Abstract
Background Platelet concentrate application with added thrombin improves Achilles tendon repair in the rat. Upon tissue injury, platelets are activated by thrombin, which has many biological properties in common with growth factors. We wanted to differentiate the effect of platelets from that of thrombin.
Methods The Achilles tendon was transected in 50 rats. Platelet gel was prepared from the blood of 10 other rats. The rats were given either platelet gel with active or neutralized thrombin implanted into the defect during the operation, or a local injection 6h postoperatively with 50 μL of either platelet concentrate, thrombin or saline. The rats were killed after 14 days and the tendons were mechanically tested.
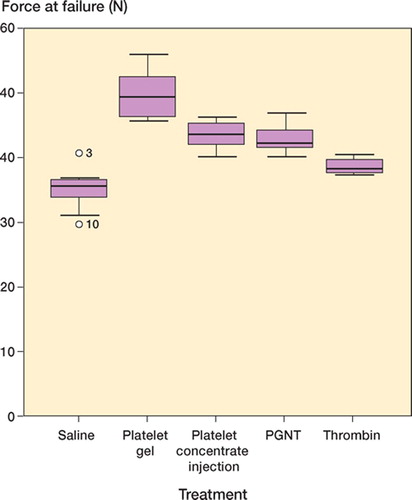
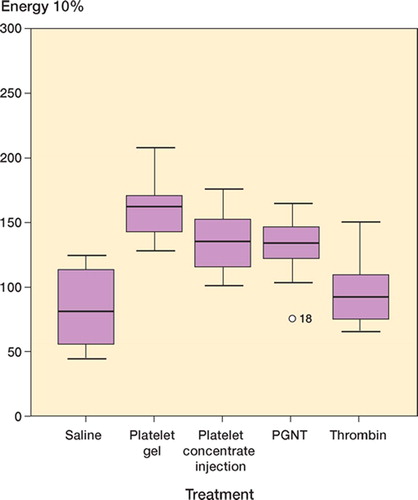
Results Compared to saline, platelet gel caused a 42% increase in force at failure, a 90% increase in energy, and a 61% increase in ultimate stress. Platelet gel with neutralized thrombin caused a 22% increase in force at failure, and energy and stress were less elevated. Injected platelet concentrate caused a 24% increase in force at failure, and thrombin caused a 10% increase. These effects and the differences between treatments were statistically significant.
Interpretation Platelets and thrombin had independent and additive stimulatory effects on tendon repair. The clinical relevance is so far unknown.
Upon injury, platelets release bioactive proteins and growth factors necessary for initiation and acceleration of repair and regeneration. Platelets concentrated in a gel have global applications in surgery, and might be especially useful for soft tissue reconstruction. Equipment for platelet concentrate production in the operating room is now being marketed, but there has been little research on the effects of platelets on repair.
Platelets can be activated by a large number of bioactive molecules. Among them, thrombin is the most powerful activator. Thrombin is also a principal factor in blood clot formation following tissue injury. Furthermore, it has many biological properties in common with growth factors, such as chemotaxis of neutrophils, monocytes, and macrophages (Chen and Buchanan Citation1975), and stimulation of fibroblast proliferation (Bar-Shavit et al. Citation1983). Thrombin accelerates wound healing (Stiernberg et al. Citation2000). The in vitro growth factor-like properties suggest that this role in wound healing is separate from that of clot formation (Glenn et al. Citation1988). Thrombin is a serine protease. Its protease activity is responsible for the transformation of fibrinogen to fibrin. Thrombin can bind and cleave so-called protease-activated cell surface receptors. Non-proteolytic parts of the molecule can activate other receptors.
We have previously found a positive effect of an exogenously applied platelet concentrate with thrombin in tendon repair (Aspenberg and Virchenko Citation2004). Since then, we have improved our handling of the plateles and our interest has been concentrated on the effect of the thrombin added. Does thrombin have an effect of its own? Is the effect on tendon repair a combined effect of platelets and thrombin? To answer those questions, we performed experiments that included not only platelets activated with thrombin, but also platelets without any activation, and activated platelets with added Hirudin—which is a specific high-affinity inhibitor of thrombin, completely blocking its mitogenic effect.
Material and methods
Overview
We compared 5 treatments: (1) platelet gel, containing thrombin-activated platelets and added calcium, (2) platelet gel supplemented with Hirudin in order to neutralize thrombin, (3) injectable platelet concentrate without thrombin, (4) thrombin (in saline), and (5) saline alone. All platelet groups received 4 × 105 platelets in a volume of 50 μL.
