Abstract
Background Non-steroidal anti-inflammatory drugs (NSAIDs) are known to have inhibitory effects on new bone formation. We wanted to analyze some effects of the NSAID indomethacin on different inductive stimuli for bone formation.
Methods We experimentally induced heterotopic new bone with demineralized allogeneic bone matrix (DABM) and with bone autografts in rats, in order to study the effects of indomethacin on new bone formation at 3 and 6 weeks.
Results Indomethacin inhibited net bone formation in DABMs by 30% at 6 weeks. At 6 weeks, the mineral accretion rate was unaffected, indicating that it is at the early phase of the inductive process that mineral accretion is sensitive to inhibition by indomethacin, but not at the later stages.
In traumatized skeletal bone, the 45Ca-specific activity was higher than in non-traumatized bone, while indomethacin significantly reduced the 45Ca uptake at 3 weeks, but not at 6 weeks.
In the autografts, a net mineral loss occurred, but neither mineral content nor 45Ca incorporation was affected by Indomethacin treatment.
Interpretation Indomethacin inhibited the early phase of new bone formation in heterotopic DABMs and the early bone healing process in traumatized skeletal bone, but did not affect resorption or bone formation in heterotopic autografts. ▪
Non-steroidal anti-inflammatory drugs (NSAIDs) have important effects on new bone formation by reducing bone induction, delaying fracture healing and trauma-induced bone turnover (Rø et al. Citation1976, Törnkvist et al. Citation1985, Keller et al. Citation1987, Citation1989a, Simon et al. Citation2002). In clinical studies, NSAIDs have been shown to increase the risk of nonunion in diaphyseal femoral fractures and in spinal fusions (Glassman et al. Citation1998, Giannoudis et al. Citation2000). In addition, NSAIDs have pronounced inhibitory effects on heterotopic ossification (HO) after total hip arthroplasty, indicating a clinically relevant and potentially important effect (Ritter and Sieber Citation1985, Sodemann et al. Citation1988).
Heterotopic ossification is an interesting phenomenon; a fully differentiated tissue forms at a location where bone was not meant to be (Brooker et al. Citation1973, Nilsson and Persson Citation1999). HO may occur in a variety of conditions, while trauma seems to be the eliciting factor (Puzas et al. Citation1989). The pronounced effect of NSAIDs on clinical HO after hip surgery is in contrast to the relatively limited effect on bone formation in other clinical and experimental situations. One explanation for this discrepancy could be the strength of the inductive stimulus, i.e., the size of the inductive particles, or the type of inductive implant (graft). We wanted to analyze the mechanism of action of NSAIDs on HO further, using two experimental models: bone induction by DABM, and by autografting to an intramuscular site in rats. In addition, we analyzed the effects of the treatment on orthotopic bone, and on femurs traumatized by harvesting bone autografts.
Animals and methods
Preparation of grafts
DABM was prepared from the long bones (tibia, femur, humerus) of Sprague-Dawley rats (200 g body weight) by removing soft tissue, demineralizing in 0.6 N HCl for 24 h at 4°C, defatting with 1:1 chloroform/methanol (v/v) at room temperature for 1 h, and washing in cold water (Törnkvist et al. Citation1983). The physes were cut with a scalpel, and the diaphyses and metaphyses were used after lyophilization. The DABM implants weighed 10–12 mg. The autografts were taken from the distal femur condyles using a thin biopsy needle (Craig-Kogler). These contained the two cortices with trabecular bone in between. Each autograft had an approximate wet weight of 25 mg.
Surgery
The implants of DABM and the autografts were placed in muscle pouches in the abdominal wall of male Sprauge-Dawley autograft donor rats under neurolept analgesia (Hypnorm, Leo, Helsingborg, Sweden; 1.0 mL/kg body weight).
