Abstract
Background In some countries, commercially available antibiotic powder-loaded acrylic bone cement is routinely used in joint replacement, while, in others, “off-label” formulations are used in selected procedures (where the antibiotic powder is blended manually with the powder of a plain cement in the operating room/theater by either the surgeon or approved personnel). In the latter situation, an arbitrary rather than a rational approach is used for deciding on the amount of the antibiotic that is blended with the cement powder (herein referred to as “the antibiotic powder loading”).
Methods and results The first objective of this study was to present two methods for estimating the optimum loading of gentamicin sulfate powder that may be blended manually with the powder of a commercially available acrylic bone cement, ABC (Wopt). The second objective was to define the challenges associated with each of these methods. The loading (W) was optimized with respect to two key properties of the cured cement that were obtained simultaneously, namely (1) fatigue life in phosphate-buffered saline solution (PBS) at 37°C, and (2) the rate of elution of the gentamicin from the cement (E) in that medium. Three sets of specimens were used, containing 2.25, 4.25 and 11.50 wt/wt% of the gentamicin that was blended manually with the cement powder. The fatigue tests involved determining the number of cycles at which a specimen fractured (Nf) when subjected to fully-reversed tension-compression sinusoidal load, ± 15 MPa at 2 Hz. E was determined from the concentration of gentamicin in the PBS solution when the specimen fractured, using fluorescence polarization immunoassay for the measurement. Consistent with a priori expectations, it was found that, with increase in W, Nf decreased while E increased. Two approaches for obtaining an estimate of Wopt are described, a mathematical method and an empirical one, that lead to Wopt values of 6.50 wt/wt% and 4.78 wt/wt%, respectively. Thus, this antibiotic loading ranges from about the same to about 92% greater than that in SmartSet GHV Gentamicin, which is a commercially available bone cement used clinically and which has the same composition as SmartSet HV except for the presence of gentamicin sulfate in its powder (blended in by the manufacturer).
Interpretation We present and critically compare two rational methods of determining the optimum loading of an antibiotic powder in an acrylic bone cement, which should serve as a guide when using “off-label” antibiotic powder-loaded acrylic bone cements in cemented joint replacements.
Antibiotic powder-loaded acrylic bone cements (APLBCs) are frequently used either adjunctively or prophylactically in joint replacements (Engesaeter Citation2003). The practice in Europe is to use pre-blended commercially available APLBCs. In the United States, the practice for primary arthroplasties is to use an “off-label” APLBC (where an antibiotic powder is blended manually with the powder of a plain cement by a surgeon or nurse, at the time of the operation), while for revisions, either an “off-label” or a pre-blended commercially available APLBC is used. No pre-blended commercially available APLBC has been approved by the Food and Drug Administration (FDA) for primary arthroplasties and only a few pre-blended commercially available APLBCs are FDA-approved for use in the second stage of two-stage revision cases after an infection (Food and Drug Administration Citation2003).
In the majority of APLBCs, the antibiotic powder that is blended with the cement powder (either in pre-blended commercially available formulations or in “off-label” mixtures) is gentamicin sulfate. Although there is a sizeable literature on the in vitro properties of gentamicin sulfate-loaded acrylic bone cements (Kuhn Citation2000), the issue of the optimum loading of the gentamicin sulfate in the cement powder has not been addressed. (Note that the antibiotic loading, W, is herein defined as the mass of the gentamicin sulfate blended into the cement powder, C, expressed as a percentage of the total mass of the blended cement powder, G; that is, W = 100 × C/G %.) To do so would require (a) determining the in vitro values of a select number of properties of the curing and cured cement, as a function of C, and (b) treating the several property values-versus-C data appropriately. The assumption implicit in this approach is that there exists a value of W for which there is an optimum mix of the values of the properties determined. That value of W is herein designated Wopt.
We present two methods for estimating Wopt from the fatigue life and the elution rate of the gentamicin sulfate when blended with a commercially available acrylic bone cement, SmartSet HV (DePuy Orthopaedics, Warsaw, IN).
Material and methods
Material
The materials used were SmartSet HV (DePuy Orthopaedics, Warsaw, IN) and gentamicin sulfate (Sigma-Aldrich, St. Louis, MO).
