Abstract
Background and purpose Poor bone ingrowth into the porous coating of tibial components has been reported. We hypothesized that iliac marrow grafting might be useful to enhance bone ingrowth into a porous-coated implant. The first part of this study was to examine the presence of fibroblast colony-forming units (CFUF) containing osteogenic precursor cells in tibial bone marrow and iliac bone marrow. The second aim was to compare the clinical and radiographic results after bilateral total knee arthroplasty (TKA) with and without autologous bone marrow transplantation to the bone-implant interface.
Methods Simultaneous bilateral TKA was performed in 21 patients with osteoarthritis. Aspirated iliac bone marrow was transplanted to the interface of one randomly selected porous-coated tibial component in each patient, and contralateral knees served as controls. All of the 21 patients were followed for 5 years.
Results The average number of CFU-F was significantly lower in tibial marrow than in iliac marrow (p = 0.008). The final fluoroscopically-guided radiographs revealed a decrease in the number of knees with radiolucent lines after marrow grafting compared to those without grafting (p = 0.004).
Interpretation Iliac bone marrow is useful as a bone grafting material to enhance the biological fixation in porous-coated implants.
Uncemented total knee arthroplasty (TKA) was developed to obtain biological fixation between a porous-coated implant and bone. Although the short-term clinical results of cementless TKA are comparable to those of uncemented TKA (Vince et al. Citation1989, Rosenbergh et al. Citation1990), most human retrieval studies have shown poor ingrowth of bone into the porous coating, especially of the tibial components (Sumner at al. Citation1995, Bloebaum et al. Citation1997). It has been reported that bone ingrowth into porous coating is dependent on adequate initial stability, implant design, and surgical technique (Dempsy et al. Citation1989). In addition, the presence of osteogenic precursor cells in bone marrow is essential for bone ingrowth to occur (Spector Citation1988). Accordingly, it is important to determine whether there is a disadvantageous microenvironment in the proximal tibial marrow that impairs bone ingrowth into porous-coated tibial components. It has been reported that there is no active bone marrow in any limb bone distal to the femur in normal adults (Hashimoto Citation1962). On the other hand, iliac bone marrow has been shown to be active and to contain abundant osteogenic precursor cells in animal studies (Friedstein et al. Citation1987, Beresford Citation1989). In addition, several clinical studies have suggested that aspirated iliac bone marrow is useful as a bone grafting material (Connolly et al. Citation1989, Citation1991, Connolly Citation1995).
Our hypothesis was that transplantation of iliac marrow to the interface between a porous-coated tibial component and bone might enhance biological fixation after TKA. We first examined the presence of fibroblast colony-forming units (CFU-F), which may contain osteogenic precursor cells, in human tibial bone marrow and iliac bone marrow. Secondly, we compared the clinical and radiographic results after bilateral TKA with and without autologous bone marrow transplantation to the bone-implant interface.
Material and methods
All 21 patients (mean age 66 (57–69) years, 15 women) with osteoarthritis of the knee who were scheduled to have bilateral TKA at our university hospital between January 1998 and March 1999 were included in the study. All patients had bilateral osteoarthritis of the knee. The study protocol was approved by the institutional board at our hospital. The purpose of the study was explained, and a detailed informed consent form was signed by each patient.
All patients underwent simultaneous bilateral TKA, and bone marrow transplantation was performed in one knee of each patient. The knee that received marrow transplantation was decided by tossing a coin and the contralateral knee received no transplantation. All patients were followed for an average of 5.6 (5–6.5) years.
