Abstract
Introduction Local application of growth factors to stimulate wound and fracture healing is attracting increasing interest. We studied the effect of local application of a potent angiogenic growth factor, basic fibroblast growth factor (bFGF), on resistance to local infection after soft tissue trauma.
Methods For in-vitro and in-vivo experiments, we used recombinant human bFGF. The in-vitro investigations were performed by isolation of human leukocyte fractions, cytokine analysis, phagocytosis assay, flow cytometry, and LDH assay. For the in-vivo investigation, a paired comparison of infection rates was carried out on Sprague-Dawley rats after standardized, closed soft tissue trauma and local, percutaneous bacterial inoculation of different concentrations of Staphylococcus aureus (2 × 104 to 2 × 107 colony-forming units (cfu)). The lower leg was treated with 1, 10 or 100 ng bFGF (16 animals for each concentration) and without bFGF (16 animals).
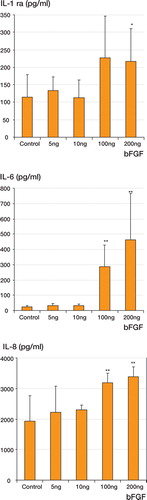
Results Cytotoxic reactions due to the concentrations of bFGF used could be excluded in the in-vitro tests since incubations of isolated peripheral blood mononuclear cells (PBMCs) with increasing concentrations of bFGF for 24 h did not lead to an increase in the release of lactate dehydrogenase in the culture supernatants compared to corresponding control incubations without any bFGF added. A significant increase in cytokine release was observed after the co-incubation of PBMCs with 100 or 200 ng of the same bFGF that was used for the animal experiments. Furthermore, the capacity of phagocytes in whole blood to phagocytose bacteria was suppressed in the presence of 100 ng exogenously added bFGF. We found continuously reduced granulocytic phagocytosis in FGF-supplemented blood compared to non-supplemented blood.
In the in-vivo investigation, the infection rate for the group without bFGF was 0.25. In the groups with 1, 10 and 100 ng bFGF, the infection rates were 0.5, 0.7 and 0.8, respectively. A dose-dependent increase in infection rate was observed after local application of bFGF, compared to the untreated control group. The difference in infection rates for the groups in which 10 and 100 ng bFGF was used, relative to the group without bFGF, was statistically significant.
Interpretation If these initial results are confirmed for other potent angiogenic growth factors, then the local use of growth factors for stimulation of wound and bone healing—a main focus of current research in traumatology—will have to be reconsidered and preceded with a strict evaluation of the risks and benefits.
Regarding the current pathophysiological model, local posttraumatic infection of the locomotor system is the result of an imbalance between the amount, virulence and pathogenicity of the microorganisms introduced on the one hand, and insufficient capacity of the host immune response on the other. The injury-induced structural and functional damage to the local tissue leads to an impairment in humoral immunocompetence (cytokines, growth factors and others) and cellular immunocompetence (granulocytes, macrophages, lymphocytes, etc.) due to devascularization, reduced perfusion, disturbed endothelial permeability, hypoxia, acidosis, hematoma, and edema, together with increased intracompartmental. Thus, rapid and specific activation of the immune system at both the local and the systemic levels is of prime importance for an adequate resistance to infection after trauma. A prerequisite for the rapid effect of immunocompetent cells at the trauma site is the earliest possible restoration of vascularization/perfusion of the damaged tissue—to ensure the permanent recruitment of immunocompetent cells for protection against infection within the traumatized tissue and to induce rapid wound healing by the formation of granulation tissue.
Clinical and animal experiments have shown that local application of growth factors may accelerate this restoration of vascularization/perfusion and lead to improvement of wound and fracture healing (Mustoe et al. Citation1987, Sprugel et al. Citation1987, Rifkin andMoscatelli Citation1989, Klingbeil et al. Citation1991, Yasko et al. Citation1992, Albertson et al. Citation1993, Lind et al. Citation1993, Kawaguchi et al. Citation1994, Nash et al. Citation1994, Nielsen et al. Citation1994, Steenfos et al. Citation1994, Critchlow et al. Citation1995, Heckman et al. Citation1995, Nagai et al. Citation1995, Richard et al. Citation1995, Okumura et al. Citation1996, Wang and Aspenberg Citation1996, Beer et al. Citation1997, Davidson et al. Citation1997, Leunig et al. Citation1997, Bostrom and Camacho Citation1998, Kato et al. Citation1998, McGee et al. Citation1998, Nakamura et al. Citation1998, Wieman et al. Citation1998, Corral et al. Citation1999, Radomsky et al. Citation1999, Bouxein et al. Citation2001, Govender et al. Citation2002, Luppen et al. Citation2002, Einhorn et al. Citation2003, Hom and Manivel Citation2003).
