Abstract
Background Recombinant human (rh) bone morphogenetic protein 13 (BMP13) has been shown to induce the formation of tendon and ligament tissues in animal experiments. The role of BMP13 in tissue regeneration in human tendons remains unexplored, however.
Material and methods We collected healthy human patellar tendon samples for histological examination and tendon fibroblast culture. The cultured cells were incubated in the presence and absence of rhBMP13 and the effect of the protein on cell proliferation was measured using 5-bromo-2’-deoxyuridine uptake.
Results BMP13 was detectable by immunohisto-chemical staining in healthy patellar tendon samples, and was located exclusively in active tenoblasts and perivascular mesenchymal cells but not in interstitial tenocytes. The expression of proliferating cell nuclear antigen (PCNA) and pro-collagen type I showed a similar distribution. In vitro studies showed that rhBMP13 can increase proliferation of tendon fibroblasts and increase the gene expression of pro-collagen type I in tendon fibroblast culture.
Interpretation Our findings indicate that BMP13 may be involved in the matrix remodeling process in adult tendon, and that it may play a role in tissue regeneration in tendons.
Bone morphogenetic proteins (BMPs) are a family of highly related molecules that form a subgroup within the transforming growth factor beta (TGF-β) superfamily. The BMP family is comprised of more than 10 proteins, many of which have been cloned (Celeste et al. Citation1990). Functionally, most BMPs are capable of inducing bone and cartilage formation in animals by influencing the differentiation of mesenchymal progenitor cells along the cartilage, or bone lineage (Ducy and Karsenty Citation2000). On the other hand, BMP13, also known as growth and differentiation factor (GDF) 6 or cartilage-derived morphogenetic protein (CDMP) 2, does not induce bone or cartilage formation (Wozney and Rosen Citation1998). In contrast, intramuscular injection of BMP13-transfected adenovirus has been shown to induce the formation of tendon and ligament-like connective tissue rich in collagen I in a rat model (Helm et al. Citation2001). Moreover, Forslund and Aspenberg (Citation2001) reported that injection of BMP13 could strengthen tendon tensility and also stimulate tendon healing in injured Achilles tendon in rats.
Healing of an injured tendon can trigger reconstitution of extracellular matrices such as collagens and proteoglycans, which is similar to the healing process of other connective tissue such as skin. However, tendon heals at a much slower rate and involves many complications (Jozsa and Kannus Citation1997, Wong et al. Citation2002). It is thus worthwhile to study cellular and molecular events in tendon healing in order to devise strategies to promote healing, especially the regulation of tendon healing by cytokines such as BMPs. We studied the expression of BMP13 in human patellar tendons and its involvement in physiological activities in adult tendon tissues. Although some of the in vivo effects of BMP13 have been reported, the outcomes of such studies have provided no information on how BMP13 mediates the formation of tendon and ligament-like tissue. We also examined the in vitro effects of recombinant human BMP13 (rhBMP13) on cultured human tendon fibroblasts, with respect to the proliferative response and to the gene expression levels of extracellular matrix components such as biglycan, decorin, and pro-collagen types I and III, in order to assess whether BMP13 is active in stimulating proliferation and matrix deposition in human tendon fibroblast cultures. Decorin and biglycan are major core proteins of the proteoglycans present in the extracellular matrix of tendon tissue. Decorin is known to inhibit collagen fibrillogenesis (Nakamura et al. Citation2000), while biglycan may play a role in modulating the biological activities of decorin, probably through the action of TGF-β. Pro-collagen types I and III are precursor proteins which form the structural scaffold in the early stages of tendon healing, finally resulting in mature collagen fibres by matrix remodeling. Thus, investigation of the effect of BMP13 on the expression level of biglycan, decorin, and pro-collagen types I and III may reveal its role in the tendon healing process.
Methods
Sample collection and preparation
All sampling procedures were approved by the Human Research Ethics Committee of the authors’institution. All subjects were recruited from this institution. 12 anterior cruciate ligament (ACL) deficient patients (8 men) with an average age of 31 (16–38) were operated using the healthy patellar tendon as an autograft. None of the subjects had a previous history or clinical signs of patellar tendon injury. After being thoroughly informed as to the procedures involved, all subjects completed consent forms prior to operation. A piece of healthy patellar tendon measuring 0.2 × 0.5 × 0.2 cm was removed from the remnant of the patellar tendon autograft during ACL reconstruction. All patellar tendon biopsies were taken within the deep layers of the tendons in its central portion to avoid region variation (Abrahamsson and Lohmander Citation1996, Kang and Kang Citation1999). All specimens were cleansed in sterile saline, fixed in buffered formalin and then used to prepare 5 μm-thick paraffin-embedded sections.