Preparation of bone marrow
Bone marrow was obtained using a standard aspiration needle (Lee-Lok, Mineapolis, MN), as described elsewhere (Hernigou and Beaujean Citation1997). Briefly, the bone marrow aspiration needle was advanced into the medullary cavity at a site 2 cm posterior and 1 cm distal to the anterior superior iliac spine. Then, a 10-mL syringe containing 1 mL of heparin solution (1,000 units per mL) was connected to the needle. 5 mL of bone marrow was aspirated, and subsequently an additional 2 × 5 mL was aspirated from 2 sites adjacent to the first. The first and the second bone marrow aspirates (total volume 10 mL) were stored on a sterilized table at room temperature for the transplantation. The third marrow aspirate was separated by centrifugation on a Ficoll-Hypaque density gradient (1.077 g/mL) and the fraction of mononuclear cells thus obtained was washed 3 times with phosphate-buffered saline (pH. 7.4) and used for the subsequent assay of colony-forming units. In addition, 5 mL of bone marrow was aspirated before surgery from the metaphysis of the tibia scheduled for marrow transplantation and was also prepared for assay of colony-forming units.
Colony formation assay for stromal precursors
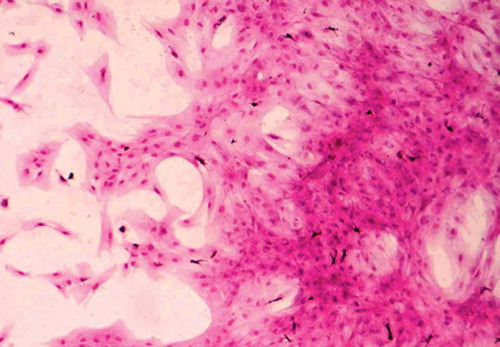
Fibroblast colony-forming units (CFU-F) were assayed in the iliac and tibial aspirates of the 21 patients according to the adherent cell colony assay method of Nara et al., with minor modifications (Nara et al. Citation1984). Briefly, 4 × 105 bone marrow mononuclear cells were cultured in 2 mL of alpha-minimal essential medium (Gibco, Grand Island, NY) supplemented with 20% fetal calf serum (Gibco) in 35-mm diameter dishes (Falcon; Nippon Becton Dickinson, Tokyo, Japan) at 37°C in a humidified atmosphere of 5% CO2 in air. The cultures were done in triplicate and the medium was totally replaced every 3 days. On day 14, the medium was discarded and the culture dish was dried and stained with Wright's stain. Colonies of more than 50 fibroblasts were counted ().
Figure 1. Photomicrograph of fibroblast colony-forming units obtained from cultured iliac bone marrow mononuclear cells (Wright's stain, magnification × 160).
The mean number of CFU-F obtained from iliac bone marrow was compared with that from tibial bone marrow in each patient using the Wilcoxon signed-rank test, and correlations between the number of CFU-F and sex or age were analyzed by calculating Pearson's correlation coefficients.
Operative procedure and comparison of clinical results
All patients received the same posterior cruciate sparing prosthesis (Anatomic Modular Knee; Depuy, Warsaw, IN). All operations were performed by, or under the direct supervision of one senior surgeon (KJK). The knee allotted to bone marrow grafting was operated first, and subsequently the contralateral knee without bone marrow grafting was treated. These procedures were randomized in terms of bone marrow grafting, but not randomized in terms of which side should be operated first. This could be a possible bias. Femoral components were placed with pressfit fixation without cement. The tibial cut was made using an extramedullary guide, and a hole for the central stem was drilled. After placing a bone plug (obtained from the resected tibial bone) at the base of the drill hole, cement was inserted with a special pusher so that it did not flow out over the tibial surface, and the tibial component with 5 mL of bone marrow applied to its surface pore was fixed to the tibia without screws or further cement. If any cement escaped from the hole onto the tibial surface, it was removed before inserting the tibial component. Just before a final impaction of the tibial tray, another 5 mL of bone marrow was injected onto the tibial bone surface. Originally, this procedure was developed to achieve the rigid fixation of porous-coated tibial components with optimum bone ingrowth by fixing the central stem with cement for osteopenic bone in rheumatoid patients (Kim et al. Citation1994). The patella was resurfaced with a cemented polyethylene component in all knees; lateral release was also performed. All patients were allowed to perform full weight bearing 2 weeks postoperatively.
