Abstract
Background All metal implants—and metal-on-metal bearings in particular—corrode and cause a release of metal ions. Because cobalt and chromium have been shown to be carcinogenic and mutagenic in human and animal models, systemic toxicity and cancer risk are considered to be possible disadvantages of the metal-on-metal articulation.
This study was designed to investigate the serum concentration profiles of chromium, cobalt and molybdenum after implantation of a Birmingham hip resurfacing arthroplasty (BHR) and a cementless total hip replacement with a 28-mm Metasul articulation (MTHR), over the first 2 years after implantation.
Methods We analyzed profiles of metal ion serum levels in 111 patients implanted with a BHR, in 74 patients implanted with an MTHR, and in 130 implant-free probands control subjects using atomic absorption spectrophotometry.
Results Chromium and cobalt concentrations (in μg/L) of all BHR and MTHR patients differed significantly from those of control subjects (chromium: < 0.25; cobalt: 0.25). The median chromium and cobalt concentrations in BHR patients had increased to 5.1 and 4.3 μg/ L 2 years after surgery. Concentrations in BHR patient exceeded those in the unilateral MTHR patients. Molybdenum serum concentrations hardly changed over time in either group and were not significantly different from the concentrations seen in the control subjects.
Interpretation During the first 2 years after surgery, the Birmingham hip resurfacing arthroplasty leads to a significantly greater increase in serum chromium and cobalt levels than the 28-mm metal-on-metal MTHR. Observation of patients over a longer period will be necessary in order to evaluate any chronic adverse effects to the system due to elevated chromium and cobalt serum concentrations.
Periprosthetic tissue reactions to wear debris, in particular to polyethylene and cement, are the major current limitations in joint alloarthroplasty. In particular, polyethylene debris causes a cascade of cellular and humoral events that induce a granulomatous foreign body reaction, which often leads to osteolysis and implant loosening. Thus, reduction of wear debris became an important issue for improvement of the long-term results of total hip replacement, and has re-stimulated interest in alternative bearing materials. Cobalt, chromium, and molybdenum alloys, and also ceramics, have a higher resistance to wear than polyethylene. Studies of retrieved historical metal-on-metal prostheses have shown extremely low wear rates, even 10–20 years after implantation (Amstutz and Grigoris Citation1996). Also, only a mild (if any) foreign body reaction to metal debris was found (Willert et al. Citation1996).
Today, several designs of modern-generation metal-on-metal total hip replacement and hip resurfacing devices are available, with satisfying short- to mid-term results. Only Bachfischer et al. (Citation2000) reported a higher rate of heterotopic ossifications and considered the metal-on-metal bearing to be responsible. The amount of wear found on retrieved implants turned out to be as low as suggested by hip simulator tests (Schmidt et al. Citation1996, Streicher et al. Citation1996, Kremling et al. Citation2004).
All metal implants, in particular metal-on-metal bearings, do however corrode at a rate determined by their surface area and cause a release of metal ions. In addition to the corrosion potential of the implant itself, wear debris elevates the ion release due to the increased exposure of metal surface. In a number of studies, the release of metal ions from total hip arthroplasties (both locally and systemically) has been documented (Betts et al. Citation1992, Basle et al. Citation1996, Doorn et al. Citation1996, Schaffer et al. Citation1999, Prohaska et al. Citation2000, Harding et al. Citation2002, Maezawa et al. Citation2002, Savarino et al. Citation2002, Adami et al. Citation2003, Clarke et al. Citation2003, Lhotka et al. Citation2003, MacDonald et al. Citation2003).
Because both cobalt and chromium have been shown to be carcinogenic and mutagenic in human and animal models, systemic toxicity and cancer risk have been considered to be possible disadvantages of the metal-on-metal articulation (Rae Citation1981, Amstutz et al. Citation1996, Visuri et al. Citation1996, Willert and Semlitsch Citation1996).
Conservative metal-on-metal hip resurfacing arthroplasty is an attractive concept, particularly in young and active patients. Two unique advantages are retention of upper femoral bone stock and avoidance of stress shielding in the proximal femoral shaft. Additionally, based on probable lubrication from the fluid film production of wear debris is thought to be less likely in metal-on-metal articulations of large diameter than in conventional 28-mm metal-on-metal bearings (Smith et al. Citation2001).