Tendon fibroblasts were prepared from samples taken from 10 other patients (7 men) undergoing reconstruction of the ACL using the patellar tendon as healthy autograft (mean age 23.8 (17–40)). Immediately after surgical removal, the fresh samples were stored in sterile phosphate-buffered saline (PBS) for primary fibroblast culture.
Immunohistochemical staining
5-μm-thick consecutive sections of healthy patellar tendon tissues were mounted on 3-aminopro-pyl-triethoxysilane (Sigma-Aldrich, St Louis, MO) coated slides and dried overnight at 40°C. After removal of paraffin and dehydration, consecutive paraffin sections from each sample were quenched with 0.5% hydrogen peroxide for 20 min and transferred to 10 mmol/L citrate buffer solution (pH 6) and boiled in a microwave oven (700 W) for 1 min. After brief digestion with 0.05% trypsin for 5 min and neutralization with 1% bovine serum albumin (BSA) in PBS for 20 min, the sections were incubated with primary antibody in a humid chamber at 4°C overnight. Mouse monoclonal antibodies against human BMP13 (provided by Genetic Institute, Cambridge, MA), against human proliferating cell nuclear antigen (PCNA) (Oncogene Research Products, Boston, MA) and against human pro-collagen type I (Oncogene Research Products, Boston, MA) were used at a dilution of 1:500, 1:400, and 1:400, respectively. Negative controls for non-specific staining were prepared by omitting the primary antibody. After thorough washing with PBS, the sections were incubated with biotinylated anti-body against mouse IgG (DAKO, Glostrup, Denmark) prepared in goat. After 3 washes in PBS the sections were incubated in avidin-biotin complex (DAKO) for 60 min. Finally, 3,3'-diaminobenzidine tetrahydrochloride was used as substrate in the presence of H2O2 and the reaction was allowed to proceed for3 min at room temperature. The sections were rinsed in distilled water, counterstained in Mayer’s hematoxylin, dehydrated through graded alcohol, and mounted with p-xylene-bis-pyridinium bromide (DPX) (Sigma-Aldrich). Light microscopy was then performed at a final magnification of 400×.
Culture of human patellar tendon fibroblasts
In brief, immediately after surgical removal the patellar tendon samples were cut (under sterile conditions) into small pieces of approximately 1 mm3. They were trypsinized at 37°C for 5 min, and the enzyme activity was then neutralized with 10% fetal bovine serum (FBS) in Dulbecco’s modified Eagle medium (DMEM). The tissue explants were placed in a 35-mm culture dish (Corning, NY) in medium with 10% FBS and incubated at 37°C, 95% O2, and 5% CO2. After the fibroblasts had migrated out from explant tissues and formed confluent monolayers (in approximately 4 weeks), they were harvested by trypsinization and centrifuged at 1 500 rpm for 5 min. For further culture, the tendon fibroblasts were resuspended in medium with 10% FBS and 2 × 105 cells were seeded onto a 25 cm2 culture flask. Cell proliferation assay was performed at the second passage (see below).
Proliferation assay
Tendon fibroblasts were seeded onto a 96-well microtiter plate at a density of 3000/well with serum-free DMEM and with or without 5, 25 and 50 ng/mL rhBMP13 (provided by Genetic Institute, Cambridge, MA). They were examined after 48 h. The effect of rhBMP13 on cell proliferation was quantified using the 5-Bromo-2’-deoxyuridine Labeling and Detection Kit III (Roche Molecular Biochemicals, Indianapolis, IN), which uses 5-bromo-2’-deoxyuridine (BrdU) to measure the number of dividing cells. At the end of the 48-h incubation period, cells were washed 3 times with PBS and BrdU labeling reagent was introduced in the cultures at 37°C for 5 h. After washing for 3 times in PBS, the cells were then fixed and labeled with anti-BrdU-Peroxidase for 60 min at room temperature. BrdU incorporated into the DNA of the proliferating cells was identified and quantified by enzyme-linked immunosorbent assay (ELISA) with tetramethylbenzidine (TMB) substrate. Optical density (OD) was measured at 450 nm.
