Abstract
Background We examined the effects of ultra-high molecular weight polyethylene (UHMWPE) particles on mononuclear cell proinflammatory gene expression in a novel murine model. We hypothesized that mononuclear cell gene trainscription of tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), interleukin-6 (IL-6) and macrophage chemoattractant protein-1 (MCP-1) would be upregulated by the addition of polyethylene particles in this murine intramedullary rod model.
Material and methods The model involved a stainless steel Kirschner wire inserted retrograde with a line-to-line fit in bilateral femora of C57bl/6 mice. Additionally, the right femora were injected with 3 × 109 UHMWPE particles. Mononuclear marrow cells were isolated by bone marrow aspiration and Ficoll-Paque centrifugation at 2, 4 and 10 weeks post-surgery. Total RNA was isolated and real-time RT-PCR was performed to quantify gene expression. Histological specimens of explanted femora were also analyzed to track the changes in periprosthetic tissue.
Results UHMWPE particles stimulated gene transcription in mononuclear cells when examined at 2, 4 and 10 weeks post-surgery, compared to the rod-only group. Relative levels of IL-1βand MCP-1 mRNA increased in a linear fashion over the 10-week time-course. IL-6 mRNA showed increased expression which peaked at 4 weeks. TNF-αmRNA expression was variable and reached a minimum at 4 weeks. The addition of UHMWPE particles stimulated ingress of macrophages and multinuclear cells of macrophage origin to the bone-implant interface.
Interpretation In this model, a single bolus of UHMWPE particles had a long-term effect on gene transcription in mononuclear cells which perpetuated a chronic inflammatory state. This murine model of intramedullary particle-induced inflammation simulates periprosthetic events associated with implant wear in humans, and may contribute to a more mechanistic understanding of wear-debris associated prosthesis failure.
The long-term outcome of total joint replacement surgery remains compromised by wear-debris associated inflammation, bone loss and implant loosening (Harris et al. Citation1976, Callaghan et al. Citation1998, Orishimo et al. Citation2003). The bone-implant interface in arthroplasty failure is characterized by a foreign-body and chronic inflammatory reaction (Santavirta et al. Citation1990, Jiranek et al. Citation1993, Neale et al. Citation1999, Al-Saffar and Revell Citation2000). Macrophages and multinuclear cells of macrophage origin contribute, in part, to the expression of inflammatory factors implicated in chronic inflammation, osteoclastogenesis and bone resorption. Macrophages contained within periprosthetic tissues retrieved from failed total joint arthroplasties (TJA) are associated with high levels of proinflammatory cytokines such as interleukin- 1 beta (IL-1β), tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) (Jiranek et al. Citation1993, Chiba et al. Citation1994, Goodman et al. Citation1998), each of which has been shown to stimulate bone resorption (Merckel et al. Citation1999, Kudo et al. Citation2003, Xing et al. Citation2003).
Several proinflammatory cytokines have been implicated in osteoclastogenesis and bone resorption. TNF-αis expressed by many cell lines, including activated monocytes. TNF-αcan be stimulated by IL-1 and is able to inhibit or induce its own synthesis in an autocrine fashion. TNF-αstimulates the production of proinflammatory adhesion molecules and increases the differentiation and maturation of osteoclasts, resulting in bone resorption in vitro and in vivo (Algan et al. Citation1996, Schwartz et al. Citation2000, Kudo et al. Citation2002). Inhibition of TNF-αreduces particle-induced inflammation and connective tissue destruction in in-vitro and invivo models of particle disease (Merckel et al. Citation1999, Clohisy et al. Citation2002).
IL-1 is produced by a variety of cell types, including macrophages and osteoclasts. It is a proinflammatory cytokine that activates lymphocytes, chemokines such as MCP-1, and proinflammatory cytokines such as TNF-αand IL-6. IL-1, similar to TNF-α, is thought to operate through the NFκB transcription factor.IL-1 receptor antagonists have been shown to reduce the effects of particle-induced inflammation and connective tissue destruction in invivo models of particle disease (Sud et al. Citation2001, Yang et al. Citation2002).
