogastrulae had diameters ranging from 991 to 1134 µm. Embryos developed a stomodeum (b, d, e). The differentiation of the stomodeum began with the proliferation of ectodermal cells and their invagination towards the center of the embryo (b, d, e). Mesentery differentiation started with the invagination of the mesogleal layer towards the center of the embryo, followed by the formation of the mesenterial filament via apposition of endodermal cells to the mesentery's free edge.
Figure 5. Astroides calycularis. Embryonic development. (a) Localization of the embryos in the coelentric cavity. Note the presence of vitellogenic oocytes in the mesenteries. (b) Stereoblastula in the coelenteric cavity: the cleavaged superficial layer surrounds the central yolk mass. (c) Stereogastrula. At this stage of development, the ectoderm is clearly distinct from the yolk endoderm. The mesogleal layer is well defined. (d) Sagittal section of the stomodeal invagination. (e) Transversal section at the oral pole of the embryo showing the stomodeal invagination (b, blastoderm; cc, coelentric cavity; ec, ectoderm; em, embryo; en, endoderm; m, mesoglea; mf, mesenteric filament; o, oocyte; s, stomodeal invagination; y, yolk).
Released larvae () had completed ontogenesis (differentiated mouth and pharynx, the gastrovascular cavity was divided into compartments by mesenteric septa). In the field, larvae were observed in July and had a demersal behaviour and an orange–yellow colour similar to that of adult polyps (). Larvae were able to contract and become more spherical or to stretch out and become more cylindrical. Larvae length ranged from 1700 to 2000 µm.
Physical data and reproduction
Mature spermaries and oocyte were observed in April and May 2004 and 2005 when the seawater temperature was 16–17°C, and photoperiod 13.5–14.6 h. Planulation took place between May and July 2004 and 2005 when seawater temperature was 17–23°C, and photoperiod 14.6–15.0 h.
Discussion
This study is the first to investigate the reproductive biology of Astroides calycularis in detail. In this paper, the morphological aspects of gametogenesis, ontogenesis and larval stage are described.
The individuals examined were gonochoric both at the polyp and colony level. This is in contrast with the hermaphroditism proposed by Lacaze-Duthiers (1873) in samples from the Algerian coasts and by Cirino et al. (Citation1993) after aquarium observations on the reproductive behaviour of Mediterranean samples of undetermined origin. In particular, Lacaze-Duthiers (1873), from observations of dissected polyps with a magnifying glass, described the presence of hermaphroditic colonies formed by sex separated polyps (monoic hermaphroditism) as a dominant condition, with some rare cases of simultaneous hermaphroditic polyps. The gonochorism found in the present study reflects the sexual condition expected for the family Dendrophylliidae, since hermaphroditism (found in 32% of the species studied) is an infrequent reproductive condition in this taxon (Goffredo et al. Citation2005; ). Species that change between gonochorism and hermaphroditism in different populations are known among the alcyonarians: Heteroxenia elizabethae Kölliker, 1874, a soft coral of the Xeniidae family, is gonochoric in the Great Barrier Reef, but hermaphroditic in the Red Sea (Hwang & Song Citation2007); in Sarcophyton glaucum (Quoy & Gaimard, 1833) of the family Alcyoniidae, a low level of hermaphroditism is found in South Africa, whereas in the Red Sea only gonochorism is present (Schleyer et al. Citation2004). The possibility that A. calycularis might express different sexuality in different populations, as an adaptation to different environments, cannot be excluded. In Palinuro, A. calycularis has high population densities, covering up to 90% of the rocky substrate. Here the population has an increased probability of fertile encounters and thus gonochorism would be advantageous, since it would ensure cross-fertilization and maintain the genetic variability of the population. If in Algeria A. calycularis has low population densities reducing the probability of fertile encounters, then simultaneous hermaphroditism of colonies would become adaptive, maximizing their fertilization rate (Ghiselin Citation1969).
Table IV. Reproductive traits in Dendrophylliid corals (h, hermaphroditic; g, gonochoric; –, unknown; b, brooder; bs broadcast spawner).
