Abstract
Introduction The survival of total hip arthroplasties (THAs) has been considered to be poor in young patients. We evaluated the population-based survival of uncemented THA for primary osteoarthritis (OA) in patients under 55 years of age and the factors affecting survival.
Methods The Finnish Arthroplasty Register was established in 1980. Between that year and 2003, 92,083 primary THAs were entered in the register, 5,607 of which were performed for primary OA in patients under 55 years of age. Using records from these 5,607 THAs, we selected uncemented femoral and acetabular components that had been used in more than 100 operations during the study period. Survival of both components (cup/stem) and their combinations were analyzed separately with the Kaplan-Meier analysis and the Cox regression model.
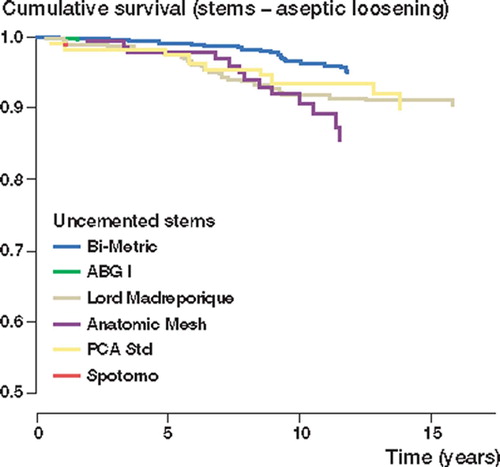
Results All uncemented stems studied showed a survival rate of over 90% at 10 years. The Biomet Bi-Metric stem had a 95% (95% CI 93– 97) survival rate even at 15 years. Overall survival of the extendedly porous-coated Lord Madréporique stem (p = 0.003) and the proximally porous-coated Anatomic Mesh stem (p = 0.0008) were poorer than that of the Biomet Bi-Metric stem. When endpoint was defined as stem revision for any reason, results were generally similar; there was no difference, however, between the survival rates of the Lord Madréporique stem and the Bi-Metric stem.
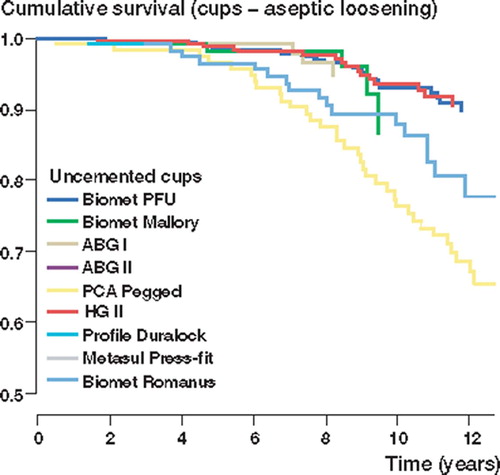
Of the acetabular components, the Biomet Universal, the ABG Il and the Harris-Galante II cups showed < 90% survival rates at 10 years with aseptic loosening as endpoint; at 13 years the corresponding survival rates were 94% (95% CI 91–97) for the Biomet Universal and 95% (95% CI 91–98) for the Harris-Galante II cups with aseptic loosening as endpoint. The PCA Pegged porous-coated uncemented cup showed a poor 13-year survival rate of 68% (95% CI 59–78) with aseptic loosening as endpoint. However, when endpoint was defined as any revision (including exchange of liner), the 10-year survival rates of all brands of cup except Harris-Galante II declined to under 80%.
Interpretation Modern second-generation uncemented stems, with proximal circumferential porous- or HA-coating, seem to be a good choice for young patients with primary OA. Similarly, modern press-fit porous- and HA-coated cups appear to have good endurance against aseptic loosening in these young patients. However, liner revisions were common; thus, survival rates of uncemented cups were unsatisfactorily low. Polyethylene wear and unfavorable locking mechanisms between the metal shell and the polyethylene liner and their sequelae remain matters of concern in this young and active group of patients. ▪
Generally speaking, survival of THAs is considered to be poorer in young patients (Herberts and Malchau Citation2000, Furnes et al. Citation2001). Eskelinen et al. (Citation2005) have recently reported population-based results of different implant concepts in young patients. To our knowledge, however, nationwide results of single brands of modern uncemented THA concepts in young patients have not been published.
A good long-term outcome has been recorded for patients under 55 years of age with modern uncemented (porous- and/or HA-coated) femoral (McLaughlin and Lee Citation2000, Kim et al. Citation2002, Aldinger et al. Citation2003, Capello et al. Citation2003, Jacobsen et al. Citation2003, Kim et al. Citation2003a) and acetabular (Kim et al. Citation2003b) components in THA. Most of these reports, however, have been from highly specialized centers and refer to only one brand of implant. Only a few register-based studies have reported the results of THA in young patients at a population-based level (Havelin et al. Citation2000, Malchau et al. Citation2002, Eskelinen et al. Citation2005). Despite the good results of modern uncemented implants in young patients, some authors still consider the use of uncemented implants in THA to be experimental (Thanner et al. Citation1999, Havelin et al. Citation2000).
We used the population-based Finnish Arthroplasty Register to analyze the outcome of modern (porous-and HA-coated) uncemented THAs in patients under 55 years of age with primary osteoarthritis (OA).