We designed the experiment to study the effects of Indomethacin on bone induction in bone autografts and allografts (DABM), and also in femurs traumatized by the autograft harvesting procedure. Thirty-six adult male Sprague-Dawley rats (weight 500 g) each had 2 autografts (Auto) taken from the distal femur condyles. In addition, 4 samples of allogeneic, demineralized bone matrix (DABM) were implanted. Thus, each animal was implanted with 4 allografts and 2 autografts. The rats were divided into 4 groups of 9 animals and treated for 3 or 6 weeks with indomethacin (2 mg/kg/day) or placebo (NaCl) given in daily, subcutaneous injections. The animals were randomized as to the different treatments. 24 h before death, the rats were each given a single intramuscular injection of carrier-free 45Ca (10 μCi/kg body weight) (Amersham AB, Solna, Sweden). The animals were killed by carbon dioxide 3 or 6 weeks after surgery. The implants, femurs, tibias and humerus were retrieved. Mineral content and isotope activity were analyzed in the 2 types of grafts, and in one tibia, femur, and humerus from each rat.
Analyses
The implants (allografts and autografts) and the femurs, tibias and one humerus were ashed in a muffle furnace at 600° for 24 h and weighed. The ash was dissolved in 1N HCl. Aliquots of 1 mL were added to 10 mL of Aquasol scintillation fluid (Amersham). The radioactivity of the samples was counted in a liquid scintillation counter. The ash weight, the absolute 45Ca activity, and the 45Ca specific activity (CPM/mg ash) were calculated for the different implants and for the different orthotopic bones in each rat. The mean values of ash weight and 45Ca activity for the two types of grafts were calculated for each rat. The mean values of the two types of grafts and the values for the orthotopic bone were then used to calculate the mean and standard deviation for each of the 4 groups. In addition, the ratio of 45Ca-specific activity of the DABMs, autografts and femurs to the 45Ca-specific activity of the humerus (osteoquantum index) was determined in each rat as a measure of relative calcium accretion rate.
Statistics
The Wilcoxon rank sum test was used for all statistical analyses.
The experiments were approved by the Animal Experiment Ethics Committee of Uppsala University.
Results
3 rats died during the first postoperative week, leaving 8 or 9 animals in each group. During the first postoperative week there was a slight weight loss in all groups, followed by a weight gain during the remaining experimental period. The growth rates were not affected by indomethacin treatment; there was a 20% weight gain in both groups over 6 weeks (from 500 g to 600 g).
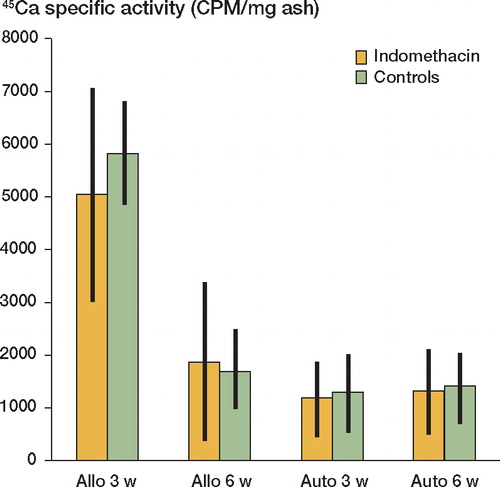
The DABMs exhibited a rapid net gain in bone (ash weight), while the autografts showed a net loss in mineral content between 3 and 6 weeks. Indomethacin treatment induced reduced net bone formation (ash weight) by 30% (p < 0.05) in the allografts at 6 weeks (). The mineral accretion (uptake of 45Ca) was similarly inhibited in the allografts by the indomethacin treatment at 6 weeks (data not shown). The treatment did not affect the 45Ca-specific activity of the grafts (). Between weeks 3 and 6, the autografts underwent a 40% loss of bone mineral and had a low absolute uptake of 45Ca, resulting in a low specific 45Ca activity. However, mineral loss and 45Ca-specific activity were not affected by indomethacin treatment.
Figure 1. Mineral (ash) content of the bone grafts.Values are mean (mg ash/implant) ± 95% confidence limits.N = 8–9. Allo: allografts at 3 or 6 weeks; auto: autografts.