The stages of male gametogenesis corresponded to those described in other species of the family Dendrophylliidae, for example Heteropsammia aequicostatus Fisk, 1981 and Heteropsammia cochlea (Spengler, 1781) (gonochoric, broadcast spawner; Harriott Citation1983), Leptopsammia pruvoti and Balanophyllia elegans Verrill, 1864 (gonochoric, brooder; Goffredo et al. Citation2005), Balanophyllia europaea (hermaphroditic, brooder; Goffredo et al. Citation2002), or in corals of different families such as Fungiacyathus marenzelleri (Vaughan, 1906) (gonochoric, broadcast spawner; Fungiacyathidae; Waller et al. Citation2002), Monomyces rubrum (Quoy & Gaimard 1833) (gonochoric, brooder; Flabellidae; Heltzel & Babcock Citation2002), Mussimilia hispida (Verril, 1902) (hermaphroditic, broadcast spawner; Mussidae; Neves & Pires Citation2002) and in the genus Madracis sp. Milne Edwards and Haime, 1849 (hermaphroditic, brooder; Pocilloporidae; Vermeij et al. Citation2004).
Concerning female gametogenesis, a particular process of differentiation was noted in the shape of the nucleus during the last phase of development of the oocyte. After the migration of the nucleus to the cell outskirts, which is a process that generally occurs during oogenesis in scleractinians and more generally in anthozoans (Szmant-Froelich et al. Citation1985), the nucleus adhered closely to the oolemma and changed shape from circular to a particular dome- or U-shape. The frequency of this nuclear morphology in the female gametogenesis of the scleractinians is not clear. A nucleus defined as falciform or dome-shaped or U-shaped and adherent to the oolemma of the mature oocyte was described in other species, such as the hermaphroditic broadcast spawners Acropora cervicornis (Lamark, 1816) (Acroporidae; Vargas-Angel et al. Citation2006), Gardineroseris planulata (Dana, 1846) (Agariciidae; Glynn et al. Citation2000), Pocillopora damicornis (Linneo 1758) and P. elegans Dana, 1846 (Pocilloporidae; Glynn et al. Citation1991), the hermaphroditic brooders B. europaea (Dendrophylliidae; Goffredo et al. Citation2002), Favia fragum (Esper, 1795) (Faviidae; Szmant-Froelich et al. Citation1985) and the gonochoric brooders L. pruvoti (Dendrophylliidae; Goffredo et al. Citation2006), M. rubrum (Flabellidae; Heltzel & Babcock Citation2002) and Porites porites Pallas, 1766 (Poritidae; Tomascik & Sander Citation1987). This nuclear morphology is therefore present across sexual condition, reproductive mode and taxonomic classification and might facilitate fertilization (Szmant-Froelich et al. Citation1985). In brooder corals fertilization may occur when the oocyte is still present in the mesentery and the mesenterial gastrodermis adjacent to the indentation of the nucleus may be easily penetrated by spermatozoa (Szmant-Froelich et al. Citation1985). In this study, mature oocytes were found exclusively inside the mesentery and never in the gastrovascular cavity. Therefore the union of gametes must occur when the oocytes are still in the mesentery. In addition, in M. rubrum (brooder species whose mature oocytes have an indented nucleus) the oocytes remain inside the mesoglea of the mesentery until fertilization, before migrating to the gastrovascular cavity of the polyp (Heltzel & Babcock Citation2002). Another possible explanation for nuclear migration and shape change may be that they are oogenetic phases involved in the determination of the oral–aboral axis of the future embryo (Goffredo et al. Citation2005). Several studies indicate that in anthozoans, hydrozoans and ctenophores the peripheral area of the mature oocyte where the nucleus is placed might represent the animal pole and thus correspond to the future oral pole of the embryo (Momose & Houliston Citation2007).
The stages of female gametogenesis in A. calycularis differ from those of other species of the family Dendrophylliidae in the presence of lipid vesicles, which have been described in other families, such as Acroporidae, Mussidae, Pocilloporidae, Poritidae (Vargas-Angel et al. Citation2006). In brooder species of the genus Madracis (Pocilloporidae), it has been suggested that the lipid, vesicle-rich yolk contributes to the energy available to the planula, thus increasing larval dispersion (Vermeij et al. Citation2004). Although the primary function of lipid vesicles has been attributed to floating in some species (Lee et al. Citation2006), the demersal behaviour observed in the planulae of A. calycularis suggest they have a trophic function.
