Abstract
Background The intrinsic healing capacity of articular cartilage remains poor, despite various attempts that have been undertaken to treat cartilage defects. This study describes the experimental use of a double-layer bioimplant consisting of a bone-substitute layer and a cartilage-substitute layer.
Animals and methods In group A, 12 implants were placed into osteochondral defects of the load-bearing area of rabbit femoral condyles. In group B, 12 implantations were done in the same manner, with a separating membrane consisting of cement between both layers to investigate ingrowth of mesenchymal stem cells from the subchondral marrow space. Group C, with 12 similar defects but without treatment, served as control. Investigations by microscopy and immunohistochemistry were done after 12 weeks.
Results All bioimplants showed coverage of the defect with a regeneration tissue that contained cartilage-like regions. Implants with a cement layer showed less cartilage and more fibrous tissue. The bioimplant group showed more cartilage-like regeneration tissue than the control groups, which only showed incomplete fibrous filling of the defects. Results from the second group supported the hypothesis that the subchondral space must be opened for adequate regeneration. Additional examinations were done using an established semiquantitative score. The bioimplant group showed a significant improvement in results compared to the group with the separating layer and the control group.
Interpretation Our findings indicate that cartilage repair by resorbable bioimplants seems to be a promising new approach, especially if mesenchymal stem cells are present and can differentiate under mechanical load.
In the past, several attempts have been made to improve the healing ability of hyaline cartilage. These methods have ranged from debridement, Pridie drilling or microfracture to autologous osteochondral transplants (OCT) and the implantation of autologous chondrocytes (ACI). Unfortunately, none of these procedures has been able to ensure a complete repair of the defect. Furthermore, studies exist that show that the “advanced” techniques are no better than the established ones. For example, Horas et al. (Citation2000) compared the clinical outcome of OCT to the outcome of ACI. No signigficant difference could be seen in their randomized trial but the OCT group showed marginally better results. Even when compared to microfracture, there was no significant difference in the results of the patients treated with microfracture and those who received ACI, although the patients who received microfracture showed significantly better results in the SF-36 physical component scores (Knutsen et al. Citation2004).
One common problem of both ACI and OCT is the need for a donor site within a joint. It is doubtful whether one can remove part of the cartilage of a joint without creating future problems at the donor site. A number of authors have described the capability of mesenchymal stem cells to differentiate to chondrocytes if subjected to intermittent mechanical loading (Tägil and Aspenberg Citation1998a, Citationb). Unfortunately, the stem cells migrating into the defect will be destroyed if full weight bearing is allowed early after the operation. We developed a protective structure that allows the stem cells to differentiate to chondrocytes without being destroyed by mechanical loads that are too high. In order to allow for this differentiation without disturbing the organization of normal cartilage architecture, we deduced the following requirements: 1. The implant should consist of 2 layers with different mechanical properties, thus paying heed to the different properties of the replaced bone and cartilage tissues. 2. The implant should allow full weight bearing immediately after the implantation to induce cell differentiation. 3. No precultured and potentially differentiated cells should be used. 4. In order to allow ingrowth of mesenchymal stem cells, adherence to the implant, and differentiation to hyaline cartilage under the influence of bioactive substances, the subchondral plate must be opened.
Some authors have hypothesized that the regeneration of the articular cartilage is not due to ingrowth of mesenchymal stem cells but to redifferentiation of fibroblasts or to the ingrowth of the neighboring cartilage. To investigate this theory, we blocked the possible migration of cells from the underlying bone to the regenerated cartilage in another control group. This animal study examines whether a new resorbable implant is capable of improving the repair of osteochondral defects in rabbits as compared to either empty defects or to a group in which the ingrowth of cells from bone marrow into the cartilage replacement layer has been blocked by means of a cement layer. This was done in order to determine the origin of the immigrating cells.