Animal model
The study included 60 female Sprague-Dawley rats with a mean body weight of 184 g (170–210), housed 2 per cage at 21°C in a 12-h light and dark cycle and given food and water ad libitum. The regional ethics board had approved the study, and institutional guidelines for the care and treatment of laboratory animals were adhered to.
Preparation of platelet concentrate
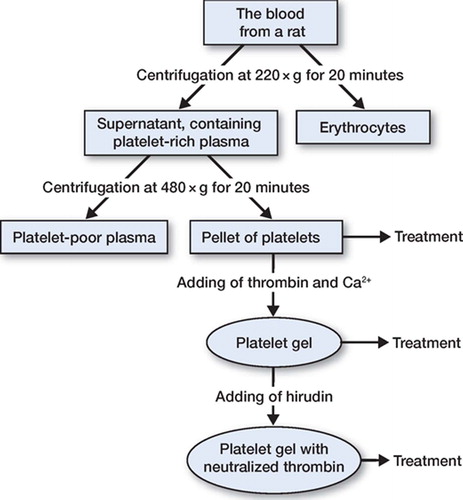
Whole blood was collected from 10 female Sprague-Dawley rats (200 g, M&B, Ry, Denmark). The rats were anesthetized with isoflurane, and 4–6 mL of whole blood was collected by cardiac puncture using a 10-mL syringe containing 1.5 mL of anticoagulant citrate phosphonate dextrose (CPD) buffer (0.15 mg CPD/mL) and a 1.2-mm needle. After blood collection, the animals were killed with an intracardiac injection of an overdose of pentobarbital. The anticoagulated blood was then centrifuged at 220 × g for 20 min. The supernatant, containing platelet-rich plasma, was used for a second centrifugation at 480 × g for 20 min to form a pellet of rat platelets. The platelets were then resuspended in plasma and the cell density was adjusted to 8.3 × 109 platelets/L ().
The platelet concentrate was dispersed in 20 microwells, 50 μL in each, and activated by adding 0.25 U thrombin (1.25 μL) from bovine plasma (Sigma Chemical Co., St. Louis, MO) and 5 IE (5 μL) calcium chloride (Braun Melsungen) at 37°C. The gels in 10 of the microwells received Hirudin (recombinant from yeast; Fluka Chemie GmbH, 20.75 U/vial) at 0.5 U per microwell to neutralize thrombin. The platelet concentrate and platelet gel were irradiated at 25 Gy according to international blood banking standards, to inactivate any remaining white blood cells. The platelet concentrate and platelet gel were stored at 4°C for a maximum period of time of 24 h, and then applied to the transsected Achilles tendons (see below).
Operative procedure and treatment
The rats (n = 50) were anesthetized with 5% isoflurane gas (Forene; Abbot Scandinavia, Solna, Sweden) in an anesthetic induction chamber and then 3.5% in a mask. The skin on the right hind limb was shaved. Antibiotics by way of 5 mg intramuscular injections of tetracycline (Engemycin; Intervet, Boxmeer, Holland), and analgesics in the form of 0.015 mg buprenorphine (Temgesic; Schering-Plough, Brussels, Belgium) were given preoperatively. The animal was placed prone on a warm pad (38.2°C) and the right hind leg was stretched backwards and washed with chlorhexidine (5 g chlorhexidine digluconate, 600 g ethanol, sterile water). A 3-mm transverse incision was made in the skin lateral to the right Achilles tendon. The surrounding fascia was opened and the Achilles tendon complex was exposed. Approximately 7 mm of the plantaris tendon was then removed to simplify force measurements at the end of the experiment. Subsequently, the Achilles tendon was cut transversely 1.5 and 4.5 mm proximal to the calcaneal insertion. Thus, a 3-mm segment was removed. The tendon was left unsutured with a gap between the tendon stumps, and the skin was sutured. There was no postoperative immobilization. After the operation, the animals were placed in clean cages under a heating lamp for constant temperature of 30°C until they awoke completely.
The rats were divided into 5 groups for treatment by a lottery, randomizing each rat groups of 10 at a time. This was done by an assistant during the operation. The rats either received 50 μL (1 piece) of platelet gel in the defect at the operation, or a local injection 6h postoperatively of 50 μL of the platelet concentrate or control solution.