One of these growth factors, basic fibroblast growth factor (bFGF), has particularly high potency. It has been possible to show in in-vitro experiments that the mobilization and proliferation of endothelial cells can be increased by a factor of 10 by administration of bFGF (Joseph-Silverstein and Rifkin Citation1987). Rifkin et al. (Citation1989) showed that the local application of bFGF promoted more rapid neovascularization of the rabbit cornea and of the yoke in hen's eggs. In studies of McGee et al. (Citation1998) and Sprugel et al. (Citation1987) local application of bFGF in incision wounds and subcutaneous wound chambers was found to lead to increased accumulation of fibroblasts, macrophages and capillaries (Sprugel et al. Citation1987, McGee et al. Citation1998). In a similar model, antiserum against bFGF was found to prevent the production of granulation tissue (Broadley et al. Citation1989). Investigation of skin transplants has shown that the survival rate of an implant after administration of bFGF rises, due to an increase in revascularization (Burges Citation1989). In animal models with diminished wound healing (mice with diabetes, with obesity, or in receipt of steroid treatment), bFGF increased the rate of wound healing (Richard et al. Citation1995). Likewise, local application of bFGF in patients with diabetic ulcer led to an increase in healing rates (Albertson et al. Citation1993, Okumura et al. Citation1996). However, no studies investigating whether the local application of bFGF has a favorable effect on the course and extent of bacterial infections of the soft tissues have been reported to date. Based on the pathophysiological model of wound healing, and taking the biological function of bFGF into account, local application of bFGF after severe soft tissue trauma may minimize the infection risk or promote more rapid healing of manifest infection. The effect of bFGF on the risk of local infection would be an indirect one. An increase in neovascularization may lead to more rapid mobilization of both the immunocompetent cells required to fight infection and the factors required for granulation. The possibility of a direct stimulatory and/or suppressive effect of bFGF on the growth of Gram-positive bacteria has been excluded in in-vitro experiments (Okumura et al. Citation1996). Systemic effects and associated side effects following local application of bFGF have not been shown in any of the existing studies.
Material and methods
For the in-vitro and in-vivo experiments, recombinant human basic fibroblast growth factor (bFGF; the 146-amino acid form) was obtained from R&D Systems (Wiesbaden, Germany). The endotoxin level in this preparation was <0.1 endotoxin unit/ L of protein (R&D Systems). We selected bFGF as angiogenic growth factor because, besides its angiogenic potency, it possesses other characteristics that might lead to a reduction in infection rates. Moreover, bFGF increases the mobilization and proliferation of endothelial cells, leads to local accumulation of fibroblasts and macrophages, and accelerates wound granulation.
In-vitro investigation
Isolation of human leukocyte fractions
Peripheral blood mononuclear cells (PBMCs) were isolated from EDTA-anticoagulated peripheral blood (9 mL Monovette; Sarstedt, Nürnbrecht, Germany) by a single-step procedure based on a discontinuous double Ficoll gradient as described by Köller et al. (Citation2001). This method led to viable PBMCs with more than 95% purity. Isolated cells were adjusted to 1 × 106cells/mL RPMI 1640 cell culture medium (Invitrogen, Eggenstein, Germany) supplemented with L-glutamine (0.3 g/L) and sodium bicarbonate (2.0 g/L), 10% fetal calf serum (FCS) (Invitrogen), and 20 mm N-(2-hydroxyethyl)-piperazine-N′-(2-ethanesulfonic acid) (Sigma-Aldrich, Deisenhofen, Germany).
Cytokine analysis
Cytokine concentrations (interleukin-1 receptor antagonist (IL-1ra), IL-6, IL-8) in the supernatants of PBMCs that were incubated in the absence and presence of different concentrations of bFGF (5 ng/mL, 10 ng/mL, 100 ng/ mL, and 200 ng/mL) were quantified by enzymelinked immunosorbant assay (ELISA). Antibodies and recombinant human cytokines (standards) were supplied by R&D Systems. Volumes of 100 μL were used in all ELISA procedures, except for 200 μL of blocking solution (PBS containing 1% BSA) and washing buffer (PBS-Tween 0.05%). Quantification was done following the manufacturer's ELISA protocol. ELISA microtiter plates were read with an ELISA reader (MRX Revelation; Dynex Technologies, Denkendorf, Germany) set to 450 nm. Quantification of cytokine concentrations was performed using recombinant human cytokines as standards. The sensitivities of the immunoassays were 25 pg/mL for IL-1ra, 0.6 pg/mL for IL-6, and 10 pg/mL for IL-8.
Phagocytosis assay
After preincubation of whole heparinized blood (Li-Heparin-Monovette; Sarstedt) for 10 min at 37°C in the absence or presence of different concentrations of bFGF (5 ng/mL, 10 ng/mL, 100 ng/mL, 200 ng/mL) the phagocytic function of granulocytes was analyzed using a fluorescein-labeled opsonized Escherichia coli diagnostic kit (PHAGOTEST; Orpegen, Heidelberg, Germany). This assay allowed the quantitative determination of cells that ingested bacteria, and also of ingested bacteria, while excluding bacteria attached to cellular surfaces. The assay procedure was followed according to the manufacturer's instructions. Fluorescence signals were measured using a flow cytometer (FACSCalibur; see below). Fluorescence signals greater than fluorescence signals obtained after an identical incubation of corresponding polymorphonuclear neutrophil leukocytes (PMN) fractions with E. coli in an ice/water mixture were scored as a positive phagocytosis.
Flow cytometry
Flow cytometry was performed using a FACSCalibur (BD Biosciences, Heidelberg, Germany) and fluorescence data were analyzed using CELLQuest 1.2.2 software (BD Biosciences). Calibration reagents and solutions for flow cytometry were also from BD Biosciences. For each analysis, 104 cells were acquired. Fluorescence values were expressed as mean fluorescent intensity (MFI).
