Abstract
Background We developed a total hip system using osseointegration guidelines, a metaphyseal-loading proximal femoral replacement in the retained neck and a dual-geometry titanium shell in the acetabulum.
Patients and methods A randomized controlled clinical trial was undertaken in 52 patients (53 hips), using the cemented Spectron stem and cementless Harris-Galante II cup as control implants (24 patients in experimental group, 29 control patients). Clinical measures of Harris Hip Score (HHS), pain score and radiostereometric analysis (RSA) at regular intervals for up to three years were used to monitor progress.
Results No statistically significant differences were found in HHS and pain score; the stability of the cementless experimental implant was also comparable to that of the cemented controls by RSA. 3 revisions were required for migration in the experimental group and 1 was required for component dislocation in the control group.
Interpretation Our findings indicate the practicality of osseointegration of titanium implants, but suggest that current performance is inadequate for clinical introduction. However, the stable fixation achieved in the retained neck in the majority of patients is indicative of osseointegration. This finding will encourage technical and design improvements for enhancement of clinical osseointegration and should also encourage further study. Periprosthetic osteolysis might be avoided by the establishment and maintenance of direct implant-bone connection: “osseointegration”.
Although total hip replacement is a highly effective treatment for painful joint diseases, the long-term survival and performance of implant systems continue to be restricted by periprosthetic osteolysis (Malchau et al. Citation2000, Citation2002). The causes of osteolysis are the subject of debate, but activation of macrophages by wear particles (Schmalzried et al. Citation1992, Citation1994, Jasty et al. Citation1994, Sundfeldt et al. Citation2002), pulsatile fluid pressures developed by cyclic joint loading (Aspenberg and Herbertsson Citation1996, Van Der Vis et al. Citation1998), reduced bone strains due to the altered mechanics, or stress-shielding (Huiskes et al. Citation1989, Engh et al. Citation1992) have all been implicated. Periprosthetic osteolysis leads to loss of implant-bone support, to micromotion and to fibrous tissue at the implant-bone interface, and eventually to instability of the prosthetic components.
In dental and maxillofacial practice, “osseointegration” has been shown to give longstanding, reliable connection between implant and bone (Albrektsson et al. Citation1981, Citation1988, Brånemark Citation1985, Albrektsson and Albrektsson Citation1987). Adoption of “osseointegration” principles might therefore prevent the onset of periprosthetic osteolysis, by achieving good bone-implant contact early and maintaining that state (Albrektsson Citation1993). Secure implant-bone contact immediately postoperatively would enable implant design of a more physiological nature, and enhance long-term stability, but the materials and forms required for osseointegration would require a major shift in design paradigm and not simply minor adjustments of existing forms.
The introduction of new joint replacement systems has been justly criticized for unscientific and unethical experimentation on patients (Murray et al. Citation1995). The preferred approach is a methodical, stepwise investigation of each aspect of the reliability and efficacy of any new implant system (Malchau Citation1995), thus minimizing the risk to patients. The present work describes such an approach applied to a novel total hip arthroplasty, enabling ethical development of an osseointegrated system.
Patients and methods
Preclinical development
Once the applicability of osseointegration principles to orthopedic implants was established (Carlsson Citation1989, Röstlund Citation1990), a total hip arthroplasty system was designed and evaluated (Albrektsson et al. Citation1998) using laboratory and cadaver tests (Macdonald et al. Citation2002, Citation2003). The postoperative stability and mechanics of the femoral implant (Macdonald et al. Citation2002) and the acetabular component (Macdonald et al. Citation1999a,Citationb) were tested in cadaver bone, as were the instruments and surgical procedure.
Clinical development
After thorough preclinical investigations had been concluded, a limited clinical trial was started in 1992 at one center with small groups (4 or 5 patients) using radiostereometric analysis (RSA). Each group of patients was operated on and followed for 6–12 months, after which the implant design and surgical technique were reviewed and any improvements implemented before starting the next group. 4 such groups were included consecutively (19 patients in total).