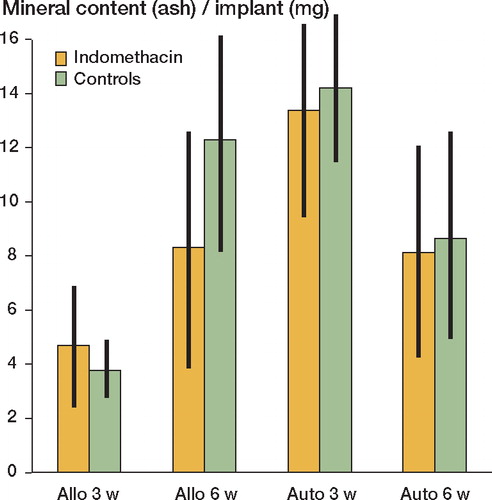
Figure 2. 45Ca-specific activity of the bone grafts.Values are mean (CPM/mg ash) ± 95% confidence limits.N = 8–9.Allo: allografts at 3 or 6 weeks; auto: autografts.
In the orthopic skeleton, the mineral content and the 45Ca uptake were not affected by indomethacin treatment. However, in the traumatized femurs (where the bone grafts had been harvested from the femur chondyles), indomethacin treated rats had a significantly lower uptake of 45Ca, and consequently lower specific 45Ca activity and osteoquantum index at 3 weeks (p < 0.05), but not at 6 weeks (data not shown).
Discussion
The inhibitory effect of NSAIDs on bone is a matter of debate and concern. This is not surprising, since NSAIDs have been shown to exert inhibitory effects on fracture healing and bone turnover, while they have also been proven to be highly effective in the treatment of acute and postoperative pain. Several experimental investigations have shown NSAIDs to have inhibitory effects on trauma-induced bone regeneration, such as fracture healing and bone induction (Rø et al. Citation1976, Sudmann et al. Citation1979, Nilsson et al. Citation1986). NSAIDs also cause reduced bone ingrowth into porous implants and inhibition of bone remodeling (Keller et al. Citation1989a, Citationb). Clinically, NSAIDs have negative effects on the healing of long bone fractures and spinal fusions (Glassman et al. Citation1998, Giannoudis et al. Citation2000). A strong additional indication of the clinical relevance of such inhibitory effects on new bone formation is given by the pronounced inhibition of NSAIDs on heterotopic ossification after total hip arthroplasty (Nilsson and Persson Citation1999).
Experimental bone induction may be induced by transplants of inductive substances and different types of bone grafts, and can be used to study different aspects of bone formation under conditions that may be regarded as intermediate between in vitro and in vivo. Morphological and chemical analysis of heterotopic bone has shown an intense turnover and a high content of growth factors, indicating a metabolically active tissue (Urist et al. Citation1983). Clinical HO may occur in various conditions, but by far the most common is in muscle and soft tissues following surgical trauma, especially after total hip arthroplasty (Brooker et al. Citation1973, DeLee et al. Citation1976, Nilsson and Persson Citation1999). Clinical and experimental HO have many similarities. In both cases the hematoma is replaced by granulation tissue, followed by mesenchymal cell proliferation, development of cartilage and then bone (Urist et al. Citation1983). The similarity of this process to fracture healing makes it a useful model for analysis of the effects of different treatments on bone. In addition, both experimental and clinical HO is inhibited by treatment with NSAIDs. There are, however, also important differences in terms of the response to treatment with NSAIDs between experimental and clinical HO. Clinical HO after THA is almost completely abolished by treatment with NSAIDs during the early postoperative period, while experimental HO is only inhibited by approximately 30%.