LDH release
The cytosolic enzyme lactate dehydrogenase (LDH) is released when the cells are damaged. Release of LDH into cell supernatants was measured with a microtiter plate-based assay (CytoTox 96; Promega, Madison, WI).
In-vivo investigation
For the in-vivo investigation, female Sprague-Dawley rats were used. We decided to use the rat because it is used routinely in experiments that address immunological and molecular-biological issues and those dealing with infection, and because the rat is an animal for which the necessary reagents (growth factors, primers, antibodies, etc.) are available most readily and at relatively low cost. This aspect of animal selection should not be disregarded, especially when looking ahead to other essential investigations that will give a better understanding of pathophysiological changes after soft tissue trauma.
We performed a paired comparison of local infection rates after standardized, closed soft tissue trauma to the anterior tibial muscle and local, percutaneous inoculation of bacteria at different concentrations (1 × 105, 2 × 105, 1 × 106 and 2 × 107 cfu) in female Sprague-Dawley rats without administration of bFGF (group I) and with 1, 10 and 100 ng bFGF (groups II, III, and IV). The bacteria and the bFGF were injected into the anterior tibial muscle immediately after trauma to the soft tissues. We used a computer-assisted, controlledimpact technique powered by an electric motor, as used by Kälicke et al. (Citation2003) and Schaser et al. (Citation1999), to produce a standardized degree of closed soft tissue trauma. An impulse of 0.28 kgm/s was applied to the anterior tibial muscle of the lower leg. The diameter of the piston was 10 mm. The right lower leg was shaved and positioned in a plastic mold to avoid passive movement, and to ensure optimal energy transmission. Immediately after contusion and injection of the bacteria and bFGF, we performed radiographic controls of the right tibia in two views to exclude fractures and preexisting pathologies in the tibia. The impulse was selected so that it would not fracture the bone beneath the soft tissues, and would not cause compartment syndrome. The procedure did not result in an open wound. The clinically visible signs of soft tissue inury were swelling of the soft tissues and bruising.
The study was approved by the National Animal Protection Authority (number 50.8720 3.68, Bezirksregierung Arnsberg, Germany).
Bacterial inoculum
We obtained a human-pathogenic beta-hemolysing phage-typed Staphylococcus aureus strain (V 8189-94) from an infected hip prosthesis and prepared a broth inoculum suspension as described by Melcher et al. (Citation1994). We used bacterial concentrations of 1 × 105 to 2 × 107 cfu per 100 mL.
Recombinant human bFGF was aseptically dissolved in 0.1 mL NaCl. For groups II, III and IV, 1 ng, 10 ng or 100 ng amounts (respectively) were dissolved in 0.1 mL NaCl and subsequently administered to the anterior tibial muscle. In group I (the control group), 0.1 mL NaCl only was administered to the anterior tibial muscle.
Anesthesia
The animals were anesthetized before application of the standardized closed soft tissue trauma, and inoculation of Staphylococcus aureus and bFGF. We used isofluran in an induction chamber followed by intraperitoneal application of 2.0–2.5 mL/kg midazolam/fentanyl/fluanison. Postoperative analgesia was performed with 0.2 mg/kg buprenorphine in a flavored gelatine base.
Microbiology
After 7 days, the animals were killed by CO2 inhalation in an induction chamber. The anterior tibial muscle and the underlying tibia were removed under sterile conditions and quantitatively and qualitatively evaluated for bacterial growth. To achieve this, the soft tissue and the bone were plated out onto agar plates. The samples obtained were then weighed, crushed in a sterile mortar, and poured into liquid tryptone soya agar. After hardening of the agar, all the samples were incubated for 24 h at 37°C. The aim was to count the colonies growing in the filled Petri dishes with a counter pen, and to relate the results to the initial weight of the sample in grams. Infection was defined as positive bacterial growth in the soft tissues and/or bone. From a theoretical standpoint, this has to be regarded as an all-or-nothing definition, whereby even proof of a single bacterial colony is rated as positive bacterial growth. Lysotyping of positive bacterial growth was conducted according to standard international phenotyping procedures (International bacterioPhage Set).
Experimental procedure and statistics
We applied a grouped sequential experimental procedure with an “up-and-down” dosage technique. As described in previous publications, this technique allows the determination of the level of bacterial concentrations at which differences in the infection rates of the groups being compared are most evident (Melcher et al. Citation1994, Citation1995, Hauke et al. Citation1996, Arens et al. Citation1996, Citation1999a, Citationb, Kälicke et al. Citation2003). This concentration is close to the level of the infection dose of 50% (ID50) (Reed and Münch Citation1938). In this procedure, the bacterial concentration is sequentially adapted to the predicted ID50 in each test phase, whereby a high bacterial concentration was used initially in the first test phase in which the groups with and without soft tissue trauma both developed an infection. In subsequent test phases, the bacterial concentration in both groups was gradually reduced until negative test results were achieved in both. Having established the upper and lower thresholds, approximately 1 ID50 was applied to the remaining animals in the subsequent test phases. The experiment consisted of 6 phases.
