Abstract
Background Surgibone Unilab is prepared from bovine bone and contains hydroxyapatite and protein. It is supposed to be immunogenically inert but the protein could be antigenic in man.
Patients and methods We followed 27 patients for an average of 2.5 (1–5) years, all of whom had received Surgibone mixed with autograft to fill in defects in the acetabulum and the proximal femur in revision hip surgery.
Results In 17 patients, there was apparently complete incorporation of the bone graft within 6 months. In 3 of these patients, the graft was incorporated after 3 months. In 3 patients, however, there was no incorporation of the graft as late as 3 years after the operation. 3 other patients appeared to have a type of graft rejection (pseudoinfection). 1 other patient suffered MRSA deep infection of the prosthesis which resulted in removal of the implants 1 month postoperatively.
Interpretation Use of Surgibone xenograft in revision hip surgery, even in combination with autograft, resulted in failure and the need for rerevision in at least one quarter of the cases studied.
Kiel bone, first used by Maatz et al. (Citation1952), was used for various indications during the 1960s. Salama (Citation1983) achieved good results when the Kiel bone was mixed with autograft in 44 of 52 pseudarthrosis cases.
Surgibone (Unilab Inc., NJ) is prepared from Canadian calf bone, and is thought to be completely free of Bovine Spongiform Encephalopathy (BSE) infection. The exact method of preparation is not stated by the manufacturer, but is described as being similar to the preparation of Kiel bone (Maatz et al. Citation1952) in which protein denaturation with 20% hydrogen peroxide is followed by ether extraction of lipids. It is said to be composed of 71–80% hydroxyapatite and 20–29% protein. It is sterilized in ethylene oxide. The function of Surgibone is to provide an osteoconductive latticework of dead trabeculae, on which the osteoblasts of the host can lay down osteoid. The graft bone may eventually be replaced by the process of “creeping substitution”.
In the United Kingdom, the National Health Service (NHS 1998) Executive has laid down guidelines regarding the use of xenograft transplantation (NHS Executive 1998). Any procedure involving the use of living cells, tissues and organs from a non-human animal source, transplanted or implanted into a human or used for ex vivo perfusion, should be approved by the Secretary of State for Health prior to use. This regulation is not applicable to Surgibone xenograft, which does not contain any living cells.
We used Surgibone in revision hip surgery over a 6-year period to augment autograft in filling defects in the acetabulum and proximal femur when there was insufficient autograft available. Here, we present the results of a prospective study of 27 patients who received Surgibone graft during this period.
Patients and methods
Between January 1994 and December 1999, among 220 revision hip arthroplasties performed in our hospital, massive bony defects in the acetabulum or proximal femur—requiring supplementation of autograft—occurred in 27 cases. There were 26 patients with acetabular defects: cavitary (11), segmental (6), or combined (9) as defined by the AAOS classification (d'Antonio et al. Citation1989), including 3 patients with a proximal femoral defect also. There was only 1 patient with a femoral defect only (all grade III as defined by the AAOS classification) (d'Antonio et al. Citation1993). 3 of the femoral defects were in Gruen regions 1 and 2, and 1 was in Gruen region 7.
Preoperative assessment was carried out both clinically and radiographically. Any medical history of increased inflammatory markers, diabetes, immunodeficiency or prolonged steroid intake, allergy or infection was recorded. We performed the revision arthroplasty in an operating theatre with vertical laminar airflow, using the lateral transgluteal approach. All patients underwent full hematological screening and we performed culture of peroperative wound swabs to exclude infection.
Between 10 and 50 mL of Surgibone was mixed with 10–20 mL fresh autograft reamed from the acetabular bed and packed firmly into the defect before placement of a PFC (Johnson and Johnson, Raynham, MA) porous-coated cobalt-chrome hemispherical cementless cup. The cup was secured in place with 4 to 6 screws into the best-quality acetabular bone remaining. The femoral component used was either the S-ROM (Johnson and Johnson) in 8 cases, the Wagner (Sulzer Medica, Baar, Switzerland) in 6 cases, cemented Ultima collared or straight stems (Johnson and Johnson) in 11 cases, and the CAD/CAM (Stanmore Implants Worldwide, Stanmore, Middlesex, UK) in 2 cases. All patients were given antibiotic prophylaxis with amoxicillin 0.5 g and flucloxacillin 0.5 g intravenously at induction of anesthesia and orally every 6 hours for 3 days. Antithrombotic prophylaxis was with warfarin given on the second postoperative day, and for 2 weeks postoperatively to maintain the International Normalised Ratio (INR) between 1.5 and 2.5. The patients were mobilized without weight bearing for 6 weeks using crutches or frame, and then with partial weight bearing for 6 weeks.