IL-6 is released from periprosthetic membranes of failed arthroplasties (Neale et al. Citation1999, Konttinen et al. Citation2002) as well as from macrophages challenged with prosthetic wear debris (Nakashima et al. Citation1999b, Niki et al. Citation2003). Furthermore, IL-6 has been shown to promote osteoclastogenesis (Ishimi et al. Citation1990, Jilka et al. Citation1992, Neale et al. Citation1999) in concert with other bone-resorbing agents.
MCP-1 is a chemotactic protein produced by monocytes and fibroblasts, and is involved in monocyte/macrophage recruitment to sites of inflammation (Boring et al. Citation1997). MCP-1 responds to IL-1 in vitro and may potentiate the release of proinflammatory cytokines from macrophages (Rahimi et al. Citation1995, Nakashima et al. Citation1999a, Biswas and Sodhi Citation2002).
Although animal models are available to study the mechanisms of joint failure, few models simulate the intramedullary long-bone environment representative of joint replacement in humans. Also, even though the roles of individual cytokines such as TNF-á, IL-1â, IL-6 and MCP-1 have been studied in short-term experiments using other models, the temporal relationship of these cytokines in the intramedullary murine environment has not been addressed.
In order to study the effects of intramedullary UHMWPE in the murine femoral intramedullary space, we have developed a technique of rod implantation, particle addition and mononuclear cell isolation that can be used to improve the quality of mechanistic in vivo-studies over extended periods of time. To identify the relationship betweenvarious proinflammatory cytokines over time, we performed real-time RT-PCR analysis on periprosthetic mononuclear cells isolated from the femoral space after 2, 4 and 10 weeks of implantation with a rod, both with and without UHMWPE particles. We hypothesized that a single bolus of 3 × 109 UHMWPE particles would induce a chronic inflammatory and foreign-body reaction characterized by increased transcription of TNF-α, IL-1β, IL-6 and MCP-1 over the course of 10 weeks.
Material and methods
Experimental design
We studied the effects of an intramedullary rod and UHMWPE particles placed in the femora of wild-type C57BL/6 mice. Each mouse was implanted with a rod and particles in the right femur (experimental side), and a rod only in the left femur (control side). The mononuclear cell RNA from 5 experimental femora implanted with UHMWPE particles and intramedullary rods was pooled to make up 1 experimental group sample, and pooled mononuclear cell RNA from the 5 contralateral rod-only femora of the same mice comprised the corresponding control group sample. 3 pooled RNA samples and 3 pooled controls, derived from 15 mice, were studied at each of 3 time points: 2, 4 and 10 weeks after surgery. Thus, 45 mice were used.
Animals
Adult (12–14 week-old) male C57BL/6 wild-type mice were obtained from the university in-house breeding colony. The animals were cared for according to the NIH institutional guidelines for the care and use of laboratory animals.
Surgical procedure
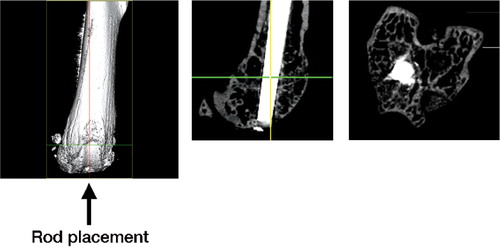
Animals were anesthetized with a 1:1 ketamine/xylazine cocktail prepared at 20 mg/mL and administered subcutaneously. The distal femora were accessed through a medial parapatellar arthrotomy and lateral displacement of the quadriceps-patellar complex. A 27-gauge needle was used to manually drill through the intercondylar notch and into the medullary cavity of the distal femur (). This was followed by insertion of a 25-gauge needle. The carrier solution with UHMWPE particles was then injected into the right femur, whereas the left femur received carrier solution alone, with no particles. A 10-mm long, 25-gauge stainless steel Kirschner wire was then inserted retrograde with a line-to-line fit in the femoral canal (). The quadriceps-patellar complex was repositioned and the medial quadriceps arthrotomy was repaired with 5.0 Vicryl sutures. Femora were harvested bilaterally at 2, 4 and 10 weeks. The mice were killed in a CO2 chamber.
Figure 1. Microcomputed tomographic images of: (A) a mouse femur with no implanted rod. The arrow indicates the orientation of rod placement. (B) A 25-gauge rod inserted retrograde in a line-to-line fit in the distal femur. (C) Cross-section taken at the level of the distal femoral metaphysis showing the location of histological analysis.