All knees were evaluated before and after arthroplasty using the knee score devised by the Knee Society (Insall et al. Citation1989). To assess the tibial tray-bone interface, fluoroscopically-guided anteroposterior and lateral radiographs were obtained at 2 weeks, 3 months, 6 months, and 1 year postoperatively, and then annually thereafter. The radiographs were analyzed to detect subsidence of the component and radiolucent lines at the implant-bone interface. The area of any gap or radiolucent line at the implant-bone interface was analyzed for each zone, as defined by the Knee Society system (Insall et al. Citation1989). We defined any gap or radiolucent line on AP and lateral radiographs taken 2 weeks postoperatively as initial gap. Evaluation of the knee scores was performed by one independent physiotherapist, and the radiographic findings for each knee were evaluated by 6 independent observers who had no discussions about the findings and no knowledge of the operative findings. For the femoral and patellar components, routine weight-bearing lateral radiographs and Merchant radiographs were evaluated, because positioning of the X-ray beam parallel to the interface of each component was difficult. All of the observers and the physiotherapist were blinded as to which knee had the bone marrow graft. Failure was defined as the removal of any components at revision total knee arthroplasty.
Statistics
Student's t-test was used to compare the preoperative and postoperative knee scores of the knees with and without marrow grafting. The number of knees with radiolucent lines or gaps in each period was compared between the knees with and without marrow grafting using the McNemar exact test, with the level of significance set at p = 0.05. Intraobserver variance was assessed by analyzing the same radiographs 10 times and interobserver variance was also assessed for each analysis. Statistical analyses were done using SPSS for Windows, version 11. Intraobserver and interobserver reliability were evaluated using two-way mixed effect model, and average-measure interclass correlation coefficients (ICCs) were calculated for the prevalence of radiolucent lines or gaps.
Results
Comparison of the number of fibroblast colony-forming units in tibial and iliac bone marrow
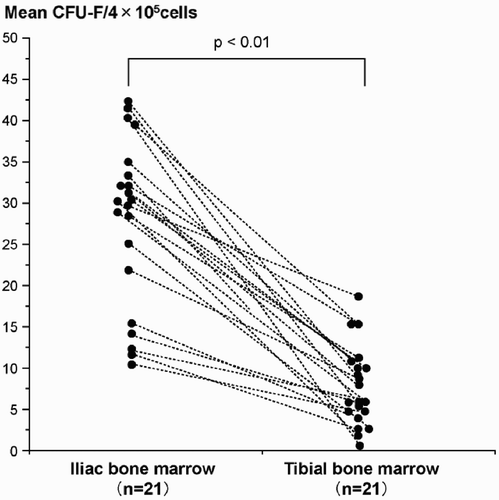
Fibroblast colony-forming units (CFU-F) were detected in all of the samples of iliac bone marrow, and were also detected in all samples of tibial marrow. However, the number of CFU-F in tibial marrow was lower than that in iliac marrow for each patient: mean 8.2 (SD 2.1) and 29 (SD 6.0), respectively (p = 0.008) (). Although there was no correlation between the number of CFU-F in tibial bone marrow and age and sex, there was a negative correlation between the number in iliac marrow and age (correlation coefficient 0.51; p = 0.03).
Figure 2. Graph showing a comparison of the mean number of fibroblast colony-forming units (CFU-F) per 4 × 105 bone marrow mononuclear cells in iliac and tibial marrow from each patient. Each point shows the mean number of CFU-F for triplicate assays. Data from individual patients are connected by broken lines.
Clinical results of bilateral TKA with and without marrow transplantation
There was no significant difference between the knees with marrow grafting and those without it with regard to the knee scores before or after the operation. The average scores without bone marrow grafting were 35 (20–59) points preoperatively and 90 (77–100) points postoperatively. For the knees with bone marrow grafting, the average scores were 36 (21–60) points preoperatively and 91 (78– 100) points postoperatively. The average function scores were 35 (21–58) points preoperatively and 80 (72–100) points postoperatively.