For a priori calculation of the necessary sample size based on a statistical power of 0.8 and a type-I error probability a <0.05, we had to estimate the size of the effect that the closed soft tissue trauma might be expected to have on infection resistance compared to in the absence of such a trauma. Adequate data were not available from the literature to assist us in this prediction. However, we assumed that the effect of bFGF administration would be at least as significant as the effects of various osteosynthesis materials on local infection resistance. In previous investigations with the same experimental design, approximating to 1 ID50 and incorporating the previously mentioned parameters, an infection rate for the titanium dynamic compression plate of 35% had been demonstrated in contrast to 75% for the steel DCP (Arens et al. Citation1996). In keeping with these values, we assumed an effect size of 0.4, corresponding to an infection rate of 30% (50–20) for at least one of the groups with bFGF treatment and 70% (50 + 20) for the group without bFGF treatment. On this basis, we calculated a required sample size of 16 animals per group and decided to perform an a priori statistical evaluation based on a sample size of 64 animals in all (16 animals per group), in order to identify the differences between the group without bFGF and the groups in which bFGF was used.
The statistical evaluation was based on the twosided Fisher exact test (with p < 0.05 as the level of significance).
Results
In-vitro investigation
Cytotoxic reactions due to the concentrations of bFGF used could be excluded since incubations of isolated PBMCs with increasing concentrations of bFGF (5 ng/mL, 10 ng/mL, 100 ng/mL, and 200 ng/mL) for 24 h did not lead to an increase in the release of lactate dehydrogenase (LDH) in the culture supernatants, compared to respective control incubations without added bFGF (data not shown). Because of possible interference of exogenous bFGF with host defense mechanisms, we performed a series of in-vitro experiments on leukocyte activation (cytokine release) and on the phagocytic capacity of PMN using either isolated leukocyte fractions or whole blood cells.
We observed a significant increase in cytokine release after the coincubation of PBMCs with 100 or 200 ng of the same bFGF that was used for the animal experiments (). FGF at lower concentrations did not elicit a significant increase in cytokine release.
Figure 1. Cytokine release from PBMCs (1 × 106/mL) in the presence of exogenously added bFGF (5, 10, 100, and 200 ng/mL) after 24 h of incubation. For culture conditions, see Material and methods. Control: no exogenously added bFGF. * p < 0.05; ** p < 0.01.
Furthermore, the capacity of phagocytes (mainly granulocytes) within whole blood to phagocytose FITC-labeled bacteria was suppressed in the presence of 100 ng exogenously added bFGF (100 ng/ mL). Reduced granulocytic phagocytosis in FGFsupplemented blood compared to non-supplemented blood was continuously observed.
In-vivo investigation
There were no complications relating to the procedure or the anesthesia. All animals recovered from the anesthesia within a few hours. None of the animals died or suffered from open wound infection. Lysotyping of positive bacterial growth confirmed that no bacteria were present in any of the specimens other than the inoculated strain of Staphylococcus aureus. Regrettably, only a sufficient qualitative analysis of bacterial growth could be performed. It was impossible to achieve a detailed quantitative analysis because a complete blanket of bacteria had formed in every case of a positive bacterial finding, so that the bacterial colonies could not be counted. The main emphasis had to be placed on quantitative analysis for this reason
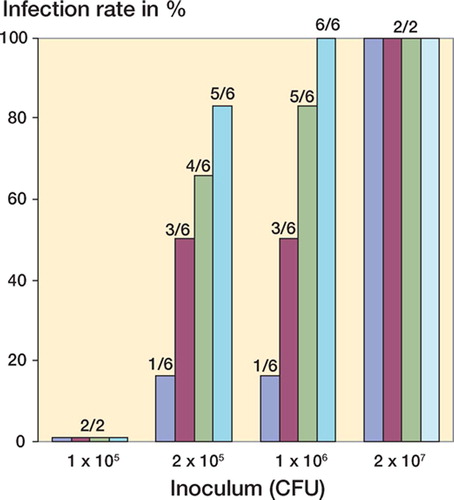
The overall infection rate was 0.6. For the group without bFGF, the infection rate was 0.25 (4/16 animals). In the groups that received 1, 10, and 100 ng bFGF, the infection rates were 0.5 (8/16 animals), 0.7 (11/16 animals) and 0.8 (13/16 animals). There was a dose-dependent increase in the infection rates due to local injection of bFGF into the anterior tibial muscle, as compared to the control group that had not been treated with bFGF. Here the differences in infection rates for the groups in which 10 and 100 ng bFGF had been administered were highly significant relative to the control group (p = 0.03 and p = 0.004). In the group in which only 1 ng bFGF was used, there was a 25% difference in infection rate relative to the control group, but this difference was not statistically significant (p = 0.3). The differences became more apparent when comparing ID50. In the group without bFGF, the ID50 was 2.5 × 106 cfu, as opposed to an ID50 of 1.9 × 105 and 1.8 × 105 CFU in the groups in which 10 and 100 ng bFGF was used, i.e. a more than 10 fold difference. In the group with 1 ng bFGF, the ID50 was recorded at 5.30 × 105 CFU, which was likewise clearly below the ID50 for the group without bFGF (). Apart from phase I, a higher rate of infection associated with the bFGF application was apparent in all phases of the experiment ().