Fabrication of test specimens
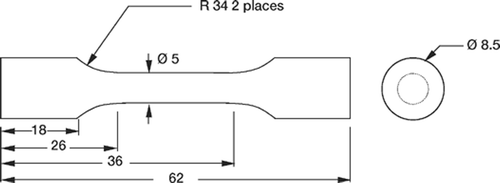
The relevant amount of the gentamicin sulfate powder was blended manually with the powder of SmartSet HV cement, using a sturdy polyethylene spatula. After that, the powder mixture and the cement's liquid monomer were mixed manually, for about 2 min, in a polyethylene bowl that was open to the air (temperature, 20 ± 1°C; relative humidity, 55 ± 2%). The resulting dough was then poured into a cement gun, from which it was extruded into a four-celled silicone rubber mold, yielding “dog-bone”-shaped fatigue test specimens (), according to ASTM F 2118-03 (ASTM Citation2004). After about 20 min in the mold, the specimens were gently pushed out.
3 sets of specimens were fabricated. In the first set, 0.9 g of gentamicin sulfate was blended with the powder of the cement (corresponding to an antibiotic loading of 2.25 wt% of gentamicin sulfate in the powder) (the HV2.25 set). In the second set, 1.7 g of gentamicin sulfate were blended with the powder of the cement (corresponding to an antibiotic loading of 4.25 wt% of gentamicin sulfate in the powder) (the HV4.25 set). In the third set, 4.6 g of gentamicin sulfate were blended with the powder of the cement (corresponding to an antibiotic loading of 11.50 wt% of gentamicin sulfate in the powder) (the HV11.50 set). These 3 gentamicin sulfate loadings were selected because they cover a wide spectrum of plausible loadings. In each set, 25 specimens were fabricated and examined both visually and radiographically for surface and internal flaws (with diameter greater than 0.5 mm) in their central-reduced and transition sections. Only those specimens that had no such flaws were used in the tests. In each set, between 18 and 20 specimens passed this inspection and, out of these, 12 were selected randomly and used as the test specimens.
Fatigue testing
The specimen was gripped in a custom-built fatigue test machine, with the specimen immersed in 1.3 L of PBS (pH 7.4; Gibco Invitrogen, Grand Island, NY) at 37°C that was contained in an environmental test chamber. During the test, the specimen was subjected to fully-reversed tension-compression loading, corresponding to a stress of ± 15 MPa at a frequency of 2 Hz, until it fractured at number of cycles Nf (ASTM Citation2004). During the test, a waxed paper skirt was placed snugly on top of the environmental test chamber to limit the amount of PBS that evaporated from it. Thus, when necessary, more PBS was added to the chamber to ensure that the volume of PBS in it was always 1.3 L.
The Nf results were treated using a statistical method that is widely used to analyze extreme value data (the three-parameter Weibull method) (Weibull Citation1951), with the aid of commercially available software ((Matlab7; The MathWorks, Natick, MA), to yield estimates of 3 parameters: (i) b, which is a measure of the dispersion of the Nf results, such that the smaller b is, the larger is the dispersion, (ii) No, the minimum or guaranteed fatigue life, which is the number of fatigue stress cycles above which all specimens have 100% probability of survival, and (iii) Na, the characteristic fatigue life, which is the number of stress cycles at which 63.2% of the specimens survive. These estimates were then combined to compute an overall index of the material's fatigue performance, NWM (Shigley and Mischke Citation2001).
Elution tests
At the end of a fatigue test, 1 mL of the PBS was collected from the environmental test chamber and the concentration of gentamicin sulfate in that assay (Q, in μg/mL) was then measured using fluorescence polarization immunoassay (FPIA) (TDx; Abbot Laboratories, Abbott Park, IL). The elution rate of the gentamicin sulfate into the PBS (E) was computed as Q/T, where T was the total amount of time that the specimen was in the PBS solution; that is, the sum of the time to fracture in the fatigue test and the time between the fracture of the specimen and when the assay was collected for the FPIA.
