Abstract
Background Native BMP extracts from reindeer effectively induce ectopic new bone formation in vivo, but their bone healing properties have not yet been evaluated. We investigated the effect of reindeer BMP extracts on the healing of long bone defects.
Methods The implants tested contained 5 mg or 10 mg of unsterilized BMP extract from reindeer and 10 mg of gamma-sterilized BMP extract administered with collagen carrier (Lyostypt, B. Braun, Germany). 70 μg of rhBMP-2 with collagen carrier (InductOs; Wyeth Europa) served as positive control, and collagen implants (Lyostypt) and untreated defects served as negative controls. New Zealand White rabbits with 1.5 cm of critical-size radius bone defects were used, with 8 weeks of follow-up.
Results Radiographic analysis showed bone formation (BF) to be higher in all groups containing BMPs than in the untreated controls. BF was also higher in the rhBMP-2 group, and marginally higher in the group treated with 10 mg of unsterilized reindeer BMP extract (p = 0.06) as compared to the collagen controls. Bone union (BU) was better in the unsterilized BMP extract groups and rhBMP-2 group than in the untreated controls. BU was also better in the implants with 10 mg of unsterilized reindeer BMP extract and rhBMP-2 than in the collagen-treated implants. The mean area of new bone at the site of the defect proved to be higher in all implants containing BMP than in the untreated defects. It was also higher in the groups with 10 mg of unsterilized reindeer BMP extract and rhBMP-2 than in the collagen-treated controls. Mechanical tests showed torsional stiffness of the bones to be higher in the group with 10 mg of unsterilized BMP extract than in the collagen group. The mean cross-sectional bone area measured by pQCT densitometry was higher in the rhBMP-2 group than in the collagen group. The mean bone density at the defect area was higher in the group with 10 mg of unsterilized BMP than in the rhBMP-2 group.
Interpretation We conclude that both reindeer BMP extract and rhBMP-2 induced improved healing of the rabbit radius bone defects at the doses used. Gamma sterilization of reindeer BMP extract reduced osteoinductivity slightly, but not significantly.
Bone morphogenetic proteins (BMPs) stimulate regeneration of bone and cartilage (Jortikka et al. Citation1993a,b, Cook Citation1999, Govender et al. Citation2002, Wozney Citation2002, Cheng et al. Citation2003, Pekkarinen et al. Citation2003). The bone-healing properties of different recombinant and extracted BMPs have been studied using long bone defect models in rabbits (Hollinger et al. Citation1998, Kokubo et al. Citation2003), dogs (Sciadini and Johnson Citation2000, Tuominen et al. Citation2000), rats (Takagi and Urist Citation1982, Chen et al. Citation2002, Matsuo et al. Citation2003) and sheep (Gao et al. Citation1996, Citation1997). Favorable results in preclinical and clinical trials have also led to the regulatory approval of different commercial recombinant BMP products for some clinical purposes by government agencies (Burkus et al. Citation2002a,b, Govender et al. Citation2002, Valentin-Opran et al. Citation2002, Matsuo et al. Citation2003). Considering that there are about 20 BMPs known, products consisting of single recombinant BMPs cannot be regarded as being ideal. Also, our knowledge of their long-term effects and of how safe they are is still limited. BMPs occur as a mixture in living organisms, and native BMPs are also a mixture when extracted. Thus, studies aiming at safe and effective products from native BMPs are still needed. We have shown previously that reindeer BMP extracts effectively induce ectopic new bone formation in vivo (Pekkarinen et al. Citation2003, Citation2004, Citation2005a,Citationb). However, the bone healing properties of reindeer BMP extract have not yet been evaluated.
We investigated the effects of reindeer BMP extracts on the healing of critical-size bone defects (CSDs) in the rabbit radius. The bone-inducing capacity of reindeer BMP extracts was compared to untreated controls and collagen-treated controls, while rhBMP-2 (InductOs) was studied as a reference because its bone-healing capacity is well documented (Hollinger et al.Citation1998, Sciadini and Johnson Citation2000, Yudell and Block Citation2000, Govender et al. Citation2002, Li et al. Citation2002).
