Abstract
Background Osteogenic growth factors have been suggested to enhance the fixation of implants used in joint replacement. We examined the effect of locally delivered transforming growth factor-β1 and insulin-like growth factor-1 in a biodegradable poly (D, L-lactide) coating.
Material and methods In a paired study using 9 dogs, unloaded titanium implants surrounded by a 1-mm gap were inserted into the proximal humerus. The growth factors were incorporated in a poly (D, L-lactide) coating at a 1% (w/w) ratio of TGF-β1 and a 5% (w/w) ratio of IGF-1. Control implants were uncoated. After 4 weeks, the implants were evaluated by mechanical push-out test and by histomorphometry.
Results A twofold increase was seen in mechanical fixation (strength, stiffness, energy absorption) for the growth factor-treated implants (p = 0.04). Similar results were seen in histomorphometry, as bone ongrowth was 2.5 times higher (p = 0.02), and gap healing was 30–110% higher (p = 0.04) for the growth factor-treated implants than for the control implants. Ongrowth of fibrous tissue was eliminated by the treatment.
Interpretation TGF-beta-1 and IGF-1, locally delivered in a biodegradable poly(D,L-lactide) coating, enhance the mechanical fixation and osseointegration of titanium implants in cancellous bone, and no fibrous tissue is produced in the growth factor treated implants.
Cementless total hip replacements (THRs) require a rapid bony integration to ensure long-term fixation. This is even more important in the revision setting, where bone stock is diminished and healing potential reduced compared with primary surgery (Glassman et al. Citation1987, Engh et al. Citation1988, Engelbrecht et al.Citation1990, Nicolas et al.Citation1994, An et al. Citation2000).
One strategy for improving osseointegration is the administration of osteogenic/osteotropic growth factors. Applications employing one growth factor have been extensively investigated (Aspenberg et al. Citation1989, Thaller et al. Citation1993, Lind et al. Citation1996b, Zellin et al. Citation1998, Laffargue et al. Citation2000, Tielinen et al. Citation2001, Soballe et al. Citation2004). Applications combining two or more growth factors may be more favorable, due to their additive or synergistic effects on bone formation. Transforming growth factor beta 1 (TGF-β1) and insulin-like growth factor 1 (IGF-1) are of special interest as they are highly expressed during bone growth (Dequeker et al. Citation1993, Nicolas et al. Citation1994, Lammens et al. Citation1998, Pfeilschifter et al. Citation1999, Eingartner et al. Citation1999, Kveiborg et al. Citation2001).
To minimize potential systemic side effects, the growth factors should be delivered locally. A controlled release system should prevent washing of growth factors off the implant by bleeding. Applications used in other studies for local growth factor delivery have included growth factor adsorption to calcium phosphates, mixtures of calcium phosphates and collagen, collagen sponges, hydro-gels, immobilization of growth factors on implant surfaces, and use of various polymers (Lee et al. Citation2000, Park et al. Citation2000, Vehof et al. Citation2002, Barboza et al. Citation2004, Rose et al. Citation2004, Ito et al. Citation2005). Lactide-based polymers such as poly (D, L-Lactide) (PDLLA) have been used clinically for decades as material for screws and plates, and PDLLA has been used successfully as a drug carrier (Nicolas et al. Citation1994, An et al. Citation2000, Schmidmaier et al. Citation2001b, Rose et al. Citation2004). A few cases of inflammatory reactions have been seen, but only with bulky-design PDLLA implants (Bostman Citation1998). The lactic acid released from the PDLLA coating by hydrolysis is metabolized via the TCA cycle to carbon dioxide and water. The combination of TGF-β1 and IGF-1 in PDLLA has not been investigated previously in an implant model. We hypothesized that the combination of TGF-β1 and IGF-1 in PDLLA would enhance the osseointegration and mechanical fixation of unloaded cylindrical porous-coated titanium implants surrounded by a gap.