The embryos were observed in the gastrovascular cavity of female polyps during May, suggesting a spring fertilization. The formation of blastocoel was not found in the embryos observed; embryonic development proceeded with the formation of stereoblastulae and subsequent gastrulation by delamination. Generally, the type of embryonic development is associated to the reproductive mode in scleractinians: brooder corals tend to have stereoblastulae, whereas broadcast spawner corals mostly have coeloblastulae (Goffredo et al. Citation2005). There are some exceptions to this model: in Manicina areolata Linneo 1758 coeloblastulae form both in conditions of broadcasting and brooding (Wilson Citation1888); Fungia scutaria Lamarck, 1801, broadcast spawner, produces stereoblastulae (Krupp Citation1983). The differences in embryonic development might be correlated to the availability of space during ontogenesis: in brooders the physical restrictions to embryonic development compel the formation of a stereoblastula, whereas in broadcast spawners, without physical restrictions, the development of coeloblastulae is allowed (Heltzel & Babcock Citation2002). Understanding the relationship between the models of embryogenesis and the reproductive mode in scleractinians needs further investigations.
Larval development in brooding coral species tends to be complete at the time of planulation. Released larvae have a clearly differentiated mouth and pharynx and the coelenteron is divided into mesenterial septa (Richmond & Hunter Citation1990). The plasticity of the planulae and their changeable shape from contracted to extended are a common feature of Anthozoa larvae (see Chia & Crawford Citation1973 for pennatulaceans; Benayahu Citation1989 for alcyonaceans; Hand & Uhlinger Citation1992 for actiniarians; Gutiérrez-Rodrìguez & Lasker Citation2004 for gorgonaceans; Goffredo & Zaccanti Citation2004 for scleractinians). The larvae of A. calycularis assumed mainly a cylindrical shape and had a demersal behaviour. Their size was intermediate compared with that of the larvae of other brooder corals of the same family: the oral–aboral axis of planulae reaches 2000 µm against a maximum of 1600 µm in L. pruvoti (Goffredo et al. Citation2005), 2200 µm in B. europaea (Goffredo & Zaccanti Citation2004) and 4000 µm in B. elegans (Beauchamp Citation1993).
A significant number of polyps were inactive (33.8%) and a significant number of colonies were indeterminate (24.4%). Inactive polyps and indeterminate colony sizes were not different from those of sexually active polyps or colonies. Thus, these inactive elements might have been in a state of seasonal quiescence. In particular, the 11 indeterminate colonies, found in the summer–autumn period, from July to October, when only female colonies were found, might have been quiescent males after the period of spring fertilization. The quantitative analysis of the annual cycle of gonadal development and its relationship with environmental parameters, which will be presented in a separated paper, will clarify the aspects of the annual cycle of male and female gametogenesis.
In conclusion, A. calycularis in Palinuro (1) was gonochoric and brooding, (2) had oocytes with a yolk rich in lipid vesicles and an embryonic development that proceeded via stereoblastula and stereogastrula, and (3) released larvae that had completed ontogenesis and had a mainly demersal behaviour.
Editorial responsibility: Ole S. Tendal
Acknowledgements
We wish to thank P. Agresta, V. Airi, V. Bernardelli, M. Galli, V. Guglielmo, E. Manzardo, C. Mattei, M. Meteori, W. Micheli, R. Navarra, F. Oliaro, I. Saurini and B. Zoli for their valuable SCUBA assistance. A special thank to the diving Pesciolino Sub (www.pesciolinosub.it) at Palinuro for the monthly sampling. Field coral photographs by G. Neto (www.giannineto.it). R. Falconi (University of Bologna) gave us valuable assistance in defining laboratory guidelines. N. Kirk (Auburn University), J. Bilewitch (State University of New York), and E. Caroselli (University of Bologna) revised and significantly improved this paper. We thank F. Uiblein, O. S. Tendal and two anonymous referees for valuable comments that improved the manuscript. The Marine Science Group (www.marinesciencegroup.org) and the Scientific Diving School (www.sdseducational.org) gave scientific, technical and logistical support. Our research was supported by the Ministry of Education, University and Research, by the Association of Italian Tour Operator, Scuba Schools International Italy, Project Aware Foundation, Egyptian Ministry of Tourism, Scuba Nitrox Safety International, the Canziani Foundation. The experiments complied with current Italian laws.
Notes
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
References
- Abe , N. 1937 . Postlarval development of the coral Fungia actiniformis var. palawensis Doderlein . Palao Tropical Biological Station Studies , 1 : 73 – 93 .
