Abstract
Background Cartilage degeneration often occurs after osteosynthesis of a devascularized intermediary fragment in a joint fracture, in mosaicplasty or in whole-joint toe-to-finger transplantation. Hypothetically, the degeneration is secondary to a collapse of the transferred subchondral bone as it remodels during high mechanical load. Bisphosphonates are used to reduce resorption of necrotic bone. We tested a systemic pretreatment before harvesting the graft in order to protect the bone and cartilage against collapse and secondary arthrosis.
Methods Rats were given one zoledronate injection and bone grafts were harvested. The grafts were frozen, thawed and placed into bone chambers, and implanted into another batch of rats. Graft resorption and new bone formation was measured by histomorphometric analysis and compared with untreated grafts.
Results In the remodeled area of the controls, the graft was almost totally resorbed and replaced by bone marrow. In the zoledronate-treated specimens, the graft remained and the graft trabeculas were lined with new bone. By histomorphometry, the total amount of bone (graft plus new bone) within the remodeled area was 16% in the zoledronate-treated grafts and 5% in the controls (p = 0.003).
Interpretation A bone graft can be pretreated with bisphosphonate and remain protected against resorption once implanted again. ▪
Necrotic bone is often resorbed once it is reached by vascular ingrowth, but the resorption is more or less balanced by simultaneous bone formation in the normal healing as well as in the remodeling of a bone graft or an osteonecrosis. A resorption that is too fast or too extensive may cause a problem, especially in mechanically loaded bone, for example adjacent to a joint. The imbalance between the resorption and formation of new bone may cause a temporary mechanical weakening as the revascularization front advances into the necrotic bone. A collapse of the load-bearing subchondral bone might be the cause of cartilage degeneration after intraarticular fractures or any nonvascularized osteochondral transplant. In order to avoid the collapse and secondary arthrosis, it would be advantageous to be able to treat the necrotic bone or graft with an antiresorptive agent such as a bisphosphonate during the whole evascularization and remodeling period, which can sometimes last for years.
Bone resorption is mediated by osteoclasts and occur during or following the revascularization of the necrotic bone. Bisphosphonates bind to the bone mineral and when bone is resorbed by the osteoclasts, the bisphosphonates are internalized by the cell and interfere with the cell metabolism, leading to apoptosis (Rogers Citation2003). Bisphosphonates have a strong affinity for calcium phosphate and adhere to the bone mineral also when applied directly to living or dead bone. We investigated whether the strong binding retains the anti-resorptive effect also after a surgically induced necrosis such as a bone grafting procedure. If so, a novel way of administering bisphosphonate for large grafts can be proposed.
Methods
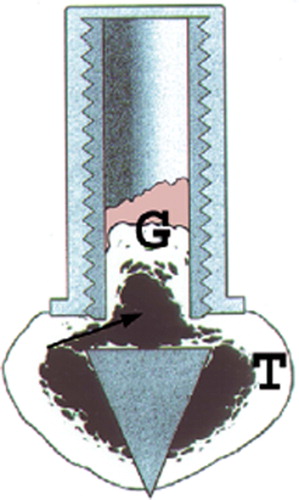
We used a model with a cancellous graft in a bone conduction chamber (Aspenberg and Wang Citation1993; BCC, ). The chamber consists of a threaded titanium cylinder, formed out of two half-cylinders held together by a hexagonal screw cap. The interior of the chamber is 7 mm long and has a diameter of 2 mm. One end of the implant is screwed into the proximal tibia of a rat. At this end, there are two ingrowth openings where tissue can grow in from the subcortical bone.
Figure 1. The bone conduction chamber (BCC) in situ in the proximal tibia (T). The graft (G) is placed in the chamber and mesenchymal tissue grows from the bottom upwards into the bone graft, which is subsequently remodeled. Arrows point to ingrowth openings. (Reproduced from Eur J Exp Musculoskel Res 1993; 2: 70 with permission).
Grafts. 4 donor rats (female, approx. 200 g) were given a single dose of 0.7 mg zoledronate subcutaneously and another 4 donor rats were given a saline injection. After 24 h, the rats were killed and bone grafts harvested from the proximal tibias. The knee joints were opened and the epiphyses and growth plates were discarded. A cylindrical cancellous bone rod was taken out from each tibia in the axial direction using a hole cutter, and frozen at –70°C. The grafts were thawed and placed in the chambers, which then were inserted in the recipient animals.
Surgical procedure. 16 male Sprague-Dawley rats (382–425 g) received one chamber each, containing either a treated graft or a control graft. Under aseptic conditions, a longitudinal incision was made over the anteromedial aspect of the proximal tibial metaphysis. Holes were made with a drill and the chambers were screwed into position with the ingrowth holes situated subcortically. The wound was closed, leaving the entire chamber subcutaneous.
