Abstract
Background Mechanical stimulation improves the repair of ruptured tendons. Injection of a platelet concentrate (platelet-rich plasma, PRP) can also improve repair in several animal models. In a rat Achilles tendon transection model, 1 postoperative injection resulted in increased strength after 4 weeks. Considering the short half-lives of factors released by platelets, this very late effect calls for an explanation.
Methods We studied the effects of platelets on Achilles tendon regenerates in rats 3, 5 and 14 days after transection. The tendons were either unloaded by Botulinum toxin A (Botox) injections into the calf muscles, or mechanically stimulated in activity cages. No Botox injections and ordinary cages, respectively, served as controls. Repair was evaluated by tensile testing.
Results At 14 days, unloading (with Botox) abolished any effect of the platelets and reduced the mechanical properties of the repair tissue to less than half of normal. Thus, some mechanical stimulation is a prerequisite for the effect of platelets at 14 days. Without Botox, both activity and platelets increased repair independently of each other. However, at 3 and 5 days, platelets improved the mechanical properties in Botox-treated rats.
Interpretation Platelets influence only the early phases of regeneration, but this allows mechanical stimulation to start driving neo-tendon development at an earlier time point, which kept it constantly ahead of the controls.
Subcutaneous tendon repair starts with an organizing hematoma and ends with a remodeled tendon-like structure. Mechanical loading stimulates this development. Yet, mechanical stimulation is seldom used clinically in non-synovial tendon repair, obviously because of the fear of overloading and distracting the tendon. Other methods of stimulating repair are therefore called for. One possibility is the injection of growth factors or of a platelet concentrate into the hematoma. (Kurtz et al. Citation1999, Aspenberg and Forslund Citation2000, Forslund Citation2003, Zhang et al. Citation2003, Aspenberg and Virchenko Citation2004, Virchenko et al. Citation2005,) In rat and rabbit models, this increases tendon regenerate strength by one or two thirds, at a stage when the tendon is about half-healed. Remarkably, a single postoperative platelet injection in a loaded rat model causes improved material properties and histological maturity of the tendon regenerate as late as 3 weeks after the transection, well into the remodeling stage of repair (Aspenberg and Virchenko Citation2004). This is a remarkable effect, and it appears counter-intuitive that short-lived growth factors, released from the platelets into the hematoma, should influence the properties of the remodeling tendon regenerate after such a long time. In order to understand this phenomenon, we varied the mechanical conditions during repair, with the idea that some kind of interplay between the effects of mechanics and platelets might explain it.
Because the enigmatic effect was seen after a matter of weeks, we started by analyzing the 2week time point. Our first goal was to see whether the effects of the platelet injection would disappear if mechanical stimulation were eliminated. We then went on to study the effects of increased loading. The results of these experiments then led us to hypothesize that mechanical stimulation starts first when healing has reached a threshold level, and that a platelet injection makes the early callus reach this level at an earlier time point. This would explain how a short-lived effect of the platelets could have long-term consequences. If this hypothesis is correct, there would be an early effect of platelets in unloaded tendons also, although it would not last. We finally tested whether this was the case.
Animals and methods
Overview of experiments
We used 130 Sprague Dawley rats. Platelet concentrate was prepared from the blood of 10 rats that were killed. Normally there were 10 rats in each treatment group. All experiments included tendon transection with spontaneous healing and were evaluated mechanically. All experiments except the final one were evaluated after 14 days. Firstly, 20 rats with unloading by Botox-induced paralysis of the calf muscles were randomized to receiving either the platelet or control injection. Then 20 rats were randomized to increased loading in activity cages or the control treatment (normal cages). We then repeated this experiment with addition of groups that received Botox and were randomized to activity cages or control. Finally, rats were randomized to platelet treatment or control (no treatment), and evaluated after either 3 or 5 days.
The study was approved by the regional animal ethics committee.