Only after there had been encouraging results with these pilot studies, a more extensive multicenter trial was planned using a controlled randomized protocol with the novel Gothenburg osseointegrated titanium hip (GOT) system Mk. I (Astra Tech AB, Gothenburg, Sweden) and a control system consisting of the Harris Galante II cup (Zimmer, IN) and the Spectron EF femoral component (Smith and Nephew Richards, IN). These control implants were selected because of their excellent clinical record in the Swedish National Hip Registry (Malchau et al. Citation2000). A hybrid hip (uncemented cup with cemented stem) was recommended by the National Institutes of Health Consensus Statement (NIH Citation1994) as being the optimal system. Clinical monitoring by RSA enables reliable prediction of long-term results after only two years of follow-up with small patient numbers (Selvik Citation1974, Kärrholm Citation1989, Valstar et al. Citation2005). Groups of 30 patients were considered a good balance between adequate statistical power and general patient safety.
With ethical approval from the Ethics Committee of the host institution (Sahlgrenska University Hospital), the study was started in accordance with US Food and Drug Administration guidelines. From 1996 to 1998, 52 patients were enrolled under specific inclusion and exclusion criteria (including full informed written consent), and were randomized by a call-center just before surgery using the Minimization method (Pocock Citation1983), to receive either the GOT or Harris-Galante II/Spectron arthroplasty. 1 patient was bilaterally operated with a GOT device on one side and the control for the contralateral hip. In total, 24 patients received the experimental system and 29 control systems were implanted. The mean age at the time of surgery was 59 (44–71) years.
Patients were included if scheduled for primary hip replacement due to osteoarthrosis: aged 40–75 years old, weighing less than 106 kg, amenable to regular radiographs on all follow-up occasions, and on completion of witnessed verbal informed consent. Exclusion criteria were: previous infection in the affected joint, local or general osteoporosis, administration of cortical steroids for more than 3 months during the previous year, drug or medication abuse liable to influence follow-up, mental disorders or other illnesses normally considered contraindications for hip arthroplasty, or current involvement in other clinical studies.
The operations were performed individually by the 5 participating surgeons at 4 centers, in a clean-air theater using personal exhaust gowns. The patients were operated under spinal anesthesia with antibiotic and anti-thromboembolic prophylaxis. Partial weight bearing with crutches was required for the first 6 weeks postoperatively.
The condition of each patient was monitored by clinical evaluation preoperatively, postoperatively, at 3, 6, and 12 months, and then annually, using a standard data sheet, the Harris Hip Score (HHS), and a visual analog scale for pain at rest and one for pain during activity. An independent physiotherapist, unaware of prosthesis type or radiographic appearance, undertook the clinical evaluation at each follow-up.
Preoperative radiographs of the whole pelvis, and true anteroposterior and lateral views of the hip were taken. These examinations were repeated postoperatively, at 3, 6, and 12 months, and then annually. Due to the high precision of RSA, only radiolucencies and ectopic calcification were evaluated on the conventional radiographs. These plain radiographs were analyzed by an independent observer (a radiologist not involved in the surgery or clinical follow-up of the patients) following a special protocol using an optical measurement device (X-calliper; Eisenlohr Technologies Inc., Davis, CA). Wear of the polyethylene liner was measured according to Livermore et al. (Citation1990) and using RSA. Osteolysis and radiolucent lines were analyzed by Gruen zones for the Spectron femoral component. The GOT femoral component interface was divided into 8 zones. The bone around the acetabular component was divided into three zones (DeLee and Charnley Citation1976). The presence of radiolucency was described by the maximal width and length within a zone.
Migration of the implants was measured with RSA as described elsewhere (Kärrholm Citation1989, Kärrholm et al. Citation1994b, Citation1997, Valstar et al. Citation2005). For this purpose, 8–12 tantalum balls of 0.8 mm diameter were inserted into the proximal femur and around the acetabulum during the operation. The prosthetic components were marked pre- or peroperatively with 1.0-mm tantalum balls. Radiographs for RSA were taken postoperatively, after 3, 6 and 12 months, and then annually. All RSA radiographs were then measured and calculated.