Material and methods
Implants and coating technique
We used cylindrical plasma-sprayed porous-coated implants made of titanium alloy (Ti-6Al-4V) with a nominal diameter of 6.0 mm and length of 10.0 mm (Biomet Inc., Warsaw, IN).
TGF-β1 and IGF-1 (R&D Systems, UK) were dissolved in a poly (D, L-lactide) (PDLLA) -Resomer 203 (Boehringer Ingelheim GmbH, Germany) and ethyl acetate solution, resulting in a 1% (w/w) ratio of TGF-β1 and 5% (w/w) ratio of IGF-1. The implants were dipped twice in the solution and air-dried, all under sterile conditions. Based on coating experiments, where the implants where weighed before and after coating, an estimated 140 μg IGF-1 and 28 μg TGF-β1 would be incorporated into the PDLLA coating of each implant.
Animals and surgical procedure
A controlled animal study was carried out. The study was approved by the local committee for animal care and use. Implants were inserted into cancellous bone sites in the proximal humerus under general anesthesia, using sterile technique. Unloaded implants with a circumferential 1.0-mm gap were inserted bilaterally in 9 skeletally mature mongrel dogs (25–27 kg). The study design was paired with insertion of growth factor-coated implants on one side, and identical control implants without PDLLA or growth factors on the contralateral side.
The implantation site was exposed through a lateral approach, going through a cleavage in the deltoid muscle. The periost was removed only at the site of drilling, just distal to the minor tubercle. A guide wire was inserted, and then an 8.0-mm cannulated drill was used to prepare the hole to receive the implant. Drilling was performed at approximately 2 rotations per second, to prevent thermal trauma to the bone. The implantation site was lavaged using isotonic saline prior to insertion of the implant. Bottom and top washers, 8.0 mm in diameter, were attached to stabilize the implants and prevent soft tissue ingrowth, and the implants were inserted. After insertion, the overlying soft tissue was closed in layers. During surgery, 750 mg of Cefuroxim was administered intravenously. After 4 weeks of observation, the animals were killed and the specimens harvested. Cultures were taken from all implantation sites.
Specimen preparation
Pending preparation, the proximal humerus was stored at –20°C. The bone-implant specimens were cut on a water-cooled diamond band saw (Exact Appartebau, Germany) leaving two transverse sections. The outermost section of approximately 3.5 mm was used for mechanical testing. The remaining section was sectioned for histomorphometric analysis.
Mechanical testing
Implants were tested to failure in shear by a push-out test on an Instron Universal Test Machine (Model 4302; Instron, UK). The specimens were placed on a metal support jig with a 7.4-mm circular opening. A preload of 2 N was applied, to ensure contact with the implant. The displacement rate was 5.0 mm/min. Maximum shear strength (MPa), apparent shear stiffness (MPa/mm), and energy to failure (J/m2) were calculated from the recorded load-displacement data.
Histological evaluation
The specimens were dehydrated in graded ethanol (70–100%) containing 0.4% basic fuchsine, and embedded in methylmethacrylate. After sectioning, they were counterstained with 2% light green. The preparation method allows distinction between mineralized bone, fibrous tissue and bone marrow (Gotfredsen et al. Citation1989). The embedded specimens with the implant in situ were randomly rotated around the vertical axis of the implant. In the central part of implants, 4 serial sections of 15–20 μm were produced using a Leiden microtome (Leiden, Holland) (Overgaard et al. Citation2000, Schmidmaier et al. Citation2001b) Histomorphometry was performed in a blinded fashion using an image analysis system (CAST-Grid; Olympus, Denmark). Quantification of tissue was performed using stereological principles (Gundersen et al. Citation1988).
Tissue ongrowth was defined as tissue in direct contact with the implant surface, and was determined using the cycloid intercepting technique. The number of intersections with tissue in contact with the implant surface was counted in successive adjacent fields at the tissue-implant interface. The gaps were divided into inner (0–480 μm) and outer (480– 960 μm) zones and tissue volumes in the two zones were determined by the point-counting technique.