Methods
BMP materials
Native reindeer (Rangifer tarandus) BMP extract was prepared from diaphyseal bone of the reindeer. Cortical bones from each animal were chilled immediately after death. The epiphyseal ends, bone marrow and periosteum were mechanically removed, and after freezing in liquid nitrogen, the cleaned cortical bones were ground to a particle size of 1.0 mm3. The pulverized bone was demineralized in 0.6 M HCl and extracted in 4 M guanidine hydrochloride (GuHCl) at 4°C. The GuHClextracted solution was filtered with a tangential flow system and concentrated. The concentrated solution was dialyzed against deionized water and the water-insoluble material was collected. After redissolving in 4 M GuHCl solution, the waterinsoluble material was dialyzed against 0.25 M citrate buffer, pH 3.1. The citrate-buffer-insoluble material was washed with deionized water and lyophilized (Jortikka et al. Citation1993a).
We used the commercial product of recombinant human bone morphogenetic protein-2 (Induct Os; Wyeth Europa, Maidenhead, UK). InductOs is authorized for the treatment of tibial fractures and consists of recombinant human bone morphogenetic protein-2 (rhBMP-2, dibotermin alfa), applied to an absorbable collagen sponge (ACS) matrix (type I bovine collagen) (Govender et al. Citation2002).
Reconstitution of implants and sterilization
5 or 10 mg of reindeer BMP extract was dissolved in 100 μL sterile water and pipetted onto the collagen sponge (25 × 20 mm, Lyostypt compress from native collagen of bovine origin, mainly consisting of type IV collagen; B. Braun, Tuttlingen, Germany). The doses were chosen according to our previous studies (Pekkarinen et al. Citation2003, Citation2004, Citation2005b, Citation2005c). Finally, implants were lyophilized in test tubes for 48 h.
The InductOs kit (Wyeth Europa, Maidenhead, UK) for implant contains 12 mg rhBMP-2, solvent (10 mL sterile water), and absorbable collagen sponge matrix (ACS). The solvent containing 12 mg of rhBMP-2 was made according to manufac-ture's instructions before operation. 46.7 μL of the solvent, thus containing 70 μg of rhBMP-2, was pipetted onto the collagen matrix (10 × 20 mm) to form the implant. The dose of rhBMP-2 was determined from the literature (Hollinger et al. Citation1998, Bessho et al. Citation1999, Li et al. Citation2002).
Control implants were constructed in an identical fashion, but they contained only collagen carrier (Lyostypt). All implants were made under sterile conditions.
Gamma sterilization of the reindeer BMP extract implants was performed by a specialized company (Isotron Ltd., Swindon, UK). The dose of irradiation was 4.10 MRad.
Groups
26 eight-month-old male New Zealand White rabbits were used. Each animal was treated bilaterally with two different implants randomized from the following groups: (1) collagen + 5 mg of unsterilized reindeer BMP extract (n 8), the “5 mg BMP” group; (2) collagen + 10 mg of unsterilized reindeer BMP extract (n 10), the “10 mg BMP” group; (3) collagen + 10 mg of gamma-sterilized reindeer BMP extract (n 10), the “10 mg sterilized BMP” group; (4) collagen + 70 μg of rhBMP-2 (Induct Os) (n 8), the “rhBMP-2” group; (5) collagen (n 8), the “collagen” group; and (6) no implant (n 8), the “untreated” group.