Patients and methods
Our study was based on information recorded in the Finnish Arthroplasty Register (Puolakka et al. Citation2001a) relating to patients who underwent THA between 1980 and 2003. Information on 92,083 THAs had been recorded individually for every operation since the start of the register in 1980. An English translation of the form used for this purpose has been published (Puolakka et al. Citation2001a). Revisions were linked to the primary operation using the unique personal identification number assigned to each resident of Finland.
Inclusion criteria
We used the following inclusion criteria: only patients aged less than 55 years at the time of the operation were included. In order to eliminate the effect of diagnosis as a confounding factor, only patients with primary OA as a recorded indication for operation were included. We selected uncemented femoral and acetabular components that had been used in more than 100 operations during the study period (Havelin et al. Citation1995a, Citationb). In addition, only stem designs with a mean follow-up of more than 5 years, and more than 20 patients at risk at 10 years (Dorey Citation2004) were included. The uncemented isoelastic Mathys stem (RM Mathys AG, Bettlach, Switzerland) with previously reported poor results (Niinimäki et al. Citation1994) was excluded from the study. On the acetabular side, only press-fit cup designs with porous- or HA-coating were included. The Biomet Romanus cup commonly used in Finland, with porous-coating and screwring design, was also included. Because of excessive liner wear and osteolysis, new uncemented cup brands with improved locking mechanisms between the metal shell and the polyethylene liner have been introduced onto the market in the late 1990s. Thus, these new brands of cup, the so-called “second-generation” of modern press-fit porous- or HA-coated uncemented cups (e.g. ABG II, Biomet Vision), with short-term follow-up (mean follow-up less than 5 years) were included in the study in order to determine whether the preliminary results of the new cup brands would differ from those of the older ones. Similarly, stem and cup combinations used in more than 100 operations during the study period, including new brands with a short follow-up time, were included in the study. Uncemented smooth-threaded cups with well-documented poor results were excluded (Engh et al. Citation1990, Tallroth et al. Citation1993, Simank et al. Citation1997).
Selected types of prosthesis
According to our inclusion criteria, 7 uncemented stem designs were included: ABG I (How-medica International, Staines, UK), Anatomic Mesh (Zimmer, Warsaw, IN), Bi-Metric (Biomet, Warsaw, IN), CLS Spotorno (Sulzer Orthopedics, Zürich, Switzerland), Lord Madréporique (Benoist Girard, Bagneux, France), PCA Standard (How-medica), and Profile Porous (DePuy, Leeds, UK) ().
Table 1. Design, surface and material of the femoral and acetabular components in the study
10 uncemented cup designs were included: ABG I (Howmedica), ABG II (Howmedica), Harris Galante II (Zimmer), Mallory (Biomet), Morscher Press-Fit (Sulzer Orthopedics), PCA Pegged (Howmedica), Profile Duraloc (DePuy, Warsaw, IN), Romanus (Biomet), Universal (Biomet), and Vision (Biomet) ().
8 cup-stem combinations were included (stem/cup): ABG I/ABG I, ABG I/ABG II, Anatomic Mesh/Harris-Galante II, Biomet Bi-Metric/Mallory, Biomet Bi-Metric/Romanus, Biomet Bi-Metric/Universal, Biomet Bi-Metric/Vision, and PCA Std/PCA Pegged. The Lord Madréporique stem was used mainly (n = 273, 96%) with the Lord smooth-threaded cup, which was excluded from the study. The Lord stems operated with smooth-threaded cups were, however, included in the stem analysis.
Statistics
The endpoint for survival was defined as revision when either one component or the whole implant was removed or exchanged. Both revision for any reason (including exchange of liner) and aseptic loosening served as endpoints. Revision indications included in the category “revision for any reason” are shown in . Aseptic loosening was selected as a separate endpoint, because “revision for any reason” also included nonimplantrelated re-operations. Kaplan-Meier survival data were used to construct the survival probabilities of implants at 7, 10, and 15 years for the femoral components, and 5, 10 and 13 years for the acetabular components (Kaplan and Meier Citation1958). Survival data obtained in the Kaplan-Meier analysis were compared by the log-rank test. The Cox multiple-regression model was applied to study differences between groups and to adjust for potential confounding factors (Cox Citation1972). In all models, the confounding factors were age (< 46 and 46–54 years) and sex. Estimates from Cox analyses were used to construct adjusted survival curves at mean values of the risk factors. The Wald test was applied to calculate p-values for data obtained from the Cox multiple regression analysis. Differences between groups were considered to be statistically significant if the p-values were less than 0.05 in a two-tailed test.
Table 2. Reasons for revision of components in the study. Values are no. of revisions (revision burden %)
For the statistical analyses we used SPSS statistical software version 11.0 (SPSS Inc, Chicago, IL).
Results
General
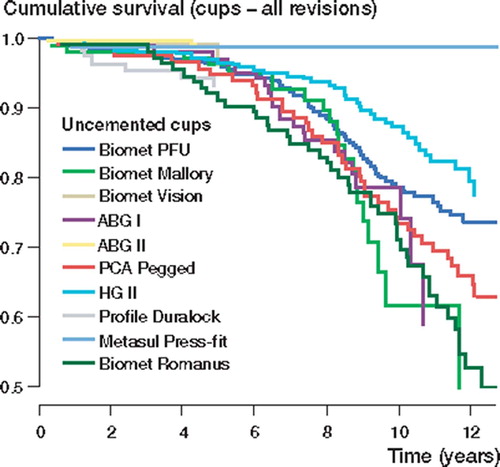
Reasons for revision of the femoral and acetabular components are shown in . Over the study period, 39 revisions of the PCA Pegged cups were performed due to aseptic loosening. On the other hand, of the 44 Biomet Romanus and 14 of the 21 Biomet Mallory cups revised were re-operated due to other reasons (including exchange of liner).