Patients were followed prospectively both clinically and radiographically at 6 weeks, 3 months, 6 months, 12 months and at annual intervals postoperatively. The minimum follow-up period was 9 months, the maximum was 5 years and 3 months, and the mean was 2 years and 7 months. 1 patient died from unrelated causes shortly after the follow-up at 9 months. 3 patients were followed for 1–2 years. The rest of the patients were followed up for more than 2 years.
Radiographic evaluation of pelvic and lateral views of the hip involved assessing graft incorporation, graft resorption, cup loosening, and cup and/or stem migration. Graft incorporation was assumed to have occurred when there was a clear increase in the bone density in an area of previous deficiency, the disappearance of lines of demarcation surrounding a grafted area, and a progressive reduction of the speckled appearance of the graft material.
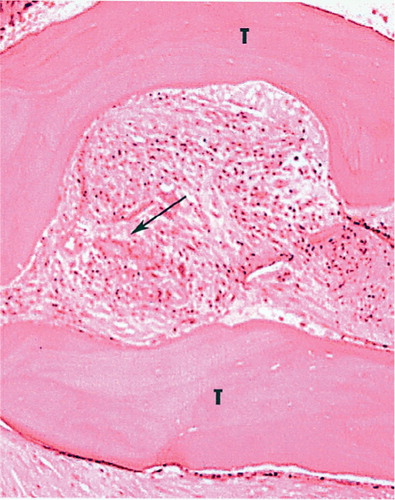
The median preoperative Harris Hip Score (HHS; Harris Citation1969) of the 27 patients was 24 (7–47). At the most recent follow-up, it was 63 (44–96). 17 patients showed radiographic evidence of at least partial graft incorporation at mean 6 (3–12) months after surgery (). Graft incorporation was seen as early as 3 months postoperatively in 3 patients. 1 of the 17 patients has since required rerevision for acetabular loosening, despite apparently good graft incorporation. Histological examination of a specimen from the grafted area showed new bone formation and evidence of the graft material as residual necrotic bony spicules (). 1 patient died 6 months after surgery. At his latest follow-up examination 3 months after surgery, he had had a satisfactory clinical result and there was no clinical or radiographic evidence of graft failure.
Results
Figure 1. Incorporation of graft. A) Preoperative view of left hip in an 86-year-old female, 8 years after primary hip replacement. B) Immediate postoperative view following reconstruction with Surgibone and autograft under PFC cup (DePuy, Warsaw, IN) and SROM stem (DePuy). C) Appearance of graft at 3 months, showing partial incorporation.
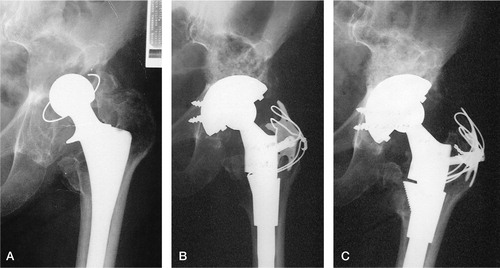
Figure 2. Histology. Evidence from the grafted area of new bone formation and residual necrotic bony spicules from the graft material.
10 hips were rerevised: 2 because of early loosening, 6 due to loosening and graft failure, and 1 because of late loosening. 1 had a proven deep infection. 2 patients suffered early failure of fixation of the acetabular component and were subject to rerevision using Burch-Schneider (Sulzer) acetabular support ring and cement. At reoperation, 3 weeks and 8 weeks after primary surgery, the appearance of the Surgibone was still granular, and there was serous fluid surrounding the graft with no evidence of ingrowth of granulation tissue. The Surgibone was removed in these cases.