Intramedullary rod
A 25-gauge stainless steel Kirschner wire (McMaster-Carr, Chicago, IL) was cut into 10-mm long rods and steam autoclaved.
Particles
UHMWPE particles with a mean diameter of 0.5 (SD 0.3) μm were harvested from in-vitro wear experiments and used in the present study. Particle size was measured using scanning electron microscopy and NIH imaging software. The particles were reconstituted to a concentration of 1 × 1011 particles/mL, resulting in the delivery of 3 × 109 ± 5 ×108 UHMWPE particles per femur. The carrier solution consisted of a 1:3 solution of sodium hyaluronate (Pharmacia Upjohn, Kalamazoo, MI) in phosphate buffered saline (Gibco BRL, Grand Island, NY). Particles were tested and confirmed negative for endotoxin using the Limulus Amoebocyte Lysate assay (BioWhittaker, Waldersville, MD).
Isolation of mononuclear cell RNA
Bone marrow cells were collected from the marrow space immediately proximal to the distal femoral condyles and at the base of the greater trochanter. The bone marrow cavity was aspirated with 3 mL of Dulbecco's Modified Eagles Medium (DMEM, Gibco) and aspirate from five femora was pooled to provide a sufficient quantity of RNA for further analysis. Bone marrow aspirate was then layered over Ficoll-Paque Plus (Amersham Biosciences, Sweden). The layered solution was centrifuged at 6,000 rpm for 30 min. The supernatant was removed and the cells washed three times with PBS. The pelleted cells were lysed in 1 mL TriReagent (Sigma, St. Louis, MO). Total RNA was isolated and cleaned using the RNeasy Mini Kit (Quiagen, Valencia, CA). RNA yield was quantified by spectrophotometry. cDNA was generated using Taqman reverse transcription reagents (Applied Biosystems, Foster City, CA). Following the manufacturer's protocol, a solution of 40 μL of RNA + reagents was incubated in a thermal cycler for 10 min at 25°C, 30 min at 48°C and 5 min at 95°C.
Real-time RT-PCR
Relative changes in TNF-α, IL-1β, MCP-1 and IL-6 mRNA levels were measured using semi-quantitative real time PCR (RT-PCR). Unlabeled commercially available primers specific for murine TNF-α, IL-1β, MCP-1 and IL-6 mRNA and 28S RNA and a FAM reporter dye probe were obtained from Applied Biosystems. 1.5 μL of primer + probe was combined with 15.5 μL per Master Mix (Applied Biosystems), 11.5 μL DNAse/RNAse free water (Sigma) and 2.0 μL DNA sample. 10 μL of this solution was loaded into wells of a 96-well-plate and run in triplicate.
Following primer binding, probe hybridization and TAQ polymerase degradation, the levels of the reporter dye present were read using a Prism 7900HT Sequence Detection System (Applied Biosystems). Levels of mRNA were normalized to 28S RNA and measured according to the ΔΔCt method of relative quantification (Livak and Schmittgen Citation2001). Briefly, ΔCt = Ctx – Ct28S where x is the RNA of interest, and ΔΔCt = Cty–Ctz where y = rod and UHMWPE particles and z = rod-only sample. The fold difference was calculated from 2–ΔΔCt and reported as fold change relative to the rod-only sample.
Histology
Each femur was cut immediately proximal to the distal condyles (5 mm from the articular surface) and the specimen was placed in 10% neutral buffered formalin (pH 7.2–7.4, Sigma) for 24 h at room temperature and then decalcified in formic acid-based decalcifier (TBD-2, Thermo-Shandon, Pittsburgh, PA) for 36 h. The intramedullary rod was carefully removed and the bone samples were embedded in paraffin. 4-μm thick sections were cut on a microtome transversely through the distal metaphysis, starting 3 mm from the distal end of the femur and proceeding distally (). Sections were stained with hematoxylin and eosin (HE).
Statistics
Statistical analysis was performed using withingroup ANOVA and Student’s paired t-test with Bonferroni’s correction. A p-value of less than 0.05 was regarded as being significant.