- Abe , N. 1939 . “ Ecological studies on Rhizopsammia minuta var. mutsuensis Yabe and Eguchi ” . In Jubilee publication for Prof H Yabe's 60th birthday, Volume 1 , 175 – 87 . Tokyo : Japan Society for the Promotion of Scientific Research .
- Alvarez-Pérez , G , Busquets , P , De Mol , B , Sandoval , NG , Canals , M and Casamor , JL. 2005 . Deep-water coral occurrences in the Strait of Gibraltar , 207 – 21 . Berlin : Springer .
- Babcock , RC , Bull , G , Harrison , PL , Heyward , AJ , Oliver , JK Wallace , CC . 1986 . Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef . Marine Biology , 90 : 379 – 94 .
- Babcock , RC , Wills , BL and Simpson , CJ. 1994 . Mass spawning of corals on a high latitude coral reef . Coral Reefs , 13 : 161 – 69 .
- Beauchamp , KA. 1993 . Gametogenesis, brooding and planulation in laboratory populations of a temperate scleractinian coral Balanophyllia elegans maintained under contrasting photoperiod regimes . Invertebrate Reproductive Development , 23 : 171 – 82 .
- Benayahu , Y. 1989 . Reproductive cycle and developmental processes during embryogenesis of Clavularia hamra (Cnidaria, Octocorallia) . Acta Zoologica , 70 : 29 – 36 .
- Bianchi , CN. 2007 . Biodiversity issues for the forthcoming tropical Mediterranean Sea . Hydrobiologia , 580 : 7 – 21 .
- Cairns , SD. 1999 . Species richness of recent Scleractinia . Atoll Research Bulletin , 459 : 1 – 12 .
- Cairns , SD , Hoeksema , BW and Van Der Land , J. 1999 . Appendix: List of extant stony corals . Atoll Research Bulletin , 459 : 13 – 46 .
- Casellato , S , Masiero , L , Sichirollo , E and Soresi , S. 2007 . Hidden secrets of the Northern Adriatic: Tegnùe, peculiar reefs . Central European Journal of Biology , 2 : 122 – 36 .
- Chia , FS and Crawford , BJ. 1973 . Some observations on gametogenesis, larval development and substratum selection of the sea pen Ptilosarcus guerneyi . Marine Biology , 23 : 73 – 82 .
- Cirino , P , Toscano , A and Bentivegna , F. 1993 . “ Reproduction of Astroides calycularis in the Naples Aquarium, Italy ” . In Proceedings of the 3rd International Aquarium Congress , Edited by: Prescott , JH . 183 – 87 . Boston, MA : New England Aquarium .
- Connell , JH and Keough , MJ. 1985 . “ Disturbance and patch dynamics of subtidal marine animals on hard substrata ” . In The Ecology of Natural Disturbance and Patch Dynamics , Edited by: Pickett , STA and White , PS . 125 – 51 . Orlando, FL : Academic Press .
- Creed , JC and De Paula , AF. 2007 . Substratum preference during recruitment of two invasive alien corals onto shallow-subtidal tropical rocky shores . Marine Ecology Progress Series , 330 : 101 – 11 .
- Edmondson , CH. 1929 . Growth of Hawaiian corals . Bulletin of Bernice P. Bishop Museum , 58 : 1 – 38 .
- Edmondson , CH. 1946 . Behavior of coral planulae under altered saline and thermal conditions . Occasional Papers of Bernice P. Bishop Museum , 18 : 283 – 304 .
- Fadlallah YH. 1981 The reproductive biology of three species of corals from central California PhD thesis University of California Santa Cruz 213
- Fadlallah , YH. 1983a . Sexual reproduction, development and larval biology in scleractinian corals: A review . Coral Reefs , 2 : 129 – 50 .
- Fadlallah , YH. 1983b . Population dynamics and life history of a solitary coral, Balanophyllia elegans, from central California . Oecologia , 58 : 200 – 07 .
- Fadlallah , YH and Pearse , JS. 1982 . Sexual reproduction in solitary corals: overlapping oogenic and brooding cycles, and benthic planulas in Balanophyllia elegans . Marine Biology , 71 : 223 – 31 .
- Fan , TY , Lin , KH , Kuo , FW , Soong , K , Liu , LL and Fang , LS. 2006 . Diel patterns of larval release by five brooding scleractinian corals . Marine Ecology Progress Series , 321 : 133 – 42 .
- Fujiwara , M and Caswell . 2001 . Demography of endangered North Atlantic right whale . Nature , 414 : 537 – 41 .