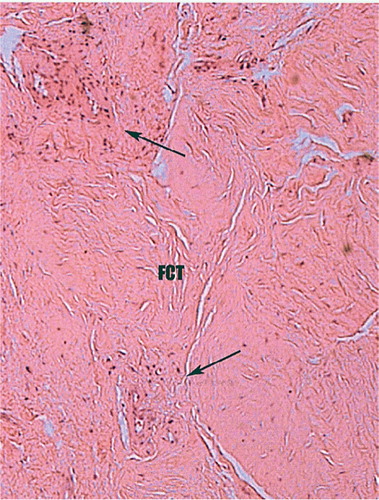
3 patients showed no radiographic evidence of graft incorporation, even up to 3 years postoperatively. 2 of these had had acetabular grafts and the other had been femoral. 1 of these patients suffered a late cup migration following fracture of a supporting screw 1.5 years after the operation (). Histopathological examination of grafted bone taken at the time of surgery revealed no sign of bone incorporation (). All 3 patients have now undergone further revision surgery.
Figure 3. Failure of graft incorporation. A) Preoperative view of right hip of a 75-year-old woman with evidence of lysis around a Ring (Zimmer, Warsaw, IN) polyethylene cementless acetabular component. B) Immediate postoperative view following reconstruction of the acetabulum with a PFC cup (DePuy) autograft and Surgibone (Unilab). C) 18 months after surgery. Extensive migration of the acetabular component, with obliteration of the graft. No evidence of infection.
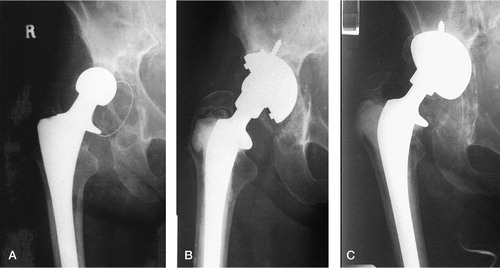
Figure 4. Histology of biopsy of an area of grafted bone obtained at rerevision for cup loosening (see ). No radiographic evidence of graft incorporation.
3 patients showed evidence of either foreign body reaction to the graft, or a low-grade infection which we were unable to identify. At rerevision surgery for persistent pain, 2 of these patients were found to have a large collection of thick yellow purulent material within the hip joint capsule surrounding the implant. After Gram staining and microscopy of this material and of multiple biopsies of tissue from around the joint, no organisms were identified and prolonged cultures revealed no growth of bacteria. Histopathological examination of granulation tissue taken at the time of surgery showed evidence of both acute and chronic inflammation, but again no infection.
The first patient, an 82-year-old woman who lived alone with two cats, was admitted to hospital with gastrointestinal bleeding and fever (39°C) 2 years after hip revision surgery. She had been suffering constant aching pain in the hip for 6 months, but was able to walk with a single stick. Her white cell count was 11.4 × 109/L, the ESR was 71 mm/hr and blood culture (one of two bottles) yielded a scant growth of the organism Pasteurella multocida. She was treated with intravenous cefotaxime, to which the organism was sensitive, and 3 days later a second blood culture was negative. She continued to be pyrexial (38°C) and radiographic examination revealed proximal cup migration indicative of loosening. Aspiration of the hip produced 70 mL of purulent material, but culture proved negative. A two-stage rerevision was undertaken successfully. Prolonged aerobic and anaerobic cultures taken during the operation were also negative for bacteria, and microscopy identified no organisms. Recovery has since been uneventful and the inflammatory markers have returned to normal.
The second of these 3 patients exhibiting foreign body reaction was a 73-year-old woman who had undergone a two-stage revision due to Staphylococcus aureus infection of the primary prosthesis. We used Surgibone in the reconstruction operation to fill in the acetabular defect. 9 months later, she complained of sudden onset of pain in the groin and radiographs revealed collapse of the medial wall of the acetabulum and migration of the cementless acetabular component into the pelvis. The patient was apyrexial, the ESR was 30 mm/h, and the Creactive protein was 60 mg/L. At rerevision surgery, copious purulent material was found in the hip joint cavity. Prolonged cultures were negative, and no organisms were found by Gram staining and microscopy.
Both of these patients have since been skin patch-tested for type 4 hypersensitivity reactions to Surgibone. No erythema or skin edema was detected after the material had been applied to the skin in the deltoid area for 72 h.