Total RNA yield (μg/mL) from mononuclear cells
Results
Yield of total RNA from mononuclear cells of femoral medulla
Total RNA was isolated from bone marrow mononuclear cells of femora with rod and UHMWPE particles and femora with rod only after 2, 4 and 10 weeks. At 2 weeks, the RNA levels (measured by spectrophotometry) were 161 and 188 μg/mL, respectively; at 4 weeks they were 192 and 200 μg/mL, respectively; and at 10 weeks they were 305 and 157 μg/mL, respectively. There were no significant differences in yield observed at 2 or 4 weeks, but at 10 weeks the total RNA was increased by 94% (p = 0.01; Table).
RT-PCR
At 2 weeks, the average expression of TNF-α, IL-1β, MCP-1 and IL-6 mRNA was not significantly altered when experimental and control sides were compared ( and ).
Figure 2. Relative mRNA expression of mononuclear cells from murine femora with UHMWPE and rod, versus rod only. (A) TNF-alpha expression at 2, 4 and 10 weeks. (B) IL-1 beta expression at 2, 4 and 10 weeks. Each time point consisted of 3 groups of mice (n = 3) with each group consisting of pooled mononuclear cells from 5 femora. * p < 0.05 using ANOVA and Fisher's post-hoc test; ** p < 0.1 using ANOVA, and p < 0.05 with a paired Student's t-test. Error bars indicate standard error of the mean.
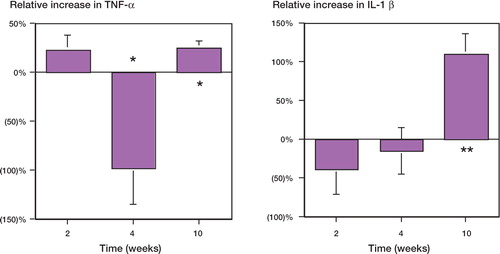
Figure 3. Relative mRNA expression of mononuclear cells from murine femora with UHMWPE and rod, versus rod only. (A) MCP-1 expression at 2, 4 and 10 weeks. (B) IL-6 expression at 2, 4 and 10 weeks. Each time point consisted of 3 groups of mice (n = 3) with each group comprised of pooled mononuclear cells from 5 femora. * p < 0.05 using a paired Student's t-test at individual time points. Error bars indicate standard error of the mean.
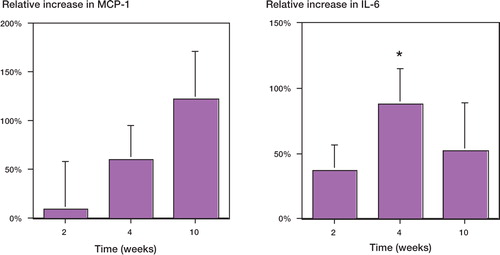
At 4 weeks, TNF-αmRNA from mononuclear cells was reduced by 95% (p = 0.02) in the femora with both rod and UHMWPE, as compared to rod-only (control) femora (). No statistically significant differences in IL-1βmRNA expression were observed (). IL-6 mRNA was elevated by 80% (p = 0.04; ). No statistically significant differences in MCP-1 mRNA expression were observed compared to the controls ().
At 10 weeks, the mononuclear cells from the rod and UHMWPE particle group showed elevated expression of TNF-αand IL-1βmRNA relative to the rod-only group, by 25% and 110%, respectively (p = 0.04, 0.01; ). MCP-1 and IL-6 expression were elevated by 120% (p = 0.03) and 50% (p = 0.05), respectively ().
Histology
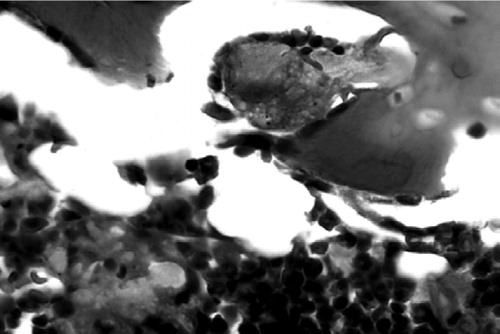
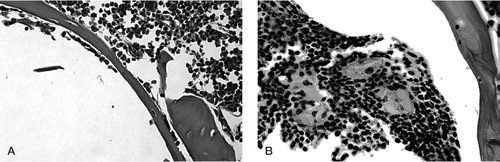
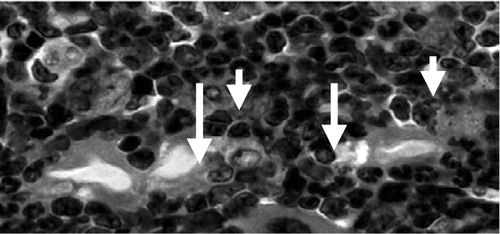
At 2 weeks, the periprosthetic tissues were extremely cellular in both treatment and control groups, and contained normal marrow elements including megakaryocytes. In the treatment group, macrophages and multinuclear cells of macrophage origin were seen to surround and engulf the polyethylene particles (). By 4 weeks, macrophages with intracellular particles were prominent in the tissues of the experimental group. Scalloped bone edges, indicating bone resorption, were observed in the marrow space adjacent to the intramedullary rod in the group with polyethylene particles (). At 10 weeks postoperatively, in the femora with rod but without particles, there were no giant cells and the marrow was less cellular in general (). In femora with rod and particles, numerous multinucleated foreign body giant cells containing polyethylene particles were located at the periprosthetic interface ().