Botulinum toxin A injections
The rats were anesthetized with 5% Isoflurane gas (Forene; Abbot Scandinavia, Solna, Sweden) in an anesthetic induction chamber and then with 3.5% Isoflurane in a mask. The skin on the right hind limb was shaved. The Botulinum toxin A (Botox, Allergan) was reconstituted in sterile saline to a final concentration of 50 U/mL before each use. The right hind leg was stretched backwards and 1 U (0.4 ng) of Botox was slowly injected into each of the lateral and medial heads, the gastrocnemius and the soleus (total dose 3 U). The injections lasted 1 min per muscle. Botox is a highly specific inhibitor of acetylcholine release in the neuromuscular endplate, and should have no effects on the tendon other than from the absence of muscle contraction. The dose, volume and method of administration of the Botox was established in pilot studies to result in obvious muscle paralysis but to cause only a small degree of inhibition of locomotory ability and no systemic changes, such as reduction of body mass.
Activity cages
The activity cages were manufactured by the workshop of our hospital. The cages consist of two floors connected by a narrow passage. The rats had nest boxes on the ground floor, from which they could climb up to the first floor, which was constructed as a maze. In two corners of the maze, food pellets were placed. Sawdust was spread both in the maze and on the ground floor and water was provided ad libitum on the ground floor (). In a study on the behavior of untreated rats in this environment, the rats spent 75% of their time in the maze, and used less time for sleeping and grooming, which suggests increased physical activity (Carlsson 2005). The standard cages were 40 × 25 cm plastic containers with a metal grating above.
Platelet concentrate and platelet gel preparation
Whole blood was collected from 10 female Sprague Dawley rats (200 g; M&B, Ry, Denmark). The rats were anesthetized with Isoflurane, and 4–6 mL of whole blood was collected by cardiac puncture using a 10 mL syringe containing 1.5 mL anticoagulant citrate phosphonate dextrose (CPD) buffer (0.15 mg CPD/mL), and a 1.2 mm needle. After blood collection, the animals were killed with CO2 gas. The blood was then centrifuged at 220 × g for 20 minutes. The supernatant, containing platelet-rich plasma, was used for a second centrifugation at 480 × g for 20 min to form a pellet of rat platelets. The platelets were then resuspended in plasma and the cell density was adjusted to 8.3 × 109 platelets/L. This preparation was used for platelet injections.
For platelet gel preparation, the same procedure was used and then the platelet concentrate was dispersed in 20 microwells, 50 μL per well, and activated by adding 0.25 U thrombin from bovine plasma (Sigma, St. Louis, MO) and 10% calcium chloride (Braun Melsungen; 1000 IE/mL CaCl2–2sg) at 37°C 4 mM. Both the platelet concentrate and the platelet gel were stored at 4°C for a maximum time of 24 h and were then applied to the transsected Achilles tendons. The two different platelet preparations were used in this work because of a planning error. However, they appear to be equally efficacious in their effect on the mechanical properties of tendon at 14 days (data not shown; submitted for publication).
Operating procedure and treatment
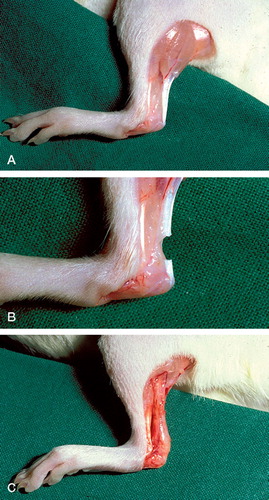
The rats were anesthetized with 5% Isoflurane gas (Forene, Abbot Scandinavia, Solna, Sweden) in an anesthetic induction chamber and then with 3.5% Isoflurane in a mask. The skin on the right hind limb was shaved. 5-mg intramuscular injections of tetracycline (Engemycin; Intervet, Boxmeer, Holland), and analgesics in the form of 0.015 mg buprenorphine (Temgesic; Schering-Plough, Brussels, Belgium) were given preoperatively. The animal was placed prone on a warm pad (38.2°C) and the right hind leg was stretched backwards and washed with chlorhexidine ethanol. A 3-mm transverse incision was made in the skin lateral to the right Achilles tendon and the Achilles tendon complex was exposed. Approximately 7 mm of the plantaris tendon was then removed to simplify mechanical measurements at the end of the experiment. Subsequently, the Achilles tendon was cut transversely 1.5 and 4.5 mm proximal to the calcaneal insertion. Thus, a 3-mm segment was removed (). The tendon was left unsutured with a gap between the tendon stumps and the skin sutured. There was no postoperative immobilization. After the operation, the animals were placed in clean cages under a heating lamp for a constant temperature of 30°C until they completely woke up.