Evaluation. The chambers were harvested after 6 weeks. The specimens were fixed in 4% formalin, decalcified, embedded in paraffin, cut parallel to the long axis of the chamber with a microtome, and stained with hematoxylin and eosin. Three sections from each specimen, at a distance of 300 μm from each other and showing the entire chamber contents, were used for histological and histomorphometric analyses.
The bone density in the remodeled area was evaluated using point counting and a Merz grid. The total number of points, points covering bone in general and points covering newly formed living bone were counted. The distinction between living and dead bone was based on matrix staining, presence of osteocytes, and trabecular shape. In each section, 6 randomly chosen areas were analyzed. For all measurements, the data were averaged to form a single value for each graft. The study was approved by the local ethics committee.
Statistics
Each donor rat was used for grafts to 2 recipients. Thus, repetitive measures had to be taken into account and an essential mixed-models approach was used to eliminate a “between-rats” effect in test comparisons between zometa-exposed rats and non-exposed control ones. In a mixed-models analysis, we used the measured bone density of each recipient as the response variable whereas the random effect was explained by the individual rats (rat identification i = 1, 2…8). The fixed effect was explained by the compared groups (j = 1 for the zometa-treated group, and j = 0 for the control group). Two analogous sets of analyses, one focusing on the density of living bone and another focusing on the density of all types of bone, were performed separately. The mixed-models facility of SPSS v.12.0 was used to perform the statistical analysis.
Results
No infections occurred. Histologically, the whole graft was invaded by vascularized fibrous tissue replacing the original bone marrow. New bone had invaded the graft but did not reach the whole way through it in any specimen, and a clear ossification front was seen in all grafts. In the remodeled area of the controls, the graft was almost totally resorbed and replaced by bone marrow. In the zole-dronate-treated specimens, the graft remained in the remodeling area and the graft trabeculas were lined with new bone. The increased bone density was obvious to the naked eye ( and ).
Figure 2. Control specimen after 6 weeks (left). At the bottom, next to the ingrowth openings (arrows), a marrow cavity (M) is formed where the bone graft trabeculas have been resorbed. Above this, the invading frontier of new ingrown bone (N) can be seen, a vascularized fibrotic marrow with remaining non-resorbed and non-remodeled bone graft (G) (hematoxylineosin, ×20). Detail of specimen (right) with the ingrowth front of new bone with the normal cellular marrow (M) below and the fibrotic marrow distally (F) (hematoxylineosin, ×40).
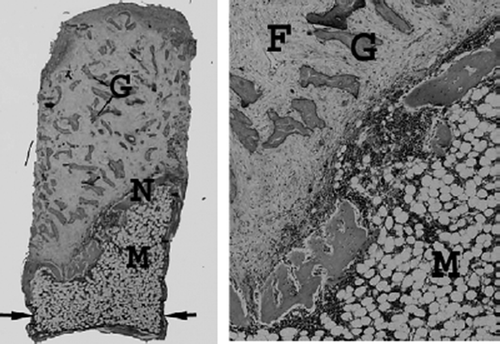
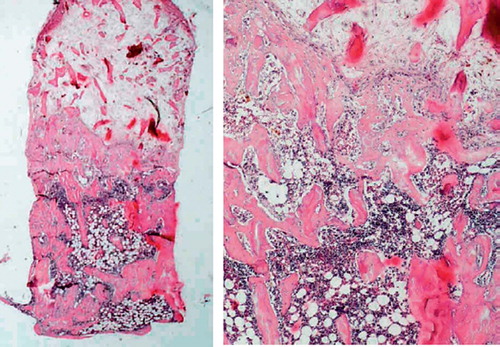
Figure 3. Zoledronate-treated specimen after 6 weeks (left). A marrow cavity has also formed, but the graft trabeculas are still remaining to a large extent. The total amount of bone, both newly formed and remaining graft bone, is higher than in the control. Detail of specimen (right).
Bone volume fraction measured with Merz grid and expressed as percentage of total tissue volume
In the bone density measurement, the total amount of bone within the remodeled area was 16% in the zoledronate-treated grafts and 5% in the controls (p = 0.03, mixed-models analysis). The total amount of living newly formed bone was 10% in the treated grafts and 4% in the controls (p = 0.02) (Table). The mixed-models analysis of zometa-treated rats vs. the control rats showed a difference regarding both the newly-formed living bone (p = 0.03) and all types of bone (p = 0.02).