Surgical procedure
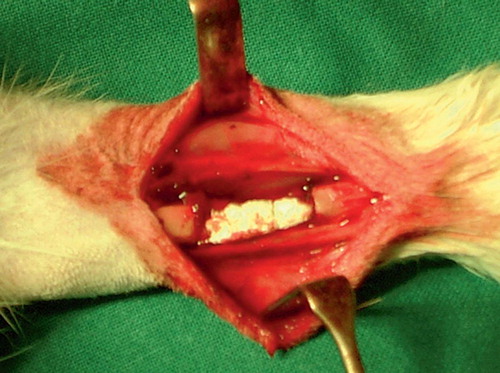
Surgery was performed under general anesthesia (Domitor 0.3 mg/kg, Ketalar 20 mg/kg). 1 mL of prophylactic antibiotic (Procapen, 300,00 IU/mL; Orion, Espoo, Finland) was given to each rabbit preoperatively. Longitudinal skin incisions were made bilaterally over the radial bones at the middle one-third of the front leg. The periosteum was separated from the surrounding muscle. A 15-mm critical-size defect (CSD) located about 2–2.5 cm proximal to the radiocarpal joint was created using a circular saw attached to a dental handpiece. After that, the defect was checked and any remaining periosteum was removed. A thorough wash with saline was done and the implant was applied to the defect (). No fixation was used because of the support from the ulna, and unrestricted weight bearing was normally allowed.
After 8 weeks, the rabbits were killed with an overdose of pentobarbital (Mebunat), 60 mg/kg intravenously. The radii were dissected out and the soft tissues were removed. The bones were wrapped in saline and frozen at –20°C until analysis.
Radiographic evaluation of bone formation
Radiographs of the radius (100 mA, 20 kV, 0,08 s/exp; Mamex de Maq, Soredex, Orion) were taken after 8 weeks of implantation (). The percentage of new bone formation (BF) within the defect and the development of the union or non-union (BU) of the bone was estimated clinically by two independent investigators according to the scoring system presented by Sciadini et al. (Citation1997). Any cases of disagreement were reviewed together. Interpretations between the different groups were done blind.
Figure 2. Healing of the critical-size bone defects: (A) no bone formation, nonunion, (B) average amount of bone formation, nonunion, (C) normal bone formation, total union.
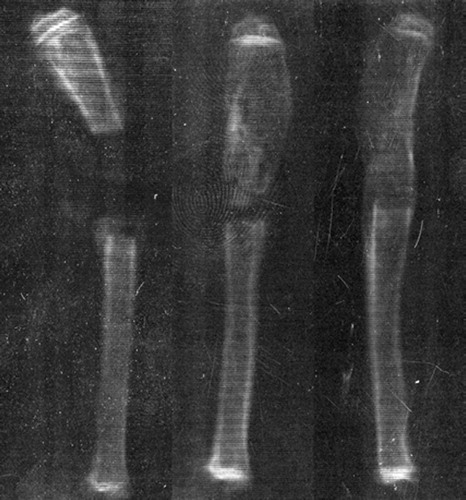
After clinical estimation, radiographic images were digitized using an optical scanner (HP Scan-Jet 4070). Bone formation was evaluated as the area (in mm2) of the calcified tissue visible on the region of the bone defect by using Scion Image Beta 4.02 (Scion Corp., MD, USA) software. The mean optical density (mmAl) of the defined area was measured with the same software. Calibration of the optical density was performed by using an aluminium wedge that was imaged with the bones.
Computed tomography
After thawing, the bones were scanned using a peripheral quantitative computed tomography (pQCT) device (Stratec XCT 960A, software version 5.21; Norland Stratec Medizintechnik GmbH, Birkenfeld, Germany). A voxel size of 0.148 × 0.148 × 1 mm3 was used. We scanned 3 slices of each sample: 1 slice in the middle of the implant and 1 at each end, 5 mm from the middle slice. The positions of the slices were defined by using the axial scout view of the pQCT system. Bone density (mg/cm3), and cross-sectional bone area (mm2) were recorded for each slice as given by the pQCT software, using an attenuation threshold of 0.4 c- 1 (169 mg/cm3) for bone. Average values for the 3 slices were used in the statistical analysis.
Mechanical tests
The radii of the rabbits were thawed to room temperature for torsional testing. During the mechanical tests, the bones were kept moist. The ends were embedded into the molds with dental stone (GC Fujirock Improved Dental Stone; G-C Dental Industrial Corp., Tokyo, Japan). Torsional shaft was 3.5 cm. After hardening of the dental stone, the bones were placed in the torque machine (Lepola et al. Citation1993) and torsionally loaded at a constant angular speed of 6 degrees per sec until failure. Maximum breaking load (Nm), torsional stiffness (Nm/degree) and energy to failure (Nm degree) were recorded (Jämsä and Jalovaara Citation1996).