We wanted to analyze the effects of NSAIDs on bone by addressing some aspects of differences in response to NSAIDs in clinical and experimental HO. For this purpose, we studied experimental induction of heterotopic new bone with demineralized allogeneic bone matrix, bone autografts, and traumatized bone in rats. We found an early inhibition of new bone formation in the allografts in rats treated with indomethacin. At 6 weeks the mineral accretion rate was unaffected, indicating that the early phase of the inductive process was sensitive to inhibition, but not later stages. This early inhibitory effect on bone formation was further substantiated by the finding of decreased mineral accretion in traumatized femurs at 3 weeks, but not at 6 weeks. Furthermore, Indomethacin has previously been shown to affect fracture healing, the regional acceleratory phenomenon after osteotomy in the rabbit, and the regeneration of strength after drilling (Törnkvist et al. Citation1984, Keller et al. Citation1989a), indicating effects also on these reparative processes.
In contrast to the findings of reduced bone formation by NSAIDs in clinical and experimental HO, and following trauma/fractures, bone formation in autografts and in orthotopic bone was unaffected by the indomethacin in our study. This finding implies that induction by allografts differs in some respects from bone formation by autografts. The process of bone formation in the two types of grafts is probably different; demineralized allogeneic bone matrix induces a stronger bone-inductive response than autografts, probably by releasing inductive factors (BMPs) to a greater extent. In addition, allogenous tissue might induce a more extensive inflammatory response due to immune reactions to the graft. This might explain the differences in the effect of indomethacin on the two types of grafts. However, the low rate of new bone formation in autografts combined with a high rate of resorption could result in non-detection of inhibitory effects on bone formation. Interestingly, bone resorption predominated in the autografts where the new bone formation was at a low level, resulting in considerable net bone loss. Since the rate of bone loss in autografts was not different in the treated and control groups, we may conclude that indomethacin does not affect bone resorption in this model. This is in agreement with previous findings of no inhibitory effects of indomethacin on bone resorption in orthotopic bone or HO in rats (Bauer et al. Citation1984).
The lack of effects of NSAIDs on bone turn-over under steady-state conditions is supported by the findings of inhibitory early effects (within 3 weeks), while no inhibition occurs later in the process (6 weeks). In addition, it cannot be excluded that the increased amount of trauma due to several implantations of grafts and the autograft harvesting can cause general effects on the experimental animals. However, such effects are similar in all groups and part of the experimental setup. There was no difference between the groups in body-weight gain or recovery after surgery, indicating that the inhibitory effect of indomethacin is specific to bone healing.
The mechanism of action of NSAIDs on bone is not fully understood, but three basic requirements must be fulfilled for new bone formation to occur: an inducing agent (condition 1) must act on inducible osteogenic precursor cells (condition 2) in an appropriate environment (condition 3). All these processes may be affected by treatment with NSAIDs. NSAIDs probably act through their well-known inhibitory effects on prostaglandin synthesis, since all cyclooxygenase inhibitors appear to share the inhibitory properties on bone formation (Nilsson and Persson Citation1999, Einhorn Citation2002, Simon et al. Citation2002). There is ample evidence of potent regulatory effects by prostaglandins on both osteo-blast and osteoclast function (Kawaguchi et al. Citation1995, Einhorn Citation2003). In addition, prostaglandins affect the inflammatory response to trauma, thus altering the local environment for the bone-forming cells. One interesting feature of this inhibition is the distinct connection to a trauma event; inhibition only occurs early in the reparative process and delay of medication of more than 4–5 days results in no inhibition, both in experimental and clinical HO (Törnkvist et al. Citation1985, Sodemann et al. Citation1988). Thus, both direct and indirect effects on the osteo-blast probably contribute to the reduced net bone formation by indomethacin and other NSAIDs.
In conclusion, we found inhibitory effects of Indomethacin on experimentally increased bone formation such as trauma to bone and in bone induction, while no inhibitory effects occurred under steady-state conditions, or when resorption predominated.
This work was supported by a grant from the Swedish Medical Research Council (grant no. 17X-06577-13B).
No competing interests declared.