Contrary to what one might expect, Skipor et al. (Citation2002) did not find lower serum concentrations of cobalt and chromium after hip resurfacing arthroplasty than in hip replacement arthroplasty with a Metasul bearing up to 2 years postoperatively. Clarke et al. (Citation2003) actually demonstrated elevation of serum cobalt and chromium levels in a small group of patients implanted with different hip resurfacings, significantly above the levels measured in a patient group implanted with a metal-on-metal THR.
We investigated the serum profiles of chromium, cobalt and molybdenum concentration in patients with successfully implanted Birmingham hip resurfacing arthroplasty (clinically and radiographically) and compared them to those of patients who underwent uni- and bilateral uncemented total hip replacement with a metal-on-metal articulation and to those of an implant-free control group over the first 2 postoperative years.
Patients and methods
Patients
111 patients implanted unilaterally with a Birmingham hip resurfacing (BHR), and 60 patients implanted unilaterally and 14 patients implanted bilaterally with a cementless total hip replacement (Metasul bearing, MTHR), were included into the investigation after obtaining informed consent. 56 BHR patients were investigated for 3 months, 50 patients for 12 months, and 23 patients for 24 months postoperatively (some patients were measured more than once). Metal ion levels of MTHR patients were obtained from 18 unilaterally implanted patients at 3 months, 13 at 12 months and 34 at 24 months. 6 bilaterally implanted MTHR patients were investigated 3 months after surgery of their second hip, 8 at 12 months and 3 at 24 months.
According to established indication criteria for hip resurfacing arthroplasty, the patient groups differed in age and sex (). The BHR patients had slightly better Harris hip scores than the MHTR samples, particularly 3 months after implantation. The BHR implants had a median articulation size of 50 mm. The cup abduction angles were of comparable size in both patient groups ().
Table 1. Characteristics of control and patient samples (medians and quartiles for continuous, relative frequencies for categorical outcome variables) and Harris hip score (HHS) assessment of patients
Among this cohort of 111 BHR-implanted patients with varying assessment times, 18 could be followed up over the first 2 assessment times, i.e. metal ion levels were obtained at 3 and 12 months after surgery.
All patients had well-functioning implants, both clinically and radiographically, without signs of loosening. None of the patients suffered from anemia, tumor disease, infection or renal insufficiency. No chronic occupational exposure to cobalt, chromium or molybdenum sources was reported by any of patient.
In addition, 130 patients awaiting hip or knee joint arthroplasty (with no previous implantation) served as control subjects for the investigation.
Implants
The prostheses used in this study were either the Birmingham hip resurfacing device (BHR; Midland Medical Technologies Ltd., Birmingham, U.K.) or a standard cementless total hip replacement device consisting of a TiAl6Nb7 (ISO 583211) alloy press-fit acetabular and femoral component and a metal-on-metal bearing (Metasul; Sulzer Orthopedics Ltd., Switzerland) with a ball head size of 28 mm.
Radiographic analysis
For all patients, follow-up radiographs of the pelvis (a.p.) were analyzed to exclude implant loosening and to determine the cup position (cup abduction angle).
Metal ion analysis
Blood samples were collected in 7-mL S-Monovette tubes (for trace metal analysis; Sarstedt AG, Germany) using a specific steel needle for trace metal analysis (Sarstedt). Serum was separated by centrifugation at 2,000 g for 10 min. Samples were stored at –20°C before analysis of chromium, cobalt and molybdenum content using a graphite furnace atomic absorption spectrometer (Z-8200) with polarization-Zeeman absorption (Hitachi Ltd., Japan). Calibration was performed by the standard addition method using 0.00 μg/L, 5.00 μg/ L and 10.00 μg/L as calibration points in triplicate for each element. The samples were diluted 1:2 in buffer (1% HNO3(Merck AG, Germany), 0.2% Triton X-100 (Sigma-Aldrich Chemie GmbH, Germany), 0.2% Antifoam B (Sigma-Aldrich); Cr and Co: additional 0.8% Pd-matrix-modifier (Merck), 0.3% Mg-matrix-modifier (Merck)). The accuracy and precision of the method was validated to < 10% using the control materials Seronorm Trace Elements Serum (SERO AS, Norway). The detection limit of the method was estimated to be 0.5 μg/ L for each element (mean + 3 SD from buffer). The Dixon test was used to eliminate aberrant values. All probes having ion levels below the detection limits were adjusted to < 0.25 μg/L.
