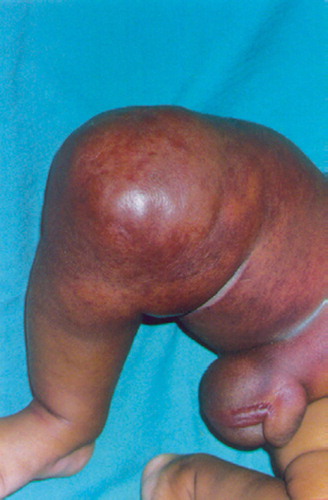
A 2-month-old child had had a right thigh swelling since birth. He presented with a sudden enormous increase in the swelling, erythema, fever and penoscrotal edema for 1 week ().
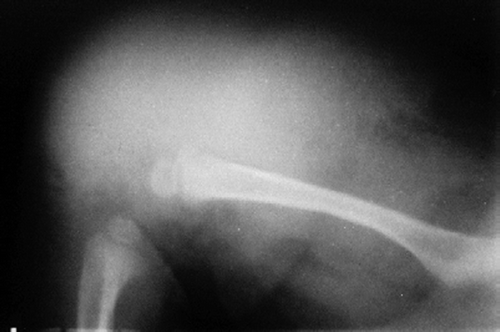
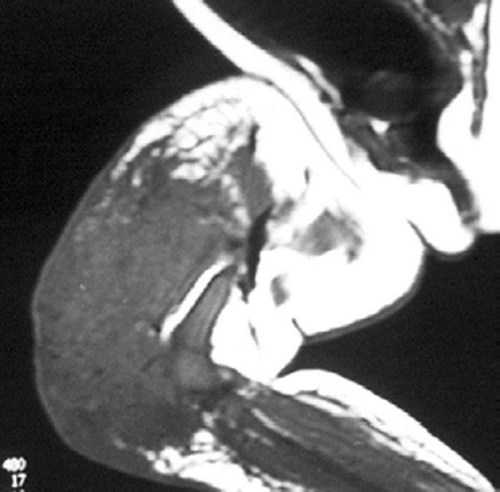
There was a high degree of suspicion of a rapidly progressing malignant tumor. The swelling was warm and indurated with moderate tenderness. He had good knee motion. Distal neurovascular status was normal. Hematological investigations were normal. Plain radiographs revealed a diffuse soft tissue shadow around the lower and middle third of the femur without any bony involvement or periosteal reaction; they were thus inconclusive (). Ultrasonographic examination showed few hypoechoic shadows with hypervascularity. MRI was inconclusive, showing circumferential mass in the quadriceps (lateral more than medial) and lateral hamstrings with homogenous density ().
Figure 3. MRI showing circumferential mass in quadriceps and lateral hamstrings with homogenous density.
The differential diagnosis therefore pointed towards subacute osteomyelitis, Ewing's sarcoma, eosinophilic granuloma, fibrosarcoma and other rare tumors. The child underwent a diagnostic through-cut biopsy. There was profuse bleeding during the procedure. Intraoperative findings revealed a fatty vascular tissue surrounding almost the entire girth of the lower right thigh. The histopathology was reported as benign juvenile intramuscular hemangioma. Angiographic embolization by cyanoacrylate glue was planned, but the child was clinically unfit at that time due to eosinophilia.
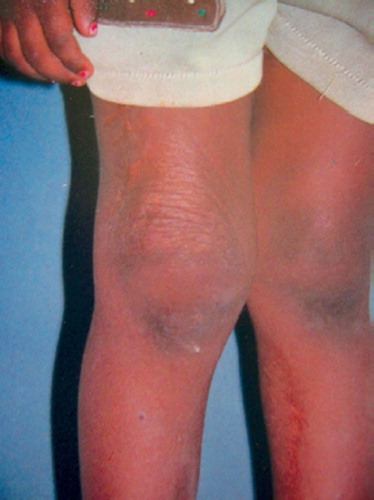
The child returned after 3 months with partial regression of the swelling. It was thus decided to observe the lesion. 80% regression had occurred by 6 months, with complete regression at 1 year. At subsequent follow-ups after 3 and 4 years, no recurrence was seen ().
Discussion
Hemangiomas are benign vascular tumors, characterized by a rapid proliferative phase (0–1 years) followed by spontaneous involution (1–5 years) with continued improvement for up to 12 years (involuted phase) (Hasan et al. Citation2001). They appear at birth, or immediately after birth. Girls are affected three times as frequently as boys. The superficial lesions arising from skin and subcutaneous tissues present as painless red masses with an occasional distinctive bluish tinge. The deep lesions from fascia and muscles seldom have any physical signs and clinically present with intermittent, persistent discomfort. Few present with a palpable mass, but typically the overlying skin is not discolored. Intramuscular hemangiomas are uncommon, accounting for 0.8% of all benign vascular tumors (Cohen et al. Citation1983). They occur most often in young children and usually involve the lower extremities, especially the thigh. Shortly after birth some grow rapidly, out of proportion to the growth of the child and often at a very alarming rate.