The third of these 3 patients was a 75-year-old woman who underwent revision hip replacement for a fractured acetabular component with prolonged hip dislocation and erosion of acetabular bone. Surgibone had been used to fill the defect. Over the following 1.5-year period, radiographs showed pronounced migration of the cementless acetabular component, pelvic osteolysis and disappearance of the graft (Figure 5). The patient had recently been treated with skin grafting of chronic venous ulcers, complicated by infection with B-hemolytic Streptococcus Group G. The white cell count was 19.0 × 109/L, but she was apyrexial. Purulent fluid aspirated from the hip joint cavity was cultured and found to be sterile. She died from a myocardial infarction shortly afterwards. Clinically, it did not appear that the patient had an acute infection in the hip joint, but possibly a foreign body reaction or ‘pseudo infection’ that led to the migration of the cup. No postmortem examination was carried out. Thus, all 6 cases in which the graft did not incorporate required rerevision surgery due to failure of the revision hip replacement.
Proven deep infection occurred in a 74-year-old woman who had a rerevision operation using Surgibone to fill a large cavitary acetabular defect. She had multiple postoperative complications. The operative wound began to discharge and a deep infection with Methicillin-resistant Staphylococcus aureus was diagnosed. The infected implant and the Surgibone were removed 24 days after the index surgery. She made a good recovery with control of infection and wound healing. She has since been able to walk adequately with a painless pseudarthrosis.
Discussion
Surgibone is reported to be biocompatible, non-antigenic and non-immunogenic, and it is described by the manufacturer as being immunologically inert (Unilab). However, in the Ramani et al. (Citation1975) series of Clowards fusions, 2 of 73 Surgibone dowels had to be removed because of infection. The pus in these cases was sterile, raising the possibility of a foreign body reaction. Surgibone, which was introduced in 1995, is used in a wide variety of bone rebuilding procedures, particularly in spinal fusions, where results have been mixed. Savolainen et al. (Citation1994) reported their experience in 101 patients undergoing Clowards cervical fusion using this material, and found a 98% success rate of fusion at an average of 3 years of follow-up. There was no difference in the frequency of complications compared with a series in which autograft bone was used. Rawlinson (Citation1994) prospectively compared 49 patients receiving Surgibone with 40 patients receiving autograft bone in Clowards fusions, and found that while the majority of the autograft group had successful fusions, the majority of the Surgibone group did not achieve satisfactory fusion and many had persistent pain. The histological findings in failed Surgibone fusions suggested an intense foreign body reaction, extensive collagen and dense lymphocytic infiltration. There was no sign of new bone formation, and no evidence of live osteoid against the dead Surgibone trabeculae.
On the other hand, Säveland et al. (Citation1994) reported that complete graft resorption occurred in 1 of 9 patients with rheumatoid arthritis who underwent occipito-cervical fusion using chips of Surgibone and wire fixation. Radiographic fusion was seen in only half of the patients.
Augmentation of autograft in the filling of massive bony defects in reconstructive hip surgery has been tried with allograft (Borja and Mnaymneh Citation1985, Gross et al. Citation1985, McGann et al. Citation1986, Garbuz et al. Citation1996, Gross Citation1999). Oonishi et al. (Citation1997) described their experience with filling of acetabular defects using hydroxyapatite granules secured with a layer of cement under a cemented acetabular component. Of 40 patients followed up for 4–10 years, 34 were found to have good incorporation of graft, without any cases of infection or graft rejection.
In our series, 1 patient was discovered to have Pasteurella bacteremia. Infection of joint replacements with Pasteurellais extremely rare. Although we cannot exclude the possibility that the infection could have arisen from a contaminated Surgibone bovine graft, a more likely source of the organism would have been the patient's pet cats—through inoculation by scratches to the skin.