Figure 2. Infection rate related to dose of inoculum (in CFU), showing the positive results as percentages of each group. The numbers indicate the number of infected animals over the total number of animals, at each dose of inoculum.
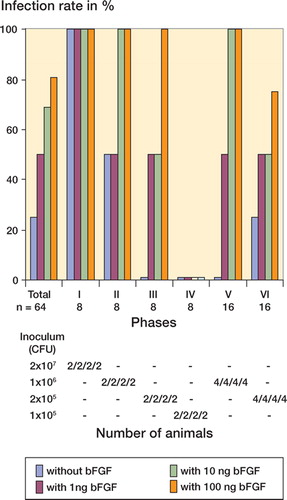
Figure 3. Infection rates for the groups with and without bFGF-application after soft tissue trauma in total and related to each of six experimental phases are graphically demonstrated in the upper section. The number of animals in total and in each phase is noted at the top. Inoculum doses between 1x105 CFU and 2x107 CFU were used. Below the results from each phase for either group, the inoculum doses and the number of animals used at these doses are indicated, reflecting the sequential “up-and-down” dosage technique
Discussion
Starting with the pathophysiological model of wound healing and the biological function of bFGF, we formulated our hypothesis that local application of bFGF after severe soft tissue trauma would lead to a minimization of infection risk, or would result in more rapid healing in the face of manifest infection. We used an established standardized trauma-infection rat model to validate our hypothesis (Kälicke et al. Citation2003). Surprisingly, local application of bFGF after soft tissue trauma did not lead to a reduction in infection rates, as would be expected from a theoretical-scientific standpoint. On the contrary, it led to a dose-dependent, sometimes highly significant increase in infection rate after soft tissue trauma; that is, the original hypothesis was disproven.
What are the possible reasons for reduced infection resistance after local application of bFGF in the rat model? One possible cause of reduced resistance to infection after soft tissue trauma and local application of bFGF is the possible toxicity of bFGF. Given at high concentrations, bFGFs and other growth factors have a toxic effect. Their biological efficacy after a one-dose local application at the concentrations we selected, namely 1–100 ng bFGF, and also at much higher doses, has been verified in numerous publications (Joseph-Silverstein and Rifkin Citation1987, Sprugel et al. Citation1987, Broadley et al. Citation1989, Burges Citation1989, Rifkin and Moscatelli Citation1989, Klingbeil et al. Citation1991, Albertson et al. Citation1993, Kawaguchi et al. Citation1994, Bland et al. Citation1995, Nagai et al. Citation1995, Okumura et al. Citation1996, Leunig et al. Citation1997, Kato et al. Citation1998, McGee et al. Citation1998, Nakamura et al. Citation1998). With the exception of one study (Bland et al. Citation1995), local application of the appropriate growth factor led to an acceleration of wound and fracture healing in all of the above studies.
bFGF is a growth factor with mitogenic potency, i.e. it can stimulate the cell so that increased DNA synthesis and cell division occur. In this context, bFGF stimulates the proliferation of all cells of mesodermal origin, and many cells of neuroectodermal, ectodermal, and endodermal origin. These cells include fibroblasts, endothelial cells, astrocytes, oligodendrocytes, neuroblasts, keratinocytes, osteoblasts, smooth muscle cells, and melanocytes. From a theoretical point of view, bFGF—because of its mitogenic potency—might also have stimulated the growth of the Staphylococcus strain used in our study. Thus, bFGF could stimulate DNA synthesis in bacteria which would lead to proliferation of the strain. No evidence for this possibility can be found in existing publications. Okumura et al. (Citation1996) excluded the possibility of a direct stimulatory or suppressive effect of bFGF on the growth of gram-positive bacteria and, therefore, on Staphylococcus aureus in their in-vitro studies in 1996. In our own in-vitro experiments, we also failed to observe an alteration in the growth behavior of Staphylococcus aureus as a reaction to the application of bFGF.
Depending on the dose of bFGF and the duration of the local application, inhibitory effects of bFGF have also been observed. In this regard, Nagai et al. (Citation1995) were able to prove both stimulatory and inhibitory effects on terminal osteoblast differentiation in the rat model, depending on the dose of bFGF and the duration of local application. Bland et al. (Citation1995) were the only research group unable to demonstrate accelerated fracture healing after the application of bFGF. They used a comparably high bFGF dose of 3 μg. Here, a possible inhibitory effect must be taken into consideration as an explanation for the test results because the mitogenic potency of bFGF means that, depending on the dose, its application may lead to increased cell stimulation or cell inhibition. This inhibitory effect of bFGF could also explain our in-vivo results. Inhibition caused by bFGF would bring with it a retarded and reduced angiogenesis and the formation of granulation tissue within the traumatized tissue, together with a reduced resistance to infection. Any inhibitory effect of bFGF can probably be excluded in the present study since accelerated wound and fracture healing was observed in all other studies using similar doses of bFGF.
Another possible cause of reduced resistance to infection after soft tissue trauma and local application of bFGF is that due to the experimental design of the study, it is not possible to exclude an effect of small amounts of contaminating lipopolysaccharide (LPS, endotoxin) in the recombinant bFGF material. LPS may evoke an overwhelming spectrum of biological activities when administered to animals or humans, or in vitro. In the interaction with myeloid leukocytes, endotoxins induce a variety of intracellular signaling cascades, finally leading to the release of endogenous mediators such as IL-1 and IL-6.