Statistics
Statistical analysis was performed using Intercooled Stata version 8.0 software (StataCorp,College station, TX). The difference between pairs did not follow a normal distribution, as indicated by the qq-plot. Thus, a paired non-parametric analysis was applied (Wilcoxon matched-pairs signed-ranks test). All results are presented as median and range. P-values less than 0.05 were considered significant.
Results
All dogs completed the study without complications. Culture swabs from implantation sites were all negative. At the time of necropsy, there were no clinical signs of infection or inflammation.
Mechanical fixation
8 of 9 treated implants had higher values in strength, stiffness, and energy than their controls (p = 0.04) (; ). There was a marked difference in the variance of the controls compared to the treated implants for all three parameters. The effect of the growth factors on fixation was relatively larger in animals in which the controls were poorly fixated.
Figure 1. Plot of the paired data for maximum shear strength. Lines connect the data pairs corresponding to each dog. The graphs for energy to failure and apparent shear stiffness were similar. *(p = 0.04).
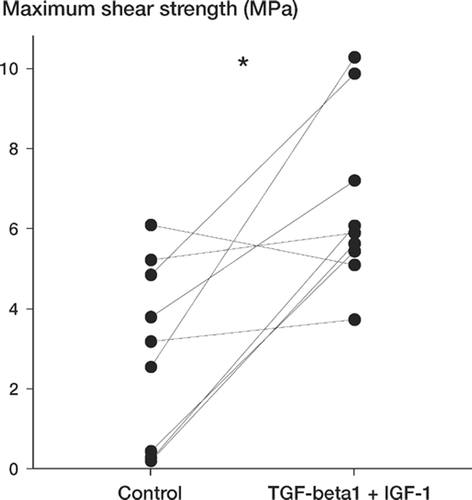
Table 1. Results from push-out test presented as median and range
Histomorphometry
There was a 2.5 fold median increase in bone ongrowth (p = 0.02), a 30% median increase in inner zone gap healing (p = 0.04), and a 112% median increase in outer zone gap healing (p = 0.02) (; ). There was practically no fibrous tissue on the treated implants (ongrowth, p = 0.01; inner zone gap healing, p = 0.01). In the control implants there was a significant difference between the healing of the inner and outer zones (p = 0.02), while there was no difference between the healing of the inner and outer zones in the growth factor-treated implants (p = 1) ().
Figure 2. A. Illustration of an uncoated control implant. Adjacent to the implant (black) there is a thick rim of fibrous tissue (red, arranged longitudinally), and there is very little bone (green) in the gap. B. Illustration of a growth factor-treated implant. There is abundant bone (green) on the implant surface, and no fibrous tissue.
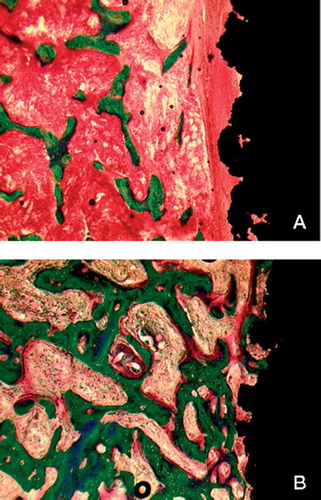
Table 2. Histomorphometric results. Tissue ongrowth results presented as median and range
Table 3. Histomorphometric results. Gap healing presented as median and range. Data on gap healing are presented as percentage of total count in the gap
Discussion
The formation of gaps around cementless implants is unavoidable. Some authors have reported that as little as 10–20% of a press-fit inserted prosthesis is in contact with the bone (Noble et al. Citation1988, Schimmel and Huiskes Citation1988, Geesink Citation2002). Our previous work with gap implants has consistently shown abundant ongrowth of fibrous tissue, inferior bony ongrowth, and poor mechanical fixation (Soballe et al. Citation1990, Elmengaard et al. Citation2005). In previous studies, the titanium implant surface has been shown to be similar to that of commercially available prostheses with a plasma spray titanium coating (Soballe et al. Citation1992, Citation1993). We hypothesized that the combination of TGF-β1 and IGF1 in PDLLA would enhance the osseointegration and mechanical fixation of unloaded cylindrical porous-coated titanium implants surrounded by a gap.
