Abstract
Background In a prospective 2-year study we have used dual-energy X-ray absorptiometry to measure periprosthetic bone mineral density (BMD) following implantation of a novel, “physiological”, acetabular component designed using composite materials.
Method The acetabular components were implanted in hydroxyapatite (HA) and HA-removed options. They were implanted in conjunction with a cemented femoral component in 50 female patients who presented with displaced, subcapital, fractures of the neck of the femur. Regions of interest (ROI) were defined according to De Lee and Charnley. BMD during follow-up was compared with immediate postoperative values for the affected limb.
Results The mean precision error (CV%) was 1.01%, 2.26% and 1.12%, for ROI I, II and III respectively. The mean change in BMD, for both cups, was analyzed. There was no significant difference between the BMD changes induced with the HA- and non-HA-coated cups.
Interpretation After an initial fall in BMD in all 3 ROI at 6 months, ROI I and ROI II showed return to baseline BMD by 2 years. ROI III showed no significant decrease in BMD beyond 6 months, but did not return to baseline levels. Statistical analysis revealed no significant decrease in BMD in ROI I and ROI II at 2 years, compared with immediate postoperative values. The changes in BMD reflect a pattern of maximally reduced stress in the non-weight-bearing zone (ROI III), with preservation of bone density in weight bearing zones ROI I and ROI II. These results support the design principles of the Cambridge cup.
During human walking, load is transferred from the spine to the lower limb via the internal trabecular lamellae of bone. They pass from the sacroiliac joint through the ilium to the roof of the acetabulum, and then from the load-bearing segment of the femoral head to the principal compressive trabecular system of the proximal femur (Humphrey Citation1888, Inman Citation1947, Kummer Citation1966). At the hip joint, the load is transmitted between segments of the hyaline articular cartilage that cover the horseshoe-shaped portion of the acetabulum and the spherical part of the femoral head (Harrison et al. Citation1953, Greenwald and O'Conner Citation1971).
On each side of the joint there are zones of changing tissue stiffness; where trabecular bone merges into the subchondral plate which then supports hyaline articular cartilage. Following hip replacement, new zones of dissimilar material stiffness are created at the interfaces between prosthetic implant and host bone. The integrity of these interfaces is critical to stability of the prosthesis and successful outcome. One criterion for improving implant design and long-term component stability may be to minimize the modulus mismatch between implant and bone.
Composite materials technology offers the potential to fabricate components with mechanical properties that are closer to those of host bone (Field Citation1988). The Cambridge acetabular component is modeled to replace the cartilage and underlying subchondral bone of the horseshoe-shaped articular portion of the acetabulum (Field Citation1988) ().
The acetabular component comprises a 3-mm-thick bearing surface of ultra-high molecular weight polyethylene (UHMWPE) moulded to a 1.5-mm backing of 30% carbon flber-reinforced polybutyleneterephthalate (CFRPBT). Full details of the Cambridge cup design have been reported elsewhere (Field et al. Citation2006). It is intended that the cup should deform in concert with the surrounding acetabular bone, when loaded, so that micromotion at the bone-prosthesis interface is reduced. Distribution of stress to the acetabulum should be close to physiological, with reduction of stress shielding and bone remodeling.
Dual energy X-ray absorptiometry is an established technique for assessment of bone mineral density (BMD) in the proximal femur (Cohen and Rushton Citation1995). However, there have been relatively few reports of changes in peri-acetabular BMD following total hip arthroplasty (Korovessis et al. Citation1994, Säbo et al. Citation1998, Wilkinson et al. Citation2001) and no reports on the changing pattern of acetabular BMD with advancing age. To date, reported studies have used the De Lee and Charnley zones to define the regions of interest (ROI) for measurement of periacetabular BMD, as in conventional radiographic assessment (De Lee and Charnley Citation1976).
As with the proximal femur, periacetabular BMD has been seen to change after total hip arthroplasty. All of the studies have demonstrated an initial fall in periacetabular bone mineral density (Korovessis et al. Citation1994, Säbo et al. Citation1998, Wilkinson et al. Citation2001). Thereafter, recovery has been uneven with maximal recovery in ROI I and permanent depression of BMD in ROI II and III. These findings are attributed to rim loading of metal-backed components.
Levenston et al. (Citation1993) reported the results of simulated, stress-related, bone remodeling around conventional uncemented acetabular components using finite element analysis. The study predicted bone loss of up to 50% medial to the prosthesis, due to stress shielding. However, they predicted increases in bone density of approximately 30% in the region of the acetabular rim. Wilkinson et al. (Citation2001), using their uncemented metal backed acetabular component, confirmed an increase in BMD around the prosthetic rim and a decrease in the central pelvic zones (ROI II).