However, the level of endotoxin in the bFGF used was <0.01 endotoxin units (EU) per 100 ng of bFGF. Nevertheless, very small amounts of endotoxin might also activate LPS-sensitive myeloid leukocytes, with a subsequent cytokine release. The threshold values for endotoxin to elicit a cytokine response are usually higher than 0.01 EU (Hartung et al. Citation2001).
Wound healing is characterized by numerous complex immunological processes. Following trauma, cascade-like activation of the clotting and complement system occurs, as does the release of growth factors that act chemotactically on the (subsequently) immigrating cells active in wound healing. Locally and systemically active, immunocompromising factors of various origins can lead to an imbalance between the activating and inhibiting wound factors and thus increase the risk of infection (Schäffer and Becker Citation1999). Every exogenous application of a growth factor will inevitably upset the natural balance of activating and inhibiting wound factors. Exogenous application of bFGF in traumatized tissue without bacterial contamination accelerates wound healing. In our standardized trauma-infection model involving the additional exogenous application of bFGF, this balance is obviously so profoundly disturbed in the presence of bacteria and due to as yet unexplained immunological processes that, depending on the dose, a highly significant increase in infection rates results in some cases. In one of the few studies performed so far in humans to investigate the application of growth factors, Govender et al. (Citation2002) found a lower infection rate in open tibial shaft fractures treated by tibial nailing and local application of rhBMP-2 than in the control group, which was treated by tibial nailing without rhBMP-2 application. These authors were able to prove a reduction in infection rates for fractures with severe soft tissue injury (Gustilo-Anderson type III). In contrast, in fractures with only slight soft tissue damage (Gustilo-Anderson type I–II) no differences in the infection rates were identified. The explanations for the reduced infection rates were, according to the authors, the acceleration of fracture healing by 39 days relative to the control group without rhBMP-2 treatment, and the effects of early definitive fracture stabilization. Why this only affected the group with severe soft tissue damage remains unclear. The authors did, however, state that the investigators were not blinded to treatment assignment, and the potential for bias cannot be excluded.
It is known that bFGF receptor expression is essential for leukocyte development, and that leukocytes are activated by bFGF (Satoshi et al. Citation2000). Our data suggest that bFGF may modulate the host defense by increasing local tissue cytokine concentration, which, in turn, may suppress local host defense mechanisms. In fact, phagocytosis is the major mechanism for combatting staphylococcal infections, and our in-vitro studies on the phagocytic capacity of leukocytes in the presence of exogenously added bFGF have demonstrated mainly suppression of phagocytosis. However, the phagocytosis results were not consistent throughout all experiments, and should be investigated in more detail by further studies.
At the very least, this study has shown that specific recombinant growth factors with proven activities on angiogenesis and tissue regeneration may have adverse effects of host defense mechanisms.
In recent years, there has been increasing interest in the local use of growth factors to achieve local stimulation of wound and fracture healing. The multitude of publications cited above gives some indication of this. In some studies, the insights gained in in-vitro and animal experiments were transferred to in-vivo testing, and led to the use of appropriate growth factors in humans. In the general euphoria surrounding the local application of growth factors, it may be that undesirable (side) effects have not been taken into account—or they may remain unrecognized. For this reason, we consider it important to communicate our study findings even though a definite and satisfactory explanation for the reduction in resistance to infection after severe soft tissue trauma and local applicatin of bFGF is lacking. The lower leg of the rat is so small that simultaneous microbiological and histological evaluations were not possible in our test series. Further studies will be dedicated to identifying the definitive cause of our findings by performing histological, immunohistochemical and, possibly, molecular-genetics investigations. If these initial results are confirmed for other potent angiogenic growth factors, then the local administration of growth factors to stimulate wound and bone healing will have to be reconsidered and subjected to a strict evaluation of the risks and benefits.
This project was supported by a grant from the research commission of the Berg-Bau-Berufsgenossenschaft, Land Nordrhein-Westfalen, Germany.
Contributions of authors
TK: concept, animal research application, leading and carrying out the in-vivo experiments, and preparation of the article. MK: leading and carrying out the in-vitro experiments. TMF: preparation of the bacterial suspension. US: development of the computer-assisted, controlled-impact technique powered by an electric motor. OS: anesthesia of the rats. GP: lysotyping. GM: director of BG Kliniken Germannsheil, Bochum, Germany, who provided space for conduction of the experiments. SA: assisted in preparing the study application and preparation of the article.