We found a significant effect of the combination of growth factors on all mechanical parameters. This was in accordance with the histomorphometric results. We saw a greater effect at the implant-tissue interface than in the surrounding gap. Bone ongrowth was generally 2–3 times higher for treated implants, while ongrowth of fibrous tissue was eliminated. In the gap, the major effect was a higher bone ingrowth for the treated implants. We used a combination of TGF-β1 and IGF-1, as a synergetic or additive effect on fracture healing has been demonstrated when combining the two growth factors as opposed to administration of either growth factor alone (Schmidmaier et al. Citation2003).
Previous studies using a similar implant model in the dog have reported varied results with regard to the effect of TGF-β1 on implant fixation. TGF-β1 adsorbed to a hydroxyapatite coating only increased bone ongrowth by one-third, while no improvement was seen in gap healing and mechanical fixation compared to the control implants (Lind et al. Citation1996a) When TGF-β1 was adsorbed to a tricalcium phosphate coating, bone ongrowth and gap healing was increased by 50% (Lind et al. Citation1996b).
For IGF-1, positive effects have been shown on obliteration of rabbit femoral defects and rat calvarial defects (Thaller et al. Citation1993, Laffargue et al. Citation2000). In a titanium chamber in a rabbit tibia, IGF1 had the effect of reducing the turnover rate of minerals, and did not change the amount of bone mineral (Aspenberg et al. Citation1989). The combination of TGF-β1 and IGF-I has been investigated in a rat fracture model and a sheep spinal fusion model (Kandziora et al. Citation2003, Schmidmaier et al. Citation2003). In both models, the combination enhances healing and mechanical strength. In a long-term study in a rat fracture model, this combination of growth factors in PDLLA accelerated fracture healing and the recovery of mechanical strength, while the long-term results for the treated group and the control group were equal. The authors conclude that this strongly indicates that this growth factor treatment does not alter the natural healing process, but only accelerates it (Schmidmaier et al. Citation2004).
We used a PDLLA coating to deliver growth factors into the gap locally. Several studies have demonstrated that PDLLA is a suitable delivery vehicle for growth factors in both fracture healing and spinal fusion studies (Schmidmaier et al. Citation2001a,Citationb). In a saline dilution model using the same coating process, PDLLA released approximately half of the growth factors within 48 h and another 25% within 6 weeks (Schmidmaier et al. Citation2001b). Although the in vivo release pattern may be accelerated, we can expect that the growth factors were indeed released to the surrounding gap.
This study did not include control implants with a PDLLA coating; thus, we cannot conclude that the positive effects were due to the growth factors alone. However, we do not expect the PDLLA coating to have had a bone stimulating effect in our model, as preliminary results from another experimental study performed at our institution have indicated that the PDLLA coating does not alter the bony integration of similar titanium implants compared to uncoated samples. This contrasts with the rat model, where a stimulating effect has been observed.
IGF-1 and TGF-β1 were administrated in 5% and 1% (w/w) ratios of the PDLLA coating, respectively. These w/w ratios have worked in several animal models (Kandziora et al. Citation2003). Our method of administration is more advantageous than the adsorption of a certain amount of growth factor to a ceramic surface, as the dose is automatically adjusted for the size of implant, and an even distribution of growth factors is easier to obtain.
The possibility of adverse effects of the PDLLA coating must also be taken into consideration. In the present study there was sporadic presence of giant cells in all PDLLA-coated implants. Our thick histological sections did not permit quantification of cells; we can only state that they existed. The long-term consequence of the presence of these giant cells is unknown. However, the finding of giant cells on implanted polymers is common (Paivarinta et al. Citation1993, Coonts et al. Citation1998, Wildemann et al. Citation2005).