It has been well documented that hemangiomas undergo a period of involution, during which they spontaneously become smaller and lighter in color. This process of involution may progress at different rates (Hasan et al. Citation2001). Accordingly, they are divided into two groups. “Fast involuters”, where involution occurs during the first 6 years of life, have favorable outcome with complete involution in 60% of cases. Hemangiomas requiring longer than 6 years, “slow involuters”, need corrective surgery in 80% of cases (Fin et al. 1983, Waner Citation1996). Some hemangiomas disappear completely and others undergo only a partial involution. The histological change during this process is the spontaneous regression of the newly formed vessels. Mast cells and mitochondrial cytochrome b gene expression has been documented to be involved in an accelerated regression of hemangioma (Hasan et al. Citation2001, Tan et al. Citation2004). Hemangiomas do not undergo malignant transformation, but occasionally they may permeate through all tissue barriers in an aggressive fashion (O'Donnell et al. Citation2001).
MRI with contrast defines the extent of the swelling and its relationship with adjacent muscles and other soft tissues (Murphey et al. Citation1995, Waner and Suen Citation1999). It is noteworthy that in our case, MRI completely failed to provide the diagnosis. No vascular channels were identifiable on the scan. This can be explained by the fact that bleeding had already occurred within the tumor—as witnessed by the sudden increase in its size. In most cases, ultrasonographic examination delineates the diagnosis accurately and also excludes a vascular malformation. Digital subtraction angiography also accurately identifies the pattern and the extent of the hemangiomatous neovasculature.
Historically, most surgeons advocate the “watchful waiting” approach, as all hemangiomas undergo partial or complete involution. This approach has recently been challenged, as even completely involuted hemangiomas can produce significant anatomical abnormalities because of atrophic skin changes, telangiectasias and fibrofatty tissue deposition (Waner et al. Citation1992). Approximately 60% of all hemangiomas require some form of surgical correction despite involution (Waner Citation1996, Hochman et al. Citation1999). Superficial lesions are usually amenable to surgical excision. Wide excision does not always lead to complete cure, and is often unjustified due to excessive morbidity. Recurrence rates following surgery can be as high as 18% (Demir et al. Citation2004). Injection with corticosteroids (systemic or local), sclerosing agents and angiographic embolization of the feeder vessels are the new modalities of treatment, which also include cryosurgery. Combined methods of embolization and cryodestruction may reduce the recurrence rate (Shafranov et al. Citation1998). Graded injections of interferon α-2a have shown a very high rate of rapid regression with the least amount of morbidity. However, potential side effects including spastic diplegia have restricted its use (Beth et al. Citation1999, John et al. 1999).
In our case, the hemangioma underwent a rapid involution phase. Watchful observation can be recommended for the lesions that do not pose lifethreatening complications or impair function.
Author contributions
KLD data collection, manuscript preparation, literature review. RM data collection, literature review. MGY guidance for manuscript preparation
- Allen P W, Enzinger F M. Hemangioma of skeletal muscle: an analysis of 89 cases. Cancer 1972; 29: 8–22
- Beth A D, Nancy B E, Illona J F. Hemangiomas in children. N Engl J Med 1999; 341: 173–181
- Cohen A J, Youkey J R, Clagett G P, Huggins M, Nadalo L, d'Avis J C. Intramuscular hemangioma. JAMA 1983; 249: 2680–2
- Demir Z, Oktem F, Celebioglu S. Rare case of intramasseteric cavernous hemangioma in a three-year-old boy: a diagnostic dilemma. Ann Otol Rhinol Laryngol 2004; 113(6)455–8
- Finn M C, Glowacki J, Mulliken J B. Congenital vascular lesions: clinical application of a new classification. J Pediatr Surg 1983; 18: 894–900
- Greinwald J H, Jr, Burke D K, Bonthius D J, Bauman N M, Smith R J. An update on treatment of hemangiomas in children with interferon Alfa-2a. Arch Otolaryngo Head Neck Surg 1999; 125: 21–7
- Hasan Q, Tan S T, Gush J, Davis P F. Altered mitochondrialcytochrome b gene expression during the regression of hemangioma. Plast Reconstr Surg 2001; 108(6)1471–6
- Hochman M, Vural E, Suen J, Waner M. Contemporary management of vascular lesions of the head and neck. Curr Opin Otolaryngol Head Neck Surg 1999; 7: 161
- Murphey M D, Fairbairn K J, Parman L M, Baxter K G, Parsa M B, Smith W S. Musc uloskeletal angiomatous lesions: radiologic-pathologic correlation. Radiographics 1995; 15: 893–917
- O'Donnell T M P, Devitt A T, Kutty S, Fogarty E E. Recurrent congenital hemipericytoma in a child. J Bone Joint Surg (Br) 2001; 83: 269–72
- Shafranov V V, Butorina A N, Borkhunova E N. Treatment of hemangiomas in children by cryomethod. Proceedings 10th World Congress of Cryosurgery, MoscowRussia. Department Russian State University, 1998
- Tan S, Wallis R A, He Y, Davis P. Mast cells and hemangioma. Plast Reconstr Surg 2004; 113(3)999–1011
- Waner M. The treatment of vascular lesions. Facial Plast Surg Clin North Am 1996; 4: 275–81
- Waner M, Suen J. Treatment options for the management of hemangiomas: Hemangiomas and vascular malformations of the head and neck. M Waner. Wiley-Liss, New York 1999; 239–41
- Waner M, Suen J Y, Dinehart S. Treatment of hemangiomas of the head and neck. Laryngoscope 1992; 102: 1123–32