Survival of the stem designs ()
Table 3. Survival and adjusted risk ratio for revision of stem brands. Endpoint was defined as revision due to aseptic loosening of the stem or stem revision for any reason. 7−, 10−, and 15–year survival rates obtained from the Kaplan–Meier analysis
With aseptic loosening as endpoint, the Bi-Metric, the CLS Spotorno, the ABG I and the Profile Porous stems all had survival rates of > 95% at 10 years. At 15 years, the survival rate of the Bi-Metric stem was still 95% (CI 93–97), and that of the PCA Std stem was 90% (CI 84–97). The 15-year survival rate of the extendedly porous-coated Lord Madréporique stem was 91% (CI 88–94). Survival rates of the other stem brands were compared with that of the Bi-Metric stem (reference stem). Cox regression analysis (with adjustment for age and sex) showed that the Lord Madréporique stem (RR 2.2, CI 1.3–3.7; p = 0.004) and the Anatomic Mesh stem (RR 2.8, CI 1.5–5.4; p = 0.002) had a higher risk of revision than the Bi-Metric stem ().
Figure 1. Cox-adjusted survival curves of 3,127 stems in patients under 55 years of age, with brand of stem as the strata factor. Endpoint was defined as stem revision due to aseptic loosening. Adjustment has been made for age and gender. The curve of the Profile Porous stem is not shown, as it had a 100% survival rate at 10 years.
With stem revision for any reason as endpoint, all brands of stem showed < 90% survival rates at 10 years. The Bi-Metric stem showed a 92% (CI 90–94) survival rate at 15 years. In the Cox model, the Anatomic Mesh stem was found to have a 2.2-fold (CI 1.3–3.7; p =; 0.004) increased risk of stem revision as compared to the Bi-Metric stem.
Survival of the cup designs ()
Table 4a. Survival and adjusted risk ratio for revision of cup brands. Endpoint was defined as revision due to aseptic loosening of the cup. 5–, 10– and 13–year survival rates obtained from the Kaplan–Meier analysis
Table 4b. Survival and adjusted risk ratio for revision of cup brands. Endpoint was defined as cup revision for any reason. 5−, 10− and 13–year survival rates were obtained from the Kaplan-Meier analysis
With aseptic loosening as endpoint, only the ABG I, the Biomet Universal and the Harris-Galante II cups showed survival rates of < 90% at 10 years. The survival rates of the Biomet Universal and the Harris-Galante II cups remained at around 90%, even at 13 years. Most recently introduced uncemented cup brands showed good survival rates at 5 years, but 10-year survival data were not yet available for these brands. At 5 years, however, there was no difference between survival rates of these new brands of cup and the reference cup (Biomet Universal). In the Cox regression analysis, the Biomet Romanus (RR 2.5, CI 1.3–4.8; p =; 0.009) and the PCA Pegged cups (RR 4.2, CI 2.4–7.3; p < 0.001) showed higher risk of revision than the Biomet Universal cup after 5 years of follow-up (). There were no other differences in survival rates between the cup brands.
Figure 2. Cox-adjusted survival curves calculated for 2,801 cups, with brand of cup as the strata factor. Endpoint was defined as cup revision due to aseptic loosening. Adjustment has been made for age and gender. The curve of the Biomet Vision cup is not shown, as it had a 100% survival rate at 5 years.
With any cup revision as endpoint, only the Harris-Galante II cup showed over 80% survival rate at 10 years. 13-year survival rates of cups with long-term follow-up (the Biomet Universal, the Harris-Galante II and the PCA Pegged) declined to under 80%. In the Cox regression analysis, before 5 years of follow-up, the ABG I (RR 0.3, CI 0.1–0.8; p =; 0.02) and the ABG II cups (RR 0.2, CI 0.0–0.7; p =; 0.02) showed lower risk of revision than the Biomet Universal cup. The difference between the ABG I and the Biomet Universal cups, however, disappeared after 5 years of follow-up. The Harris-Galante II cup had a 0.7-fold (CI 0.5–1.0; p =; 0.04) reduced risk of revision as compared to the Biomet Universal cup. The Biomet Romanus cup showed a 1.9-fold (CI 1.3–2.7; p < 0.001) increased risk of revision as compared to the reference cup (Biomet Universal) ().
Survival of the total hip replacements ()
Table 5. Survival of THR combinations and adjusted risk ratio for revision. Endpoint was defined as revision due to aseptic loosening of the stem and/or the cup or any revision. 5–, 10– and 13–year survival rates were obtained from the Kaplan–Meier analysis
With aseptic loosening of the stem and/or the cup as endpoint, the Biomet Bi-Metric/Universal (the reference brand), the ABG I/ABG I and the Ana-tomic Mesh/Harris-Galante (HG) II cup-stem com-binations showed < 95% survival rates at 10 years. The Cox regression analysis revealed that the Bi-Metric/Romanus (RR 2.8, CI 1.6 – 4.9; p < 0.001) and the PCA Std/PCA Pegged (RR 4.0, CI 2.5–6.5; p < 0.001) prostheses had significantly higher risk of revision than the Bi-Metric/Universal combination ().