In conclusion, the combination of TGF-β1 and IGF-1 in PDLLA showed some very promising results in the present study—as all the parameters examined were improved by the treatment. The finding of more bone and a dramatic reduction in the amount of fibrous tissue on the treated implants provides far better conditions for the continued osseointegration of the implant. The combination of TGF-β1 and IGF-1 in PDLLA appears to be so powerful that it should be compared with hydroxyapatite, a surface coating that is already widely used for clinical purposes.
The authors would like to thank Britt Wildemann for her help in coating the implants, and Anette Milton, Jane Pauli and Feng Ya Mei for technical assistance. Niels Trolle Andersen from the Department of Biostatistics, University of Aarhus, provided invaluable help on the statistics. Financial support was given by the Interdisciplinary Research Group, Nanoscience and Biocompatibility, the Danish Research Agency (grant no. 2052-01-0049), the Danish Rheumatism Association, the Kurt Bønnelycke and Hustru Grethe Bønnelycke Fund, and the Max and Anna Friedmann Legat til Sygdomsbekæmpelse. Biomet Inc. donated the implants used in the study.
Contributions of authors
AL protocol, surgery, specimen preparation and analysis, and writing of manuscript. BE protocol, surgery and revision of manuscript. GS protocol and revision of manuscript. KS protocol and revision of manuscript.
- An Y H, Woolf S K, Friedman R J. Pre-clinical in vivo evaluation of orthopaedic bioabsorbable devices. Biomaterials 2000; 21: 2635–52
- Aspenberg P, Albrektsson T, Thorngren K G. Local application of growth-factor IGF-1 to healing bone. Experiments with a titanium chamber in rabbits. Acta Orthop Scand 1989; 60: 607–10
- Barboza E P, Caula A L, Caula F O, de Souza R O, Geolas N L, Sorensen R G, Li X J, Wikesjo U M. Effect of recombinant human bone morphogenetic protein-2 in an absorbable collagen sponge with space-providing biomaterials on the augmentation of chronic alveolar ridge defects. J Periodontol 2004; 75: 702–8
- Bostman O M. Osteoarthritis of the ankle after foreign-body reaction to absorbable pins and screws: a three- to nine-year follow-up study. J Bone Joint Surg (Br) 1998; 80: 333–8
- Coonts B A, Whitman S L, O'Donnell M, Polson A M, Bogle G, Garrett S, Swanbom D D, Fulfs J C, Rodgers P W, Southard G L, Dunn R L. Biodegradation and biocompatibility of a guided tissue regeneration barrier membrane formed from a liquid polymer material. J Biomed Mater Res 1998; 42: 303–11
- Dequeker J, Mohan S, Finkelman R D, Aerssens J, Baylink D J. Generalized osteoarthritis associated with increased insulin-like growth factor types I and II and transforming growth factor beta in cortical bone from the iliac crest. Possible mechanism of increased bone density and protection against osteoporosis. Arthritis Rheum 1993; 36: 1702–18
- Eingartner C, Coerper S, Fritz J, Gaissmaier C, Koveker G, Weise K. Growth factors in distraction osteogenesis. Immuno-histological pattern of TGF-beta1 and IGF-I in human callus induced by distraction osteogenesis. Int Orthop 1999; 23: 253–9
- Elmengaard B, Bechtold J E, Soballe K. In vivo effects of RGD-coated titanium implants inserted in two bone-gap models. J Biomed Mater Res A 2005; 75(2)249–55
- Engelbrecht D J, Weber F A, Sweet M B, Jakim I. Long-term results of revision total hip arthroplasty. J Bone Joint Surg (Br) 1990; 72: 41–5
- Engh C A, Glassman A H, Griffin W L, Mayer J G. Results of cementless revision for failed cemented total hip arthroplasty. Clin Orthop 1988, 235: 91–110