- Albertson S, Hummel R P, Breeden M, Greenhalgh D G. PDGF and FGF reverse the healing impairment in protein-malnourished diabetic mice. Surgery 1993; 114: 368–73
- Arens S, Schlegel U, Printzen G, Ziegler W J, Perren S M, Hansis M. Influence of the materials for fracture fixation implants on the development of local infection. An experimental study of steel versus titanium DC-Plates in rabbits. J Bone Joint Surg (Br) 1996; 78: 647–51
- Arens S, Eijer H, Schlegel U, Printzen G, Perren S M, Hansis M. Influence of the design for fixation implants on local infection. An experimental study of DC-Plates vs. Point-Contact-Fixators in rabbits. J Orthop Trauma 1999a; 13: 470–6
- Arens S, Kraft C, Schlegel U, Printzen G, Perren S M, Hansis M. Susceptibility to local infection in biological internal fixation. Experimental study of open vs. minimally invasive plate osteosynthesis (MIPO) in rabbits. Arch Orthop Trauma Surg 1999b; 119: 82–5
- Beer H D, Longaker M T, Werner S. Reduced expression of PDGF and PDGF receptors during impaired wound healing. J Invest Dermatol 1997; 109: 132–8
- Bland Y S, Critchlow M A, Ashhurst D E. Exogenous fibroblast growth factors-1 and –2 do not accelerate fracture healing in the rabbit. Acta Orthop Scand 1995; 66: 543–8
- BMP-2 Evaluation in Surgery for Tibial Trauma (BESTT) Study Group, Govender S, Csimma C, Genant H K, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishany M, Börner M G, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, van der Velde D, Hardy P, Holt M, Josten C, Ketterl R L, Lindeque B, Lob G, Mathoven H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens P M, Rondia J, Rossouw W C, Daneel P J, Ruff S, Rüter A, Santavirta S, Schildhauer T A, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne R B, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg (Am) 2002; 84: 2123–34
- Bostrom M P G, Camacho N P. Potential role of bone morphogenetic proteins in fracture healing. Clin Orthop 1998, 355: 274–82
- Bouxsein M L, Turek T J, Blake C A, Dàugusta D, Li X, Stevens M, Seeherman H J, Wozney J M. Recombinant human bone morphogenetic protein-2 accelerates healing in rabbit ulnar osteotomy model. J Bone Joint Surg (Am) 2001; 83: 1219–30
- Broadley K N, Aquino A M, Wooward S C. Monospecific antibodies implicate basic fibroblast growth factor in normal wound repair. Lab Invest 1989; 61: 571–5
- Burges A N. Epidermal growth factor and transforming growth factor beta. Br Med Bull 1989; 45: 401–24
- Corral C J, Siddiqui A, Wu L, Farrell C L, Lyons D. VEGF is more important than bFGF during ischemic wound healing. Arch Surg 1999; 134: 200–7
- Critchlow M A, Bland Y S, Ashhurst D E. The effect of exogenous transforming growth factor-beta 2 on healing fractures in the rabbit. Bone 1995; 16: 521–7
- Davidson J M, Broadley K N, Quaglino D. Reversal of the wound healing deficit in diabetic rats by combined basic fibroblast growth factor and transforming growth factor-β1 therapy. Wound Rep Reg 1997; 5: 77–83
- Einhorn T A, Majeska R J, Mohaideen A, Kagel E M, Bouxsein M L, Turek T J, Wozny J M. A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture reair. J Bone Joint Surg (Am) 2003; 85: 1425–35
- Gustilo R B, Anderson J T. Prevention of infection in the treatment of 1025 open fractures of long bones: retrospective and prospective analysis. J Bone Joint Surg (Am) 1976; 58: 453–8
- Hartung T, Aaberge I, Berthold S, Carlin G, Charton E, Coecke S, Fennrich S, Fischer M, Gommer M, Halder M, Haslov K, Jahnke M, Montag-Lessing T, Poole S, Schechtman L, Wendel A., Werner-Felmayer G. Novel Pyrogen Tests Based on the Human Fever Reaction. ATLA 2001; 99–123, The Report and Recommendation of ECVAM workshop 43
- Hauke C, Schlegel U, Melcher G A, Printzen G, Perren S M. Einfluss des Implantatmaterials auf die locale Infektresistenz bei der Tibiamarknagelung. Eine experimentelle Vergleichsstudie am Kaninchen mit Marknägeln aus rostfreiem Stahl und Reintitan. Swiss Surg 1 1996, Suppl 2: 45
- Heckman J D, Aufdemorte T B, Athanasiou K A. Treatment of acute osteotomy defects in the dog radius with rhTGFbeta1. Trans Orthop Res Soc 1995; 20: 590–4
- Hom D B, Manivel J C. Promoting healing with recombinant human platelet-derived growth factor-BB in a previously irradiated problem wound. Laryngoscope 2003; 113: 1566–71
- Joseph-Silverstein J, Rifkin D B. Endothelial cell growth factors and the vessel wall. Semin Thromb Hemost 1987; 13: 504–13
- Kälicke T, Schlegel U, Printzen G, Schneider E, Muhr G, Arens S. Influence of a standardised closed soft tissue trauma on resistance to local infection. An experimental study in rats. J Orthop Res 2003; 21: 373–8
- Kato T, Kawaguchi H, Hanada K, Aoyama I, Hiyama Y, Nakamura T, Kuzutani K, Tamura M, Kurokawa T, Nakamura K. Single local injection of recombinant fibroblast growth factor-2 stimulates healing of segmental bone defects in rabbits. J Orthop Res 1998; 16: 654–9