Figure 4. Cox-adjusted survival curves calculated for 2,061 THRs, with implant combination as the strata factor. Endpoint was defined as revision due to aseptic loosening of the stem and/or the cup. Adjustment has been made for age and gender. The curve of the Bi-Metric – Vision is not shown, as it had a 100% survival rate at 5 years.
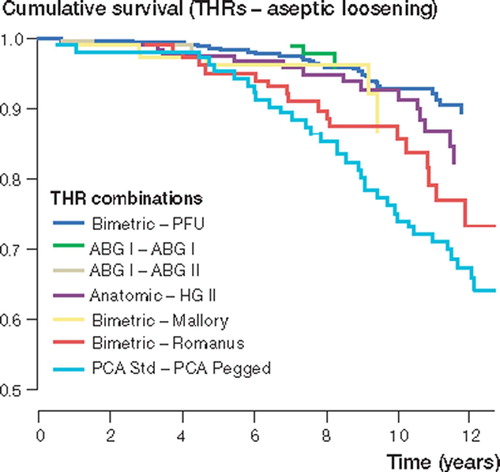
When endpoint was defined as any cup and/or stem revision, the survival rates of most brands declined markedly. Only the Anatomic Mesh/HG II prosthesis showed over 80% survival rate at 10 years. With any revision as endpoint in the Cox model, the Bi-Metric/Romanus prosthesis was found to have a 2.2-fold (CI 1.5–3.1; p < 0.001) increased risk of revision as compared to the reference brand (the Bimetric/Universal prosthesis). The ABG I/ABG II combination showed lower risk of revision (RR 0.3, CI 0.1–1.0; p =; 0.04) than the reference brand.
Discussion
We found that modern second-generation uncemented stems, with proximal circumferential porous- or HA-coating, provide young OA patients with good long-term survival rates. Similarly, the press-fit porous- or HA-coated cups studied, except the PCA Pegged cup, showed relatively low rates of revision due to aseptic loosening. It seems that when adequate primary stability is achieved with uncemented THA designs, good resistance to aseptic loosening can be expected. However, polyethylene wear, liner problems and periprosthetic osteolysis are still the main problems with modern uncemented acetabular components (Harris Citation2003).
Validity of the data
We acknowledge that the current register-based study has certain limitations. We were not, for example, able to report any subjective outcome measurements, e.g. Harris Hip Score or disease-specific quality of life measurements. Moreover, in register-based analyses with thousands of patients, it is not possible to conduct radiographic analyses. Also, where young patients are concerned, a register-based study may have diagnostic pitfalls in that a small proportion of the patients diagnosed with primary osteoarthritis may in fact have mild developmental dysplasia (DDH) (Harris 1986). It has been reported that patients with DDH of the hip may have poorer outcome of THA than other patient groups (Furnes et al. Citation2001). Follow-up of several implant designs in our material is still rather short; however, we believe it is important to report short-term results of hip implants in order to avoid the widespread use of failed designs. Register-based studies, however, provide valuable insight into the use of the THA procedure in a certain patient group, as the number of arthroplasties studied is significantly greater in register-based studies than in clinical studies from single centers. Furthermore, the results can be compared with those of other Nordic arthroplasty registers, which gives a broad overview of the results for both single implants and the methods used in THA (Havelin et al. Citation2000, Malchau et al. Citation2002). The statistical methods used in our study were valid, as we applied not only Kaplan-Meier survival analysis but also Cox multiple regression analysis to take account of confounding factors. The importance of considering confounding factors in the survival analysis of hip implants has been shown previously (Furnes et al. Citation2001). When studying the results of THAs, we should evaluate studies based on registers and also studies reported by single centers, as they complement each other.
In our study, the most frequently used components were implanted 8 to 15 times more often than the most infrequently used endoprostheses. It is notable that this may cause some bias in the results; e.g. rarely used designs may only have been implanted in one center, and if the surgical technique of the center has been suboptimal, the results for a particular implant will not reflect its true merit. As Biomet's components were so commonly used, the benefits and pitfalls of Biomet endoprostheses may be overemphasized in this study. Another form of bias in register-based studies is definition of failure: the only endpoint for failure one can evaluate is revision operation; thus, silent osteolysis, excessive wear, or clinically poor performance of an implant may remain unnoticed. Ideally, introduction of new implants and materials should always be stepwise and controlled: beginning with a randomized controlled pilot study, followed by a prospective multicenter trial (Kärrholm Citation2003).
When survival of hip implants is being evaluated, it is important to analyze and report both the independent survival rates of femoral and acetabular components, and the survivorship of the whole prosthesis. Similarly, both aseptic loosening and all revisions should serve as endpoints separately. For example, in this study the PCA total hip replacement (the PCA Std stem and the PCA Pegged cup) showed very poor performance with a 60% survival rate at 13 years. When the results were analyzed in a more precise way, it emerged that the femoral component of this implant could compete with the modern second-generation uncemented stems, but the acetabular component appeared to be a true failure. Another example is the Biomet Bi-Metric – Universal prosthesis; if we look at the 13-year survival rate of the Bi-Metric – Mallory THA (74%) in this study, it appears that this uncemented THA has an unacceptably poor performance. However, more precise analysis revealed that the Bi-Metric stem itself has a very good survival rate. On the other hand, the Universal cup had a satisfactory survival rate of 93% at 10 years with aseptic loosening as endpoint, but other revisions (including exchange of the polyethylene liner especially) markedly impaired its results. This is in accordance with the previous report of Puolakka et al. (Citation1999), based on the (non-age-dependent) material of the same register.