- Ghiselin , MT. 1969 . The evolution of hermaphroditism among animals . The Quarterly Review of Biology , 44 : 189 – 208 .
- Glynn , PW , Colley , SB , Maté , JL , Cortés , J , Guzman , HM Bailey , RL . 2008 . Reproductive ecology of the azzoxanthellate coral Tubastraea coccinea in the Equatorial Eastern Pacific: Part V. Dendrophylliidae . Marine Biology , 153 : 529 – 44 .
- Glynn , PW , Colley , SB , Ting , JH , Maté , JL and Guzman , HM. 2000 . Reef coral reproduction in the eastern Pacific: Costa Rica, Panamà, and Galapagos Islands (Ecuador). IV . Agariciidae, recruitment and recovery of Pavona varians and Pavona sp. a Marine Biology , 136 : 785 – 805 .
- Glynn , PW , Gassman , NJ , Eakin , CM , Cortés , J , Smith , DB and Guzman , HM. 1991 . Reef coral reproduction in the eastern Pacific: Costa Rica, Panamà, and Galapagos Islands (Ecuador). I. Pocilloporidae . Marine Biology , 109 : 355 – 68 .
- Goffredo , S , Airi , V , Radetic , J and Zaccanti , F. 2006 . Sexual reproduction of the solitary sunset cup coral Leptopsammia pruvoti (Scleractinia, Dendrophylliidae) in the Mediterranean. 2. Quantitative aspects of the annual reproductive cycle . Marine Biology , 148 : 923 – 31 .
- Goffredo , S , Arnone , S and Zaccanti , F. 2002 . Sexual reproduction in the Mediterranean solitary coral Balanophyllia europaea (Scleractinia, Dendrophylliidae) . Marine Ecology Progress Series , 229 : 83 – 94 .
- Goffredo , S , Caroselli , E , Mattioli , G , Pignotti , E , Dubinsky , Z and Zaccanti , F. 2009 . Inferred level of calcification decreases along an increasing temperature gradient in a Mediterranean endemic coral . Limnology and Oceanography , 54 : 930 – 37 .
- Goffredo , S , Radetić , J , Airi , V and Zaccanti , F. 2005 . Sexual reproduction of the solitary sunset cup coral Leptopsammia pruvoti (Scleractinia, Dendrophylliidae) in the Mediterranean. 1. Morphological aspects of gametogenesis and ontogenesis . Marine Biology , 147 : 485 – 95 .
- Goffredo , S and Telò , T. 1998 . Hermaphroditism and brooding in the solitary coral Balanophyllia europaea (Cnidaria, Anthozoa, Scleractinia) . Italian Journal of Zoology , 65 : 159 – 65 .
- Goffredo , S , Telò , T and Scanabissi , F. 2000 . Ultrastructural observations of the spermatogenesis of the hermaphroditic solitary coral Balanophyllia europaea (Anthozoa, Scleractinia) . Zoomorphology , 119 : 231 – 40 .
- Goffredo , S and Zaccanti , F. 2004 . Laboratory observations of larval behavior and metamorphosis in the Mediterranean solitary coral Balanophyllia europaea (Scleractinia, Dendrophylliidae) . Bulletin of Marine Science , 74 : 449 – 58 .
- Grubelic , I , Antolic , B , Despalatovic , M , Grbec , B and Beg Paklar , G. 2004 . Effect of climatic fluctuations on the distribution of warm-water coral Astroides calycularis in the Adriatic Sea new records and review . Journal of the Marine Biological Association of the United Kingdom , 84 : 599 – 602 .
- Gutiérrez-Rodrìguez , C and Lasker , HR. 2004 . Reproductive biology, development and planula behavior in the Caribbean gorgonian Pseudopterogorgia elisabethae . Invertebrate Biology , 123 : 54 – 67 .
- Hand , C and Uhlinger . 1992 . The culture, sexual and asexual reproduction, and growth of the sea anemone Nematostella vectensis . The Biological Bulletin , 182 : 169 – 76 .
- Harriott , VJ. 1983 . Reproductive ecology of four scleractinian species at Lizard Island, Great Barrier Reef . Coral Reefs , 2 : 9 – 18 .
- Harrison , PL. 1985 . Sexual characteristics of scleractianian corals: Systematic and evolutionary implications . Proceedings of the Fifth International Coral Reef Congress, Tahiti , 4 : 337 – 342 .