Data analysis
Metal ion serum levels are expressed using medians and quartiles (graphically by non-parametric box plots). Exploratory group comparisons were performed by means of pairwise two-sample Wilcoxon tests for continuous endpoints, and by pairwise Fisher tests for categorical endpoints. Intra-individual changes in serum concentrations were analyzed by means of sign tests and expressed using medians and quartiles of the intraindividual differences' distribution. The results of the exploratory significance tests are expressed with p-values, where p < 0.05 indicates a locally significant difference.
Results
Chromium
The median chromium serum level of the control samples was < 0.25 μg/L (). The highest median serum concentrations in unilaterally implanted MTHR patients were found 1 year after surgery—median 1.62 μg/L—as compared to 0.83 μg/L at 3 months. At 2 years, the median serum levels were intermediate between these values: 1.22 μg/L. Chromium levels in bilaterally implanted MTHR patients were highest 3 months after implantation of the second hip: median 4.42 μg/L. At 1 and 2 years after surgery, chromium levels became reduced to 3.62 μg/L and 2.50 μg/ L, respectively. In BHR patients, serum chromium levels rose from median 1.96 μg/L at 3 months after surgery to 4.20 μg/L after 1 year, and to 5.12 μg/L after 2 years ().
Figure 1. Nonparametric box plot for the distribution of chromium serum concentration (μg/L) 3, 12, and 24 months after total hip replacement surgery, stratified for the implant (horizontals display medians and quartiles, and circles/asterisks show statistical outliers/extreme values with more than 2-times/3-times deviation of the interquartile range from the upper quartile, respectively).
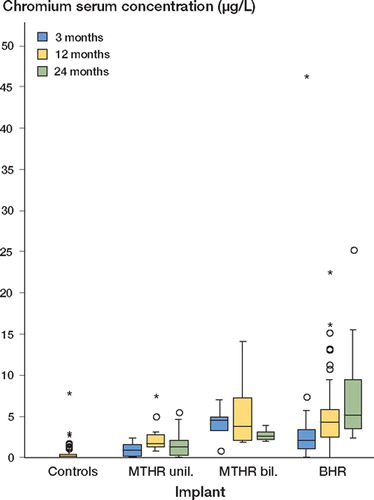
There was a statistically significant difference between each patient's serum chromium concentration and the concentration of the control individuals. Furthermore, serum chromium levels in BHR patients exceeded the concentrations in unilaterally implanted MTHR patients at each assessment time, and of the bilaterally implanted MTHR patients at 3 and 24 months after surgery ().
Table 2. P-values from exploratory pairwise comparisons of chromium serum concentration distributions by means of two-sample Wilcoxon tests at each assessment time (3, 12 and 24 months after surgery)
Cobalt
The implant-free control group had a median serum cobalt concentration of 0.25 μg/L (). Cobalt concentrations in MTHR patients were highest 2 years postoperatively, with a median of 1.70 μg/L for unilaterally implanted patients and 3.18 μg/L for bilaterally implanted patients. Cobalt profiles in BHR patients rose from median 2.17 μg/ L at 3 months to 4.28 μg/L at 2 years after surgery ().
Figure 2. Nonparametric box plot for the distribution of cobalt serum concentration (μg/L) 3, 12, and 24 months after total hip replacement surgery, stratified for the implant (horizontals display medians and quartiles, and circles/ asterisks show statistical outliers/extreme values with more than 2-times/3-times deviation of the interquartile range from the upper quartile, respectively.
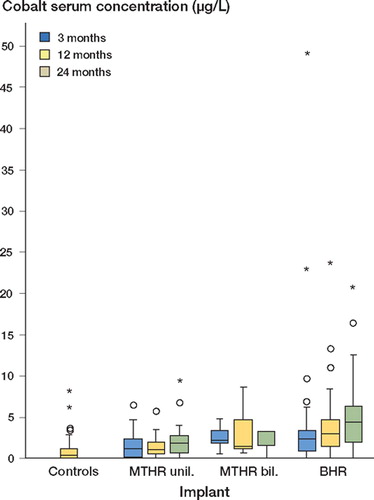
Serum cobalt levels in patients differed significantly from the control levels except for bilaterally implanted MTHR patients, at the 2-year assessment after surgery. Cobalt levels of BHR patients exceeded the levels in unilaterally implanted MTHR patients significantly at each assessment point. No statistically significant differences were found when serum cobalt levels of bilaterally implanted MTHR patients were compared with those of BHR patients and unilaterally implanted MTHR patients ().