Animals and methods
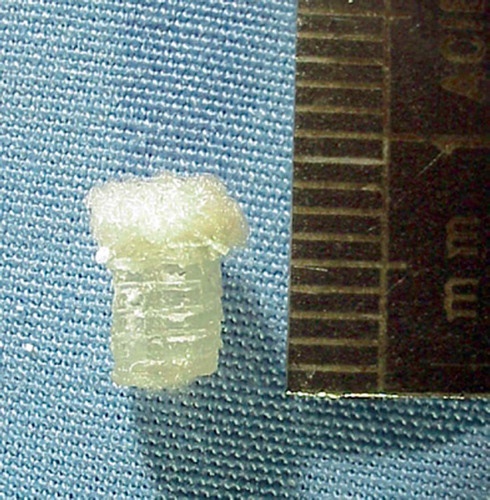
The bioimplants used consisted of 2 layers. The bone substitution layer was made of poly-D,L-lactide (Resomer R207; Boehringer Ingelheim, Ingelheim, Germany) by a jet solidification process performed in co-operation with the Fraunhofer Society, Stuttgart, Germany. Poly-D,L-lac-tide is a pure amorphous substance that is resorbed in 40–50 weeks and is known not to cause aseptic fistulae (Pistner et al. Citation1993). Using this process, an open porous cylinder 4 mm in diameter and 6 mm high was built out of single bars with a thickness of 400 μm and a pore width of 600 μm. Young's modulus was 72 MPa (measured in the linear section of the force-deformation diagram). The surface of the cylinder was curved in order to roughly resemble the physiological contour of the condyle. A cartilage substitution layer consisting of a poly-glactin/polydioxanon fleece (Ethisorb 210; Ethicon, Norderstedt, Germany) was attached to this cylinder with a resorbable glue. The fleece used is made of single filaments 10 μm wide and dissolves in about 70 days (). In order to investigate the colonization of the cartilage substitute layer, the ingrowth of cells from the bone marrow was blocked by introducing a very fine milled bone cement layer between the cartilage and the bone substitute layer (Biosorb; SBM S.A., Lourdes, France). This was done to determine whether cells from either the surrounding cartilage or the joint, e.g. synovial cells, are capable of colonizing the bioimplant and producing a regenerate.
In this animal study 3 groups were used, consisting of 6 implant sites each. Group A received bioimplants without the separating layer, group B received bioimplants with the separating cement layer, and the defects of group C were left untreated. The animals were adult chinchilla bastard rabbits (Charles River, Sulzfeld, Germany) that were at least 1 year old and with a body weight of 3–4 kg.
A round defect in the medial femoral condyle of 4 mm in diameter and 6 mm in depth was made using a hand drill. These defects were either filled with a bioimplant (with and without cement layer) or left untreated. During the drilling process, the depth was checked continuously for an accurate depth of 6 mm. The bioimplants were inserted press-fit without any additional fixation or glue. Finally, the wound was closed. During the postoperative period pain control was achieved with metamizole drops in water, and the animals were allowed full weight bearing in their cage. No animals were excluded due to infection, illness or implant failure.
After 12 weeks, the animals were killed using phenobarbital injections and the operated femoral condyle was removed. The specimens were fixed in 100% methanol for 1 week at 4°C and decalcified in 5% EDTA solution. The specimens were then cut into 12-μm sections using a cryomicrotome and stained with hematoxylin and eosin. Immunolabeling for type II collagen was done according to previously published protocols (Milz et al. Citation1999). The specimens were evaluated using the score of Pineda et al. (Citation1992). We tried to blind the histologist examining the slices to the treatment but as the remains of the implant could still be seen, the treatment was easily recognized.
Statistics
The distribution and kind of data suggested the using of non-parametric test procedures; thus, the Kruskal-Wallis test and Mann-Whitney U-test were used. In a first step, the Kruskal-Wallis test was used to check the null hypothesis. The null hypothesis was rejected at a p-value of < 0.05. Afterwards 3 U-tests were done for pair comparison without alpha inflation. The 2-sided U-test was used.
Results of histological evaluation (scores shown are averages)
Results
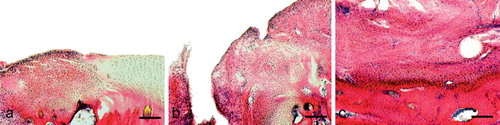
The group that received the bioimplant without the separating layer showed a mostly even articular surface with newly formed bone in the implant (). Using the semiquantitative score, this group achieved an average score of 6.0 (SD 3.2) (Table). The group with the separating layer showed next to no bony regeneration and no regeneration of articular cartilage (). This group achieved an average of 10.3 (SD 2.8) and the control group 10.0 (SD 3.8) using the score of Pineda. The control group showed a fibrous regenerate without bony or cartilaginous regeneration ().