- Harrison , PL and Wallace , CC. 1990 . “ Reproduction, dispersal and recruitment of scleractinian corals ” . In Ecosystem of the World. 25. Coral Reefs , Edited by: Dubinsky , Z . 133 – 207 . Amsterdam : Elsevier .
- Heltzel , PS and Babcock , RC. 2002 . Sexual reproduction, larval development and benthic planulae of the solitary coral Monomyces rubrum (Scleractinia: Anthozoa) . Marine Biology , 140 : 659 – 67 .
- Hizi-Degany , N , Meroz-Fine , E , Shefer , S and Ilan , M. 2007 . Tale of two colors: Cladopsammia gracilis (Dendrophylliidae) color morphs distinguished also by their genetics and ecology . Marine Biology , 151 : 2195 – 206 .
- Hwang , SJ and Song , JI. 2007 . Reproductive biology and larval development of the temperate soft coral Dendronephthya gigantea (Alcyonacea: Nephtheidae) . Marine Biology , 152 : 273 – 84 .
- Jokiel , PL , Ito , RY and Liu , PM. 1985 . Night irradiance and synchronization of lunar release of planula larvae in the reef coral Pocillopora damicornis . Marine Biology , 88 : 167 – 74 .
- Kinchington , D. 1981 . “ Organic-matrix synthesis by scleractinian coral larval and post-larval stages during skeletogenesis ” . In Proceedings of the Fourth International Coral Reef Symposium, Volume 2 , Edited by: Gomez , ED. 107 – 13 . Manila : Marine Sciences Center, University of the Philippines .
- Krupp , DA. 1983 . Sexual reproduction and early development of the solitary coral Fungia scutaria (Anthozoa: Scleractinia) . Coral Reefs , 2 : 159 – 64 .
- Kruzic P Radic I Pozar-Domac A. 2005 First record of Cladocora debilis (Cnidaria: Anthozoa) in the Adriatic Sea JMBA2 Biodiversity Records 1 1 2
- Kruzic , P , Zibrowius , H and Pozar-Domac , A. 2002 . Actiniaria and Scleractinia (Cnidaria, Anthozoa) from the Adriatic Sea: First records, confirmed occurrences and significant range extensions of certain species . Italian Journal of Zoology , 69 : 345 – 53 .
- Kruzic , P , Zuljevic , A and Nikolic , V. 2008 . Spawning of the colonial coral Cladocora caespitosa (Anthozoa, Scleractinia) in the Southern Adriatic Sea . Coral Reefs , 27 : 337 – 41 .
- Lacaze-Duthiers , H. 1873 . Développement des coralliaires. Actinaires à Polypiers . Archives de Zoologie Expérimentale Générale , 2 : 269 – 348 .
- Lacaze-Duthiers , H. 1897 . Faune du Golfe du Lion. Coralliaires, Zooanthaires, Sclérodermés . Archives de Zoologie Expérimentale Générale , 5 : 1 – 249 .
- Lee , RF , Hagen , W and Kattner , G. 2006 . Lipid storage in marine zooplankton . Marine Ecology Progress Series , 307 : 273 – 306 .
- Levy , O , Appelbaum , L , Leggat , W , Gothlif , Y , Hayward , DC Miller , DJ . 2007 . Light-responsive cryptochromes from a simple multicellular animal, the coral Acropora millepora . Science , 318 : 467 – 70 .
- Lyons , KM. 1973 . Collar cells in the planula and adult tentacle ectoderm of the solitary coral Balanophyllia regia (Anthozoa, Eupsammiidae) . Cell Tissue Research , 145 : 57 – 74 .
- Minelli A Ruffo S La Posta S. 1995 Checklist delle specie della fauna italiana Cnidaria, Ctenophora 3 Bologna Edizioni Calderini 38
- Momose , T and Houliston , E. 2007 . Two oppositely localised Frizzled RNAs as axis determinants in a cnidarian embryo . Public Library of Science-Biology , 5 ( 4 ) : 889 – 899 .
- Moseley , HN. 1881 . On the deep sea Madreporaria. Report on the scientific results of the voyage of H.M.S. Challenger (1873–1876) . Zoology , 2 : 127 – 248 .
- Neves , EG and Pires , DO. 2002 . Sexual reproduction of Brazilian coral Mussimilia hispida (Verril, 1902) . Coral Reefs , 21 : 161 – 68 .