Statistics
Statistical analysis was performed using the SPSS statistical package and the Confidence Interval Analysis software (version 2.0.0; University of Southampton). The non-parametric Kruskall-Wallis test was used to evaluate the statistical differences between the groups, and the Mann-Whit-ney test for pairwise comparison between each BMP group and the untreated and collagen groups (negative controls), and between the reindeer BMP groups and the rhBMP group (positive control). Values of p < 0.05 were considered statistically significant. Results are given as median and 95% confidence limits, using the Wilcoxon method for continuous variables and the binomial method for grading variables.
Results
Because of physeolysis, 7 rabbits had to be killed before the endpoint. Thus, since the animals were treated bilaterally, 3 samples from the 5 mg BMP group, the 10 mg BMP group, the 10 mg sterilized BMP group, and the untreated groups, and 1 sample from both rhBMP-2 and collagen groups were excluded from the study.
Bone formation and bone union evaluated clinically from radiographs
Bone formation (BF) at the defect site was higher in the groups with 5 mg BMP (p = 0.04), 10 mg BMP (p = 0.004), 10 mg sterilized BMP (p = 0.02), and rhBMP-2 (p = 0.001) than in the untreated group. BF was also higher in the rhBMP-2 group (p = 0.02) and marginally higher in the group with 10 mg BMP (p = 0.06) than in the collagen group ().
Bone formation in the defect area of rabbit radius evaluated by radiographic analysis, mechanical testing and pQCT densitometry. Data are presented as median (95% CI)
Bone union (BU) was higher in the groups with 5 mg BMP (p = 0.04), 10 mg BMP (p = 0.01), and rhBMP-2 (p = 0.01) than in the untreated group. BU was also higher in the groups with 10 mg BMP (p = 0.04) and rhBMP-2 (p = 0.04) than in the collagen group ().
There were no significant differences in bone formation and bone union between the groups containing BMPs ().
New bone area and optical density evaluated by radiography
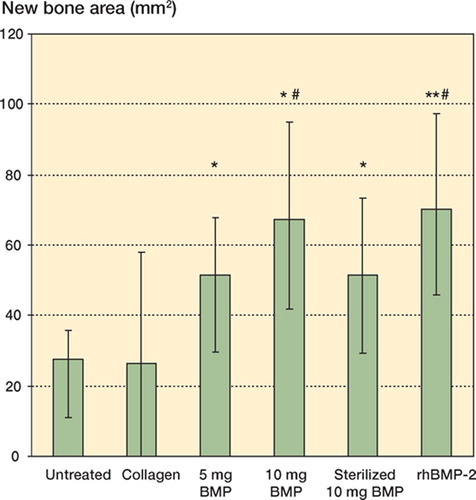
Mean new bone area (mm2) at the defect site was higher in the groups with 5 mg BMP (p = 0.03), 10 mg BMP (p = 0.02), 10 mg of sterilized BMP (p = 0.04) and rhBMP-2 (p = 0.004) than in the untreated group. Also, the new bone area was higher in the groups with 10 mg BMP (p = 0.03) and rhBMP-2 (p = 0.04) than in the collagen group. There were no significant differences in area of new bone between the groups containing BMPs ().
Figure 3. New bone areas (median, 95% CI) of rabbit radius bone defects evaluated from digitized radiographs.* p < 0.05, ** p < 0.01 vs. untreated group. # p < 0.05 vs. collagen group
There were no significant differences in mean optical density (mmAl) of new bone between the different groups ().
Mechanical testing
Mechanically unstable bones were not tested, and their values were considered to be zero in the statistical analysis. Stiffness of the bones (in Nm/ deg) was significantly higher in the group with 10 mg BMP than in the collagen group (p = 0.02). There were no other statistically significant differences between the groups in the mechanical tests ().