Measurements from RSA are calculated as translations along and rotations about the 3 cardinal axes of the body (x: transverse or medio-lateral; y: vertical or cranio-caudal; z: frontal or antero-posterior). The stem translation results are presented as maximum total point motion (MTPM), the vector sum of the translations in the 3 cardinal directions ().
Precision of the RSA technique was reassessed by performing repeat observations on patients during the trial (the patient being dismissed from the radiography suite after the first views, asked to walk around for 30 min, and then repositioned for repeat radiographs). These repeated measurements were undertaken as follows: GOT stem (16 patients), Spectron stem (13 patients), GOT cup (14 patients), Harris-Galante cup (15 patients) ( and ).
Table 1. RSA femoral accuracy at the 95% confidence interval from repeated examinations. Rotation around and translation along the cardinal axes for the GOT and Spectron stems
Table 2. Acetabular accuracy of RSA at the 95% confidence interval from repeated examinations. Rotation around and translation along the cardinal axes for the GOT and Harris Galante II cups
Description of the test device
The GOT femoral component of commercially pure titanium (c.p. Ti) is designed to fit a cavity produced by rotational cutting tools within the neck of the femur (). Proximally, a collar engages the resected femoral neck and a large-diameter threaded portion engages the endosteal surfaces within the femoral neck, reducing to a smaller diameter (10 mm) thread across the subtrochanteric medullary space, engaging and just penetrating the lateral cortex. All bone-contacting surfaces were threaded and ceramic bead-blasted to a roughness of about 1 μm Ra. The larger-diameter sections were produced in diameters of 17–24 mm in 1-mm increments, to ensure optimal fit within the femoral neck, but all were 40 mm in length. The distal (10-mm diameter) section length was varied in 5-mm increments to adjust for anatomical variation. A conventional taper trunnion above the collar, nitrogen ion implanted to reduce fretting and corrosion, engages a Partially Stabilized Zirconia (PSZ) modular head of 28 mm diameter fitted at surgery.
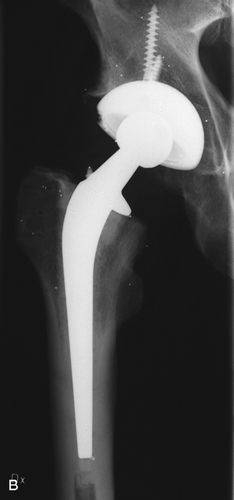
Implanted hip systems, also showing tantalum balls for RSA measurement. A. GOT system. B. Spectron femoral stem and Harris-Galante II cup
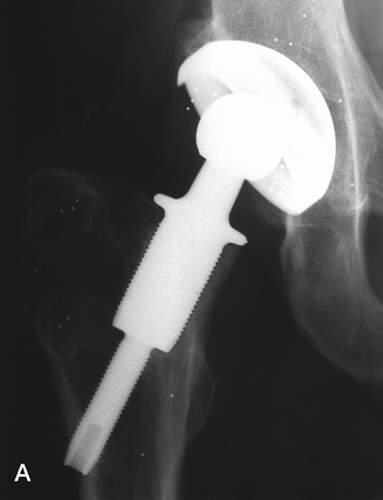
The acetabular component was also produced in c.p. titanium: a dual-geometry cup with textured and bead-blasted surfaces contacting bone. Within the hemispherical envelope of the anatomical acetabulum, the cup comprised a cylindrical section at the mouth or opening of the acetabulum and a spherical segment deeper within the cavity. The outer surfaces of the cylindrical section include buttressed threads to enhance the development of stable fixation, to permit impaction implantation and to allow bone adaptation by remodeling. The dual geometry is inherently stable under all loading modes (Macdonald et al. Citation1999a) and, in combination with specially developed reamers, enables more accurate cavity reaming (Macdonald et al. Citation1999b) and intimate contact on implantation. The modified thread form enables progressive tightening of the fixation and enhanced torsional resistance and avoids stress protection at the dome and stress concentration at the thread edges. The external surface of the shell was treated to achieve the same surface texture as the femoral component. The UHMWPE liner locked inside the cup with shape and size match, and further retention was achieved by a textured locking band and thermal expansion.