Discussion
Apart from treatment of osteoporosis and bone metastases, bisphosphonates can be used for various conditions in the orthopedics field such as loosening of implants (Hilding et al. Citation2000), resorption of bone grafts (Åstrand and Aspenberg Citation2002), deformation of joints due to osteonecrosis (Little et al. Citation2003a), or stress shielding in distraction osteogenesis (Little et al. Citation2003b, Lai et al. Citation2005). The bisphosphonates can be administered systemically, either intravenously or perorally, and bind to the bone mineral to such an extent that the binding can practically be considered to be permanent until the bone is resorbed (Rogers et al. Citation2000). However, only vascularized bone is reached by the circulating bisphosphonates, and thus local application of a bisphosphonate directly to the bone surface has been tested in animals (Aspenberg and Åstrand Citation2002). With none of the methods, the bone within the necrotic volume will be treated, until the vessels reach the central necrosis. With large grafts, the medication must be continued until full revascularization and remodeling has been achieved.
We thus propose a third way to administer the anti-resorptive drug, a method made possible with new, more potent bisphosphonates with prolonged action such as zoledronate. In human trials, one yearly injection of zoledronate has an effect comparable to daily oral medication (Reid et al. Citation2002). In the present study, we have shown that it is possible to treat the graft only once before a grafting procedure. Once implanted, the necrotic bone graft will gradually revascularize and become remodeled, and the revascularizing front will constantly reach previously unvascularized but already bisphosphonate-precoated necrotic bone. In humans, the most common indication for this grafting procedure will probably be an autografting procedure such as mosaic plasty—or it may even be possible to do nonvascularized grafts or joint transfers without subsequent joint collapse.
In conclusion, systemic pretreatment of a bone graft with a bisphosphonate can reduce the resorption of the grafted bone once in situ. This could prove useful in clinical situations: for example, to reduce the risk of failure and collapse after loadbearing osteochondral grafts such as whole-joint transfer, mosaic-plasty or hamate repair of PIP fractures (Williams et al. Citation2003).
The authors thank Inger Mårtensson for technical assistance. The project was supported by the Swedish Research Council, the Greta and Johan Kock Foundation, and the Medical Faculty of Lund University.
Author contributions
JÅ and MT planned the study and operated the animals. MT wrote the article and JÅ and PA helped in preparing the manuscript.
- Aspenberg P, Wang J S. A new bone chamber used for measuring osteoconduction in rats. Eur J Exp Musculoskel Res 1993; 2: 69–74
- Aspenberg P, Åstrand J. Bone allografts pretreated with a bisphosphonate are not resorbed. Acta Orthop Scand 2002; 73: 20–3
- Hilding M, Ryd L, Toksvig-Larsen S, Aspenberg P. Clodronate prevents prosthetic migration: a randomized radio-stereometric study of 50 total knee patients. Acta Orthop Scand 2000; 71: 553–7
- Lai K A, Shen W J, Yang C Y, Shao C J, Hsu J T, Lin R M. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surg (Am) 2005; 87: 2155–9
- Little D G, Peat R A, Mcevoy A, Williams P R, Smith E J, Baldock P A. Zoledronic acid treatment results in retention of femoral head structure after traumatic osteonecrosis in young Wistar rats. J Bone Miner Res 2003a; 18: 2016–22
- Little D G, Smith N C, Williams P R, Briody J N, Bilston L E, Smith E J, Gardiner E M, Cowell C T. Zoledronic acid prevents osteopenia and increases bone strength in a rabbit model of distraction osteogenesis. J Bone Miner Res 2003b; 18: 1300–7
- Reid I R, Brown J P, Burckhardt P, Horowitz Z, Richardson P, Trechsel U, Widmer A, Devogelaer J P, Kaufman J M, Jaeger P, Body J J, Brandi M L, Broell J, Di Micco R, Genazzani A R, Felsenberg D, Happ J, Hooper M J, Ittner J, Leb G, Mallmin H, Murray T, Ortolani S, Rubinacci A, Saaf M, Samsioe G, Verbruggen L, Meunier P J. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med 2002; 28: 653–61
- Rogers M J. New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des 2003; 9: 2643–58
- Rogers M J, Gordon S, Benford H L, Coxon F P, Luckman S P, Monkkonen J, Frith J C. Cellular and molecular mechanisms of action of bisphosphonates. Cancer (Suppl 12) 2000; 15(88)2961–78
- Åstrand J, Aspenberg P. Systemic alendronate prevents resorption of necrotic bone during revascularization. A bone chamber study in rats. BMC Musculoskelet Disord 2002; 3: 19
- Williams R M, Kiefhaber T R, Sommerkamp T G, Stern P J. Treatment of unstable dorsal proximal interphalangeal fracture/dislocations using a hemi-hamate autograft. J Hand Surg (Am) 2003; 28: 856–65