Cross-sectional bone area and bone density evaluated by pQCT
Computed tomography (pQCT) showed cross-sec-tional bone area to be higher in the rhBMP-2 group than in the collagen group (p < 0.05). Bone density was higher in the 10 mg BMP group than in the rhBMP-2 group (p < 0.05).
Discussion
BMPs combined with different carrier materials have been shown to heal experimental bone defects in many studies (Moore et al. Citation1990, Gao et al. Citation1996, Citation1997, Viljanen et al. Citation1996, Hollinger et al. Citation1998, Sciadini and Johnson Citation2000, Tuominen et al. Citation2000, Yudell and Block Citation2000, Chen et al. Citation2002, Kokubo et al. Citation2003, Barboza et al. Citation2004). Until now, there have been no studies investigating the ability of reindeer BMP extract to heal long bone defects. We have shown previously that reindeer BMP extract is an effective inducer of new bone formation in a muscle pouch model in mice (Pekkarinen et al. Citation2003, Citation2004, Citation2005b, Citationc). In the present study, we compared the bone healing properties of the reindeer BMP extract to results with untreated and collagen controls, while rhBMP-2 (InductOS) was also included in the comparisons because the bone healing capacity of rhBMP-2 has been well documented in experimental animal and clinical studies (Hollinger et al. Citation1998, Sciadini and Johnson Citation2000, Yudell and Block Citation2000, Govender et al. Citation2002, Li et al. Citation2002).
Many previous studies concerning the ability of BMPs to promote healing of long bone defects have been performed using synthetic polymer carriers or composite implants containing collagen and frames such as coral, tricalcium phosphate or hydroxyapatite (Gao et al. Citation1996, Citation1997, Tuominen et al. Citation2000, Citation2001, Hu et al. Citation2003, Kokubo et al. Citation2003). In the present study we did not use composite implants because the rabbit radius is a relatively small bone and its critical-size bone defect does not require extra fixation or a carrier that is mechanically stronger than collagen. BMPs have also been used successfully in the healing of fractures with collagen carrier alone (Bax et al. Citation1999, Blokhuis et al. Citation2001, Govender et al. Citation2002, Einhorn et al. Citation2003).
Hollinger et al. (Citation1998) used rhBMP-2 with collagen carrier in the healing of rabbit radial criti-cal-size defects. After 8 weeks, rhBMP-2 treatment regenerated osseous contours and new bone formation was significantly higher in the rhBMP-2 or autograft groups than in the collagen or untreated groups. Using a radial bone defect in dogs, Sciadini and Johnson (Citation2000) reported that rhBMP-2 with a collagen sponge carrier had potential osteoinductive activity as measured by biomechanical parameters when evaluated 12 and 24 weeks postoperatively. Defects treated with rhBMP-2 implants were comparable to those treated with autograft, and significantly stronger than those treated with placebo. Li et al. (Citation2002) enhanced bone consolidation of distraction osteogenesis in a rabbit model by using rhBMP-2 with an absorbable collagen sponge carrier. They concluded that rhBMP-2 enhances the consolidation stage of distraction osteogenesis in the rabbit tibia. Also, Yudell and Block (Citation2000) showed that rhBMP-2 with absorable collagen sponge carrier has the potential to stimulate bone gap healing in the zygomatic arch osteotomies.
Our results are in line with those of previous studies. Here, rhBMP-2 effectively stimulated the healing of the rabbit critical-size bone defect. Reindeer BMP extract also induced bone healing effectively and its effect was comparable to that of rhBMP-2 at the doses used. These findings confirm our previous results showing that reindeer BMP extract has good osteoconductivity in the muscle pouch model of mice (Pekkarinen et al. Citation2003, Citation2004, Citation2005a, Citationb). Bessho et al. (Citation1999) showed the osteoinductivity of rhBMP-2 to be less than one-tenth of the osteoinductivity of human BMP extract, but that conclusion does not match the findings of our study.