Characterization of the gentamicin sulfate powder
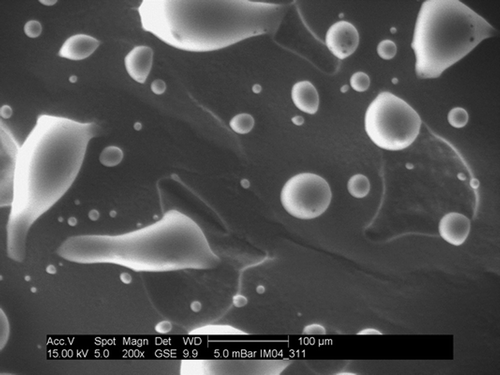
The particle size distribution and the morphology of the gentamicin sulfate powder used were determined using a laser diffraction system (Sympatec Particle Size Analyzer, Model HDD200; Sympatec GmbH, Golar, Germany) and an environmental scanning electron microscope (Model XL30; Philips, Achtsewed, the Netherlands), operated at an acceleration voltage of 15 kV, respectively.
Statistics
Statistical analysis of the effects of the added gentamicin sulfate, performed using analysis of variance (ANOVA) with Bonferroni correction/adjustment, was applied to detect specific differences between the three study groups, in terms of both ln Nf and ln E. The level of significance was set at p < 0.05 (or 0.017, after adjustment). All the tests were conducted using commercially available software (SAS 8.02; SAS Institute, Cary, NC).
Results
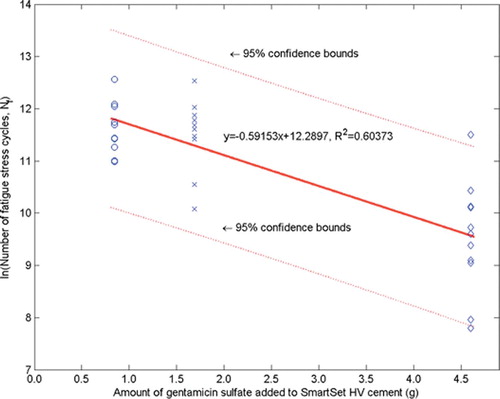
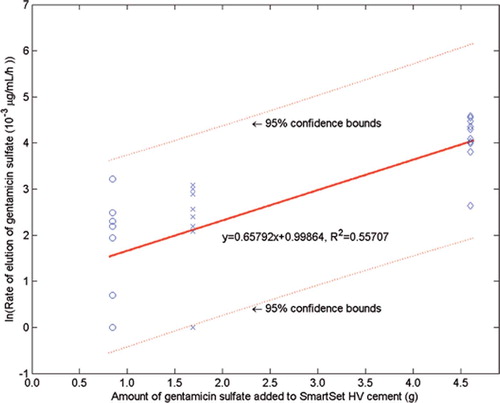
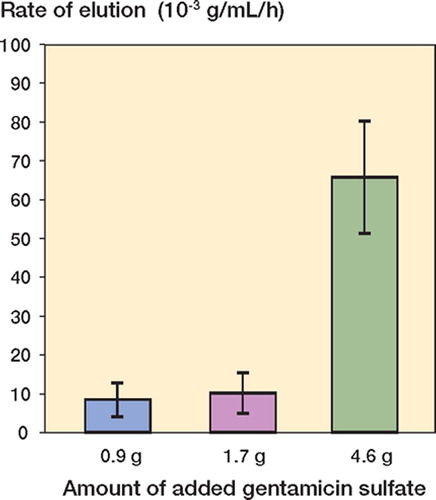
The Nf and E results are presented in and . Statistical analyses of the log transformation of these results showed two clear trends. First, there was no significant difference in the means of ln Nf for the HV2.25 and HV4.25 sets (corrected p = 1.00), but the difference was significant for the HV2.25 set compared to the HV11.50 set (corrected p < 0.003), and for the HV4.25 set compared to the HV11.50 set (corrected p < 0.003). Second, there was no significant difference in the means of ln E for the HV2.25 and HV4.25 sets (corrected p = 1.00), but the difference was significant for the HV2.25 set compared to the HV11.50 set (corrected p < 0.003), and for the HV4.25 set compared to the HV11.50 set (corrected p < 0.003).
Figure 2. Variation in number of fatigue cycles (Nf) with the amount of gentamicin sulfate added to the cement powder. (Mean, 95% CI).