- Ocaña , A , Sànchez Tocino , L and Lòpez-Gonzàlez , PJ. 2000 . Faunistic and biogeographical observations concerning the Anthozoa (Cnidaria: Anthozoa) of the Granada coast (Sea of Alboran) . Zoological baetica , 11 : 51 – 65 .
- Penland , L , Kloulechad , J , Idip , D and van Woesik , R. 2004 . Coral spawning in the western Pacific Ocean is related to solar insolation: Evidence of multiple spawning events in Palau . Coral Reefs , 23 : 133 – 40 .
- Peres , JM. 1967 . The Mediterranean benthos . Oceanography and Marine Biology, an Annual Review , 5 : 449 – 533 .
- Petersen , D , Falcato , J , Gilles , P and Jones , R. 2007 . Sexual reproduction of scleractinian coral in public aquariums: current status and future perspectives . International Zoo Yearbook , 41 ( 1 ) : 122 – 37 .
- Richmond , RH and Hunter , CL. 1990 . Reproduction and recruitment of corals: comparisons among the Caribbean, the tropical Pacific, and the Red Sea . Marine Ecology Progress Series , 60 : 185 – 203 .
- Rossi , L. 1971 . Cnidari e Ctenofori d'Italia . Quaderni della Civica Stazione Idrobiologica di Milano , 2 : 77 – 86 .
- Schleyer , MH , Kruger , A and Benayahu , Y. 2004 . Reproduction and the unusual condition of hermaphroditism in Sarcophyton glaucum (Octocorallia, Alcyoniidae) in KwaZulu-Natal, South Africa . Hydrobiologia , 530/531 : 399 – 409 .
- Solomon S Qin D Manning M Chen Z Marquis M Averyt K et al. 2007 Climate Change 2007: The Physical Science Basis Cambridge Cambridge University Press 996
- Szmant-Froelich , A , Reutter , M and Riggs , L. 1985 . Sexual reproduction of Favia fragum (Esper): Lunar patterns of gametogenesis, embryogenesis and planulation in Puerto Rico . Bulletin of Marine Science , 37 ( 3 ) : 880 – 92 .
- Szmant-Froelich , A , Yevich , P and Pilson , MEQ. 1980 . Gametogenesis and early development of the temperate coral Astrangia danae (Anthozoa: Scleractinia) . The Biological Bulletin , 158 : 257 – 69 .
- Tomascik , T and Sander , F. 1987 . Effects of eutrophication on reef-building corals. III. Reproduction of the reef-building coral Porites porites . Marine Biology , 94 : 77 – 94 .
- Vargas-Angel , B , Colley , SB , Hoke , SM and Thomas , JD. 2006 . The reproductive seasonality and gametogenic cycle of Acropora cervicornis off Broward County, Florida, USA . Coral Reefs , 25 : 110 – 22 .
- Vermeij , MJA , Sampayo , E , Broker , K and Bak , RPM. 2004 . The reproductive biology of closely related coral species: Gametogenesis in Madracis from the southern Caribbean . Coral Reefs , 23 : 206 – 14 .
- Waller , RG , Tyler , PA and Gage , JD. 2002 . Reproductive ecology of the deep-sea scleractinian coral Fungiacyathus marenzelleri (Vaughan, 1906) in the northeast Atlantic Ocean . Coral Reefs , 21 : 325 – 31 .
- Willis , BL , Babcock , RC , Harrison , PL , Oliver , JK and Wallace , CC. 1985 . Patterns in the mass spawning of corals on the Great Barrier Reef from 1981 to 1984 . Proceedings of the Fifth International Coral Reef Congress, Tahiti , 4 : 343 – 48 .
- Wilson , HV. 1888 . On the development of Manicina aereolata . Journal of Morphology , 2 : 191 – 282 .
- Wilson , JR and Harrison , PL. 2003 . Spawning patterns of scleractinian corals at the Solitary Islands – a high latitude coral community in eastern Australia . Marine Ecology Progress Series , 260 : 115 – 23 .
- Yonge , CM. 1932 . A note on Balanophyllias regia, the only eupsammiid coral in the British fauna . Journal of the Marine Biological Association of the United Kingdom , 28 : 219 – 24 .
- Zibrowius , H. 1995 . The ‘southern’ Astroydes calycularis in the Pleistocene of the northern Mediterranean an indicator of climatic change (Cnidaria, Scleractinia) . Geobios , 28 : 9 – 16 .