Säbo et al. (Citation1998) reported the BMD changes following implantation of a titanium-threaded acetabular component. They reported a 19% decrease in overall BMD for female patients at 6 months that persisted to 2 years. They also reported results for individual zones and showed a 17% reduction in BMD in ROI II at 2 years. However, they noted that the Mecron component has been associated with poor clinical outcomes and Säbo et al. do indicate that prolonged depression of BMD may be indicative of fixation failure.
Our hypothesis was that a cup designed to match the modulus of bone would reduce the loss of acetabular bone after implantation.
Patients and method
The Cambridge cup study was undertaken between December 1994 and May 1997, with implantation of 50 acetabular components. The patients all had a preoperative diagnosis of a displaced sub-capital femoral neck fracture suitable for treatment by implantation of a Thompson-type hemiarthroplasty. All subjects were female over the age of 70, with a mean age of 81 years at the time of implantation. Full details of the trial protocol and clinical results have been reported elsewhere (Field and Rushton Citation2005).
11 consecutive patients were selected from the study to undergo the prospective 2-year DEXA analysis. Ethical approval was obtained for the DEXA protocol prior to the study, the aim of the trial was explained to each patient and informed consent was obtained. Of the 11 cups included in the DEXA study, 5 were prepared with a plasma-sprayed coating of hydroxyapatite (HA) on the outside surface. The other 6 cups had had the HA coating removed to simulate the effects of resorption of the HA.
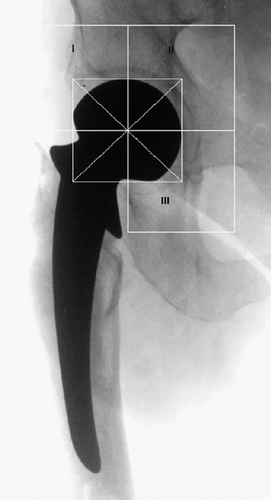
We used the Hologic QDR 1000 densitometer (Hologic Inc., Waltham, MA) in pencil beam mode to measure periprosthetic acetabular BMD. A baseline investigation was performed in the immediate postoperative period. Follow-up scans were performed at 6 weeks, 6 months, 12 months, 18 months and 2 years postoperatively. To obtain the DEXA scans, the subject was positioned supine and parallel to the table. The patient's legs were abducted and positioned using a hip positioner, with the toes pointing vertically. The scan-to-focus distance was set at 76 cm. A scout scan was performed initially to check the ROI. When this was confirmed, the appropriate zones were selected within the ROI and these regions analyzed using standard periprosthetic software, which provides a pixel size of 1 mm × 0.96 mm. With each individual scan, analysis was performed using the metal removal algorithm on the defined ROI and mean BMD was automatically calculated. We defined the regions of interest as ROI I, ROI II and ROI III using the conventional De Lee and Charnley zones (De Lee and Charnley Citation1976). The individual sizes of the regions are shown in .
Figure 2. Regions of interest (ROI) in the periacetabulum using the De Lee and Charnley (Citation1976) zones.
DEXA validation
The DEXA precision was validated using repeat measurements from a single subject. Following the baseline postoperative scan, one subject was asked to move off the scanning table and walk around the room. The subject was repositioned and a second scan was taken. The cycle was repeated to obtain a total of 5 scans.
Results
DEXA validation
Precision estimates in vivo are useful in determining whether a significant change in patient BMD has occurred. Precision estimates are usually given as the standard deviation (SD) or the percent coefficient of variation (CV%). The CV% is calculated as follows:
CV% = 100 × ((SD/√2) / mean)
Knowing the precision of a measurement assists in the interpretation of serial measurements made on one individual. The magnitude of change which must be measured to ensure that the change is real, and not simply the result of a measurement error, may be referred to as the least significant change. This represents the 95% confidence limits for the measured change.
The least significant change was calculated as follows:
Least significant change % = 2.8 × CV%
The results of DEXA validation are shown in .
Table 1. DEXA validation
Of the 11 patients, 8 had DEXA scans up to 2 years, 1 patient up to 1 year and 2 patients up to 6 months. Clinical outcomes were assessed in parallel over the 2-year period and are presented in .