Table 3. P-values from exploratory pairwise comparisons of cobalt serum concentration distributions by means of two-sample Wilcoxon tests at each assessment time (3, 12 and 24 months after surgery)
Molybdenum
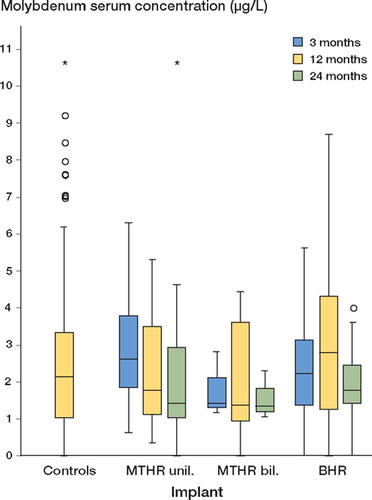
The median serum molybdenum level of the controls was 2.11 μg/L. The molybdenum levels of the patient groups investigated showed no statistically significant differences from the serum concentrations of the controls at any assessment point ().
Figure 3. Nonparametric box-plot for the distribution of molybdenum serum concentration (μg/L) 3, 12, and 24 months after total hip replacement surgery, stratified for the implant (horizontals display medians and quartiles, and circles/asterisks show statistical outliers/extreme values with more than 2-times/3-times deviation of the interquartile range from the upper quartile, respectively.
Intraindividual follow-up
For the subsample of 18 BHR patients with intraindividual follow-up at the 3- and 12-month assessment times, the chromium serum levels increased significantly between 3 and 12 months postoperatively by median 3.36 μg/L (1.10–5.74 μg/L; n = 18; p = 0.008, sign test) and between 1 and 2 years by median 4.40 μg/L (3.49–6.48 μg/L; n = 3). Cobalt serum concentration increased significantly from 3 to 12 months after surgery by median 0.71 μg/L (-0.03–2.52 μg/L; n = 18; p = 0.03, sign test) and from 1 to 2 years by median 9.84 μg/L (4.08–10.02 μg/L; n = 3).
Implant- and patient-related factors
Neither the head size of the implant (BHR patients) and the cup abduction angle nor the patients' age and BMI showed an association with the chromium, cobalt or molybdenum serum concentration profiles in the groups under investigation.
Extreme values
6 BHR patients showed extreme serum levels of metal ions, with more than three-times deviation of the interquartile range from the upper quartile ( and ). 1 male patient had a serum chromium level of 46 μg/L and a serum cobalt level of 49 μg/L at the 3-month assessment after surgery. 2 years after surgery, his levels had normalized to 2.7 μg/L and 1.6 μg/L. 3 years later, he showed a Harris hip score of 98 points without clinical or radiographic signs of implant loosening. Metal ion levels of the other 5 patients were not checked again, but 4 of them had clinical and radiographic follow-ups 1–3 years after their respective serum concentration assessment times without signs of aseptic loosening. 3 of these patients had Harris hip scores of 97–100 points. 1 woman showed a score of 40 points due to nerve palsy. All these patients had cup abduction angles between 39° and 61°.
Discussion
We found that metal-on-metal bearings lead to a significant increase in serum levels of chromium and cobalt (particularly when implanted bilaterally). Also, hip resurfacing arthroplasty showed an even greater increase in serum chromium and cobalt levels than a 28-mm metal-on-metal THR. Serum molybdenum concentration was not affected by the implants; the corresponding alloy content may to be too low to cause an alteration in serum levels.
The serum levels of chromium and cobalt for the 28-mm Metasul bearing were similar to those found in previously reported investigations for the modern, second-generation metal-on-metal bearings. Investigations that have been conducted to date have uniformly shown substantial elevations in serum, blood, erythrocyte and/or urine serum levels relative to preoperative values, and/or relative to levels measured after implant of metal-on-polyethylene or ceramic-on-polyethylene bearings (Betts et al. Citation1992, Basle et al. Citation1996, Doorn et al. Citation1996, Schaffer et al. Citation1999, Prohaska et al. Citation2000, Harding et al. Citation2002, Maezawa et al. Citation2002, Savarino et al. Citation2002, Adami et al. Citation2003, Clarke et al. Citation2003, Lhotka et al. Citation2003, MacDonald et al. Citation2003).