All proximally porous-coated uncemented stems studied showed good (< 90%) 10-year survival rates when either aseptic loosening or any stem revision was used as endpoint. Furthermore, the Biomet Bi-Metric stem showed an excellent 15-year survival rate. Good results have been reported previously with the Bi-Metric stem (Jacobsen et al. Citation2003, Meding et al. Citation2004) and with the Profile Porous stem (Kim et al. Citation2003b) from single centers; these results have now been confirmed by this nationwide study. Archibeck et al. (Citation2001) recently reported on 78 hips (in 74 patients with a mean age of 52 years at the time of the arthroplasty) treated with the Anatomic Mesh uncemented stem and found a 100% survival rate at 10 years. In our material, however, survival of the Anatomic femoral component was clearly poorer than previously reported by Archibeck; in addition, it was significantly worse than that of the Bi-Metric stem when either aseptic loosening or any stem revision was used as endpoint. The reason for this difference remains unclear. Thanner et al. reported an 84% 10-year survival rate for the PCA Std stem in patients with a mean age of 50 years (Thanner et al. Citation1999). In another series, Bojescul et al. reported a 93% survival rate for the PCA Std stem at 15 years (Bojescul et al. Citation2003). In our study, performance of the PCA Std stem was comparable to those previous reports, as well as to that of the reference stem brand (the Biomet Bi-Metric).
Keisu et al. (Citation2001) reviewed the results of 114 consecutive THAs with the Lord Madréporique femoral component, followed for at least 10 years, and found a 94% survival rate at 13 years. In another series with 107 hips, 10-year survival rate for the Lord Madréporique stem was 98% using revision as endpoint, but the combined clinical and radiographic 10-year survival rate declined to 81% (Malchau et al. Citation1996). In our study, the extendedly porous-coated Lord Madréporique stem showed a good 15-year survival rate of 91% with aseptic loosening as endpoint. Overall survival of the Lord Madréporique stem was significantly worse than that of the Bi-Metric stem, when aseptic loosening was defined as endpoint. With all revisions as endpoint, however, the difference disappeared. In our study, the Lord Madréporique stem was the only extendedly porous-coated uncemented stem. Thus, any conclusions about the possible differences in performance between the extendedly and proximally porous-coated stems in general cannot be drawn.
The only proximally HA-coated uncemented stem in our study, namely the ABG I stem, performed well with an excellent 10-year survival rate. In studies from single centers, good mid-term results have been reported with the ABG I stem (Tonino et al. Citation2000, Giannikas et al. Citation2002, Blacha Citation2004). It is unclear, however, whether HA coating improves osseointegration in the short term and resistance to aseptic loosening of porous-coated stems in the longer term. In their prospective randomized trial of 100 hips, Kim et al. (Citation2003a) recently compared porous-coated stems with and without additional HA coating; the authors concluded that after mid-term follow-up, a hydroxy-apatite coating on porous surfaces did not improve or diminish the results of total hip arthroplasty with the femoral component design used in their study. Park et al. (Citation2003) reported the results of 24 patients with bilateral arthroplasties, who received a porous-coated femoral component in one hip and an HA-coated femoral component in the other; with a minimum follow-up of 4 years, the authors found no differences between the groups—either in clinical or in radiographic results.
The CLS Spotorno stem, with a completely different concept from the other modern uncemented stem brands (the only grit-blasted macro-porous stem in the study), showed an excellent 10-year survival rate. This is in accordance with previous reports from single centers (Schramm et al. Citation2000, Aldinger et al. Citation2003). The double-wedge design of the CLS Spotorno stem seems to provide a good primary stability as well as osseointegration. Although the number of CLS stems implanted was rather small (108), these operations were performed in several hospitals and by several orthopedic surgeons. Thus, good results with the CLS Spotorno stem seem to be reproducible on a national level. The CLS Spotorno stem may offer a good alternative to modern porous-coated or HA-coated uncemented stems.
Of the press-fit porous-coated uncemented cups, both the Biomet Universal and the Harris-Galante II showed good endurance against aseptic loosening, with over 90% survival rates at 10 years. However, the 10-year survival rates of all press-fit porous-coated cup designs declined markedly when the endpoint was defined as any cup revision. This was caused by multiple revisions, performed mainly because of excessive wear of the polyethylene liner. The Harris-Galante II cup was the only cup design with a 10-year survival rate of more than 80%, when cup revision for any reason was defined as endpoint. For example, the 10-year survival rate of the Biomet Mallory cup declined from 88% to 61%. The Biomet Universal cup showed similar figures, with a major decline in survival rates: from 93% to 79% at 10 years.