Dedicated instrumentation was developed to enable accurate and reproducible surgical preparation of the implant site, with minimal bone trauma, when used by surgeons with a wide range of skills.
Surgical technique
The Gothenburg osseointegrated titanium hip. The optimal positions and sizes of the implants were estimated by preoperative planning on the radiographs with templates. We used the antero-lateral approach according to Hardinge (Citation1982). Use of specially designed instruments for femoral neck resection level and angle enabled us to ensure restoration of leg length and placement of the femoral component on the calcar in accordance with the patient's neck-diaphysis angle. A trephine of 10 mm diameter was guided axially along the femoral neck by a centralizer, and was advanced until the lateral cortex was penetrated. Then the femoral neck was reamed in one action, reaming to the endosteal border and leaving at least 4 mm thickness of cortical bone to accommodate the femoral implant. Reaming diameter confirmed or amended the preoperative estimate of implant major diameter; measurement of the cavity length as prepared confirmed the implant length required.
Before acetabular reaming, a trial reduction was performed to ensure the optimum position of the cup and to minimize hazards of dislocation or impingement. A special reamer was used to accurately prepare the cavity geometry and size in the host bone bed (Macdonald et al. Citation1999b). The acetabular component was impacted into position, producing a “press-fit”. The polyethylene liner, cooled to 0°C before insertion, warmed to body temperature after insertion into the metal shell, developing thermal locking to augment the mechanical fit. The femoral component was screwed firmly into position, a partially-stabilized zirconia (PSZ) head of 28 mm with the appropriate neck length was firmly fitted onto the taper, and the hip was reduced.
Statistics
The Mann-Whitney U-test was used for comparison between groups at 3 years. A repeated measures ANOVA was used for statistical testing of RSA results, to cope with multiple comparisons at different time intervals.
Results
Clinical results
No patients were lost to follow-up, but 1 patient died from unrelated causes (stroke) 2 years postoperatively. The remaining 52 hip arthroplasties in 51 patients were followed according to the protocol. The present analysis is based on the first 3 years.
There were no intraoperative complications. In the GOT group (n = 24), 2 patients developed superficial wound infections, which healed with antibiotics alone. 3 patients in the control group (n = 29) had clinically and venographically verified deep venous thromboses and 1 other patient developed a pulmonary embolism. 1 patient with GOT arthroplasty incurred late dislocation twice.
There were 4 revisions, 3 in the GOT group and one in the control group. In the GOT group, 2 patients were revised for aseptic loosening of the femoral component at 1 and 2 years. The third was revised due to resorption of the neck and femoral component fracture at 1 year. When the component fracture occurred, the trial was closed immediately (i.e. no further patients were recruited) and all radiographs and clinical records were reviewed.
1 patient in the control group was revised when the insert of the HG II cup dissociated. A new liner was cemented into the intact metal shell (Bensen et al. Citation2000), but the patient still has a considerable amount of pain 2 years after revision.
There were no statistical differences between the groups in terms of Harris Hip Score or either of the pain measures ().
Table 3. Harris Hip Score and pain using Visual Analog Scale
Analysis of plain radiographs
In the GOT group, 14 of the 24 patients displayed a thin zone of radiolucency adjacent to the proximal cranial fixation region of the femoral component (superiorly), accompanied by a “reactive line”.
In the control group, no radiolucencies were observed around any of the implant interfaces, although rounding of the osteotomized femoral neck surfaces just under the implant collar was often observed.
No radiolucencies were observed around any of the acetabular components.
RSA measurements
The migration rates for all components, stems and cups in both groups, were generally very small—of the same order as the limits of accuracy ( and ). The means were well within the stability thresholds of 0.85 mm (MTPM) at 6 months or 1.2 mm at 2 years reported by Kärrholm and co-workers (Kärrholm et al. Citation1994a,Citationb). For translations, there were no statistically significant differences between the two groups ( and ).