In 17 of our patients there was radiographic evidence of at least partial bony incorporation of the graft. Our results are less favorable than those of Oonishi et al. (Citation1997) who reported radiographic union in 34 out of 38 patients, in hip revision surgery using hydroxyapatite. They are also less favorable than the results of Gross (Citation1999) who used allograft and reported 90% success. Again, our results are less favorable than the results of Savolainen et al. (Citation1994) who found a graft incorporation rate of 98% using Surgibone in cervical spine fusions. Rawlinson (Citation1994), in a prospective study comparing Surgibone with autograft in anterior cervical spine fusions, concluded that Surgibone was inferior. He also found a florid host reaction to Surgibone in 1 case, which under histological examination showed no evidence of new bone formation, as occurred in our series. Säveland et al. (Citation1994) also described disappearance of Surgibone in some cases where it was used for fusion of the atlantoaxial joint. The poor results in our study cannot—with full certainty—be attributed to the use of Surgibone, but we cannot recommend this material until controlled clinical studies have proven that it is safe and effective.
Surgibone is manufactured by UNILAB, Inc., 764 Ramsey Ave. Hillside, NJ 07275, USA and is supplied in the UK by HY-MED Ltd. Medical Products Infield House, Daggons Road, Alderholt, Fordingbridge, Hants SP6 3DL. Our study has raised some concerns about the use of Surgibone in revision hip surgery. UNILAB has been informed about the results of this study.
We wish to thank Dr. Louis Temple, consultant histopathologist at Epsom General Hospital, for his help in preparation of the histopathology illustrations and reports.
No benefits or funds were received in support of this study.
- Borja F J, Mnaymneh W. Bone allografts in the salvage of difficult hip arthroplasties. Clin Orthop 1985, 197: 125–31
- D'Antonio J A, Capello W N, Borden L S, Bargar W L, Bierbaum B F, Boettcher W G, Steinberg M E, Stulberg S D, Wedge J H. Classification and management of acetabular abnormalities in total hip arthroplasty. Clin Orthop 1989, 243: 126–37
- D'Antonio J A, McCarthy J C, Bargar W L, Borden L S, Capelo W N, Collis D K, Steinberg M E, Wedge J H. Classification of femoral abnormalities in total hip arthroplasty. Clin Orthop 1993, 296: 133–9
- Garbuz D, Morsi E, Mohamed N, Gross A E. Classification and reconstraction in revision acetabular arthroplasty with bone stock deficiency. Clin Orthop 1996, 324: 98–107
- Gross A E. Revision arthroplasty of the acetabulum with restoration of bone stock. Clin Orthop 1999, 369: 198–207
- Gross A E, Lavoie M V, McDermott P, Marks P. The use of allograft bone in revision of total hip arthroplasty. Clin Orthop 1985, 197: 115–22
- Harris W H. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end result study using a new method of result evaluation. J Bone Joint Surg (Am) 1969; 51: 737–75
- Maatz R, Lentz W, Graff R. Die Knochenbilbungsfahigkeit Konservierter Spanne. Ein Bietrag zur Knochenbank. Zentralbl Chir 1952; 77: 1376
- McGann W, Mankin H J, Harris W H. Massive allografting for severe failed total hip replacement. J Bone Joint Surg (Am) 1986; 68: 4–12
- NHS Executive. Clinical procedures involving Xenotransplantation. Health Service Circular HSC 1998/126.
- Oonishi H, Iwaki Y, Kin N, Kushitani S, Murata N, Wakitani S, Imoto K. Hydroxyapatite in revision of hip replacements with massive acetabular defects. J Bone Joint Surg 1997; 79(1)87–92
- Ramani P S, Kalpag R M, Sengupta R P. Cervical spinal interbody fusion with Kielbone. J Bone Joint Surg (Br) 1975; 62: 147–50
- Rawlinson J N. Morbidity after anterior cervical decompression and fusion. The influence of the donor site on recovery, and the results of a trial of Surgibone compared to autologous bone. Acta Neurochir (Wien) 1994; 131: 106–18
- Salama R. Xenogeneic bone graft in humans. Clin Orthop 1983, 174: 113–20
- Säveland H, Aspenberg P, Zygmunt S, Herrlin K, Christensson D, Rydholm U. Bovine bone grafting in occipito-cervical fusion for atlantoaxial instability in rheumatoid arthritis. Acta Neurochir (Wien) 1994; 127: 186–90
- Savolainen S, Usenius J P, Hernesniemi J. Iliac crest versus artificial bone grafts in 250 cervical fusions. Acta Neurochir (Wien) 1994; 129: 54–7
- Unilab Surgibone for Surgical Implant-Product Information. UNILAB Inc., 764 Ransey Avenue, Hillside, NJ 07205 USA.