Figure 2. Histology of an osteochondral defect 12 weeks after insertion of the bioimplant. Hematoxylin-eosin staining. A. Regenerate 12 weeks after implantation of the bioimplant. The surface is mainly even, with a little step in the transition zone to normal cartilage (at the left). Bar represents 220 μm. B. Higher-power image of a cartilage regenerate. The rest of the polyglactin/polydioxanon is still visible (arrow). Bar represents 200 μm. C. Higher-power image of the bone replace ment Bar represents 90 μm.
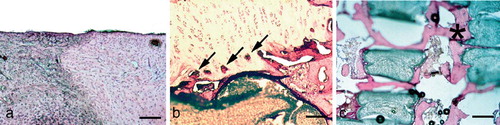
Figure 3. Histology of an osteochondral defect 12 weeks after insertion of the bioimplant modified with a cement separation layer. Hematoxylin-eosin staining. Bar represents 180 μm. A. Border of a defect. The defect zone on the right shows no covering with regenerated tissue, but exposed subchondral bone. The morphology of the cartilage at the border shows signs of degeneration such as clustering. B. At the center of the defect, under the level of joint cartilage there is hypercellular fibrous tissue almost without bony new formation. C. At the base of the defect, there is a sudden transition from fiber-rich tissue to cancellous bone. There is some hypercellular tissue in the bone marrow.
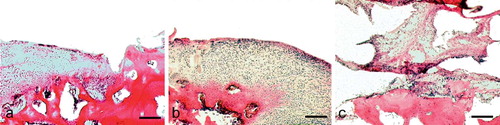
Figure 4. Histology of an osteochondral defect 12 weeks after operation without bioimplant (defect control). Hematoxylineosin staining. Bar represents 180 μm. A. Border of the defect, showing integration of the hypercellular regenerate to the original cartilage on the right. B. At the center of the defect, under the level of the joint cartilage, there has been regeneration of deep craters of fibrous tissue. No continuity of the joint surface remains. C. At the base of the defect, there is an abrupt transition from fibrous tissue to bone stock. There is no cartilaginous regeneration of the defect.
We found differences between the bioimplant group (group A) and both the group with the separating layer (group B) (p = 0.04) and the control group (group C) (p = 0.03). No significant difference was found between groups B and C (p = 0.8).
Discussion
Isolated cartilage defects are a major problem because cartilage does not show a significant healing potential, and isolated defects commonly progress to degeneration and destruction of the joint (Buckwalter et al. Citation2004).
Both mesenchymal stem cells and cells from the perichondrium are believed to be able to differentiate to chondrocytes under the right conditions. Based on this idea, we developed a biodegradable implant which allows cells from the subchondral space to grow within it and to form new cartilage and bone under the mechanical load from normal joint movement. The implant was therefore designed to have a defined stiffness, in order to transmit some load to the induced tissue. It had to be an open porous structure to allow ingrowth of cells and vessels from the subchondral marrow cavity. To avoid induction of tissue degeneration and to allow the formation of normal tissue, the bioimplant had to be resorbable. Because of the different elasticity and the different loading patterns of cartilage and bone, the implant had to consist of two different layers. The elasticity of the bone substitute layer had to assure a certain amount of stability to transmit load onto the cartilage substitute layer, to allow differentiation of ingrown cells to cartilage under local compression. At the same time, load transmission to the bone substitute layer was necessary to facilitate ossification. Thus, a modulus of elasticity of the bone substitute layer was chosen which was smaller than that of cancellous bone in order to increase the load onto the ingrown new bone with time. If the hypotheses on functional adaptation proposed by Kummer (Citation1995), Carter et al. (Citation1991) and Huiskes et al. (Citation1987) are true, the amount of regenerated bone should create a biomechanical balance where the stiffness of the bone substitute layer/newly formed bone construct equals the physiological conditions of the adjacent bone tissue. In this study we have demonstrated that by and large, this approach leads to the results expected.
Our results are also in line with other reports in the literature. Niederauer et al., for example, could show a good regeneration of osteochondral defects when comparing different multiphase implants in goats. The histological samples reached average scores of 15.3–16 out of 25 points in the semiquantitative score used (Niederauer et al. Citation2000). Unfortunately, no control group was examined—so the results cannot be easily compared to those of other groups, but, while Niederauer and colleagues stated that the lack of blood within the defect is important for the healing, our control group has shown the importance of ingrowth of cells from the marrow.