Figure 2. A. Normal rat Achilles tendon. B. Achilles tendon transection. C. Callus 14 days after transection.
The rats were divided into experimental and control groups by a lottery. For platelet treatment, this was done by an assistant during the operation. The rats either received 50 μL (1 piece) of platelet gel in the defect at the operation or a local injection of 50 μL of platelet concentrate 6h postoperatively, or control solution (saline).
Evaluation
After 3, 5 or 14 days, the rats were killed with CO2 gas. The tendon with the attached calcaneal bone was dissected free from other tissues and removed. Sagittal and transverse diameters were measured with a digital calliper. For clamping, the muscle was scraped off the tendon substance by blunt dissection to produce a fan of tendon fibers, which was then sandwiched between fine sandpaper in metal clamps. The calcaneus was fixated in a custom-made clamp in 30° dorsiflexion, relative to the direction of traction. Finally, the clamps were attached to a materials-testing machine (100R; DDL Inc., Eden Praire, MN) and the tendon was pulled at a constant speed of 1 mm/s until failure. Mechanical parameters measured were force at failure, stiffness, and energy uptake at 10% droop of the curve. Transverse area and stress at failure were calculated.
Statistics
Statistical analysis was performed with StatView for Windows version 5.0.1 (SAS Institute, Cary, NC). Experiments with two groups were evaluated with Student's t-test. When 4 groups were compared, this was done by 2-way analysis of variance.
Results
5 rats were lost from mechanical follow-up because of various technical errors.
No effect of platelets without mechanical loading at 14 days
20 rats received Botox injections and underwent tendon transection 7 days later. At that time, the paralysis appeared complete. 6 h after transection, the animals were randomized to receive an injection of platelet concentrate into the hematoma (10 rats) or to receive buffer (10 rats). With unloading by Botox, all stimulatory effects of platelets were lost (), and the tendon regenerates were less than a third as strong as with normal loading. Stiffness and energy uptake were only a quarter of normal, transverse area half of normal, and stress at failure two thirds of normal (for normal data, see and ). Thus, loading is a prerequisite for stimulation by platelets.
Table 1. No effect of platelets (PC) after 14 days in rats with calf muscles paralyzed with Botox
Table 2. Effect of activity with or without platelets*
Table 3. Combined data from activity cages, excluding Botox-treated groups
Increased cage activity improves repair but not the response to platelets
20 rats were randomized to normal cages or activity cages. After 1 week for acclimatization, they underwent tendon transection. Evaluation was done 14 days later. 2 tendons were excluded because of a fracture in the calcaneus at the mechanical test. Rats from the activity cages had increased force, energy uptake and transverse area ().
We next repeated this experiment with addition of platelet gel. Thus, the rats were randomized to 4 groups: normal cage with control rats without any treatment; normal cage with platelet gel; activity cage without any treatment; and activity cage with platelet gel. There was no significant effect on force. Platelets increased stiffness, reduced area and increased stress at failure. Activity increased area and reduced stress. No variable showed that cage activity influenced the response to platelets ().
Because of the methodological similarity, the first and the second activity cage experiments (platelet injections excluded) were combined to increase statistical power. We then found that activity increased force, energy uptake and area (). The 2 experiments differed regarding stiffness and energy. This is unexplained.