Table 4. Femoral component translation measured by RSA; MTPM of the center of the head for the GOT and Spectron stems. MTPM (maximal total point motion) is the vector sum of migration measured in the 3 axial directions. In mm, mean (standard deviation)
Due to the different component forms, the only truly comparative feature was the spherical center of the head, and because of the different migratory motions of the 2 implants, MTPM of the femoral head is the most meaningful measure of stem translation ().
For rotation, the only statistically significant difference was rotation about the x-axis at 3 years (p = 0.004) for the stems (). The GOT stem exhibited a larger rotation (but less than half a degree), although no difference was seen at the 1- and 2-year investigations. These rotations are only just greater than the limits of accuracy (). No significant differences were seen for rotation about the y- or z-axes. No significant differences were found between the GOT cup and the Harris-Galante II cup; all the values measured were small ().
Table 5. Component rotation as measured by RSA around the x-, y- and z-axes for the GOT and Spectron stems. In degrees, mean (standard deviation)
Discussion
Before the system was put into clinical practice, laboratory tests were undertaken to verify the design assumptions and to ensure patient safety with the Gothenburg osseointegrated titanium hip.
Whilst wear particles are widely accepted as contributing to periprosthetic osteolysis, the multifactorial nature of osteolysis gives significant importance to mechanical redesign to improve the load transfer to the supporting bone and prevent biomechanical resorption (or “stress-shielding”). There is uncertainty over the balance between increased component flexibility (inducing optimal bone loading) and excessive component flexibility (causing unacceptable micromotion or system failure). Whilst these can be tested in vitro using cadaver bone or glass fiber-reinforced plastic (GFRP) bone analogs, there are design decisions implicit in them which will only be validated in clinical trials.
Likewise, the importance of implant surface chemistry (“biocompatibility”) and surface morphology, and their influence on the bone reaction and hence stability (immediate and long-term) can only be elucidated in clinical trials. Surgical accuracy and trauma to bone also affect immediate stability, and may thus contribute to long-term outcome. Clinical verification of component stability is best achieved by the use of radiostereometric analysis (RSA) (Kärrholm Citation1989, Kärrholm et al. Citation1997, Valstar et al. Citation2005), which has been shown to give reliable indications at 2-year follow-up which are predictive of the 10-year stability of implant systems (Valstar et al. Citation2005).
The feasibility of applying osseointegration principles to orthopedic implants has long been established (Carlsson Citation1989, Röstlund Citation1990), and we have designed and evaluated such a total hip arthroplasty system (Albrektsson et al. Citation1998). Application of the principles of osseointegration to prosthetic design offers the possibility of immediate secure fixation (as achieved with cemented fixation) combined with long-term physiological loading, but requires forms achievable in bone with sharp minimally traumatic surgery. Such a form was designed, and, using finite element analysis, the stresses induced in the bone and component and the overall integrity of the femoral component were verified and confirmed in laboratory and cadaver tests (Macdonald et al. Citation2002, Citation2003). Indeed, the immediate stability and micromotion were shown to be comparable to that of an optimal cemented femoral system, while the femoral loading was shown to be more physiological than the cemented system (Macdonald et al. Citation2002). At each stage and for both the femoral and the acetabular component, instruments, procedure and component stability were tested in cadaver bone (Macdonald et al. Citation1999a,Citationb).
In the clinical series, we now report that improved stability of the GOT prosthesis Mk. I was generally achieved, with less than 1.2 mm migration in most patients at the 2-year follow-up (the mean MTPM in this study being 0.65 mm). 1.2 mm at 2 years, and 0.85 mm at 6 months are the thresholds suggesting long-term stability (Kärrholm et al. Citation1994a,Citationb). However, at the 1-year follow-up, 5 patients did demonstrate migration above the significance level for RSA. Of these, 3 were revised within 2 years and 2 continue to be monitored. The latter two have performed well clinically (no pain, HHS of 100), the migration rates have decreased and condensation of the bone in the calcar region has been seen. Thus, the means in the study were stable, but the statistical outliers went on to instability and confirmed the predictive value of RSA.