The Biomet Universal cup has a metallic shell of titanium alloy with plasmas-prayed porous coating, multiple screw holes and a Hexloc-liner. The Biomet Mallory cup has a metallic shell of titanium alloy with plasma-sprayed porous coating, fins to provide initial stability, multiple screw holes and a Hexloc-liner. The Biomet Vision is the newest of the Biomet cups analyzed; it has a closed metallic shell of titanium alloy with plasma-sprayed porous coating (plugged screw holes) and a Ringloc-liner. In this study, the Biomet Vision cup showed excellent short-term results. These results did not, how-ever, differ from those of the older designs of press-fit porous-coated uncemented cups. Thus, longer follow-up is required to determine whether the Vision cup with a modern uncemented cup concept and a Ringloc liner produces less wear and osteolysis than Mallory and Universal cups. The cup/liner incongruity of the two-piece acetabular designs seems to be a common denominator in most brands (Barrack et al. Citation1997, Malchau et al. Citation1997, Puolakka et al. Citation2001b, Young et al. Citation2002, Blacha Citation2004, von Schewelov et al. Citation2004). This problem has been emphasized in our study due to the large proportion of Biomet cups. The critical problems of the Hexloc liner have been reported previously from the Finnish Arthroplasty Register (Puolakka et al. Citation1999). The Morscher Press-Fit uncemented cup with titanium fiber-mesh and monoblock design showed promising short-term results. Even so, longer follow-up is required to determine the true long-term performance of this implant.
The PCA Pegged acetabular component showed poor results in our study; 76% survival rate at 10 years and 64% survival rate at 15 years is unacceptably low. The poor resistance of this cup to aseptic loosening has been reported previously (Heekin et al. Citation1993, Malchau et al. Citation1997, Thanner et al. Citation1999). In general, failure of the PCA acetabular component has been reported to result from the combination of a poor polyethylene locking mechanism, polyethylene wear, acetabular osteolysis and migration (Astion et al. Citation1996, Malchau et al. Citation1997, Elfick et al. Citation1998).
Long-term results of press-fit HA-coated uncemented cups have not been reported to date. Good mid-term results have already been reported for the ABG I acetabular component (Tonino and Rahmy Citation2000, Giannikas et al. Citation2002, Oosterbos et al. Citation2004). Giannikas et al. (Citation2002) reported good medium-term results with the ABG hip, but polyethylene wear of the acetabular insert was noted with concern (Giannikas et al. Citation2002). In addition, alarming wear and periacetabular osteolysis has recently been reported with the ABG I cup (Duffy et al. Citation2004). In a recent study of 56 patients with a mean-age of 44 years, Blacha (Citation2004) reported poor results with the ABG I cup; the 9-year survival rates were 69% for the ABG I cup and 59% for the polyethylene liner. This is in accordance with our results; the 10-year survival rate of the ABG I cup was 95% with aseptic loosening as endpoint, but only 79% with any cup revision (including exchange of liner) as endpoint. The ABG II cup showed a 99% survival rate at 5 years; survival of the ABG II cup, however, was not any better than that of the ABG I cup over the first five years postoperatively. It must be remembered that implant survival may decline markedly after 7 to 8 years (Puolakka et al. Citation1999, Thanner Citation1999); thus, longer follow-up is still needed to determine how the HA-coated ABG II cup will perform in the long term.
Uncemented smooth-threaded cups, commonly used in Finland in the 1980s, were not analyzed in this study, as their poor results have already been well documented (Engh et al. Citation1990, Tallroth et al. Citation1993, Simank et al. Citation1997).
In conclusion, the outcome of cementless total hip arthroplasty depends on many factors, including component design, patient selection, and surgical technique. As survivorship in patients older than 70 years of age is so good—from 97% to 98% at 15 years—the concern lies with younger and more demanding patients. Particulate debris from polyethylene wear and resultant osteolysis remain the primary factors limiting the longevity of hip prostheses (Harris Citation1994, Harris Citation2003). Surgeons still debate the optimal cementless stem for primary THA in the young patient; in this large nationwide material, the proximally circumferentially porous or HA-coated stems performed better than the extendedly porous-coated Lord Madréporique stem. The concepts of proximal porous coating and HA coating showed good results in young patients in a recent study from the Finnish Arthroplasty Register (Eskelinen et al. Citation2005). The results of the present study suggest that the modern second-generation uncemented stems with proximal circumferential porous or HA coating seem to be a good choice for young osteoarthritic patients.
An uncemented cup needs adequate primary stability to gain osseointegration; modern uncemented cups appear to achieve this goal. However, the cup/liner incongruity and back-side wear problems must be resolved—or the possible benefits of porous-coated modular cup designs will be lost (Engh et al. Citation1997, McAuley et al. Citation2004). Modern press-fit porous-coated and HA-coated cups seem to have good endurance against aseptic loosening in young patients. However, polyethylene wear and its sequelae remain matters of concern in this active group of patients. Highly cross-linked polyethylene and optional surface bearings, such as ceramic and metal-on-metal articulations, may reduce wear and improve the results of uncemented cups. Long-term results are still needed to determine whether they actually provide a solution to the wear problem.
This study was supported by the Research Foundation of Orion Corporation, Ortopedian ja traumatologian tutki-mussäätiö (the Foundation for Orthopedic and Traumato-logic Research in Finland), the Instrumentarium Research Foundation, the Duodecim Foundation, and the Pär Slätis Foundation for Joint Surgery. The authors thank all ortho-pedic surgeons in Finland for their assistance with the Arthroplasty Register.
No conflict of interests declared.
Author contributions
AE: study design, data analysis and writing.VR and IH: study design and writing. PPu: statistical supervisor of the Finnish Arthroplasty Register: data analysis. JN: supervisor of the Finnish Arthroplasty Register: writing. PPa: head of the Research Group: study design and writing.