Radiographic findings
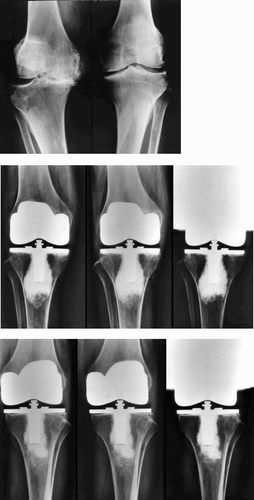
A serial comparison of the number of tibial trays with radiolucent lines and gaps between with the knees with marrow grafting and those without it is given in the . At the final examination, no complete radiolucent lines were observed around any of the components, and there was no evidence of subsidence or osteolysis. However, the number of knees with radiolucent lines or gap at the tibial tray-bone interface on anteroposterior radiographs was 4 in the knees with bone marrow grafting and 16 in the knees without it (). On lateral radiographs, the corresponding results were 2 in the knees with marrow grafting and 13 in the knees without it. There was a significant difference in both cases (p = 0.004 and p = 0.003, respectively). Although the number of knees with initial gaps was no different between the two groups, the number with radiolucent lines was significantly different by 6 months postoperatively. In the knees without bone marrow grafting, initial gaps of more than 0.5 mm persisted throughout follow-up and new radiolucent lines were observed by 1 year postoperatively. However, most of these radiolucent lines were not progressive and remained less than 2 mm wide. On the other hand, some of the initial gaps in the knees with bone marrow grafting disappeared by 1 year postoperatively, and no new radiolucent lines were observed at any time. All radiolucent lines were partial in both groups, and were not present in more than 3 zones on anteroposterior or lateral radiographs. No radiolucent lines were observed around the central stem of the tibial the 21 knees with bone marrow grafting and in 2 component, which was inserted with cement. As of the 21 knees without grafting. No radiolucent for the femoral component, a partial radiolucent lines were noted around the patellar components line less than 2 mm wide was observed in 3 of in either group.
Figure 3. A. Preoperative radiograph of the knees of a patient with osteoarthritis who underwent simultaneous bilateral total knee arthroplasty. B. Serial postoperative radiographs of the knee that received total knee arthroplasty with autologous bone marrow grafting. Note the presence of an initial gap at the tibial tray-bone interface at 2 weeks after surgery (left panel). However, the gap is no longer seen 6 months (middle panel) and 1 year after surgery (right panel). C. Serial postoperative radiographs of the contralat C.Serial postoperative radiographs of the contralateral knee, which received total knee arthroplasty without autologous bone marrow grafting. Note the presence of an initial gap at the tibial tray-bone interface at 2 weeks after surgery (left panel). This gap persisted 6 months (middle panel) and 1 year (right panel) after surgery.
Comparison of the number of prostheses with radiolucent lines and gaps in serial fluoroscopically-guided anteroposterior or lateral radiographs between the knees that underwent bone marrow grafting and those that did not
The intraobserver and interobserver variability in the assessment of radiolucent lines and gaps was not significant for either AP or lateral radiographs; average-measure ICC was 0.91 (0.82–0.96), which can be considered a high degree of reliability.
Complications
There was no infection in any of the 42 knees. 1 patient had recurrent intraarticular hematoma in 1 knee without bone marrow grafting; however, it resolved with rest and medication by 1 month postoperatively.
Discussion
We found that the number of fibroblast colony forming units (CFU-F), which contain osteogenic precursor cells, was lower in marrow harvested from the tibial metaphysis when compared to iliac marrow in patients with osteoarthritis. We also found that application of autologous bone marrow to the porous tibial component-bone interface caused a reduction in the number of knees with radiolucent lines after TKA.