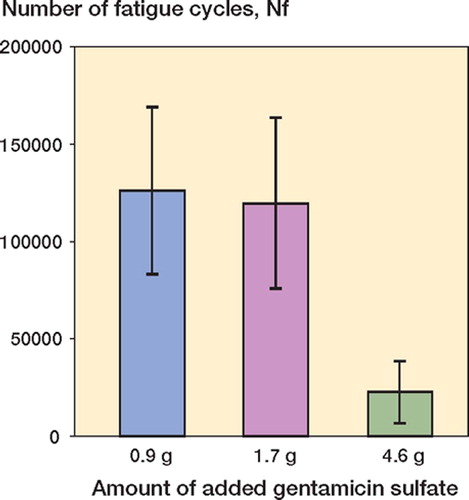
Figure 3. Variation in the rate of elution of gentamicin sulfate into the PBS solution with the amount of gentamicin sulfate added to the cement powder. (Mean, 95% CI).
The trends in the ln Nf results were confirmed by the trends in the estimates of the Weibull parameters ().
Summary of the estimates of the Weibull parameters
Plots of the ln Nf-vs.-C and ln E-vs.-C results (and the best-fit expressions) are given in and , respectively. From these results, Copt (and, hence, Wopt) were estimated using two methods. The first (“the mathematical method”) involved determining the value of C at which the percentage decrease of ln Nf with C is the same as the percentage increase of ln E with C. With this definition and the best-fit expressions given in and , we estimated Copt to be 2.60 g; hence, Wopt is 6.50 wt/wt% (= 2.60 g gentamicin in 40 g of the blended powder mixture). The upper and lower 95% confidence intervals of this estimate were found to be 3.34 g (hence, Wopt = 8.35 wt/wt%) and 1.74 g (hence, Wopt = 4.35 wt/wt%). In the second method (“the empirical method”), we define a range of mixtures that is bounded on one side by a value of C that leads to an unacceptably low fatigue life (call this value C1) and on the other side by a value of C that leads to an unacceptably low elution rate (call this value C2). In the present study, the average value of the expression: ratio of the mean of the parameter measured to the computed confidence interval for that parameter—taken for both the fatigue life and the elution rate results ( and )—is 45%. This leads us to suggest that C1 is 2.53 g gentamicin (= 0.55 × 4.6 g gentamicin), while C2 is 1.30 g of gentamicin sulfate (= 1.45 × 0.9 g of gentamicin). Thus, if Copt is defined as the midpoint of the range C1–C2, we see that, in this case, Copt is 1.91 g gentamicin or Wopt = 4.78% (= 1.91 g gentamicin in 40 g of the blended powder mixture).
Discussion
It is important to recognize the challenges in defining Wopt. These arise principally because there are a number of approaches that may used, each with its own unique set of attractions and difficulties. Thus, the mathematical method we used has the advantage that it is based on notions from optimization theory. However, it is beset with three main drawbacks. The first is that the computational effort involved is high, because it involves determination of the best-fit functions to the experimental results, differentiation of each of these functions with respect to C (for some functions, the differentiation may be difficult), and use of a numerical method to solve for Copt (and, hence, Wopt) from the final set of equations. The second drawback is that the Wopt obtained is influenced by the best-fit expressions used. For example, with the present results, when power Nf-vs.-C and linear E-vs.-C fits are used, Wopt of 6.18 wt/wt% (= 2.47 g of gentamicin in 40 g of the blended powder mixture) is obtained, whereas when power Nf-C and second-order polynomial E-C fits are used, Wopt of 4.90 wt/wt% (= 1.96 g of gentamicin in 40 g of the blended powder mixture) is obtained. The third drawback is that it is difficult to relate the definition of Wopt to the clinical use of an APLBC. The advantage of the empirical approach used in the present work is its simplicity, thus making it accessible to all workers. It does, however, suffer from the drawback that first-principles notions are not used—either in the choice of the values of W at the two extremes of the range of W (they are selected based on a subjective premise, that of unacceptably low fatigue life and unacceptably low elution rate) or in the determination of a single value of Wopt from within that range, if that is desired.
Keeping in mind the points made above, we point out that two of the Wopt estimates obtained in this study (the lower bound result from the mathematical method and the result from the empirical method) are about the same value as the loading of gentamicin sulfate in SmartSet GHV Gentamicin (DePuy Orthopaedics, Warsaw, IN) (4.22 wt/wt%), which is a commercially available APLBC that is used clinically and has the same composition as SmartSet HV, except for the inclusion of the gentamicin sulfate that is pre-blended by the manufacturer using a proprietary method. On the other hand, the other Wopt estimates from the mathematical method are 54–98% larger than the loading in SmartSet GHV Gentamicin.