Table 2. Clinical data on the 11 patients
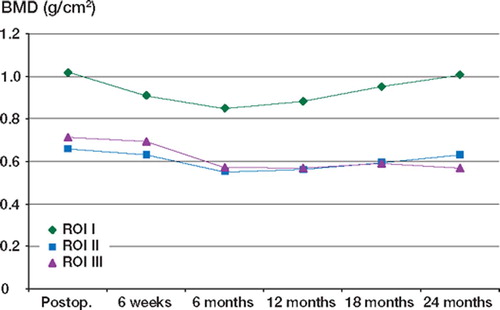
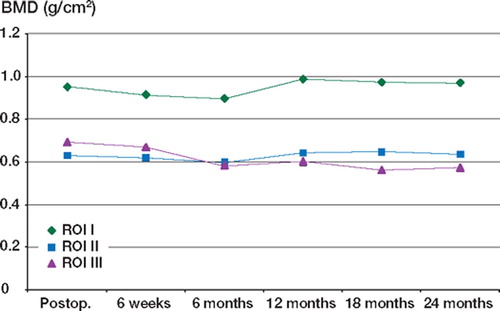
The BMD measurements, over 2 years, in the periacetabulum are presented in for HA-coated and non-HA-coated cups. The mean values are shown in and , and and for the HA and non-HA patient groups, respectively.
Table 3. Bone Mineral Density measured in the acetabulum using the HA–coated cup over 2 years
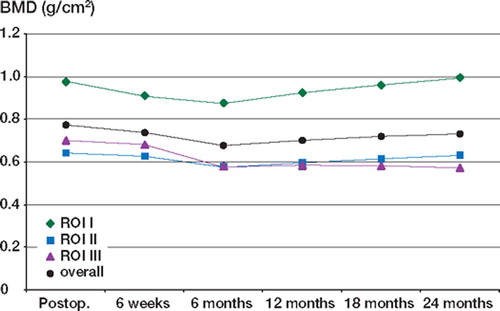
Using the least significant change as a measure of significance, we found that for both groups the BMD was reduced significantly in all 3 ROI at 6 months, but by 2 years the BMD in ROI I and ROI II had returned to the baseline level. In ROI III, the BMD continued to fall for 1 year, after which time there was no further significant decrease. The combined results for HA and non-HA patients are shown in and .
Table 4. Mean BMD for all Cambridge cups with and without HA over 2 years
When the 3 ROI were averaged to gain an overall BMD for the periacetabulum, the overall BMD fell significantly until 6 months, and then improved up to the 2-year level ( and ). At 2 years, the overall BMD was still significantly reduced, owing to the magnitude of the decrease in ROI III.
Table 5. Summary of mean per cent change in BMD changes measured with the Cambridge cup
Discussion
In our study the BMD measured during follow-up was compared with early postoperative values for the affected limb. Following our validation study, we defined our mean precision error (CV%) as 1.01%, 2.26% and 1.12% for region of interest I, II and III, respectively. These figures are comparable to those reported by Säbo et al. (Citation1998) (1.3% ± 0.9%) and slightly lower than those reported by Wilkinson et al. (Citation2001), 2.5–3.6%.
The rationale for using an early postoperative scan of the hip under study, rather than a scan of the contralateral hip or a preoperative scan of the affected hip, has been explained by Säbo et al. (Citation1998). However, all previous studies have been undertaken on patients who underwent hip replacement for degenerative joint disease. These patients have varying patterns of altered BMD, either elevated secondary to arthrosis (Nevitt et al. Citation1995), or reduced due to bone demineralization from disuse (LeBlanc et al. Citation1987, Niinimaki and Jalovaara Citation1995). Our patients underwent surgery for displaced sub-capital fracture of the femoral neck. While we recognize that these patients would have been suffering preoperative osteoporosis, subjects in our study group were all active and independently mobile before their fracture. As all regained good, independent mobility, the changes that we have observed should represent the pattern of early reduced activity during recovery from surgery and then acetabular bone remodeling in response to any distortion of loading created by the prosthetic implant.
Unlike previous studies, our patients all received components of identical size. In all cases, a 45mm modified Thompson hemiarthroplasty was implanted in conjunction with a Cambridge acetabular component with an outside diameter of 54 mm. The acetabular component was uncemented, so there was no confounding influence due to uneven permeation of cement into the periacetabular bone. These factors improved the accuracy of the automated metal-removing algorithm and minimized any component-related variation in our results.
Wilkinson et al. (Citation2001) queried the use of the 3-ROI template of De Lee and Charnley (Citation1976) and have advocated a 4-ROI template to assist in the delineation of local changes in BMD. We have also experimented with a variety of templates and can agree to some extent with the concerns expressed by Wilkinson et al. However, the 4-ROI template that they advocated only provides a single ROI across the superior aspect of the acetabular component, and this may compromise delineation of BMD changes in the main trabecular columns passing upwards from the load-bearing segment of the acetabular roof. Rather than introduce a further template for acetabular analysis, we have used the De Lee and Charnley (Citation1976) template so that our results can be compared with all previous reports.