Figure 4. Tissue surrounding the rod in a femur that received polyethylene particles. 2 weeks post-surgery. Arrows indicate engulfed polyethylene particles in phagocytic cells. Hematoxylin and eosin (HE) stain. Magnification: 80 ×.
Discussion
We have taken advantage of a newly developed intramedullary model to examine the effects of UHMWPE particles on gene expression of 4 proinflammatory mediators in mononuclear cells. Our data have demonstrated that a single bolus of 3 × 109 UHMWPE particles in the presence of an intramedullary rod stimulates a sustained local inflammatory reaction in the murine medullary cavity. In our experiments, the effect was most pronounced at the final sampling time of 10 weeks.
We specifically developed a murine model in order to facilitate mechanistic studies of the bone-prosthetic interface which could easily incorporate genetic techniques. The rod used in this model is press-fit in an intramedullary location and the implant is exposed to the joint space, simulating the scenario of joint replacement in humans. The intramedullary rod is a load-sharing device, but it is not directly weight-bearing. Although it cannot exactly replicate a weight-bearing prosthesis, this model provides a means of observing temporal changes in the local periprosthetic intramedullary environment in response to the presence of UHMWPE particles.
We chose UHMWPE particles because they are clinically relevant to processes of wear and osteolysis. The particles are of such a size (0.5 (SD 0.3) μm diameter) that they can be taken up by macro-phages, and this size is similar to that of particles generated in vivo. Since the number of particles exposed to phagocytic mononuclear cells has been shown to be the major determinant of mononuclear cell activation, we selected a dosage in the order of 109. A 100:1 ratio of 0.5 μm diameter particles to phagocytic mononuclear cells has been shown to modulate gene expression in vitro (Green et al. Citation2000, Matthews et al. Citation2000). The average number of total nucleated cells in a C57bl/6 murine femur is 2 × 107 (according to acetic acid counts, Stem-Cell Technologies, BC, Canada). Since a relatively small proportion of mononuclear cells is phagocytic, the ratio of 100 particles to every phagocytic cell was deliberately exceeded by a substantial amount.
IL-1 appears to have a role at the later stages of chronic inflammation induced by UHMWPE particles. The expression of IL-1β mRNA in experimental femora was elevated at 10 weeks and expression of TNF-α mRNA was lowest at 4 weeks. Considering that TNF-α is expressed within 30 minutes in murine in vitro cultures (Algan et al. Citation1996, Merckel et al. Citation1999) and has been shown to be upregulated within 1 week in the murine calvarial model (Schwartz et al. Citation2000), the decreased expression of TNF-α mRNA at 4 weeks may be a result of early upregulation of transcription and negative feedback at a later time. In addition, Kaar et al. (Citation2001) observed that the calvarial model simulates particle-induced osteolysis at early time points, but later time points were not examined. Our results support the assertion that the complex interplay of inflammatory mediators may be better understood through the use of more extended time points. The increased presence of total mononuclear cell RNA in the femora that were exposed to particles compared to femora that were not exposed to particles at 10 weeks may support this argument.