- Bauer F C, Nilsson O S, Törnkvist H. Formation and resorption of bone induced by demineralized bone matrix implants in rats. Clin Orthop 1984, 191: 139–43
- Brooker A F, Bowerman J W, Robinson R A, Riley L H, Jr. Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg (Am) 1973; 55: 1629–32
- DeLee J, Ferrari A, Charnley J. Ectopic bone formation following low friction arthroplasty of the hip. Clin Orthop 1976, 121: 53–9
- Einhorn T A. Do inhibitors of cyclooxygenase-2 impair bone healing. J Bone Miner Res 2002; 17(6)977–8
- Einhorn T A. Cox-2: Where are we in 2003? The role of cyclooxygenase-2 in bone repair. Arthritis Res Ther 2003; 5(1)5–7
- Giannoudis P V, MacDonald D A, Matthews S J, Smith R M, Furlong A J, De Boer P. Nonunion of the femoral diaphysis. The influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Joint Surg (Br) 2000; 82(5)655–8
- Glassman S D, Rose S M, Dimar J R, Puno R M, Campbell M J, Johnson J R. The effect of postoperative nonsteroidal anti-inflammatory drug administration on spinal fusion. Spine 1998; 23(7)834–8
- Kawaguchi H, Pilbeam C C, Harrison J R, Raisz L G. The role of prostaglandins in the regulation of bone metabolism. Clin Orthop 1995, 313: 36–46
- Keller J, Bunger C, Andreassen T T, Bak B, Lucht U. Bone repair inhibited by indomethacin. Effects on bone metabolism and strength of rabbit osteotomies. Acta Orthop Scand 1987; 58(4)379–83
- Keller J, Bayer-Kristensen I, Bak B, Bunger C, Kjaersgaard-Andersen P, Lucht U, Melsen F. Indomethacin and bone remodeling, Effect on cortical bone after osteotomy in rabbits. Acta Orthop Scand 1989a; 60(1)119–21
- Keller J C, Trancik T M, Young F A, St Mary E. Effects of indomethacin on bone ingrowth. J Orthop Res 1989b; 7(1)28–34
- Nilsson O S, Persson P E. Heterotopic bone formation after joint replacement. Curr Opin Rheumatol 1999; 11(2)127–31
- Nilsson O S, Bauer H C, Brosjö O, Törnkvist H. Influence of indomethacin on induced heterotopic bone formation in rats. Importance of length of treatment and of age. Clin Orthop 1986, 207 239–45
- Puzas J E, Miller M D, Rosier R N. Pathologic bone formation. Clin Orthop 1989, 245 269–81
- Ritter M A, Sieber J M. Prophylactic indomethacin for the prevention of heterotopic bone formation following total hip arthroplasty. Clin Orthop 1985, 196 217–25
- Rø J, Sudmann E, Marton P F. Effect of indomethacin on fracture healing in rats. Acta Orthop Scand 1976; 47(6)588–99
- Simon A M, Manigrasso M B, O'Connor J P. Cyclooxygenase 2 function is essential for bone fracture healing. J Bone Miner Res 2002; 17(6)963–76
- Sodemann B, Persson P E, Nilsson O S. Prevention of periarticular heterotopic ossification following total hip arthroplasty. Clinical experience with indomethacin and ibuprofen. Arch Orthop Trauma Surg 1988; 107(6)329–33
- Sudmann E, Dregelid E, Bessesen A, Morland J. Inhibition of fracture healing by indomethacin in rats. Eur J Clin Invest 1979; 9(5)333–9
- Törnkvist H, Nilsson O S, Bauer F C, Lindholm T S. Experimentally induced heterotopic ossification in rats influenced by anti-inflammatory drugs. Scand J Rheumatol 1983; 12(2)177–80
- Törnkvist H, Lindholm T S, Netz P, Stromberg L, Lindholm T C. Effect of ibuprofen and indomethacin on bone metabolism reflected in bone strength. Clin Orthop 1984, 187: 255–9
- Törnkvist H, Bauer F C, Nilsson O S. Influence of indomethacin on experimental bone metabolism in rats. Clin Orthop 1985, 193: 264–70
- Urist M R, DeLange R J, Finerman G A. Bone cell differentiation and growth factors. Science 1983; 220(4598)680–6