Our findings differ from those of previous reports in several respects. Firstly, we observed reversal of an early decline in BMD across the entire load-bearing segment of the acetabulum. The mean BMD, of all cups, fell by 11.6% and 10.4% in ROI I and II respectively, at 6 months. Thereafter, recovery occurred—with an overall rise of 1.4% in ROI I and an overall fall of 1.7% in ROI II at 2 years. These figures suggest a near-physiological load transfer from acetabular component to host bone in these regions, with avoidance of the rim loading reported by other authors.
We hypothesize that the persistent decline in BMD observed in ROI III may be due in part to the horseshoe shape of the Cambridge cup. The component has no contact with the bony floor of the cotyloid fossa and may be underloading the pubic and ischial elements of the acetabular socket, which are adjacent to the extremities of the horseshoe. In these regions the polyethylene bearing has been relieved, to avoid binding with the head of the femoral component. To date, we have not identified any clinical, radiographic or post-mortem features that can be correlated with this decline.
The concept of a flexible acetabular component remains attractive, and further clinical experience will be required to evaluate the long-term performance of such devices in patients undergoing total hip arthroplasty for degenerative joint disease.
The authors were responsible for the conception and design of the study, the collection, analysis and interpretation of the data, and the writing of the manuscript. Progress on the project was reported to Howmedica and subsequently Stryker. The companies reimbursed the authors for the costs that they incurred to attend these meetings and also paid developmental consultancy fees to Field and Rushton for work undertaken on the successor of the Cambridge Cup (currently known as the MITCH PCR cup). Neither company influenced any of the steps detailed in the preparation of our paper.
Contributions of authors
REF implant designer, implanting surgeon, study design, manuscript preparation. MDC data analysis and manuscript preparation. PJS study, design, patient scanning, data analysis and manuscript preparation. CB study design and patient scanning. NR implant designer, study design and manuscript preparation
- Cohen B, Rushton N. Accuracy of DEXA measurement of bone mineral density after total hip arthroplasty. J Bone Joint Surg (Br) 1995; 77: 479–83
- De Lee J G, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop 1976, 12: 20–32
- Field R E. The design and preliminary evaluation of a femoral capital epiphyseal replacement arthroplasty. 1988, PhD Thesis, University of Cambridge
- Field R E, Rushton N. 5 year results of the Cambridge acetabular cup. Submitted to J Bone Joint Surg (Br) 2005
- Field R E, Jones E, Nuijten P, Storer A, Cronin M D, Rushton N. Preclinical testing of the Cambridge Cup. Journal of Materials Science July, 2006, Materials in Medicine. Acc
- Greenwald A S, O'Conner J J. The transmission of load through the human hip joint. J Biomech 1971; 4: 439–41
- Harrison M H M., Schajowicz F, Trueta J. Osteoarthritis of the hip: A study of the nature and evolution of the disease. J Bone Joint Surg (Br) 1953; 35: 598–626
- Humphry G M. The angle of the neck of the thigh bone with the shaft at various ages and under various circumstances. Lancet 1888; 2: 971–2
- Inman V T. Functional aspects of the abductor muscles of the hip. J Bone Joint Surg (Br) 1947; 29: 607–19
- Korovessis P, Piperos G, Michael A. Periprosthetic bone mineral density after Mueller and Zweymueller total hip arthroplasties. Clin Orthop 1994, 309: 214–21
- Kummer B. Photoelestic studies on the functional structure of bone. Folia Biotheoretica 1966; 6: 31–40
- LeBlanc A, Schneider V, Krebs J, Evans H, Ajhingran S, Johnson P. Spinal bone density after five weeks of bed rest. Calcif Tissue Int 1987; 41: 259–61
- Levenston M E, Beaupre G S, Schurman D J, Carter D R. Computer simulations of stress-related bone remodelling around noncemented acetabular components. J Arthroplasty 1993; 8: 595–605
- Nevitt M C, Lane N E, Scott J C, Hochberg M C, Pressman A R, Geneant H K, Cummings S R. Radiographic osteoarthritis of the hip and bone mineral density. Arthitis Rheum 1995; 38: 907–16
- Niinimäki T, Jalovaara P. Bone loss from the proximal femur after arthroplasty with an isoelastic femoral stem. Acta Orthop Scand 1995; 66: 347–51
- Säbo D, Reiter A, Simank H G, Thompson M, Lukoschek M, Ewerbeck V. Study and cross-sectional study on titanium threaded acetabular cup and cementless Spotorno Stem with DEXA. Calcif Tissue Int (USA) 1998; 62(2)177–82
- Wilkinson J M, Peel N F A., Elson A, Stockley I, Eastell R. Measuring bone mineral density of the pelvis and proximal femur after total hip arthroplasty. J Bone Joint Surg (Br) 2001; 83: 283–8