IL-6 was persistently elevated in the presence of UHMWPE particles, which is consistent with a prolonged inflammatory effect of UHMWPE particles within the marrow space. The persistent upregulation of MCP-1 suggests that as UHMWPE particles are engulfed by phagocytic mononuclear cells, the recruitment of macrophages is persistent and increases over time, even without the continued addition of wear debris. Our data support the conclusion that IL-6 and MCP-1 are released by mononuclear cells in response to stimulation by UHMWPE particles, and may contribute substantially to the persistence of the chronic inflammatory state.
The acute inflammation that accompanies surgery is generally resolved by approximately 2 weeks. Suva et al. (Citation1993) found that in the rat tibia, acute inflammation and repair were resolved within 14 days. Shimizu et al. (Citation1998) employed a far more invasive model of murine femoral bone marrow ablation and found general resolution by 2 weeks and complete resolution by 4 weeks. The focus of the present study is on the response to particles after the acute inflammation to the surgery itself has subsided. Our RNA expression analysis is based on the relative expression in femora exposed to particles versus those not exposed to particles. The time points selected reflect the post-acute inflammatory response to the surgical procedure alone, as defined by the current literature.
Histological analysis showed a persistent inflammatory and foreign body reaction over a time course of 10 weeks. The giant cells were larger and in greater numbers at 10 weeks than at 2 weeks, presumably because of increased cellular recruitment and chronic inflammation.
Non-murine animal models of various designs have been examined to provide insight into the pathophysiology of particle-induced inflammation. Dog (Shanbhag et al. Citation1997, Jones et al. Citation2001), rabbit (Sundfeldt et al. Citation2002, Hallab et al. Citation2003) and rat (Kim et al. Citation1998, Millet et al. Citation2002) models that allow examination of the intramedullary response to a prosthetic and wear debris have been designed, but to our knowledge, there have been no femoral intramedullary mouse models described. Amongst others, Childs et al. (Citation2001) and Merckel et al. (Citation1999) have used the murine calvarial model to examine the role of TNF-α in particle-induced osteolysis. In addition, Yang et al. (Citation2002) have used the air pouch model to examine the effects of gene therapy on particle-induced inflammation.
The differential upregulation of proinflammatory mediators in the present model suggests that the process of particle-induced prosthesis failure is a complex one and may involve multiple path-ways that are not yet fully understood. Because the response to particulate debris involves multiple cell types interacting through autocrine and paracrine mechanisms, this model can be used to complement in vitro studies. The strength of this model lies in its ability to allow us to examine the dynamic process of wear debris-induced inflammation in the femoral intramedullary microenvironment, using several analytical methods over extended time periods. Murine models are advantageous in the study of genetic mechanisms particularly because of their low cost and the ease of access to genetic assays. One shortcoming of small-animal models, however, is the need to use pooled sampling techniques for RNA isolation. Even so, through continued improvements in modern genetic technology, this murine model of intramedullary particle-induced inflammation may contribute to a better understanding of the mechanisms behind wear debrisassociated prosthesis failure and provide a useful means of evaluating pharmacological interventions.
This work was supported in part by grants from Saal Medical Scholars Fund, Zimmer and The Stanford University Orthopedic Research Fund.
- Al-Saffar N, Revell P A. Differential expression of transforming growth factor-alpha and macrophage colony-stimulating factor/colony-stimulating factor-1R (c-fins) by multinucleated giant cells involved in pathological bone resorption at the site of orthopaedic implants. J Orthop Res 2000; 18: 800–7