1 of the 3 revised patients showed a stably fixed device at 6 months, but painful arthritis of the contralateral hip resulted in weight bearing mainly on the GOT-operated side after 6 months, leading to the sudden onset of pain at 10 months postoperatively. For the second patient, technical difficulties at operation resulted in the femoral component being implanted with weak cancellous support (not the intended cortical support) and experiencing excessive migration. The third was revised due to resorption of the neck and femoral component fracture at 1 year. This patient was heavy (105 kg) and actively involved in sports. No revisions were performed due to cup loosening. When the component fracture occurred, the trial was closed immediately and all radiographs and clinical records were reviewed. The results of the trial were assessed independently by two separate experts in hip arthroplasty, both of whom supported closure of the trial but encouraged redesign on the basis of the lessons learnt and continuation of system development.
Because the trial was closed as soon as clinical failure was experienced, patient exposure was limited to just 24 patients in the experimental group. Even so, the accuracy of RSA enables valid conclusions with groups as small as 15 (Valstar et al. Citation2005). Thus, clinical introduction of the system was undertaken with a minimum of patient risk, but maximal scientific validity.
The RSA measurements () indicated that stability of the experimental cementless system was comparable to that of the hybrid control system, with femoral component MTPM () and cup rotation () less than those of the controls (without attaining statistical significance). Likewise, translation and wear of the cups () were not significantly different from the controls.
Table 6. RSA measures of cup rotation for the GOT and Harris-Galante II implants. In degrees, median (standard deviation)
Table 7. RSA measures of cup translation for the GOT and Harris-Galante II implants. In mm; median (standard deviation)
Rotation of the femoral components about the x-axis at 3 years showed a significant difference in favor of the control implant (). The rotation of the GOT implant was actually less than half a degree (only just measurable) and showed no dependence on the side of the hip replacement. As implanted, rotation about the x-axis is not axial revolution of the threaded implant; but is movement into less (or more) anteversion. So, it can be concluded that this motion was not due to unscrewing; the GOT implants were evenly distributed between right and left hips (one would unscrew and the contralateral one would tighten with gait loading if this were the problem).
A common radiographic observation in the GOT group was a radiolucency adjacent to the cranial proximal portion of the implant, with a thin “reactive line”. In most cases, this could not be attributed to subsidence—as the RSA values indicated migration of well below 0.3 mm (the detection level for radiolucency of a soft tissue interface (Carlsson et al. Citation1986)). Freeman et al. (Citation2003) concluded that such a “reactive line” is evidence of a fine fibrous tissue layer at the implant surface, but not associated with particles or osteolysis. In the present series, we consider that the radiolucency might have been the result of perpendicular micromotion at the superior bone-implant interface (movement of the bone away from the implant surface), or hydrostatic pressure in this region.
Although this trial indicated that our implant could generally achieve good bone-implant contact and secure fixation, the reliability and certainty of achieving such fixation was not shown in all cases. Under such circumstances, wider clinical introduction of the system could not be supported, and the clinical trial was closed. Nevertheless, the remaining patients continue to function well. Technical and design improvements made to the system components included flared geometry just inferior to the collar (to better engage the endosteal femoral neck), rougher blasting and fluoride treatment of the bone-contacting surfaces to enhance bone reaction (Wennerberg et al. Citation1998), and an altered transition between the two threaded diameters strengthening and stiffening the implant. Changes in surgical instrumentation were also introduced to improve the initial apposition to bone and the development of proximal bone-implant contact.
The present trial and its preclinical preparation progressively tested the orthopedic applicability of osseointegrated implant technology, both thoroughly and responsibly (Malchau Citation1995). All the preclinical indications were favorable, including small trials involving groups with limited numbers of patients (unpublished data), but the clinical trial proved inconclusive. Stable fixation and good results were achieved for most GOT Mk. I implants, but the reproducibility was not adequate so we ended the trial prematurely. Even so, patient exposure to experimental procedures was minimized and the clinical relevance of the experimental system could be tested after modification. Then it was ethically acceptable to embark on a clinical trial of the improved implant (Mk. II).