In conclusion, activity increased size (and thereby force and energy) and platelets improved material properties, so that the regenerates appeared to have come further ahead in the maturation process. No synergism between activity and platelets was found.
Platelets have an early effect also without loading
40 rats received Botox injections and underwent tendon transection 7 days later. They were randomized to platelet or control treatment. After 3 and 5 days, the platelets increased force, stiffness and area ().
Table 4. Effects of platelet gel in rats killed on days 3 and 5
Discussion
The basis of this study was the finding of a stimulatory effect of a single platelet injection on tendon repair, which can be traced with mechanical testing as late as 4 weeks afterwards (Aspenberg and Virchenko Citation2004). This late effect was surprising. We next noted that this effect (at 14 days) disappeared when the tendon was unloaded using Botox, which suggested a connection between the platelet effect and mechanical stimulation. This was the motivation for the experiments that ensued.
Platelets had an early stimulatory effect in tendons unloaded with Botox, but loading was a prerequisite for this effect to remain at 14 days. Activity increased regenerate size, whereas loaded and platelet-treated specimens had improved material properties at 14 days.
The development of the tendon callus starts with an organizing blood clot. Mechanical stimulation can occur first when a sufficient number of fibroblasts is present and a matrix capable of transferring some load has been produced. A possible explanation for the long-lasting effect of platelets on the maturity of a regenerate (Aspenberg and Virchenko Citation2004) could be that platelets improve the very early callus properties so that the cells can perceive and respond to mechanical loading at an earlier time point. Once the non-platelet controls reach a sufficient stage of healing (at a later time point), they too will be responsive to loading, but the platelet-treated specimens will be further ahead in their development. This hypothesis occurred to us when we saw the 14-day data. This would explain the absent effect of platelets in the Botox-treated group: without mechanical stimulation, early responsiveness to loading is of no advantage. The hypothesis would also explain the lack of synergism between increased loading and platelets, because the factors act in sequence rather than at the same time (platelets during the first days, and loading thereafter). If the hypothesis is correct, one should be able to measure an early effect of platelets in the absence of loading, i.e. different callus properties at the time that loading would start exerting its stimulatory effects. Indeed, this is what we finally found. At the early time points, platelets stimulated callus formation.
This study could only give indirect proof of the principle. In order to show more directly at what time point loading starts to stimulate repair, one would need a model in which loading could be turned off and then on at defined time points, which is something which we do not have.
The clinical implication of this study is that mechanical stimulation is of utmost importance. This has been pointed out before in several studies (Enwemeka Citation1992, Maffulli and King Citation1992, Almekinders et al. Citation1995, Kannus et al. Citation1997, Iwuagwu and McGrouther Citation1998, Zeichen et al. Citation2000). However, the use of Botox for unloading of the healing tendon appears to be a new and practical model. As long as the muscle is relaxed and pliable, traction forces in the tendon callus should be low. Earlier models have used immobilization (Murrell et al. Citation1994, Ishida et al. Citation1996, Iwuagwu and McGrouther Citation1998, Yamamoto et al. Citation1999, Palmes et al. Citation2002, Matsumoto et al. Citation2003). In animals, it is unclear to what extent immobilization really leads to unloading. Most animals will probably exert considerable traction forces by muscle contraction in spite of a brace or internal fixation. It is unknown how patients load their injured Achilles tendons while in plaster, but the situation in humans probably corresponds better to complete absence of muscle contraction with Botox than to animal cage activity with a brace. Thus, the dramatic inhibition of repair by unloading in the Botox model suggests that placing a patient in plaster with complete unloading could impair the repair process. Furthermore, platelet treatment for tendon ruptures may not be efficacious, unless it is combined with early physiotherapy. This must be early enough to benefit from the increased callus growth induced by the platelets.