- Aldinger P R, Thomsen M, Mau H, Ewerbeck V, Breusch S J. Cementless Spotorno tapered titanium stems: excellent 10-15-year survival in 141 young patients. Acta Orthop Scand, 2003; 74: 253–8
- Archibeck M J, Berger R A, Jacobs J J, Quigley L R, Gite-lis S, Rosenberg A G, Galante J O. Second-generation cementless total hip arthroplasty. J Bone Joint Surg (Am), 2001; 83: 1666–73, Eight to eleven-year results
- Astion D J, Saluan P, Stulberg B N, Rimnac C M, Li S. The porous-coated anatomic total hip prosthesis: failure of the metal-backed acetabular component. J Bone Joint Surg (Am), 1996; 78: 755–66
- Barrack R L, Folgueras A, Munn B, Tvetden D, Sharkey P. Pelvic lysis and polyethylene wear at 5–8 years in an uncemented total hip. Clin Orthop, 1997; 335: 211–7
- Blacha J. High osteolysis and revision rate with the hydroxy-apatite-coated ABG hip prostheses. Acta Orthop Scand, 2004; 75: 276–82
- Bojescul J A, Xenos J S, Callaghan J J, Savory C G. Results of porous-coated anatomic total hip arthroplasty without cement at fifteen years; a concise follow-up of a previous report. J Bone Joint Surg (Am), 2003; 85: 1079–83
- Capello W N, D'Antonio J A, Feinberg J R, Manley M T. Ten-year results with hydroxyapatite-coated total hip femoral components in patients less than fifty years old. J Bone Joint Surg (Am), 2003; 85: 885–9, A concise follow-up of a previous report
- Cox D R. Regression models and life tables. J Roy Stat Soc, 1972; 34: 187–220
- Dorey F J. Survivorship analysis of surgical treatment of the hip in young patients. Clin Orthop, 2004; 418 23–8
- Duffy P, Sher J L, Partington P F. Premature wear and oste-olysis in an HA-coated, uncemented total hip arthroplasty. J Bone Joint Surg (Br), 2004; 86: 34–8
- Elfick A P, Hall R M, Pinder I M. Wear in retrieved acetabular components: effect of femoral head radius and patient parameters. J Arthroplasty, 1998; 13: 291–5
- Engh C A, Griffin W L, Marx C L. Cementless acetabular components. J Bone Joint Surg (Br) 1990; 72: 53–9
- Engh C A, Jr, Culpepper W J, 2nd, Engh C A. Long-term results of use of the anatomic medullary locking prosthe-sis in total hip arthroplasty. J Bone Joint Surg (Am) 1997; 79: 177–84
- Eskelinen A, Remes V, Helenius I, Pulkkinen P, Nevalainen J, Paavolainen P. Total hip arthroplasty for primary osteoarthritis in young patients in the Finnish arthroplasty register. Acta Orthop 2005; 76: 28–41, 4661 primary replacements followed for 0–22 years
- Furnes O, Lie S A, Espehaug B, Vollset S E, Engesæter L B, Havelin L I. Hip disease and the prognosis of total hip replacements. J Bone Joint Surg (Br) 2001; 83: 579–86, A review of 53 698 primary total hip replacements reported to the Norweigan arthroplasty register 1987–99
- Giannikas K A, Din R, Sadiq S, Dunningham T H. Medium-term results of the ABG total hip arthroplasty in young patients. J Arthroplasty 2002; 17: 184–8
- Harris W H. Osteolysis and particle disease in hip replace-ment. Acta Orthop Scand 1994; 65: 113–23, A review
- Harris W H. Results of uncemented cups: a critical appraisal at 15 years. Clin Orthop 2003; 417: 121–5
- Havelin L I, Espehaug B, Vollset S E, Engesaeter L B. Early aseptic loosening of uncemented femoral components in primary total hip replacement: A review based on the Norwegian Arthroplasty Register. J Bone Joint Surg (Br) 1995a; 77: 11–7
- Havelin L I, Vollset S E, Engesæter L B. Revision for aseptic loosening of uncemented cups in 4,352 primary total hip prostheses. Acta Orthop Scand 1995b; 66: 494–500
- Havelin L I, Engesæter L B, Espehaug B, Furnes O, Stein A L, Vollset S E. The Norweigan arthroplasty register. Acta Orthop Scand 2000; 71: 337–53, 11 years and 73,000 arthroplasties
- Heekin R D, Callaghan J J, Hopkinson W J, Savory C G, Xenos J S. The porous-coated anatomic total hip prosthe-sis, inserted without cement. J Bone Joint Surg (Am) 1993; 75: 77–91, Results after five to seven years in a prospective study
- Herberts P, Malchau H. Long-term registration has improved the quality of hip replacement. Acta Orthop Scand 2000; 71: 111–21, A review of the Swedish THR register comparing 160,000 cases
- Jacobsen S, Jensen F K, Poulsen K, Sturup J, Retpen J B. Good performance of a titanium femoral component in cementless hip arthroplasty in younger patients. Acta Orthop Scand 2003; 74: 375–9, 97 arthroplasties followed for 5–11 years
- Kaplan E L, Meier P. Nonparametric estimation from incom-plete observations. J Am Stat Assoc 1958; 53: 457–81