Bone marrow has been found to contain osteogenic precursor cells in many animal studies (Ashton et al. Citation1980, Citation1985, Ohgushi et al. Citation1989). However, only a few studies have investigated CFU-F (which contain osteogenic precursor cells) in human bone marrow, and with varied findings (Haynesworth et al. Citation1992, Beresfordey al. Citation1994, Rickard et al. Citation1996, Muschler et al. Citation1997, Hernigou and Beaujean Citation1997). It is impossible to compare results directly in these studies because of differences in patient profile, culture medium, incubation time, and cell fraction. However, it appears that the number of osteogenic precursor cells in the iliac bone marrow from the osteoarthritis patients is within the range of variation reported for normal subjects. In contrast, the number of CFU-F in marrow from the tibial metaphysis was far lower than in iliac bone marrow from the same subject. This indicates that the osteogenic activity of bone marrow is low at the tibial metaphysis. Several clinical studies have demonstrated that bone marrow aspirated from humans is useful bone graft material (Connolly et al. Citation1989, Citation1991, Connolly Citation1995). We applied autologous bone marrow to the interface between porous-coated tibial tray and the tibial bone surface. Our findings suggest that the presence of osteogenic precursor cells has an important role in achieving biological fixation of the porous-coated tibial component.
From a clinical point of view, there might be several points of criticism regarding our technique for fixation of tibial components using a cemented stem—as well as for the clinical relevance of marrow grafting. One supposed benefit of our fixation technique is obtaining optimum bone ingrowth by minimizing the micromotion and subsidence of uncemented implants through rigid fixation of central stems with cement. The other supposed benefit is avoiding the generation of polyethylene debris through a third-body wear mechanism at the bonecement interface of tibial trays. On the other hand, the major concern with this fixation method has been stress-shielding at the bone-implant interface. Our results clearly demonstrate that marrow grafting reduces radiolucent zones and gaps that may be induced by initial gaps or stress-shielding at the bone-implant interface. Our technique of evaluating the interface using fluoroscopy may impose potential problems regarding accuracy, as already suggested by Freeman et al. (Citation1982). Thus, a more accurate method such as radiostereometry (RSA) should be used in future studies to detect implant displacement (Ryd et al. Citation1983). However, we believe that the radiographic data—analyzed by the independent observers who were blinded as to which side had the bone marrow grafting—are reliable because of the high degree of intra-and interobserver agreement.
There are some issues that must still be addressed to establish a standard method of marrow grafting to the porous implants. These issues include the volume of bone marrow, the method of promoting adhesion of marrow to the bone-implant interface, and possible limitations imposed by clinical factors such as age, sex, and underlying disease (Shigeno and Ashton Citation1995, Hernigou and Beaujean Citation1997). In addition, it is important to investigate whether or not the manipulation of bone marrow cells before transplantation can improve bone formation at the bone-implant interface in humans (Kim et al. Citation1997). Furthermore, we must confirm the true benefit of the present method in all uncemented TKAs, because implant subsidence and poor bone ingrowth at the interface still remain to be solved.
No competing interests declared.
Contributions of authors
KJK and MI: did surgery and wrote the paper. SK and TI: did the in vitro study (CFU-F measuring).