Evaluation
After 14 days, the rats were killed with CO2 gas. The tendon with the attaching calcaneal bone was dissected free of other tissues and removed. Sagittal and transverse diameters were measured with a digital calliper. For clamping, the muscle was scraped off the tendon substance by blunt dissection, to produce a fan of tendon fibers, which was then sandwiched between fine sandpaper in metal clamps. The calcaneus was fixated in a custommade clamp in 30° dorsiflexion, relative to the direction of traction. Finally, the clamps were attached to a materials testing machine (100R; DDL Inc., Eden Praire, MN), and the tendon was pulled at a constant speed of 1 mm/s until failure. Mechanical parameters measured were force at failure, stiffness and energy uptake at 10% droop of the curve. Stress and transverse area were calculated.
Statistics
One-way ANOVA was used for statistical analysis. Post hoc comparisons for differences between groups were made using Bonferroni-Dunn test. A p-value less than 0.05 was considered significant. Statistical analysis was performed with StatView for Windows version 5.0.1 (SAS Institute, Cary, NC).
Results
Compared to saline, platelet gel caused a 42% increase in force at failure, a 90% increase in energy and a 61% increase in ultimate stress. Platelet gel with neutralized thrombin caused a 22% increase in force at failure, a 57% increase in energy and a 35% increase in stress. Platelet concentrate caused a 24% increase in force and a 62% increase in energy. Thrombin caused a 10% increase in force at failure. All these results were statistically significant relative to the saline control (; and ).
Table 1. Effects of platelet gel, platelet gel with neutralized thrombin, platelet concentrate, and thrombin injections compared with saline
The gel had the greatest effect, followed by platelet gel with Hirudin and platelet concentrate— which were similar. Thrombin alone had less effect than the other active treatments. Almost all intergroup comparisons were significant ().
Table 2 A. Intergroup comparisons for force at failure (p-values)
Table 2 B. Intergroup comparisons for energy 10% (pvalues)
Table 2 C. Intergroup comparisons for stress (p-values)
The transverse area of the tendon callus was not significantly different in any of the experimental groups.
Discussion
Our results show that both platelets alone and thrombin alone can improve tendon repair in rats. There is also an additive effect when combining them. The effect of platelets is probably due to the growth factors that are released during activation. The thrombin probably functions by stimulating mitogenic events through interaction with cell surface receptors (Lundblad et al. Citation2004). We have previously found improvement of tendon repair in the same model following one injection of platelet concentrate (Aspenberg and Virchenko Citation2004). Our previous results showed a 27% increased force at failure by platelet concentrate treatment, as compared to a 42% increase by using platelet gel in this study. This difference is probably caused by a difference in dosage (1.5 × 109 platelets/mL in the first experiments and 8.3 × 109 platelets/mL in this experiment) and platelet handling. Preparatory experiments for the present study showed no difference between saline-injected and uninjected controls (data not shown).
None of the tendon regenerates increased in transverse area. The increased strength was instead caused by improved material characteristics. In our previous study, this could also be confirmed by a significantly higher histological scoring for tissue maturity (Aspenberg and Virchenko Citation2004). This effect is quite different from the effects of the growth factor CDMP-2 (cartilage-derived morphogenic protein-2). In the same model, CDMP2 improved strength and stiffness, but this was entirely due to an increase in tendon regenerate size (Forslund and Aspenberg Citation2002).
When the platelet concentrate is activated by thrombin, a gelatinous structure is formed and the wound-healing growth factors are released from the alpha-granules (Whitman et al. Citation1997). These growth factors are involved in cell growth and differentiation, including the normal processes of development and tissue repair. Several growth factors have been found to have roles in tendon healing. Improved mechanical properties of a healing tendon or ligament have been shown after exogenous application of platelet-derived growth factor (PDGF) (Hildebrand et al. Citation1998) and at least 3 members of the transforming growth factorß (TGF-ß) superfamily, i.e. the cartilagederived morphogenic proteins (CDMPs) (Forslund et al. Citation2003). Other growth factors are apparently involved in tendon repair, e.g. vascular endothelial growth factor (VEGF) (Zhang et al. Citation2003), insulinlike growth factor (IGF) (Kurtz et al. Citation1999), and basic fibroblast growth factor (bFGF) (Chan et al. Citation2000).