There are, however, limited data available on systemic metal concentrations in patients implanted with hip resurfacing devices. Skipor et al. (Citation2002) reported substantial elevations in cobalt and chromium serum levels in 25 patients up to 1 year after metal-on-metal hip resurfacing arthroplasty (Conserve plus; Wright Medical Technology Inc., Arlington, TN, USA), but they did not find statistically significant differences when comparing them to levels in patients who had undergone metal-on-metal total hip replacement. In contrast, Clarke et al. (Citation2003) described elevations in serum cobalt and chromium in a group of patients implanted with different hip resurfacings, median 16 months postoperatively (16 BHR patients and 6 patients implanted with a Cormet 2000; Corin Surgical, Cirencester, UK). Similarly to the above findings, they reported concentrations that were significantly higher than the serum levels of a patient group implanted with a metal-on-metal THR.
While it is certain that the metal ions must be released because of the combined effect of corrosion of the implant surface and wear particles, the exact reasons for the differences in metal ions between different hip resurfacings and 28-mm articulations are not yet clear. Large-diameter metal-on-metal articulations are thought to benefit from fluid film lubrication and thus reduced generation of wear particles, in comparison to smaller bearings (Jin et al. Citation1997, Smith et al. Citation2001). On the other hand, hip simulator studies and BHR explant investigations have demonstrated wear behavior similar to that of Metasul bearings (McMinn Citation2003, Kremling et al. Citation2004). In contrast to 28-mm articulations, hip resurfacing devices (in particular the BHR with the Porocast structure of the cup) constitute a much larger surface area of the implant itself, which may lead to increased ion release. Other possible causes may include differences in lubrication regimes due to metallurgy, diametric clearance, sphericity, surface roughness and different carbide content of the alloys. The BHR device consists of cast alloy containing a high carbide content of about 5%, which is responsible for the hardness and thus the wear resistance of the alloy. However, a problem with enhanced carbide levels is the reduced corrosion resistance (Montero-Ocampo et al. Citation1996).
Retrieval studies of the new-generation metal-on-metal bearings have reported a higher degree of running-in wear (15–20 μm per year) during the first year after surgery and a reduction to 2–5 μm per year after the second or third year (Schmidt et al. Citation1996, Sieber et al. Citation1999). Skipor et al. (Citation2002) reported an increase in serum chromium and cobalt concentrations up to 6 months postoperatively, followed by a slight decrease 1 year after surgery—compatible with a higher running-in wear followed by lower steady-state wear behavior of the articulation. We found the highest levels at the latest assessment point in the above recall design (2 years after surgery) complemented with a significant intraindividual increase at least up to 1 year, possibly caused by a prolonged running-in phase or an increase of the implant surface due to solution of the hydroxyapatite coating during that time period.
6 BHR patients showed extreme (up to 10-fold) elevated serum levels of chromium and cobalt ions with more than 3-times deviation of the interquartile range, usually 3 or 12 months postoperatively. A temporarily higher amount of wear due to episodes of third-body wear or a higher degree of running-in wear with these pairings may be possible explanations.
Historical McKee-Farrar metal-on-metal implants were manufactured of carbide-rich as-cast alloy and were of similar size to BHR implants. Jacobs et al. (Citation1996) showed very low serum levels of chromium and cobalt in a group of patients with McKee-Farrar implants in situ for over 20 years. Thus, a decrease in previously elevated chromium and cobalt serum concentrations in patients implanted with the BHR may be expected after the first 2 years, but this has not been proven yet. Furthermore, it should be noted that only a few BHR patients in our series showed cobalt serum concentrations in excess of the exposure limits for industrial carcinogens (5 μg/L whole blood) (Forschungsgemeinschaft Citation2004). It should be borne in mind that these exposure limits relate to blood levels following respiratory exposure, and cannot therefore be automatically translated to the levels produced by implants and wear within the body.