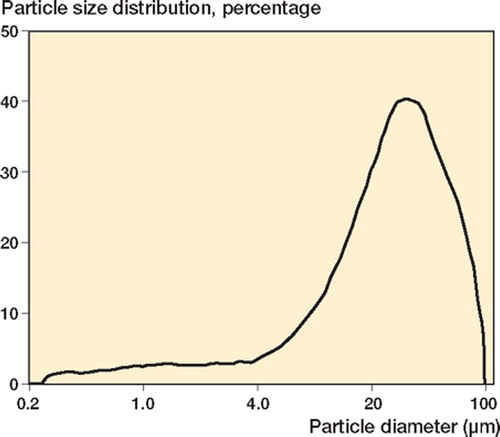
In using the results we have obtained, four points must be borne in mind. First, only one cement formulation was used. The properties of acrylic bone cement are known to be formulation-specific (Lewis Citation1997). Second, only one gentamicin sulfate powder with a specific powder size distribution, of particle size 27.13 ± 1.40 mm, and a specific morphology () was used. Third, only one mixing method was used (that is, the method used to mix the blended powder mixture with the cement's liquid monomer). It is well known that the mixing method has a marked influence on the properties of cured acrylic bone cement (Lewis Citation1997). Fourth, the Wopt was estimated based on only two properties of the cured cement, albeit very important ones. It is unknown how this Wopt estimate would be affected by the properties of the cement that were not considered, such as setting time and fracture toughness. In recognition of the first two points mentioned, we reiterate that the results obtained in this study were for the SmartSet-based cements only, and not for acrylic bone cement as a group. Thus, future studies should be conducted on all cements used in clinical practice for cemented arthroplasty.
Figure 6. A. The particle size distribution of gentamicin sulfate powder used in the study (left). (The ordinate represents the percentage of the total volume of the particles). B. The morphology of the gentamicin sulfate used (right).
In conclusion, the finding of our study was that on the basis of fatigue life and elution rate results, the estimate of the optimum loading of gentamicin sulfate in SmartSet HV acrylic cement (with the antibiotic being blended manually into the cement powder using a sturdy polyethylene spatula) depends on the methodology used. Thus, with a mathematical method, the estimate ranged from 4.35 to 8.35 wt/wt%, while with an empirical method it was 4.78 wt/wt%. This specific finding and the results of the study in general have important ramifications. The specific finding should aid orthopedic surgeons and nurses in preparing an “off-label” gentamicin sulfate-loaded SmartSet HV acrylic bone cement for use in cemented arthroplasties. The study in general highlights the challenges in obtaining Wopt for an antibiotic powder-acrylic bone cement system.
The cement used, SmartSet HV, was donated by DePuy, Inc., Warsaw, IN, who also provided funding for the study in the form of a research grant. The sponsor had no role in the design of the study, the collection and interpretation of data and the writing of the manuscript. The authors thank Mr. Jie Xu, Department of Mechanical Engineering, University of Memphis, Memphis, TN, for help with the statistical analysis of the results.
- ASTM. Standard F 2118-03, Test method for constant amplitude force controlled Fatigue testing of acrylic bone cement materials. 2004 Annual Book of ASTM Standards, Volume 13.01, Medical and surgical materials and devices; anesthetic and respiratory equipment. ASTM International, West Conshochocken, PAUSA 2004; 1199–1206
- Engesaeter L B. Antibiotic prophylaxis in total hip arthroplasty. Acta Orthop Scand 2003; 74: 644–51
- Food and Drug Administration (FDA). FDA approves Stryker Howmedica's antibiotic bone cement. August, 2003
- http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm
- Kuhn K-D. Bone cements: up-to-date comparison of physical and chemical properties of commercial materials. Springer-Verlag, BerlinGermany 2000
- Lewis G. The properties of acrylic bone cement: a state-of-the-art review. J Biomed Mater Res: Appl Biomater 1997; 38: 155–82
- Shigley J E, Mischke C R. Mechanical engineering design. 6th edn. McGraw-Hill, Inc., New York 2001
- Weibull W. A statistical distribution function of wide applicability. J Appl Mech 1951; 18: 293–7