Platelet products have found clinical applications in orthopedic surgery, maxillofacial surgery, dental implant surgery, and plastic surgery (Anitua et al. Citation2004). Still, there have been few publications reporting enhancement of healing by platelets. In a bone grafting study in goats, platelet-rich plasma (PRP) appeared to enhance bone healing considerably (Fennis et al. Citation2004). Histological analyses of bone defects surrounding titanium implants in dogs showed that PRP increased local bone formation (Kim et al. Citation2002). Human platelet concentrate was found to improve bone ingrowth distance into porous hydroxyapatite in nude rats (Siebrecht et al. Citation2002). Other studies on bone regeneration have failed to show an effect (Jensen et al. Citation2004). Muscle regeneration in rabbits was found to be improved by rabbit platelet concentrate (Jodczyk et al. Citation1986), and in skin wounds, platelet concentrate was found to increase granulation tissue and fibrous tissue formation and epithelial growth (Ksander et al. Citation1990, Carter et al. Citation2003, Henderson et al. Citation2003).
There have been a number of uncontrolled clinical studies reporting a good effect of platelet concentrate in both bone and soft tissue repair (Tischler Citation2002). To our knowledge, there have been no controlled clinical studies on soft tissue repair, and only two controlled clinical studies showing an effect of PRP on bone regeneration. Marx et al. (Citation1998) showed that combining PRP with autogenous bone in mandibular continuity defects in human patients resulted in faster radiographic maturation and a histomorphometrically denser bone regenerate. PRP and bovine porous bone mineral combined with guided tissue regeneration has been more effective in the treatment of human intrabony periodontal defects than guided tissue regeneration alone (Camargo et al. Citation2002).
There are risks associated with thrombin in clinical use. Serious coagulopathies have been reported, after topical exposure to bovine thrombin used as a hemostatic agent in orthopedic, neurosurgical, and cardiovascular surgeries (Cmolik et al. Citation1993, Spero Citation1993, Christie et al. Citation1997). In several cell types such as neurons, astrocytes (Smirnova et al. Citation1998), or tumor cells (Zain et al. Citation2000), thrombin has been shown to induce cell proliferation at low concentrations and apoptosis at high concentrations. We found a positive effect of thrombin in tendon healing, but the concept that “if a little is good, a lot may be better” may not apply. The thrombin concentration needed for optimal platelet aggregation (0.5 U/mL) is much lower than the thrombin concentrations used in other clinical applications (140 U/mL) (Marx et al. Citation1998). The amount of thrombin used in this study appears to be less than would be expected to cause complications. Because exogenous thrombin has a stimulatory effect, one might suspect that inhibition of exogenous thrombin activity (e.g. by low molecular weight heparin) delays healing.
In conclusion, the use of thrombin to activate a platelet gel adds to its efficacy in tendon repair.
Contributions of authors
OV did all animal work, analyzed data and wrote the paper. MG participated in planning, platelet preparation and writing. PA planned the study, analyzed data, and wrote the paper together with OV.
Ali Sodeifi, MSc in biomedical laboratory science, assisted with rat surgery. The study was supported by the Swedish National Center for Sports Research (“Materials in Medicine”), Linköping, Sweden, and by the Swedish Research Council (project no. 2031).
No competing interests declared.
- Anitua E, Andia I, Ardanza B, Nurden P, Nurden A T. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost 2004; 91: 4–15
- Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand 2004; 75: 93–9
- Bar-Shavit R, Kahn A, Fenton J W, 2nd, Wilner G D. Chemotactic response of monocytes to thrombin. J Cell Biol 1983; 96: 282–5
- Camargo P M, Lekovic V, Weinlaender M, Vasilic N, Madzarevic M, Kenney E B. Platelet-rich plasma and bovine porous bone mineral combined with guided tissue regeneration in the treatment of intrabony defects in humans. J Periodontal Res 2002; 37: 300–6
- Carter C A, Jolly D G, Worden C E, Sr, Hendren D G, Kane C J. Platelet-rich plasma gel promotes differentiation and regeneration during equine wound healing. Exp Mol Pathol 2003; 74: 244–55