These findings are important since there is concern that chronic increases in serum chromium and cobalt may result in long-term adverse biological effects such as immune modulation, chromosomal damage and carcinogenesis (Vahey et al. Citation1995, Visuri et al. Citation1996, Savarino et al. Citation1999, Granchi et al. Citation2000). On the other hand, cobalt, chromium and molybdenum are cofactors for a number of enzymes and are essential trace metals, but can become toxic after a minimal increase in concentration (Rae Citation1981, Vyskocil and Viau Citation1999). Despite the concern that chronically elevated serum chromium and cobalt concentrations may result in adverse long-term biological effects, the issue of clinical relevance must be critically discussed. Although total hip replacement and hip resurfacing arthroplasty using a metal-on-metal articulation are widely used procedures with well-documented clinical success, patient observation over a longer period will be necessary to evaluate any evidence of adverse chronic systemic effects in patients due to prolonged, elevated chromium and cobalt serum concentrations.
Our investigation lacked intraindividual follow-up of patients (except for the subsample of 18 BHR implanted patients, the results of which coincided very well with those found in the overall sample). From a methodological point of view, a longitudinal study design seems desirable. In addition, the choice of follow-up period of the latter should be sufficiently large to allow monitoring of long-term developments in serum concentrations, and possibly associated changes in the health of patients.
No competing interests declared.
Contributions of authors
WCW was involved in study design, gathering of data, and writing of initial drafts; JZ was involved in gathering of data; FK performed data analysis; VN performed data analysis and checked the accuracy of the data and the analysis; KPG checked the accuracy of the data and the analysis.
- Adami G, Smarrelli D., Martinelli B, Acquavita A, Reisenhofer E. Cobalt blood levels after total hip replacement (THR): a new follow-up study in Trieste (Italy). Ann Chim 2003; 93: 1–10
- Amstutz H C, Grigoris P. Metal on metal bearings in hip arthroplasty. Clin Orthop 1996, 329: S11–34
- Amstutz H C, Campbell P, McKellop H, Schmalzreid T P, Gillespie W J, Howie D, Jacobs J, Medley J, Merritt K. Metal on metal total hip replacement workshop consensus document. Clin Orthop 1996, 329: S297–303
- Bachfischer K, Karpf P M, Schwarz R. An unusual observation of total hip replacement with metal on metal gliding bearings. Z Orthop Ihre Grenzgeb 2000; 138: 230–4
- Basle M F, Bertrand G, Guyetant S, Chappard D, Lesourd M. Migration of metal and polyethylene particles from articular prostheses may generate lymphadenopathy with histiocytosis. J Biomed Mater Res 1996; 30: 157–63
- Betts F, Wright T, Salvati E A, Boskey A, Bansal M. Cobalt-alloy metal debris in periarticular tissues from total hip revision arthroplasties. Metal contents and associated histologic findings. Clin Orthop 1992, 276: 75–82
- Clarke M T, Lee P T, Arora A, Villar R N. Levels of metal ions after small- and large-diameter metal-on-metal hip arthroplasty. J Bone Joint Surg (Br) 2003; 85: 913–7
- Doorn P F, Mirra J M, Campbell P A, Amstutz H C. Tissue reaction to metal on metal total hip prostheses. Clin Orthop 1996, 329: S187–205
- Forschungsgemeinschaft D. MAK- und BAT-Werte-Liste 2004. Wiley-VCH Verlag GmbH, Weinheim 2004
- Granchi D, Ciapetti G., Filippini F, Stea S, Cenni E, Pizzoferrato A, Toni A. In vitro cytokine production by mononuclear cells exposed to bone cement extracts. Biomaterials 2000; 21: 1789–95
- Harding I, Bonomo A, Crawford R, Psychoyios V, Delves T, Murray D, McLardy-Smith P. Serum levels of cobalt and chromium in a complex modular total hip arthroplasty system. J Arthroplasty 2002; 17: 893–5