Optimization of PCR
After 48 h of incubation (see above), total RNA from tendon fibroblasts (both treated with rhBMP13 and untreated) was isolated using TRIzol reagent (Invitrogen,Carlsbad, CA). This RNA was then reverse transcribed to cDNA using the First Strand cDNA kit (Promega, Madison, WI). The conditions for PCR were optimized to suit each primer pair using various annealing temperatures (55–60°C) in a conventional PCR machine (GeneAmp 9700, Applied Biosystems, Foster City, CA). The primer sequences are given in .
Table 1. PCR primers used in the study
The sizes of PCR products expected from the biglycan, decorin, pro-collagen type I and type III cDNAs are given in . PCR products were separated in a 2% ethidium bromide prestained agarose gel and the signals were analyzed with the Image Documentation System (Ultra-Violet Products, Cambridge, UK). The optimized results were transferred with the following real-time quantitative PCR protocol to quantitate cDNAs corresponding to the specific mRNAs of interest (for biglycan, decorin, pro-collagen type I and type III).
Measurement of gene expression using real-time PCR
The real-time PCR machine (LightCycler, Roche Diagnostics, Penzberg, Germany), the reaction kits and the software used in the following procedures were purchased from Roche Diagnostics (Penzberg, Germany). The master mix for each real-time quantitative PCR was prepared using 6.7 μL water, 0.8 μL MgCl2 (4 mM), 0.25 μL of forward primer (0.5 μM), 0.25 μL of reverse primer (0.5 μM) and 1.0 μL Fast Start DNA Master SYBR Green in glass capillaries. 1 μL of 2 ng sample cDNA was then added to 9 μL master mix as the PCR template. The following PCR protocol was used: denaturation at 95°C for 10 min, each amplification and detection cycle at 95°C for 2 s, at 55°C for 5 s, and at 72°C for 20 s for 40 cycles, and finally a melting curve analysis program with a heating rate of 0.1°C per second (60–95°C) and a continuous fluorescence measurement and a cooling step to 40°C. The sizes of the PCR products were confirmed by agarose gel electrophoresis. The results of real-time PCR were analyzed using relative quantification software and expressed as ratios of cDNA copies relative to β-actin.
Statistics
We used SPSS software version 11.0 (SPSS Inc., Chicago, IL) for all statistical tests. The results from the BrdU assay and real-time quantitative RT-PCR were analyzed using non-parametric Kruskal-Wallis tests. P-values of less than 0.05 were considered statistically significant.
Results
Immunohistochemistry
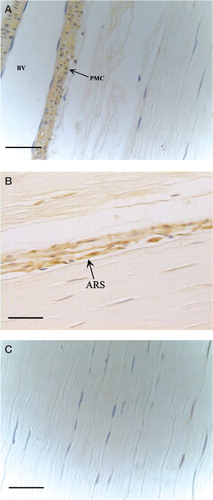
The expression of BMP13 in human patellar tendon samples was located in perivascular mesenchymal cells and tenoblasts in the regions of active remodeling, while the interstitial tenocytes were BMP13-negative ().
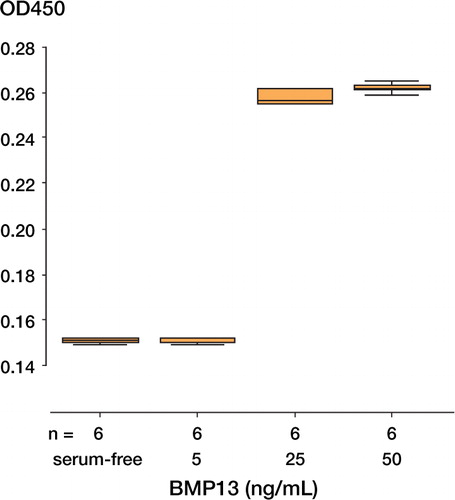
Cell proliferation ()
It was found that rhBMP13 could stimulate the cell proliferation of human tendon fibroblasts. Addition of 25 ng/mL or 50 ng/mL rhBMP13 significantly increased BrdU incorporation into the DNA of proliferating cells (p < 0.001), but no statistically significant difference was detected between 5 ng/mL BMP13 and serum-free control.
Figure 2. Increased cell proliferation of human tendon fibroblasts when cultured in the presence of recombinant human BMP13, as detected by BrdU proliferation assay.The data are expressed in O.D.units (450 nm) and measure BrdU incorporation into the DNA of proliferating cells (p < 0.05 by Kruskal Wallis test).