Contributions of authors
The principal investigators involved in the design and testing of the system components before clinical trials, in experimental design and implementation of the clinical trial (including surgical implantation and data intepretation), and in preparation of the manuscripts were LVC, TA, CMJ and WM. Associate investigators providing advice during system development, clinical trial preparation and interpretation, and surgical implantation were BEJ, BGA, LR, TR, LRW. RSA analysis and interpretation was done by LR.
Clinical trial management and monitoring were undertaken independently by Clinical Data Care of Lund, Sweden, and RSA determinations were performed independently by the Orthopaedics Department, Sahlgrenska Hospital, Gothenburg. System development was undertaken at the Department of Biomaterials Research, University of Gothenburg, independently of, but with financial support from, Astra Tech AB.
- Albrektsson T. On long term maintenance of the osseointegrated response. Aust Prosthodont J (Suppl) 1993; 7: 15–24
- Albrektsson T, Albrektsson B. Osseointegration of bone implants. Acta Orthop Scand 1987; 58: 567–77
- Albrektsson T, Brånemark P-I, Hansson H-A, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand 1981; 52: 155–70
- Albrektsson T, Dahl E, Enbom L, Engevall S, Engquist B, Eriksson R A, Feldmann G, Freiberg N, Glantz P-I, Kjellman P, Kristersson L, Kvint S, Köndell P-Å, Palmquist J, Werndahl L, Åstrand P. Osseointegrated oral implants. A Swedish multicenter study of 8139 consecutively inserted Nobelpharma implants. J Periodon 1988; 59: 287–96
- Albrektsson T, Carlsson L V, Jacobsson M, Macdonald W. Gothenburg osseointegrated hip arthroplasty. Clin Orthop 1998; 352: 81–94
- Aspenberg P, Herbertsson P. Periprosthetic bone resorption. Particles versus movement. J Bone Joint Surg (Br) 1996; 78(4)641–6
- Bensen C V, Del Schutte H J, Weaver K D. Mechanical stability of polyethylene liners cemented into acetabular shells. Crit Rev Biomed Eng 2000; 28(1-2)7–10
- Brånemark P-I. Introduction to osseointegration. Tissue integrated prosthesis. Osseointegration in clinical dentistry, P-I Brånemark, G A Zarb, T. Albrektsson. Quintessence Publ. Comp, Berlin, Chicago, Tokyo 1985; 11–76
- Carlsson L V. On the development of a new concept for orthopaedic implant fixation. Göteborgs Universitet, Gothenburg 1989, Dept. of Handicap Research & Dept. of Orthopaedics
- Carlsson L V, Röstlund T, Albrektsson B, Albrektsson T, Brånemark P-I. Osseointegration of titanium implants. Acta Orthop Scand 1986; 57: 285–9
- DeLee J G, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop 1976, 121: 20–32
- Engh C A, McGovern T F, Bobyn J D, Harris W H. A quantitative evaluation of periprosthetic bone-remodelling after primary cementless total hip arthroplasty. J Bone Joint Surg (Am) 1992; 74: 1009–20
- Freeman M A R, MacInnes T, Revell P A. The histology of “reactive lines” in well-fixed components. J Arthroplasty 2003; 1(2)224–6
- Hardinge K. The direct lateral approach to the hip. J Bone Joint Surg (Br) 1982; 64: 17–9
- Huiskes R, Weinans H, Dalstra M. Adaptive bone remodelling and biomechanical design considerations for non-cemented total hip arthroplasty. Orthopedics 1989; 12: 1255–67
- Jasty M, Bragdon C, Jiranek W, Chandler H, Maloney W, Harris W H. Etiology of osteolysis around porous-coated cementless total hip arthroplasties. Clin Orthop 1994, 308: 111–26
- Kärrholm J. Roentgen stereophotogrammetry. Review of orthopaedic applications. Acta Orthop Scand 1989; 60: 491–503
- Kärrholm J, Borssen B, Löwenheim G, Snorrason F. Does early micromotion of femoral stem prostheses matter? 4-7 year stereoradiographic follow-up of 84 cemented prostheses. J Bone Joint Surg (Br) 1994a; 76(6)912–7