- Ashton B A, Allen T D, Howlett C R, Eaglesom C C, Hattori A, Owen M. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop 1980, 151: 294–307
- Ashton B A, Abdullah F, Cave J, Williamson M, Sykes B C, Couch M, Poser J W. Characterization of cells with high lkaline phosphatase activity derived from human bone and marrow: preliminary assessment of their osteogenicity. Bone 1985; 6: 313–9
- Beresford J N. Osteogenic stem cells and the stromal system of bone and marrow. Clin Orthop 1989, 240: 270–80
- Beresford J N, Joyner C J, Devlin C, Triffitt J T. The ffects of dexamethasone and 1,25-dihydroxyvitamin D3 on osteogenic differentiation of human bone marrow stromal cells in vitro. Arch Oral Biol 1994; 39: 941–7
- Bloebaum R D, Bachus K N, Jensen J W, Hofmann A. A postmortem analysis of consecutively retrieved asymmetric porous-coated tibial components. J Arthroplasty 1997; 12: 920–9
- Connolly J F. Injectable bone marrow preparations to stimulate osteogenic repair. Clin Orthop 1995, 313: 8–15
- Connolly J F, Guse R, Lippiello L, Dehne R. Development of an osteogenic bone marrow preparation. J Bone Joint Surg (Am) 1989; 71: 684–91
- Connolly J F, Guse R, Tiedeman J, Dehn R. Autologous marow injection as a substitute for operative grafting of tibial nounions. Clin Orthop 1991, 266: 259–70
- Dempsy A J, Finlay J B, Bourne R B, Rorabeck C H, Scott M A, Millman J C. Stability and anchorage considerations for cementless tibial components. J Arthroplasty 1989; 4: 223–30
- Freeman M A R, Bradley G W, Revell P A. Observation upon the interface between bone and polymethylmethacrylate cement. J Bone Joint Surg (Br) 1982; 64: 489–93
- Friedstein A J, Chailakhyan R K, Gerasimov U V. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet 1987; 20: 263–72
- Hashimoto M. Pathology of bone marrow. Acta Haematol 1962; 27: 193–216
- Haynesworth S E, Goshima J, Goldberg V M, Caplan A I. Characterization of cells with osteogenic potential from human marrow. Bone 1992; 13: 81–8
- Hernigou P, Beaujean F. Abnormalities in the bone marrow of the iliac crest in patients who have osteonecrosis secondary to corticosteroid therapy or alcohol abuse. J Bone Joint Surg (Am) 1997; 79(7)1047–53
- Insall J N, Dorr L D, Scott R D, Scott W N. Rationale of the Knee Society clinical rating system. Clin Orthop 1989, 48: 13–4
- Kim K J, Itoh T, Kashiwazaki S, Komatsu T. Hybrid fixation for porous coated tibial components in patients with rheumatoid arthritis: A clinical and fluoroscopically guided roentgenographic study. Orthop Trans 1994–1995; 18(4)1195–6
- Kim K J, Itoh T, Kotake S. Effects of recombinant human bone morphogenetic protein-2 on human bone marrow cells cultured with various biomaterials. J Biomed Mater Res 1997; 35: 279–85
- Muschler G F, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg (Am) 1997; 79(11)1699–709
- Nara N, Jinnai I, Imai Y, Bessho M, Hirashima K. Reduction of granulocyte-macrophage progenitor cells (CFU-C) and fibroblastoid colony-forming units (CFU-F) by leukemic cells in human and murine. Leukemia 1984; 72: 171–80
- Ohgushi H, Goldberg V M, Caplan A I. Heterotopic osteogenesis in porous ceramics induced by marrow cells. J Orthop Res 1989; 7: 568–78
- Rickard D J, Kassem M, Hefferan T E, Sarkar G, Spelsberg T C, Riggs B L. Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Min Res 1996; 1: 312–24
- Rosenbergh A G, Barden R M, Galante J O. Cemented and ingrowth fixation of the Miller-Galante prosthesis. clinical and roentgenographic comparison after three-to sixyear follow-up studies. Clin Orthop 1990; 260: 71–9
- Ryd L, Boegard T, Egund N, Lindstrand A, Selvik G, Thorngren K-G. Migration of tibial component in successful unicompartmental knee arthroplasty. Aclinical, radiographic and roentgen stereophotogrammetric study. Acta Orthop Scand 1983; 54: 408–16
- Shigeno Y, Ashton B A. Human bone-cell proliferation in vitro decreases with human donor age. J Bone Joint Surg (Br) 1995; 77(1)139–42
- Spector M. Current concepts of bone ingrowth and remodeling. Non-cemented total hip arthroplasty, R Fitzgerald, Jr. Raven Press, New York 1988; 69–85
- Sumner D R, Kienapfel H, Jacobs J J, Urban R M, Turner T M, Galante J O. Bone ingrowth and wear debris in well-fixed cementless porous-coated tibial components removed from patients. J Arthroplasty 1995; 10: 157–67
- Vince K G, Insall J N, Kelly M A. The total condylar prostheis. 10-to 12-year results of a cemented knee replacement. J Bone Joint Surg (Br) 1989; 71(5)793–7