We thank Ali Sodeifi for assistance with rat surgery. This study was supported by the Swedish National Center for Sports Research, the Strategic Research Program “Materials in Medicine” (Östergötlands läns landsting, Linköping university), and by the Swedish Research Council (project no. 2031).
Contributions of authors
Both authors participated in all parts of the study, but OL did most of the experimental work and PA most of the writing.
- Almekinders L C, Baynes A J, Bracey L W. An in vitro investigation into the effects of repetitive motion and nonsteroidal antiinflammatory medication on human tendon fibroblasts. Am J Sports Med 1995; 23(1)119–23
- Aspenberg P, Forslund C. Bone morphogenetic proteins and tendon repair. Scand J Med Sci Sports 2000; 10(6)372–5
- Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand 2004; 75(1)93–9
- Carlsson L. Behavioural effects of two different types of environmental enrichment on laboratory mice and rats, Master thesis. http://cms.ifm.liu.se/biology/master_projects/2005/liska144/thesis/Final%20thesis.pdf
- Enwemeka C S. Functional loading augments the initial tensile strength and energy absorption capacity of regenerating rabbit Achilles tendons. Am J Phys Med Rehabil 1992; 71(1)31–8
- Forslund C. BMP treatment for improving tendon repair. Studies on rat and rabbit Achilles tendons. Acta Orthop Scand (Suppl 308) 2003; 74: 1–30
- Ishida H, Yasuda K, Hayashi K, Yamamoto N, Kaneda K. Effects of resumption of loading on stress-shielded autografts after augmentation procedures. An experimental study. Am J Sports Med 1996; 24(4)510–7
- Iwuagwu F C, McGrouther D A. Early cellular response in tendon injury: the effect of loading. Plast Reconstr Surg 1998; 102(6)2064–71
- Kannus P, Jozsa L, Natri A, Jarvinen M. Effects of training, immobilization and remobilization on tendons. Scand J Med Sci Sports 1997; 7(2)67–71
- Kurtz C A, Loebig T G, Anderson D D, DeMeo P J, Campbell P G. Insulin-like growth factor I accelerates functional recovery from Achilles tendon injury in a rat model. Am J Sports Med 1999; 27(3)363–9
- Maffulli N, King J B. Effects of physical activity on some components of the skeletal system. Sports Med 1992; 13(6)393–407
- Matsumoto F, Trudel G, Uhthoff H K, Backman D S. Mechanical effects of immobilization on the Achilles' tendon. Arch Phys Med Rehabil 2003; 84(5)662–7
- Murrell G A, Lilly E G, 3rd, Goldner R D, Seaber A V, Best T M. Effects of immobilization on Achilles tendon healing in a rat model. J Orthop Res 1994; 12(4)582–91
- Palmes D, Spiegel H U, Schneider T O, Langer M, Stratmann U, Budny T, Probst A. Achilles tendon healing: long-term biomechanical effects of postoperative mobilization and immobilization in a new mouse model. J Orthop Res 2002; 20(5)939–46
- Virchenko O, Fahlgren A, Skoglund B, Aspenberg P. CDMP2 injection improves early tendon healing in a rabbit model for surgical repair. Scand J Med Sci Sports 2005; 15(4)260–4
- Yamamoto E, Hayashi K, Yamamoto N. Mechanical properties of collagen fascicles from stress-shielded patellar tendons in the rabbit. Clin Biomech (Bristol, Avon) 1999; 14(6)418–25
- Zeichen J, van Griensven M, Bosch U. The proliferative response of isolated human tendon fibroblasts to cyclic biaxial mechanical strain. Am J Sports Med 2000; 28(6)888–92
- Zhang F, Liu H, Stile F, Lei M P, Pang Y, Oswald T M, Beck J, Dorsett-Martin W, Lineaweaver W C. Effect of vascular endothelial growth factor on rat Achilles tendon healing. Plast Reconstr Surg 2003; 112(6)1613–9