- Geesink R G. Osteoconductive coatings for total joint arthroplasty. Clin Orthop 2002, 395: 53–65
- Glassman A H, Engh C A, Bobyn J D. Proximal femoral osteotomy as an adjunct in cementless revision total hip arthroplasty. J Arthroplasty 1987; 2: 47–63
- Gotfredsen K, Budtz-Jorgensen E, Jensen L N. A method for preparing and staining histological sections containing titanium implants for light microscopy. Stain Technol 1989; 64: 121–7
- Gundersen H J, Bendtsen T F, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard J R, Pakkenberg B, Sorensen F B, Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 1988; 96: 379–94
- Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater J J, Carmouche J, Zhang X, Rubery P T, Rabinowitz J, Samulski R J, Nakamura T, Soballe K, O'Keefe R J, Boyce B F, Schwarz E M. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med 2005; 11: 291–7
- Kandziora F, Pflugmacher R, Scholz M, Schafer J, Schollmeier G, Schmidmaier G, Duda G, Raschke M, Haas N P. Dose-dependent effects of combined IGF-I and TGFbeta1 application in a sheep cervical spine fusion model. Eur Spine J 2003; 12: 464–73
- Kveiborg M, Flyvbjerg A, Eriksen E F, Kassem M. Transforming growth factor-beta1 stimulates the production of insulin-like growth factor-I and insulin-like growth factor-binding protein-3 in human bone marrow stromal osteoblast progenitors. J Endocrinol 2001; 169: 549–61
- Laffargue P, Fialdes P, Frayssinet P, Rtaimate M, Hildebrand H F, Marchandise X. Adsorption and release of insulin-like growth factor-I on porous tricalcium phosphate implant. J Biomed Mater Res 2000; 49: 415–21
- Lammens J, Liu Z, Aerssens J, Dequeker J, Fabry G. Distraction bone healing versus osteotomy healing: a comparative biochemical analysis. J Bone Miner Res 1998; 13: 279–86
- Lee Y M, Park Y J, Lee S J, Ku Y, Han S B, Klokkevold P R, Chung C P. The bone regenerative effect of platelet-derived growth factor-BB delivered with a chitosan/tricalcium phosphate sponge carrier. J Periodontol 2000; 71: 418–24
- Lind M, Overgaard S, Nguyen T, Ongpipattanakul B, Bunger C, Soballe K. Transforming growth factor-beta stimulates bone ongrowth. Hydroxyapatite-coated implants studied in dogs. Acta Orthop Scand 1996a; 67: 611–6
- Lind M, Overgaard S, Soballe K, Nguyen T, Ongpipattanakul B, Bunger C. Transforming growth factor-beta 1 enhances bone healing to unloaded tricalcium phosphate coated implants: an experimental study in dogs. J Orthop Res 1996b; 14: 343–50
- Nicolas V, Prewett A, Bettica P, Mohan S, Finkelman R D, Baylink D J, Farley J R. Age-related decreases in insulin-like growth factor-I and transforming growth factor-beta in femoral cortical bone from both men and women: implications for bone loss with aging. J Clin Endocrinol Metab 1994; 78: 1011–6
- Noble P C, Alexander J W, Lindahl L J, Yew D T, Granberry W M, Tullos H S. The anatomic basis of femoral component design. Clin Orthop 1988, 235: 148–65
- Overgaard S, Soballe K, Gundersen HJ. Efficiency of systematic sampling in histomorphometric bone research illustrated by hydroxyapatite-coated implants: optimizing the stereological vertical-section design. J Orthop Res 2000; 18: 313–21
- Paivarinta U, Bostman O, Majola A, Toivonen T, Tormala P, Rokkanen P. Intraosseous cellular response to biodegradable fracture fixation screws made of polyglycolide or polylactide. Arch Orthop Trauma Surg 1993; 112: 71–4
- Park Y J, Lee Y M, Lee J Y, Seol Y J, Chung C P, Lee S J. Controlled release of platelet-derived growth factor-BB from chondroitin sulfate-chitosan sponge for guided bone regeneration. J Control Release 2000; 67: 385–94