Previous studies on the bone healing capacity of purified BMP extracts have been done using different extracts and different carrier types than used here, but the results have still been more or less in line with ours (Moore et al. Citation1990, Gao et al. Citation1996, Citation1997, Viljanen et al. Citation1996, Tuominen et al. Citation2000, Citation2001). Moore et al. (Citation1990) showed bovine BMP extract and Viljanen et al. (Citation1996) moose BMP extract with collagen carriers to be effective in the healing of experimental bone defects. Gao et al. (Citation1996) healed segmental tibial defects of sheep by using composite implants containing sheep BMP extract, type IV collagen, and tricalcium phosphate cylinder. They concluded that this composite implant possessed good osteoinductivity, osteoconductivity and mechanical strength. Tuominen et al. (Citation2000) used a canine ulnar defect model to evaluate the bone healing capacity of composite implants containing bovine BMP extract, coral and collagen. They concluded that the BMP composite enhanced bone healing but the effect was not as good as that of corticocancellous autograft.
Our previous studies have shown that gamma sterilization of the reindeer BMP extract does not reduce its osteoinductivity significantly in vivo (Pekkarinen et al. Citation2004, Citation2005a, Citationc). This study is by and large in line with our previous findings, and no significant difference between unsterilized or gamma-sterilized reindeer BMP extract could be seen. However, even though the gamma-sterilized reindeer BMP extract healed bone defects well, it appeared to be slightly inferior than unsterilized reindeer BMP extract or rhBMP-2.
In this animal model, it is advisable to use adult rabbits to prevent epiphyseal slipping. We used 8-month-old rabbits, but some animals underwent physeolysis during the evaluation time. These animals had to be killed before the endpoint, and they were therefore excluded from the study. Also, we observed only slight differences between the study groups in the mechanical properties of the bones. This was mainly due to the small sample number caused by physeolysis and the limited ability of mechanical testing to discriminate bones with nonunion. In spite of this, the results were quite obvious.
The theoretical risk of transmissible spongiform encephalopathy has limited the use of materials of natural origin. Thus, there might be concern about the use of native reindeer proteins. However, prion disease has never been described in reindeer. Furthermore, the manufacturing process and gamma sterilization of the BMP extract destroys prions and virus particles (Miekka et al. Citation2003).
We conclude that both reindeer BMP extract and rhBMP-2 (InductOs) effectively induced the healing of rabbit radius bone defects at the doses used. Gamma sterilization of the reindeer BMP extract did not reduce its osteoinductivity substantially, but the results were slightly inferior to those with unsterilized reindeer BMP extract or rhBMP-2.
The study was financially supported by the Finnish Funding Agency for Technology and Innovation (TEKES), Finnish Cultural Foundation, the Regional Fund of Northern Ostrobothania and BBS-Bioactive Bone Substitutes Ltd. The sponsors did not influence the methods of evaluation, interpretation of results or writing of the report.
The authors wish to thank Mrs Marja Juustila for her kind assistance with the preparation of the implants and surgical assistance.
Contributions of authors
PJ, TP and TJ: planned the study. PJ and TJ: conducted the study. OH: participated in the preparation of the BMP extract. TJ and OH: prepared the implants. TP: performed the surgery. TP and PJ: radiographic evaluation. TP, MM and TJ: performed the pQCT measurements, mechanical testing and statistical analyses. TP, PJ, TJ, MM and OH: interpretated the results and wrote the manuscript.