- Kawaguchi H, Kurokawa T, Hanada K, Hiyama Y, Tamura M, Ogata E, Matsumoto T. Stimulation of fracture repair by recombinant human basic fibroblast growth factor in normal and streptozotocin-diabetic rats. Endocrinology 1994; 135: 774–81
- Klingbeil C K, Cesar L B, Fiddes J C. Basic fibroblast growth factor accelerates tissue repair in models of impaired wound healing. Clinical and experimental approaches to dermal and epidermal repair: normal and chronic wound. Wiley-Liss Inc, New York 1991; 443–58
- Köller M, Kutscha-Lissberg F, Brom J, Weidinger G, Muhr G. Influence of low molecular weight heparin (certoparin) and unfractionated heparin on the release of cytokines from human leukocytes. Inflammation 2001; 25: 331–8
- Leunig M, Yuan F, Gerweck L E, Jain R K. Effect of basic fibroblast growth factor on angiogenesis and growth of isografted bone: Quantitative in vitro-in vivo analysis in mice. Int J Microcirc Clin Exp 1997; 17: 1–9
- Lind M, Schumacker B, Soballe K. Transforming growth factor-beta enhances fracture healing in rabbit tibiae. Acta Orthop Scand 1993; 64: 553–6
- Luppen C A, Blake C A, Ammirati K M, Stevens M L, Seeherman H J, Wozney J M, Bouxsein M L. Recombinant Human bone morphogenetic protein-2 enhances osteotomy healing in glucocorticoid-treatet rabbits. J Bone Miner Res 2002; 17: 301–10
- McGee G S, Davidson I M, Buckley A. Recombinant bFGF accelerates wound healing. J Surg Res 1998; 45: 145–53
- Melcher G A, Claudi B, Perren S M, Schlegel U, Muntzinger J, Printzen G. Influence of type of medullary nail on the development of local infection. J Bone Joint Surg (Br) 1994; 76: 955–9
- Melcher G A, Metzdorf A, Schlegel U, Ziegler W J, Perren S M, Printzen G. Influence of reaming versus nonreaming in intramedullary nailing on local infection rate: experimental investigation in rabbits. J Trauma 1995; 39: 1123–8
- Mustoe T A, Pierce G F, Thomason A, Gramates P, Sporn M B, Deuel T. Accelerated healing of incisional wounds in rats induced by transforming growth factor beta. Science 1987; 237: 1333–6
- Nagai H, Tsukuda R, Mayahara H. Effects of basic fibroblast growth factor on bone formation in growing rats. Bone 1995; 16: 367–73
- Nakamura T, Hara Y, Tagawa M, Tamura M, Yuge T, Fukuda H, Nigi H. Recombinant human basic fibroblast growth factor accelerates fracture healing by enhancing callus remodeling in experimental dog tibial fracture. J Bone Miner Res 1998; 13: 942–9
- Nash T J, Howlett C R, Martin C, Steele J, Johnson K A, Hicklin D J. Effect of platelet-derived growth factor on tibial osteotomies in rabbits. Bone 1994; 15: 203–8
- Nielsen H M, Andreassen T T, Ledet T, Oxlund H. Local injection of TGF-beta increases the strength of tibial fractures in rat. Acta Orthop Scand 1994; 65: 37–41
- Okumura M, Okuda T, Nakamura T, Yajima M. Effect of basic fibroblast growth factor on wound healing in healing-impaired animal model. Arzneim Forsch 1996; 46: 547–51
- Radomsky M L, Aufdemorte T B, Swain L D, Casey Fox W, Spiro R C, Poser J W. Novel formulation of fibroblast growth factor-2 in a hyaluron gel accelerates fracture healing in nonhuman primates. J Orthop Res 1999; 17: 607–14
- Reed L J, Münch H. A simple method of estimating fifty per cent endpoints. Am J Hyg 1938; 27: 493–7
- Richard J L, Purer-Richard C, Daures J P, Clouet S. Effect of topical basic fibroblast growth factor on the healing of chronic diabetic neuropathic ulcer of the foot. Daibetes Care 1995; 18: 64–9
- Rifkin D B, Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol 1989; 109: 1–6
- Satoshi T, Kimiko T, Yoshiya K, Takeshi M, Akimichi O. Basic fibroblast growth factor modulates the surface expression of effector cell molecules and primes respiratory burst activity in human neutrophils. Acta Haematologica 2000; 103: 78–83
- Schäffer M, Becker H D. Immunregulation der Wundheilung. Chirurg 1999; 70: 879–908
- Schaser K, Vollmar B, Menger M, Schewior L, Kroppenstedt S, Raschke M, Lübbe A S, Haas N P, Mittlmeier T. In vivo analysis of microcirculation following closed softtissue injury. J Orthop Res 1999; 17: 678–85
- Sprugel K H, McPherson J M, Clowes A W, Ross R. Effects of growth factors in vivo. I. Cell ingrowth into porous subcutaneous chambers. Am J Pathol 1987; 129: 601–13
- Steenfos H H. Growth factors and wound healing. Scand J Plast Reconstr Hand Surg 1994; 28: 95–105
- Wang J S, Aspenberg P. Basic fibroblast growth factor promotes bone ingrowth in porous hydroxyapatite. Clin Orthop 1996, 333: 252–60
- Wieman T J, Smiell J M, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomised placebo-controlled doubleblind study. Diabetes Care 1998; 21: 822–9
- Yasko A W, Lane J M, Fellinger E J. The healing of segmental bone defects induced by recombinant human bone morphogenetic protein (rhBMP-2): A radiographic, histological, and biochemical study in rats. J Bone Joint Surg (Am) 1992; 74: 659–70