- Keisu K S, Mathiesen E B, Lindgren J U. The uncemented fully textured Lord hip prosthesis: a 10- to 15-year fol-lowup study. Clin Orthop 2001; 382: 133–42
- Kim Y H, Kook H K, Kim J S. Total hip replacement with a cementless acetabular component and a cemented femo-ral component in patients younger than fifty years of age. J Bone Joint Surg (Am) 2002; 84: 770–4
- Kim Y H, Kim J S, Oh S H, Kim J M. Comparison of porous-coated titanium femoral stems with and without hydroxy-apatite coating. J Bone J Surg (Am) 2003a; 85: 1682–8
- Kim Y H, Oh S H, Kim J S. Primary total hip arthroplasty with a second generation cementless total hip prosthesis in patients younger than fifty years of age. J Bone Joint Surg (Am) 2003b; 85: 109–14
- Kärrholm J. Retrospective evaluation of implants – is there a need. Acta Orthop Scand 2003b; 74: 1–3
- Lilliefors H. On the Kolmogoroff-Sminroff test for normal-ity with mean and variance unknown. J Am Stat Assoc 1967;, 62: 399–402
- Malchau H, Herberts P, Wang Y X, Kärrholm J, Romanus B. Long-term clinical and radiological results of the Lord total hip prosthesis. J Bone Joint Surg (Br) 1996; 78: 884–91, A prospective study
- Malchau H, Wang Y X, Kärrholm J, Herberts P. Scandinavian multicenter porous coated anatomic total hip arthroplasty study. J Arthroplasty 1997; 12: 133–48, Clinical and radiographic results with 7- to 10-year follow-up evaluation
- Malchau H, Herberts P, Eisler T, Garellick G, Söderman P. The Swedish total hip replacement register. J Bone Joint Surg (Am) (Suppl 2) 2002;; 84: 2–20
- McAuley J P, Szuszczewicz E S, Young A, Engh C A Sr. Total hip arthroplasty in patients 50 years and younger. Clin Orthop 2004; 418: 119–25
- McLaughlin J R, Lee K R. Total hip arthroplasty in young patients. 8- to 13-year results using an uncemented stem. Clin Orthop 2000; 373: 153–63
- Meding J B, Keating E M, Ritter M A, Faris P M, Berend M E. Minimum ten-year follow-up of a straight-stemmed, plasma-sprayed, titanium-alloy, uncemented femoral component in primary total hip arthroplasty. J Bone Joint Surg (Am) 2004; 86: 92–7
- Niinimaki T, Puranen J, Jalovaara P. Total hip arthroplasty using isoelastic femoral stems. J Bone Joint Surg (Br) 1994; 76: 413–8
- Oosterbos C J, Rahmy A I, Tonino A J, Witpeerd W. High survival rate of hydroxyapatite-coated hip prostheses: 100 consecutive hips followed for 10 years. Acta Orthop Scand 2004; 75: 127–33
- Park Y S, Lee J Y, Yun S H, Jung M W, Oh I. Comparison of hydroxyapatite- and porous-coated stems in total hip replacement. Acta Orthop Scand 2003; 74: 259–63
- Puolakka T J, Pajamaki K J, Pulkkinen P O, Nevalainen J K. Poor survival of cementless Biomet total hip: a report on 1,047 hips from the Finnish Arthroplasty Register. Acta Orthop Scand 1999; 70: 425–9
- Puolakka T J, Pajamäki K J, Halonen P J, Pulkkinen P O, Paavolainen P, Nevalainen J K. The. Finnish arthroplasty register. Acta Orthop Scand 2001a; 72: 433–41, Report of hip register
- Puolakka T J, Laine H J, Moilanen T P, Koivisto A M, Paja-maki K J. Alarming wear of the first-generation poly-ethylene liner of the cementless porous-coated Biomet Universal cup: 107 hips followed for mean 6 years. Acta Orthop Scand 2001b; 72: 1–7
- Schramm M, Keck F, Hohmann D, Pitto R P. Total hip arthroplasty using an uncemented femoral component with taper design: outcome at 10-year follow-up. Arch Orthop Trauma Surg 2000; 120: 407–12
- Simank H G, Brocai D R, Reiser D, Thomsen M, Sabo D, Lukoschek M. Middle-term results of threaded acetabular cups. J Bone Joint Surg (Br) 1997; 79: 366–70, High failure rates five years after surgery
- Tallroth K, Slatis P, Ylinen P, Paavolainen P, Paavilainen T. Loosening of threaded acetabular components. J Arthroplasty 1993; 8: 581–4, Radio-graphic manifestations
- Thanner J, Kärrholm J, Herberts P, Malchau H. Poor out-come of the PCA and Harris-Galante hip prostheses. Acta Orthop Scand 1999; 70: 155–62, Ran-domized study of 171 arthroplasties with 9-year follow-up
- Tonino A J, Rahmy A I. The hydroxyapatite-ABG hip system: 5- to 7-year results from an international mul-ticentre study. J Arthroplasty 2000; 15: 274–82, The International ABG Study Group
- Young A M, Sychterz C J, Hopper R H Jr, Engh C A. Effect of acetabular modularity on polyethylene wear and osteolysis in total hip arthroplasty. J Bone Joint Surg (Am) 2002; 84: 58–63
- von Schewelov T, Sanzén L, Önsten I, Carlsson Å. Catastrophic failure of an uncemented acetabular component due to high wear and osteolysis. Acta Orthop Scand 2004; 75: 283–94, An analysis of 154 Omni-fit prostheses with mean 6-year follow-up