Gene expression of biglycan, decorin, and pro-collagen types I and III
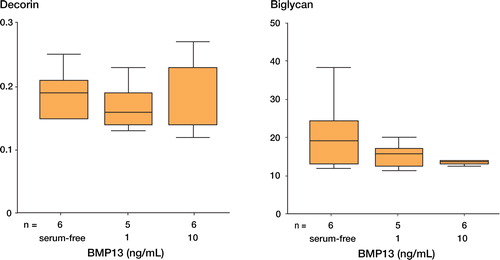
The gene expression of biglycan, decorin, pro-collagen type I and pro-collagen type III in terms of RNA synthesis, under the stimulation of 1 or 10 ng/mL rhBMP13, was analyzed by real time PCR. The relative gene expression level of pro-collagen type I was increased by the presence of 1 ng/mL rhBMP13 (p = 0.015) in human tendon fibroblasts, but rhBMP13 had no significant effects on the expression of biglycan, decorin and pro-collagen type III ( and ).
Figure 3. The effects of rhBMP13 on the gene expression of (A) pro-collagen type I and (B) type III by real-time quantitative PCR.The relative gene expression level of pro-collagen type I was significantly increased in human tendon fibroblasts by the presence of rhBMP13 in the medium (p = 0.015 by Kruskal Wallis test).The data are expressed as relative ratio with respect to β-actin.
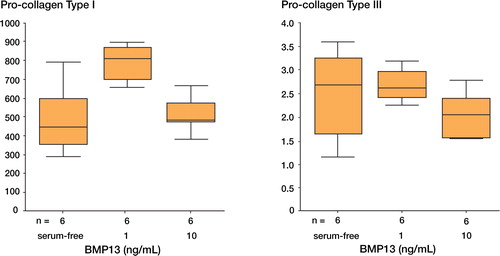
Figure 4. The effects of rhBMP13 on the gene expression of (A) decorin and (B) biglycan by real-time quantitative PCR.Recombinant human BMP13 had no significant effect on the gene expression of biglycan and decorin (p = 0.471 for decorin and p = 0.509 for biglycin by Kruskal Wallis test).The data are expressed as relative ratio with respect to β-actin.
Discussion
The apparent expression of BMP13 in healthy patellar tendons, exclusively at clustered tenoblasts in active remodeling sites and perivascular mesenchymal cells, indicates that BMP13 takes part in physiological processes in human tendons. Perivascular mesenchymal cells, also known as pericytes, are located in the endotenons. Their role has not been fully elucidated, but it has been suggested that they may act as stem cells for the healing process in connective tissues (Doherty and Canfleld Citation1999). Tenoblasts are defined as ovoid tendon cells occurring in clusters between the collagen bundles in tendons; these are morphologically distinct from the elongated tendon cells known as tenocytes. We have suggested that clustered tenoblasts may represent active remodeling sites in healthy tendons (Fu et al. Citation2004). In the active remodeling sites and perivascular mesenchymal cells, we also detected the expression of PCNA and pro-collagen type I at active remodeling sites and perivascular mesen-chymal cells (data not shown). This suggests that the effects of BMP13 in human tendons may be related to cell proliferation and collagen deposition. It is further supported by the in vitro findings that rhBMP13 can stimulate the proliferation of cultured tendon fibroblasts, and can significantly increase the gene expression of pro-collagen type I. These results imply that BMP13 may be active in adult human tendon to trigger proliferation and collagen synthesis in tendon fibroblasts.
Active remodeling represents a repair process in tendon healing where fibrogenesis occurs (Gelberman et al. Citation1990, Harwood et al. Citation1998, Rodeo et al. Citation2000). It has not been described in healthy tendons. Histologically, we have examined 111 healthy tendon samples from remnants of grafts used in ACL reconstruction with hematoxylin and eosin staining (Fu et al. Citation2004). A few patches of active remodeling sites—including perivascular mesenchymal cells—could be observed in ninetenths of tendon samples. These tendon samples were clinically diagnosed as being healthy, and the patients had no previous tendon problems. It is possible that normal tendons are under constant “remodeling” with localized healing responses to microtrauma, resulting in localized hypercellular regions. The expression of BMP13 in these regions suggests that BMP13 may play a role in mediating localized responses to microtrauma. On the other hand, the expression of BMP13 was nearly undetectable in the elongated interstitial tenocytes. Tenocytes and active tenoblasts are regarded as tendon cells within the same lineage in adult tendon tissues. Since BMP13 is expressed in tenoblasts but not in tenocytes, BMP13 may be involved in the differentiation processes of these tendon cells.