- Algan S M, Purdon M, Horowitz S M. Role of tumor necrosis factor alpha in particulate-induced bone resorption. J Orthop Res 1996; 14: 30–5
- Biswas S K, Sodhi A. In vitro activation of murine peritoneal macrophages by monocyte chemoattractant protein-1: upregulation of CD11b, production of proinflammatory cytokines, and the signal transduction pathway. J Interferon Cytokine Res 2002; 22: 527–38
- Boring L, Gosling J, Chensue S W, Kunkel S L, Farese R V, Jr, Broxmeyer H E, Charo I F. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C Chemokine receptor knockout mice. J Clin Invest 1997; 100: 2552–61
- Callaghan J J, Forest E E, Olejniczak J P, Goetz D D, Johnston R C. Charnley total hip arthroplasty in patients less than fifty years old: a twenty to twenty-five-year follow-up. J Bone Joint Surg (Am) 1998; 80: 704–14
- Chiba J, Rubash H E, Kin K J, Iwaki Y. The characterization of cytokines in the interface tissue obtained from failed cementless total hip arthroplasty with and without femoral osteolysis. Clin Orthop 1994, 300: 304–12
- Childs L M, Goater J J, O'Keefe R J, Schwarz E M. Effect of antitumor necrosis factor-alpha gene therapy on wear debris-induced osteolysis. J Bone Joint Surg (Am) 2001; 83: 1789–97
- Clohisy J C, Teitelbaum S, Chen S, Erdmann J M, Abu-Amer Y. Tumor necrosis factor-alpha mediates polymethylmethacrylate particle-induced NF-kappaB activation in osteoclast precursor cells. J Orthop Res 2002; 20: 174–81
- Goodman S B, Huie P, Song Y, Schurman D, Maloney W, Woolson S, Sibley R. Cellular profile and cytokine production at prosthetic interfaces. Study of tissues retrieved from revised hip and knee replacements. J Bone Joint Surg (Br) 1998; 80: 531–9
- Green T R, Fisher J, Matthews J B, Stone M H, Ingham E. Effect of size and dose on bone resorption activity of macrophages by in vitro clinically relevant ultra high molecular weight polyethylene particles. J Biomed Mater Res 2000; 53: 490–7
- Hallab N J, Cunningham B W, Jacobs J J. Spinal implant debris-induced osteolysis. Spine 2003; 28: 125–38
- Harris W H, Schiller A L, Scholler J M, Freiberg R A, Scott R. Extensive localized bone resorption in the femur following total hip replacement. J Bone Joint Surg (Am) 1976; 58: 612–8
- Ishimi Y, Miyaura C, Jin C H, Akatsu T, Abe E, Nakamura Y, Yamaguchi A, Yoshiki S, Matsuda T, Hirano T, Kishimoto T, Suda T. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol 1990; 145: 3297–303
- Jilka R L, Hangoc G, Girasole G, Passeri G, Williams D C, Abrams J S, Boyce B, Broxmeyer H, Manolagas S C. Increased osteoclast development after estrogen loss: Mediation by interleukin-6. Science 1992; 257: 88–91
- Jiranek W A, Machado M, Jasty M, Jevsevar D, Wolfe H J, Goldring S R, Goldberg M J, Harris W H. Production of cytokines around loosened cemented acetabular components: analysis with immunohistochemical techniques and in situ hybridization. J Bone Joint Surg (Br) 1993; 75: 863–79
- Jones L C, Frondoza C, Hungerford D S. Effect of PMMA particles and movement on an implant interface in a canine model. J Bone Joint Surg (Br) 2001; 83: 448–58
- Kaar S G, Ragab A A, Kaye S J, Kilic B A, Jinno T, Goldberg V M, Bi Y, Stewart M C, Carter J R, Greenfield E M. Rapid repair of titanium particle-induced osteolysis is dramatically reduced in aged mice. J Orthop Res 2001; 19: 171–8
- Kim K J, Kobayashi Y, Itoh T. Osteolysis model with continuous infusion of polyethylene particles. Clin Orthop 1998, 352: 46–52
- Konttinen Y T, Xu J W, Waris E, Li T F, Gomez-Barrena E, Nordsletten L, Santavirta S. Interleukin-6 in aseptic loosening of total hip replacement prostheses. Clin Exp Rheumatol 2002; 20: 485–90
- Kudo O, Fujikawa Y, Itonaga I, Sabokbar A, Torisu T, Athanasou N A. Proinflammatory cytokine (TNFalpha/IL-1alpha) induction of human osteoclast formation. J Pathol 2002; 198: 220–7
- Kudo O, Sabokbar A, Pocock A, Itonaga I, Fujikawa Y, Athanasou N A. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone 2003; 32: 1–7
- Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(Delta Delta C(T)) method. Methods 2001; 25: 402–8
- Matthews J B, Besong A A, Green T R, Stone M H, Wro-blewski B M, Fisher J, Ingham E. Evaluation of the response of primary human peripheral blood mononuclear phagocytes to challenge with in vitro generated clinically relevant UHMWPE particles of known size and dose. J Biomed Mater Res 2000; 52: 296–307
- Merkel K D, Erdmann J E, McHugh K P, Abu-Amer Y, Ross F P, Teitelbaum S L. Tumor necrosis factor- mediates implant osteolysis. Am J Pathol 1999; 154: 203–10
- Millett P J, Allen M J, Bostrom M P. Effects of alendronate on particle-induced osteolysis in a rat model. J Bone Joint Surg (Am) 2002; 84: 236–49
- Nakashima Y, Sun D, Trindade M C D, Chun L E, Song Y, Goodman S B, Schurman D J, Maloney W J, Smith R L. Induction of macrophage C-C chemokine expression by titanium alloy and bone cement particles. J Bone Joint Surg (Br) 1999a; 81: 155–62
- Nakashima Y, Sun D, Trindade M C D, Maloney W J, Goodman S B, Schurman D J, Smith R L. Signaling pathways for tumor necrosis factor-alpha and interleukin-6 expression in human macrophages exposed to titaniumalloy particulate debris in vitro. J Bone Joint Surg (Br) 1999b; 81: 603–15
- Neale S D, Sabokbar A, Howie D W, Murray D W, Athanasou N A. Macrophage colony-stimulating factor and interleukin-6 release by periprosthetic cells stimulates osteoclast formation and bone resorption. J Orthop Res 1999; 17: 686–94
- Niki Y, Matsumoto H, Suda Y, Otani T, Fujikawa K, Toyama Y, Hisamori N, Nozue A. Metal ions induce bone-resorbing cytokine production through the redox pathway in synoviocytes and bone marrow macrophages. Biomaterials 2003; 24: 1447–57
- Orishimo K F, Claus A M, Sychterz C J, Engh C A. Relationship between polyethylene wear and osteolysis in hips with a second-generation porous-coated cementless cup after seven years of follow-up. Bone Joint Surg (Am) 2003; 85: 1095–9
- Rahimi P, Wang C Y, Stashenko P, Lee S K, Lorenzo J A, Graves D T. Monocyte chemoattractant protein-1 expression and monocyte recruitment in osseous inflammation in the mouse. Endocrinology 1995; 136: 2752–9
- Santavirta S, Konttinen Y T, Bergroth V, Eskola A, Tallroth K, Lindholm T. Aggressive granulomatous lesions associated with hip arthroplasty. Immunopathological studies. J Bone Joint Surg (Am) 1990; 72: 252–8
- Schwarz E M, Lu A P, Goater J J, Benz E B, Kollias G, Rosier R N, Puzas J E, O'Keefe R J. Tumor necrosis factor-alpha/nuclear transcription factor-kappaB signaling in periprosthetic osteolysis. J Orthop Res 2000; 18: 472–80
- Shanbhag A S, Hasselman C T, Rubash H E. The John Charnley Award. Inhibition of wear debris mediated osteolysis in a canine total hip arthroplasty model. Clin Orthop 1997, 344: 33–43
- Shimizu T, Mehdi R, Yoshimura Y, Yoshikawa H, Nomura S, Miyazono K, Takaoka K. Sequential expression of bone morphogenetic protein, tumor necrosis factor, and their receptors in bone-forming reaction after mouse femoral marrow ablation. Bone 1998; 23: 127–33
- Sud S, Yang S Y, Evans C H, Robbins P D, Wooley P H. Effects of cytokine gene therapy on particulate-induced inflammation in the murine air pouch. Inflammation. 2001; 25: 361–72
- Sundfeldt M, Widmark M, Johansson C B, Campbell P, Carlsson L V. Effect of submicron polyethylene particles on an osseointegrated implant: an experimental study with a rabbit patello-femoral prosthesis. Acta Orthop Scand. 2002; 73: 416–24
- Suva L J, Seedor J G, Endo N, Quartuccio H A, Thompson D D, Bab I, Rodan G A. Pattern of gene expression following rat tibial marrow ablation. J Bone Miner Res 1993; 8: 379–88
- Xing L, Carlson L, Story B, Tai Z, Keng P, Siebenlist U, Boyce B F. Expression of either NF-kappaB p50 or p52 in osteoclast precursors is required for IL-1-induced bone resorption. J Bone Miner Res 2003; 18: 260–9
- Yang S, Wu B, Mayton L, Evans C H, Robbins P D, Wooley P H. IL-1Ra and vIL-10 gene transfer using retroviral vectors ameliorates particle-associated inflammation in the murine air pouch model. Inflamm Res 2002; 51: 342–50