- Chan B P, Fu S, Qin L, Lee K, Rolf C G, Chan K. Effects of basic fibroblast growth factor (bFGF) on early stages of tendon healing: a rat patellar tendon model. Acta Orthop Scand 2000; 71: 513–8
- Chen L B, Buchanan J M. Mitogenic activity of blood components. I Thrombin and prothrombin. Proc Natl Acad Sci U S A 1975; 72: 131–5
- Christie R J, Carrington L, Alving B. Postoperative bleeding induced by topical bovine thrombin: report of two cases. Surgery 1997; 121: 708–10
- Cmolik B L, Spero J A, Magovern G J, Clark R E. Redo cardiac surgery: late bleeding complications from topical thrombin-induced factor V deficiency. J Thorac Cardiovasc Surg 1993; 105: 222–7, discussion 27-8
- Fennis J P, Stoelinga P J, Jansen J A. Mandibular reconstruction: a histological and histomorphometric study on the use of autogenous scaffolds, particulate cortico-cancel-lous bone grafts and platelet rich plasma in goats. Int J Oral Maxillofac Surg 2004; 33: 48–55
- Forslund C, Aspenberg P. CDMP-2 induces bone or tendonlike tissue depending on mechanical stimulation. J Orthop Res 2002; 20: 1170–4
- Forslund C, Rueger D, Aspenberg P. A comparative doseresponse study of cartilage-derived morphogenetic protein (CDMP)-1, -2 and -3 for tendon healing in rats. J Orthop Res 2003; 21: 617–21
- Glenn K C, Frost G H, Bergmann J S, Carney D H. Synthetic peptides bind to high-affinity thrombin receptors and modulate thrombin mitogenesis. Pept Res 1988; 1: 65–73
- Henderson J L, Cupp C L, Ross E V, Shick P C, Keefe M A, Wester D C, Hannon T, McConnell D. The effects of autologous platelet gel on wound healing. Ear Nose Throat J 2003; 82: 598–602
- Hildebrand K A, Woo S L, Smith D W, Allen C R, Deie M, Taylor B J, Schmidt C C. The effects of platelet-derived growth factor-BB on healing of the rabbit medial collateral ligament. An in vivo study. Am J Sports Med 1998; 26: 549–54
- Jensen T B, Rahbek O, Overgaard S, Soballe K. Platelet rich plasma and fresh frozen bone allograft as enhancement of implant fixation. An experimental study in dogs. J Orthop Res 2004; 22: 653–8
- Jodczyk K J, Bankowski E, Borys A. Stimulatory effect of platelet-breakdown products on muscle regeneration. Zentralbl Allg Pathol 1986; 131: 357–61
- Kim S G, Chung C H, Kim Y K, Park J C, Lim S C. Use of particulate dentin-plaster of Paris combination with/with-out platelet-rich plasma in the treatment of bone defects around implants. Int J Oral Maxillofac Implants 2002; 17: 86–94
- Ksander G A, Sawamura S J, Ogawa Y, Sundsmo J, McPherson J M. The effect of platelet releasate on wound healing in animal models. J Am Acad Dermatol 1990; 22: 781–91
- Kurtz C A, Loebig T G, Anderson D D, DeMeo P J, Campbell P G. Insulin-like growth factor I accelerates functional recovery from Achilles tendon injury in a rat model. Am J Sports Med 1999; 27: 363–9
- Lundblad R L, Bradshaw R A, Gabriel D, Ortel T L, Lawson J, Mann K G. A review of the therapeutic uses of thrombin. Thromb Haemost 2004; 91: 851–60
- Marx R E, Carlson E R, Eichstaedt R M, Schimmele S R, Strauss J E, Georgeff K R. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998; 85: 638–46
- Siebrecht M A, De Rooij P P, Arm D M, Olsson M L, Aspenbereg P. Platelet concentrate increases bone ingrowth into porous hydroxyapatite. Orthopedics 2002; 25: 169–72
- Smirnova I V, Zhang S X, Citron B A, Arnold P M, Festoff B W. Thrombin is an extracellular signal that activates intracellular death protease pathways inducing apoptosis in model motor neurons. J Neurobiol 1998; 36: 64–80
- Spero J A. Bovine thrombin-induced inhibitor of factor V and bleeding risk in postoperative neurosurgical patients. Report of three cases. J Neurosurg 1993; 78: 817–20
- Stiernberg J, Norfleet A M, Redin W R, Warner W S, Fritz R R, Carney D H. Acceleration of full-thickness wound healing in normal rats by the synthetic thrombin peptide, TP508. Wound Repair Regen 2000; 8: 204–15
- Tischler M. Platelet rich plasma. The use of autologous growth factors to enhance bone and soft tissue grafts. N Y State Dent J 2002; 68: 22–4
- Whitman D H, Berry R L, Green D M. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg 1997; 55: 1294–9
- Zain J, Huang Y Q, Feng X, Nierodzik M L, Li J J, Karpatkin S. Concentration-dependent dual effect of thrombin on impaired growth/apoptosis or mitogenesis in tumor cells. Blood 2000; 95: 3133–8
- Zhang F, Liu H, Stile F, Lei M P, Pang Y, Oswaald T M, Beck J, Dorsett-Martin W, Lineaweaver W C. Effect of vascular endothelial growth factor on rat Achilles tendon healing. Plast Reconstr Surg 2003; 112: 1613–9