- Jacobs J J, Skipor A K, Doorn P F, Campbell P, Schmalzried T P, Black J, Amstutz H C. Cobalt and chromium concentrations in patients with metal on metal total hip replacements. Clin Orthop 1996, 329: S256–63
- Jin Z M, Dowson D, Fisher J. Analysis of fluid film lubrication in artificial hip joint replacements with surfaces of high elastic modulus. Proc Inst Mech Eng [H] 1997; 211: 247–56
- Kremling U, Franke R, Witzleb W C. Tribological investigations on metal-on-metal bearings during wear tests in a hip simulator according ISO 14 242. Mat.-wiss. u. Werkstofftech 2004; 35: 1–8
- Lhotka C, Szekeres T, Steffan I, Zhuber K, Zweymuller K. Four-year study of cobalt and chromium blood levels in patients managed with two different metal-on-metal total hip replacements. J Orthop Res 2003; 21: 189–95
- MacDonald S J, McCalden R W, Chess D G, Bourne R B, Rorabeck C. H, Cleland D, Leung F. Metal-on-metal versus polyethylene in hip arthroplasty: a randomized clinical trial. Clin Orthop 2003, 406: 282–96
- Maezawa K, Nozawa M, Hirose T, Matsuda K, Yasuma M, Shitoto K, Kurosawa H. Cobalt and chromium concentrations in patients with metal-on-metal and other cement-less total hip arthroplasty. Arch Orthop Trauma Surg 2002; 122: 283–7
- McMinn D J W. Development of metal/metal hip resurfacing. Hip International 2003; 13: 41–53
- Montero-Ocampo C, Lopez H, Salinas Rodriguez A. Effect of compressive straining on corrosion resistance of a shape memory Ni-Ti alloy in Ringer's solution. J Biomed Mater Res 1996; 32: 583–91
- Prohaska C, Pomazal K, Steffan I. ETAAS method for the determination of Cd, Cr, Cu, Mn and Se in blood fractions and whole blood. Fresenius J Anal Chem 2000; 368: 627–32
- Rae T. The toxicity of metals used in orthopaedic prostheses. An experimental study using cultured human synovial fibroblasts. J Bone Joint Surg (Br) 1981; 63: 435–40
- Savarino L, Granchi D, Ciapetti G, Stea S, Donati M E, Zinghi G, Fontanesi G, Rotini R, Montanaro L. Effects of metal ions on white blood cells of patients with failed total joint arthroplasties. J Biomed Mater Res 1999; 47: 543–50
- Savarino L, Granchi D, Ciapetti G, Cenni E, Nardi Pantoli A, Rotini R, Veronesi C A, Baldini N, Giunti A. Ion release in patients with metal-on-metal hip bearings in total joint replacement: a comparison with metal-on-polyethylene bearings. J Biomed Mater Res 2002; 63: 467–74
- Schaffer A W, Pilger A, Engelhardt C, Zweymueller K, Ruediger H W. Increased blood cobalt and chromium after total hip replacement. J Toxicol Clin Toxicol 1999; 37: 839–44
- Schmidt M, Weber H, Schon R. Cobalt chromium molybdenum metal combination for modular hip prostheses. Clin Orthop 1996, 329: S35–47
- Sieber H P, Rieker C B, Kottig P. Analysis of 118 second-generation metal-on-metal retrieved hip implants. J Bone Joint Surg (Br) 1999; 81: 46–50
- Skipor A K, Campbell P A, Patterson L M, Anstutz H C, Schmalzried T P, Jacobs J J. Serum and urine metal levels in patients with metal-on-metal surface arthroplasty. J Mater Sci Mater Med 2002; 13: 1227–34
- Smith S L, Dowson D, Goldsmith A A. The effect of femoral head diameter upon lubrication and wear of metal-on-metal total hip replacements. Proc Inst Mech Eng [H] 2001; 215: 161–70
- Streicher R M, Semlitsch M, Schon R, Weber H, Rieker C. Metal-on-metal articulation for artificial hip joints: laboratory study and clinical results. Proc Inst Mech Eng [H] 1996; 210: 223–32
- Vahey J W, Simonian P T, Conrad E U, 3rd. Carcinogenicity and metallic implants. Am J Orthop 1995; 24: 319–24
- Visuri T, Pukkala E, Paavolainen P, Pulkkinen P, Riska E B. Cancer risk after metal on metal and polyethylene on metal total hip arthroplasty. Clin Orthop 1996, 329: S280–9
- Vyskocil A, Viau C. Assessment of molybdenum toxicity in humans. J Appl Toxicol 1999; 19: 185–92
- Willert H G, Semlitsch M. Tissue reactions to plastic and metallic wear products of joint endoprostheses. Clin Orthop 1996, 329: 4–14