- Pfeilschifter J, Erdmann J, Storch S, Ziegler R, Weinreb M. Changes in the concentration of insulin-like growth factor I and transforming growth factor beta1 in rat femoral bone during growth. Calcif Tissue Int 1999; 64: 78–82
- Rose F R, Hou Q, Oreffo R O. Delivery systems for bone growth factors – the new players in skeletal regeneration. J Pharm Pharmacol 2004; 56: 415–27
- Schimmel J W, Huiskes R. Primary fit of the Lord cementless total hip. A geometric study in cadavers. Acta Orthop Scand 1988; 59: 638–42
- Schmidmaier G, Wildemann B, Bail H, Lucke M, Fuchs T, Stemberger A, Flyvbjerg A, Haas N P, Raschke M. Local application of growth factors (insulin-like growth factor-1 and transforming growth factor-beta1) from a biodegradable poly(D,L-lactide) coating of osteosynthetic implants accelerates fracture healing in rats. Bone 2001a; 28: 341–50
- Schmidmaier G, Wildemann B, Stemberger A, Haas N P, Raschke M. Biodegradable poly(D,L-lactide) coating of implants for continuous release of growth factors. J Biomed Mater Res 2001b; 58: 449–55
- Schmidmaier G, Wildemann B, Gabelein T, Heeger J, Kandziora F, Haas N P, Raschke M. Synergistic effect of IGFI and TGF-beta1 on fracture healing in rats: single versus combined application of IGF-I and TGF-beta1. Acta Orthop Scand 2003; 74: 604–10
- Schmidmaier G, Wildemann B, Ostapowicz D, Kandziora F, Stange R, Haas N P, Raschke M. Long-term effects of local growth factor (IGF-I and TGF-beta 1) treatment on fracture healing. A safety study for using growth factors. J Orthop Res 2004; 22: 514–9
- Soballe K, Hansen E S, Brockstedt-Rasmussen H, Pedersen C M, Bunger C. Hydroxyapatite coating enhances fixation of porous coated implants. A comparison in dogs between press fit and noninterference fit. Acta Orthop Scand 1990; 61: 299–306
- Soballe K, Hansen E S, Rasmussen H, Jorgensen P H, Bunger C. Tissue ingrowth into titanium and hydroxyapatite-coated implants during stable and unstable mechanical conditions. J Orthop Res 1992; 10: 285–99
- Soballe K, Hansen E S, Brockstedt-Rasmussen H, Bunger C. Hydroxyapatite coating converts fibrous tissue to bone around loaded implants. J Bone Joint Surg (Br) 1993; 75: 270–8
- Soballe K, Jensen T B, Mouzin O, Kidder L, Bechtold J E. Differential effect of a bone morphogenetic protein-7 (OP-1) on primary and revision loaded, stable implants with allograft. J Biomed Mater Res A 2004; 71: 569–76
- Thaller S R, Dart A, Tesluk H. The effects of insulin-like growth factor-1 on critical-size calvarial defects in Sprague-Dawley rats. Ann Plast Surg 1993; 31: 429–33
- Tielinen L, Manninen M, Puolakkainen P, Kellomaki M, Tormala P, Rich J, Seppala J, Rokkanen P. Inability of transforming growth factor-beta 1, combined with a bio-absorbable polymer paste, to promote healing of bone defects in the rat distal femur. Arch Orthop Trauma Surg 2001; 121: 191–6
- Vehof J W, Haus M T, de Ruijter A E, Spauwen P H, Jansen J A. Bone formation in transforming growth factor beta-I-loaded titanium fiber mesh implants. Clin Oral Implants Res 2002; 13: 94–102
- Wildemann B, Sander A, Schwabe P, Lucke M, Stockle U, Raschke M, Haas N P, Schmidmaier G. Short term in vivo biocompatibility testing of biodegradable poly(D,L-lactide)-growth factor coating for orthopaedic implants. Biomaterials 2005; 26: 4035–40
- Zellin G, Beck S, Hardwick R, Linde A. Opposite effects of recombinant human transforming growth factor-beta 1 on bone regeneration in vivo: effects of exclusion of periosteal cells by microporous membrane. Bone 1998; 22: 613–20