Other authors have tried different materials to build their implants. Carranza-Bencano et al. (Citation2000) tested the use of ready-made carbon fiber pads to help the repair of osteochondral defects in the rabbit patella. Using Wakitani's score for histological evaluation, they found a significant improvement in results when comparing the implant and control groups after a follow-up period of 1 year. These results are similar to ours, even though the follow-up time in our study was shorter. However, since other authors report osteolysis after the implantation of carbon fiber implants, their use is still controversial (Steinwachs and Kreuz Citation2003).
We conclude that the implants we used in this study give promising results when used in osteochondral lesions in rabbit knees. Due to limitations of the rabbit as a model for osteochondral defects in humans, however, a new evaluation in a more suitable animal model (e.g. sheep) is necessary to properly assess the value of this technique for the treatment of human osteochondral lesions.
No competing interests declared.
Contributions of authors
PEM: implant design, animal experiments, histological evaluation and editing of manuscript. FS: animal experiments, histological evaluation and editing of manuscript. SM: histological evaluation and editing of manuscript. JK: histological evaluation and statistical analysis. HRD: animal experiments and histology. BW: animal experiments and statistical analysis. CP: manufacture of implants. VJ implant design, animal experiments and editing of manuscript.
- Buckwalter J, Rosenberg L, Hunziker E. Articular cartilage: Composition, structure, response to injury and methods of faciliating repair. Articular cartilage and knee function: Basic science and arthroscopy 19. Raven Press, New York 2004
- Carranza-Bencano A, Armas-Padron J, Gili-Miner M, Lozano M. Carbon fiber implants in osteochondral defects of the rabbit patella. Biomaterials. 2000; 21: 2171–6
- Carter D, Wong M, Orr T. Musculosceletal ontogeny, phylogeny and functional adaptation. J Biomech 1991; 24(Suppl 1)3–16
- Horas U, Schnettler R, Pelinkovic D, Herr G, Aigner T. Osteochondral transplantation versus autogenous chondrocyte transplantation. A prospective comparative clinical study. Chirurg 2000; 7(9)1090–7, German
- Huiskes R, Weinans H, Grootenboer H J, Dalstra M, Fudala B, Slooff T J. Adaptive bone-remodeling theory applied to prosthetic-design analysis. J Biomech 1987; 20(11–12)1135–50
- Knutsen G, Engebretsen L, Ludvigsen T C, Drogset J O, Grontvedt T, Solheim E, Strand T, Roberts S, Isaksen V, Johansen O. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg (Am) 2004; 86(3)455–64
- Kummer B. Basics of Pauwels’ theory of the functional adaptation of bones. Orthopade 1995; 24(5)387–93, German
- Milz S, Putz R, Ralphs J, Benjamin M. Fibrocartilage in the extensor tendons of the human metacarpophalangeal joints. Anat Rec 1999; 256(2)139–45
- Niederauer G G, Slivka M A, Leatherbury N C, Korvick D L, Harroff H H, Ehler W C, Dunn C J, Kieswetter K. Evaluation of multiphase implants for repair of focal osteochondral defects in goats. Biomaterials 2000; 21(24)2561–74
- Pineda S, Pollack A, Stevenson S, Goldberg V, Caplan A. A semiquantitative scale for histologic grading of articular cartilage repair. Acta Anat (Basel) 1992; 143(4)335–40
- Pistner H, Gutwald R, Ordung R, Reuther J, Muhling J. Poly(L-lactide): a long-term degradation study in vivo. I. Biological results. Biomaterials 1993; 14(9)671–7
- Steinwachs M R, Kreuz P C. Combinations of different cartilage resurfacing techniques. Z Orthop Ihre Grenzgeb 2003; 141(6)625–8, German
- Tägil M, Aspenberg P. Cartilage formation by controlled mechanical stimulation. A titanium chamber study in rats. 8th annual meeting, Amsterdam. European Orthopaedic Research Society, 1998a
- Tägil M, Aspenberg P. Cartilage induction by controlled mechanical stimulation. A chmaber study in rats. 44th annual meeting, New Orleans, Louisiana. Orthopaedic Research Society, 1998b