- Barboza E P, Caula A L, Caula Fde O, de Souza R O, Geolas Neto L, Sorensen R G, Li X, Wikesjo U M. Effect of recombinant human bone morphogenetic protein-2 in an absorbable collagen sponge with space-providing biomaterials on the augmentation of chronic alveolar ridge defects. J Periodontol 2004; 75: 702–8
- Bax B E, Wozney J M, Ashhurst D E. Bone morphogenetic protein-2 increases the rate of callus formation after fracture of the rabbit tibia. Calcif Tissue Int 1999; 65: 83–9
- Bessho K, Kusumoto K, Fujimura K, Konishi Y, Ogawa Y, Tani Y, Iizuka T Comparison of recombinant, purified human bone morphogenetic protein. Br J Oral Maxillofac Surg 1999; 37: 2–5
- Blokhuis T J, den Boer F C, Bramer J A M, den Boer F C, Bakker F C, Patka P, Haarman H J, Manoliu R A. Biomechanical and histological aspects of fracture healing, stimulated with osteogenic protein-1. Biomaterials 2001; 22: 725–30
- Burkus J K, Gornet M F, Dickman C A, Zdeblick T A. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech 2002a; 15: 337–49
- Burkus J K, Transfeldt E E, Kitchel S H, Watkins R G, Balderston R A. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine 2002b; 27: 2396–408
- Chen X, Kidder L S, Lew W D. Osteogenic protein-1 induced bone formation in an infected segmental defect in the rat femur. J Orthop Res 2002; 20: 142–50
- Cheng H, Jiang W, Phillips F M, Haydon R C, Peng Y, Zhou L, Luu H H, An N, Breyer B, Vanichakarn P, Szatkowski J P, Park J Y, He T C. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg (Am) 2003; 85: 1544–52
- Cook S D. Preclinical and clinical evaluation of osteogenic protein-1 (BMP-7) in bony sites. Orthopaedics 1999; 22: 669–71
- Einhorn T A, Majeska R J, Mohaideen A, Kagel E M, Bouxsein M L, Turek T J, Wozney J M. A single percutaneous injection of recombinant human bone morphogenetic pro-tein-2 accelerates fracture repair. J Bone Joint Surg (Am) 2003; 85: 1425–35
- Gao T J, Lindholm T S, Kommonen B, Ragni P, Paronzini A, Lindholm T C, Jämsä T, Jalovaara P. Enhanced healing of segmental tibial defects in sheep by a composite bone substitute composed of tricalcium phosphate cylinder, bone morphogenetic protein, and type IV collagen. J Biomed Mater Res 1996; 32: 505–12
- Gao T J, Lindholm T S, Kommonen B, Ragni P, Paronzini A, Lindholm T C, Jalovaara P, Uris M R. The use of a coral composite implant containing bone morphogenetic protein to repair a segmental tibial defect in sheep. Int Orthop 1997; 21: 194–200
- Govender S, Csimma C, Genant H K, Valentin-Opran A. Recombinant human bone morphogenetic protein2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg (Am) 2002; 84: 2123–34
- Hollinger J O, Schmitt J M, Buck D C, Shannon R, Joh S P, Zegzula H D, Wozney J. Recombinant human bone morphogenetic protein-2 and collagen for bone regeneration. J Biomed Mater Res 1998; 43: 356–64
- Hu Y, Zhang C, Zhang S, Xiong Z, Xu J. Development of a porous poly(L-lactic acid)/hydroxyapatite/collagen scaffold as a BMP delivery system and its use in healing canine segmental bone defect. J Biomed Mater Res 2003; 67A: 591–8
- Jämsä T, Jalovaara P. A cost-effective, accurate machine for testing the torsional strength of sheep long bones. Med Eng Phys 1996; 18: 433–5
- Jortikka L, Marttinen A, Lindholm T S. Partially purified reindeer (Rangifer Tarandus) bone morphogenetic protein has a high bone-forming activity compared with some other artiodactylis. Clin Orthop 1993a, 297: 33–7
- Jortikka L, Marttinen A, Lindholm T S. Purification of monocomponent bovine morphogenetic protein in a water-soluble form. Ann Chir Gyn 1993b; 82: 25–30