It is widely accepted that normal tendon healing is accomplished in 4 phases: inflammation, proliferation, granulation and remodeling. At the early stage, extracellular matrices such as fibronectin, proteoglycan and type III collagen synthesized by fibroblasts appear sequentially. Later, type III collagen is replaced by type I collagen, which forms the ground substance of tendon to provide mechanical strength. Recombinant human BMP13 can increase the expression of the pro-collagen type I gene in tendon fibroblasts, but it does not affect the expression of RNAs for pro-collagen type III, decorin and biglycan. This indicates that BMP13 may play a less important role in reconstitution of provisional matrix, mainly composed of type III collagen and proteoglycans, in the early healing phases. The effect of BMP13 in inducing the formation of tendon and ligament-like tissue in animal models may thus be attributable to enhanced expression of pro-collagen type I, and hence deposition of type I collagen in the late phase of tendon healing, for example, the finding that injection of adenovirus-BMP13 inside tendon can stimulate the formation of tendon and ligament-like tissue in rats, and the finding that injection of BMP13 can strengthen the Achilles tendons of rats (Forslund Citation2003)). With a better understanding of the molecular mechanisms behind the activities of BMP13, it may be possible to use BMP13 to encourage tendon repair in various traumatic and pathological conditions.
The authors thank the Genetics Institute for the generous gift of the human recombinant BMP13 and specific antibodies for completion of this project. This study was supported by the University Grant Committee, Hong Kong.
No competing interests declared.
- Abrahamsson S O, Lohmander S. Differential effects of insulin-like growth factor-I on matrix and DNA synthesis in various regions and types of rabbit tendons. J Orthop Res 1996; 14: 370–6
- Celeste A J, Iannazzi J A, Taylor R C, Rosen V, Wang E A, Wozney J M. Identification of transforming growth factor [beta] family members present in bone-inductive protein purified from bovine bone. Proc Natl Acad Sci USA 1990; 87: 9843–7
- Doherty M J, Canfield A E. Gene expression during vascular pericyte differentiation. Crit Rev Eukaryot Gene Expr 1999; 9: 1–17
- Ducy P, Karsenty G. The family of bone morphogenetic proteins. Kidney Int 2000; 57: 2207–14
- Forslund C. BMP treatment for improving tendon repair. Studies on rat and rabbit Achilles tendons. Acta Orthop Scand 2003; 74: 1–30, (Suppl 308)
- Forslund C, Aspenberg P. Tendon healing stimulated by injected CDMP-2. Med Sci Sports Exerc 2001; 33: 685–7
- Fu S C, Chuk Y C, Wong Y P, Wong W N, Hung L K, Chan K M. Immunohistochemical characterization of cells in adult human patellar tendons. J Histochem Cytochem 2004, [in press]
- Gelberman R H, Woo S L, Amiel D, Horibe S, Lee D. Influences of flexor sheath continuity and early motion on tendon healing in dogs. J Hand Surg 1990; 15: 69–77
- Harwood F L, Monosov A Z, Goomer R S, Belberman R H, Winters S C, Silva M J, Amiel D. Intergrin expression is upregulated during early healing in a canine intrasynovial flexor tendon repair and controlled passive motion model. Connect Tissue Res 1998; 39: 309–16
- Helm G A, Li J Z, Alden T D, Hudson S B, Beres E J, Conningham M, Mikkelsen M M, Pittman D D, Kerns K M, Kallmes D F. A light and electron microscopic study of ectopic tendon and ligament formation induced by bone morphogenetic protein-13 adenoviral gene therapy. J Neurosurg 2001; 95: 298–307
- Jozsa L, Kannus P. Healing and regeneration of tendons. Human Tendons. Anatomy, Physiology, and Pathology. Human Kinetics, Champaign 1997, In:
- Kang H J, Kang E S. Ideal concentration of growth factors in rabbit's flexor tendon culture. Yonsei Med J 1999; 40: 26–9
- Nakamura N, Hart D A, Boorman R S, Kaneda Y, Shrive N G, Marchuk L L, Shino K, Ochi T, Frank C B. Decorin antisense gene therapy improves functional healing of early rabbit ligament scar with enhanced collagen fibril-logenesis in vivo. J Orthop Res 2000; 18: 517–23
- Rodeo S A, Seneviratne A, Suzuki K, Felker K, Wickiewicz T L, Warren R F. J Bone Joint Surg (Am or Br). Histological analysis of human meniscal allografts: a preliminary report 2000; 82: 1071–82
- Wong J, Barrass V, Maffulli N. Quantitative review of operative and nonoperative management of Achilles tendon ruptures. Am J Soprts Med 2002; 30: 565–75
- Wozney J M, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop 1998, 346: 26–37