- Kärrholm J, Malchau H, Snorrason F, Herberts P. Micromotion of femoral stems in total hip arthroplasty. A randomized study of cemeted, hydroxyapatite-coated, and porous-coated stems with roentgen sterophotogrammetric analysis. J Bone Joint Surg 1994b; 76(11)1692–705
- Kärrholm J, Herberts P, Hultmark P, Malchau H, Nivbrant B, Thanner J. Radiostereometry of hip prostheses. Methodology and prediction of end results. Clin Orthop 1997, 344: 94–110
- Livermore J, Ilstrup D, Morrey B J. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg (Am) 1990; 72: 518–28
- Macdonald W, Carlsson L V, Charnley G J, Jacobsson C M. Press-fit acetabular cup fixation: principles and testing. Proc Instn Mech Engrs 1999a; 213(Part H)33–9
- Macdonald W, Carlsson L V, Charnley G J, Jacobsson C M. Inaccuracy of Acetabular Reaming Under Surgical Conditions. J Arthroplasty 1999b; 14(6)730–7
- Macdonald W, Carlsson L V, Jacobsson M, Lee T Q. A proximal femoral implant preserves physiological bone deformation: a biomechanical investigation in cadaveric bones. Proc Instn Mech Engnrs 2002; 21(Part H)41–8
- Macdonald W, Carlsson L V, Gathercole N, Jacobsson M. Fatigue testing of a proximal femoral hip component. Proc Instn Mech Engnrs 2003; 217(pt H)137–45
- Malchau H. On the importance of stepwise introduction of new hip implant technology. Göteborgs Universitet: 64, Göteborg 1995, Dept. of Orthopaedics, Institute of Surgical Sciences
- Malchau H, Herberts P, Söderman P, Odén A. Prognosis of Total Hip Replacement. Update and Validation of Results from the Swedish National Hip Arthroplasty Registry 1979- 998. 67th Annual Meeting of the American Academy of Orthopaedic Surgeons, OrlandoUSA. Department of Orthopaedics, Göteborg University, Sweden 2000
- Malchau H, Herberts P, Garellick G, Söderman P, Eisler T. Prognosis of total hip replacement. 69th Annual meeting of the AAOS, DallasUSA, 2002
- Murray D W, Carr A J, Bulstrode C J. Which primary total hip replacement?. J Bone Joint Surg (Br) 1995; 77(4)520–7
- NIH. Total hip replacement. NIH Consensus Conference, National Institutes of Health. 1994
- Pocock S. J. Clinical trials: A practical approach. Wiley, ChichesterUK 1983
- Röstlund T. On the development of a new arthroplasty. With special emphasis on the gliding elements in the knee. Dept. of Handicap Research (Göteborg), Dept. of Orthopaedics (Mölndal Hospital). Göteborg, Göteborg 1990; 132
- Schmalzried T P, Jasty M, Harris W H. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg (Am) 1992; 74(6)849–63
- Schmalzried T P, Jasty M, Rosenberg A, Harris W H. Polyethylene wear debris and tissue reactions in knee as compared to hip replacement prostheses. J Appl Biomater 1994, 3: 185–90
- Selvik G. Roentgen stereophotogrammetry. A method for the study of the kinematics of the skeletal system. University of Lund, Lund 1974
- Sundfeldt M, Widmark M, Johansson C, Campbell P, Carlsson L. V. The effect of submicron polyethylene particles on an osseointegrated implant. Acta Orthop Scand 2002; 73(4)416–24
- Valstar E R, Gill R, Ryd L, Flivik G, Börlin N, Kärrholm J. Guidelines for standardization of radiostereometry (RSA) of implants. Acta Orthop 2005; 76(4)563–72
- Van Der Vis H M, Aspenberg P, Martii R K, Tigchelaar W, Van Noorden C J F. Fluid pressure causes bone resorption in a rabbit model of prosthetic loosening. Clin Orthop 1998, 350: 201–8
- Wennerberg A, Hallgren C, Johansson C, Danelli S. A histomorphometric evaluation of screw-shaped implants each prepared with two surface roughnesses. Clin Oral Impl Res 1998, 1: 11–9