- Kokubo S, Fujimoto R, Yokota S. Bone regeneration by recombinant human bone morphogenetic protein-2 and a novel biodegradable carrier in a rabbit ulnar defect model. Biomaterials 2003; 24: 1643–51
- Lepola V, Väänänen K, Jalovaara P. The effect of immobilization on the torsional strength of the rat tibia. Clin Orthop 1993, 297: 55–61
- Li G, Bouxsein M L, Luppen C, Li X J, Wood M, Seeherman H J, Wozney J M, Simpson H. Bone consolidation is enhanced by rhBMP-2 in a rabbit model of distraction osteogenesis. J Orthop Res 2002; 20: 779–88
- Matsuo T, Sugita T, Kubo T, Yasunaga Y, Ochi M, Murakami T. Injectable magnetic liposomes as a novel carrier of recombinant human BMP-2 for bone formation in a rat bone-defect model. J Biomed Mater Res 2003; 66A: 747–54
- Miekka S I, Forng R Y, Rohwer R G, MacAuley C, Stafford R E, Flack S L, MacPhee M, Kent R S, Drohan W N. Inactivation of viral and prion pathogens by gammairradiation under conditions that maintain the integrity of human albumin. Vox Sang 2003; 84: 36–44
- Moore J C, Matukas V J, Deatherage J R, Miller E J. Craniofacial osseous restoration with osteoinductive proteins in a collagenous delivery system. Int J Oral Maxillofac Surg 1990; 19: 172–6
- Pekkarinen T, Lindholm T S, Hietala O, Jalovaara P. New bone formation induced by injection of native reindeer BMP. Scand J Surg 2003; 92: 227–30
- Pekkarinen T, Lindholm T S, Hietala O, Jalovaara P. Influence of ethylene oxide sterilization on the activity of native reindeer bone morphogenetic protein. Int Orthop 2004; 28: 97–101
- Pekkarinen T, Lindholm T S, Hietala O, Jalovaara P. The effect of different mineral frames on the ectopic bone formation in mouse hind leg muscles induced by native reindeer bone morphogenetic protein. Arch Orthop Trauma Surg 2005a; 125: 10–5
- Pekkarinen T, Hietala O, Jämsä T, Jalovaara P. Effect of gamma irradiation on the osteoinductivity of native reindeer bone morphogenetic protein extract. Acta Orthop 2005b; 76: 231–6
- Pekkarinen T, Hietala O, Jämsä T, Jalovaara P. Gamma irradiation and ethylene oxide in the sterilization of native reindeer bone morphogenetic protein extract. Scand J Surg 2005c; 94: 67–70
- Sciadini M F, Dawson J M, Johnson K D. Bovine-derived bone protein as a bone graft substitute in a canine segmental defect model. J Orthop Trauma 1997; 11: 496–508
- Sciadini M F, Johnson K D. Evaluation of recombinant human bone morphogenetic protein-2 as a bone-graft substitute in a canine segmental defect model. J Orthop Res 2000; 18: 289–302
- Takagi K, Urist M R. The role of bone marrow in bone morphogenetic protein-induced repair of femoral massive diaphyseal defects. Clin Orthop 1982, 171: 224–31
- Tuominen T, Jämsä T, Tuukkanen J, Nieminen P, Lindholm T C, Lindholm T S, Jalovaara P. Native bovine bone morphogenetic protein improves the potential of biocoral to heal segmental canine ulnar defects. Int Orthop 2000; 24: 289–94
- Tuominen T, Jämsä T, Oksanen J, Tuukkanen J, Gao T J, Lindholm T S, Jalovaara P. Composite implant composed of hydroxyapatite and bone morphogenetic protein in the healing of a canine ulnar defect. Ann Chir Gynaecol 2001; 90: 32–6
- Valentin-Opran A, Wozney J, Csimma C, Lilly L, Riedel G E. Clinical evaluation of recombinant human bone morphogenetic protein-2. Clin Orthop 2002, 39: 5110–20
- Viljanen V V, Gao T J, Lindholm T C, Lindholm T S, Kommonen B. Xenogeneic moose (Alces alces) bone morphogenetic protein (mBMP)-induced repair of critical-size skull defects in sheep. Int J Oral Maxillofac Surg 1996; 25: 217–22
- Wozney J M. Overview of bone morphogenetic proteins. Spine 2002; 27: S2–8
- Yudell R M, Block M S. Bone gap healing in the dog using recombinant human bone morphogenetic-2. J Oral Maxillofac Surg 2000; 58: 761–6